Abstract
Purpose
Clopidogrel is a prodrug that requires transformation into an active metabolite by cytochrome P450 (CYP) in the liver in order to irreversibly inhibit the P2Y12 adenosine diphosphate platelet receptor. CYP2C19 polymorphism has been reported to correlate with reduced antiplatelet activity of clopidogrel in coronary artery disease. We assessed the association between CYP2C19 polymorphism and clopidogrel resistance in patients with cerebrovascular disease.
Materials and Methods
We retrospectively gathered data from patients who experienced cerebrovascular disease, received clopidogrel, and were tested for clopidogrel resistance and CYP2C19 polymorphism. Clopidogrel resistance was tested by the VerifyNow P2Y12 system, and the CYP2C19 polymorphism was tested by the Seeplex CYP2C19 ACE Genotyping system. Clopidogrel resistance was expressed in P2Y12 reaction units (PRU) and percent inhibition. High PRU and low percent inhibition suggests clopidogrel resistance. CYP2C19 polymorphisms were expressed as extensive, intermediate, and poor metabolizers. Clopidogrel resistance was assessed according to the subgroup of CYP2C19 polymorphism.
Results
A total of 166 patients were evaluated. The PRU values of extensive CYP2C19 metabolizers (195.0±84.9) were significantly lower than those of intermediate and poor metabolizers (237.9±88.0, 302.2±58.9). The percent inhibition of extensive metabolizers (44.6±21.8) was significantly higher than that of intermediate and poor metabolizers (30.5±21.5, 14.0±13.4).
Conclusion
Intermediate and poor metabolizing CYP2C19 polymorphism is associated with reduced clopidogrel antiplatelet activity in patients with cerebrovascular disease. The clinical implications of this finding require further investigation.
Keywords: Clopidogrel, cytochrome P450, cerebrovascular disease
INTRODUCTION
Clopidogrel is widely used for secondary prevention of ischemic cerebrovascular disease. However, a substantial number of patients experience cerebrovascular or cardiovascular attack, even with the use of clopidogrel. Several studies have reported that reduced clopidogrel response ranges from 4.8-50%.1,2 The potential mechanisms of such insufficient responses to clopidogrel are affected by several factors, including variable absorption of the prodrug, clearance of the active metabolite, potential drug-drug interactions,3 the ability of thrombin to bypass complete adenosine diphosphate (ADP) inhibition,4,5 ADP-mediated P2Y12 platelet receptor variability, genetic polymorphisms of platelet receptors, and differences in platelet signal transduction pathways. Clopidogrel is a prodrug that requires transformation into an active metabolite by cytochrome P450 (CYP) in the liver in order to irreversibly inhibit P2Y12 ADP platelet receptor. This transformation is a complex process, involving a number of CYP isoenzymes (CYP1A2, CYP2B6, CYP2C9, CYP3A4, and CYP2C19) in varying degrees.6 The CYP2C19 enzyme is known to have the most important role related to clopidogrel transformation. CYP2C19 polymorphism has been reported to correlate with clopidogrel resistance in patients with coronary angioplasty and drug-eluting stent implantation.7-10 Also, drugs such as calcium channel blockers (CCB), statins, and proton pump inhibitors which are metabolized by CYP enzymes, have been reported to competitively inhibit clopidogrel transformation, resulting in decreased clopidogrel antiplatelet activity.11-13
It is a clinical necessity to have a reliable assay to measure platelet function after clopidogrel therapy for monitoring and dose adjustment. The VerifyNow P2Y12 assay is a rapid platelet function cartridge-based assay designed to directly measure the effects of clopidogrel on the P2Y12 receptor. The novel VerifyNow P2Y12 assay was designed to overcome the limitations of the conventional optical platelet aggregation test.14 The results are expressed as P2Y12 reaction units (PRU) and percent inhibition. Although there is no consented cutoff value for either PRU or percent inhibition, low percent inhibition and high PRU are regarded as clopidogrel resistance. It has been reported that percent inhibitions below 15% and PRUs over 213 correlate with clopidogrel resistance.15 Other studies have defined percent inhibition under 20% and PRU over 240 as clopidogrel resistance.7,16 The VerifyNow P2Y12 assay is superior to traditional light transmission aggregometry because it is fast and correlates strongly with light transmission aggregometry, with an absence of limitations, such as weak reproducibility and operator-dependency.
In this study, we investigated the association between CYP2C19 polymorphism and clopidogrel resistance, as measured by the VerifyNow P2Y12 assay in patients with ischemic cerebrovascular disease. In addition, we studied the effects of CCBs on clopidogrel's antiplatelet activity.
MATERIALS AND METHODS
Subjects
We retrospectively reviewed the cases of patients with cerebrovascular disease who received clopidogrel for the prevention of ischemic stroke from January 2009 to June 2010. We selected patients who were tested for clopidogrel resistance and CYP2C19 polymorphism. We required that the patients be given clopidogrel for at least six days before the clopidogrel resistance test in order to be enrolled in this study. In all patients, CYP2C19 polymorphism testing was done after informed consent was obtained according to the National Bioethics Committee regulation. A total of 166 patients who had previously experienced cerebrovascular disease and received clopidogrel were enrolled. The patients had either cerebral infarction or intra/extracranial artery stenosis.
Clopidogrel resistance and CYP2C19 Genotyping
All patients received 75 mg/day clopidogrel for at least six days before clopidogrel resistance testing. Clopidogrel resistance was tested by the VerifyNow P2Y12 assay (Accumetrics, San Diego, CA, USA). The VerifyNow P2Y12 assay is a whole blood, light transmission-based optical detection assay that measures platelet aggregation in a cartridge containing fibrinogen-coated beads. The assay was performed according to the manufacturer's directions within 10 to 15 minutes of venous sampling. The results are expressed as percent inhibition and PRU. Percent inhibition was calculated as [1-(PRU/baseline PRU)]×100. The PRU indicates the amount of ADP P2Y12 platelet receptor mediated platelet aggregation and is inversely related to antiplatelet function. If the PRU is high, then percent inhibition and antiplatelet activity is decreased.
The CYP2C19 polymorphism was tested by the Seeplex CYP2C19 ACE Genotyping system (Seegene, Seoul, Korea). The Seeplex CYP2C19 ACE Genotyping system is a simple, innovative dual priming oligonucleotide primer-based multiplex polymerase chain reaction system having maximal specificity and sensitivity for detecting two single nucleotide polymorphisms (CYP2C19*2, CYP2C19*3 allele). The results are expressed as extensive (*1/*1 allele), intermediate (*1/*2, *1/*3) or poor (*2/*2, *2/*3, *3/*3) metabolizers.
To evaluate the correlation between CYP2C19 polymorphism and clopidogrel resistance, patients were grouped according to their CYP2C19 genotypes. PRU and percent inhibition were compared between the groups. To evaluate the effects of dihydropyridine CCB co-administration on clopidogrel resistance, the patients were divided into two groups, with or without CCB.
Statistical analysis
Statistical analysis was performed with PASW Statistics 18 (IBM, Chicago, IL, USA). Continuous variables were reported as mean with standard deviation and categorical variables were reported as frequency and percentage. The Kolmogorov-Smirnov test was used to test for normal distribution. Since all PRU and percent inhibition values were normally distributed, the analysis of variance (ANOVA) test and post hoc analysis were used to detect differences between the CYP2C19 subgroups. The chi-square test and ANOVA were used to compare demographic variables between the CYP2C19 subgroups. The student's t-test was performed to compare percent inhibition and PRU between the groups with CCB and without CCB. Deviations from Hardy-Weinberg equilibrium were tested by chi-square test. A two-tailed value of p<0.05 was considered significant.
RESULTS
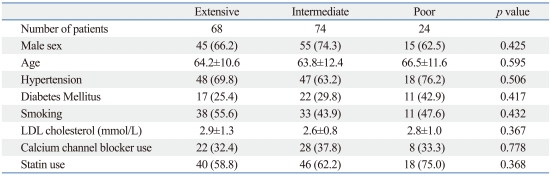
A total of 166 patients were enrolled in this study. Patients had either cerebral infarction (n=142) or intra/extracranial artery stenosis (n=24). Patient demographics, as well as clinical and laboratory findings, are shown in Table 1. The numbers of extensive, intermediate, and poor CYP2C19 metabolizers were 68 (40.9%), 74 (44.6%), and 24 (14.5%), respectively. There were no significant differences in the demographic, clinical and laboratory findings between the CYP2C19 subgroups. The numbers of patients with the CYP2C19 *1/*1, *1/*2, *1/*3, *2/*2, *2/*3, and *3/*3 genotype were 68 (40.9%), 56 (33.7%), 18 (10.8%), 14 (8.4%), 9 (5.4%) and 1 (0.6%), respectively. The CYP2C19 genotype frequencies did not deviate significantly from Hardy-Weinberg equilibrium (p=0.707 for CYP2C19*2, p=0.795 for CYP2C19*3).
Table 1.
Patient Baseline Characteristics According to CYP2C19 Metabolizer Subgroup
LDL, low density lipoprotein.
Continuous data are shown as mean±SD. Categorical data are shown as number (%).
The results of the VerifyNow P2Y12 assay in the CYP2C19 subgroups are shown in Table 2. Percent inhibition was highest in the extensive metabolizers and decreased in the intermediate and poor metabolizers (44.6±21.8, 30.5±21.5, 14.0±13.4; p<0.001). Post hoc analysis using the Bonferroni test showed significant differences between all subgroups. PRU increased from extensive to poor metabolizers (195.0±84.9, 237.9±88.0, 302.2±58.9; p<0.001). Also, post hoc analysis showed significant differences between the subgroups.
Table 2.
Clopidogrel P2Y12 Reaction Unit and Percent Inhibition of CYP2C19 Metabolizer Subgroups
PRU, P2Y12 reaction unit.
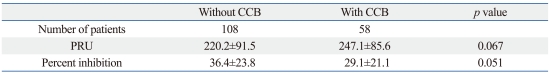
There was a tendency toward higher PRU and lower percent inhibition in patients administered a CCB than in those without CCB administration (PRU 247.1±85.6 vs. 220.2±91.5, p=0.067; percent inhibition 29.1±21.1 vs. 36.4±23.8, p=0.051) (Table 3). In the extensive metabolizer group, patients administered a CCB had higher PRU and lower percent inhibition values than patients without CCB administration (PRU 236.6±85.2 vs. 175.1±78.1, p=0.007; percent inhibition 34.4±20.5 vs. 49.4±21.0, p=0.004).
Table 3.
Clopidogrel P2Y12 Reaction Unit and Percent Inhibition in Patients with and without Calcium Channel Blocker Administration
CCB, calcium channel blocker; PRU, P2Y12 reaction unit.
DISCUSSION
This study showed that individuals with poor and intermediate CYP2C19 metabolizers exhibited significantly decreased clopidogrel antiplatelet activity than those with extensive metabolizers. We used the VerifyNow P2Y12 assay to assess the antiplatelet activity of clopidogrel, and our results were comparable with a previous study using the same assay in coronary artery disease patients.7,17 Although we did not prove that CYP2C19 polymorphism had any clinical significance in stroke recurrence, previous reports have suggested a high risk of cardiovascular events in poor and intermediate CYP2C19 metabolizer groups in coronary artery disease patients.8-10
There was also a significant difference in the PRU and percent inhibition values between the poor and intermediate metabolizers of CYP2C19. Previous studies dichotomized the results of CYP2C19 polymorphisms as carriers (poor and intermediate metabolizers) and non-carriers (extensive metabolizers) and analyzed differences between carriers and non-carriers.8,10 This method may have been used as a strategy to deal with the small percentage (2.6-3%) of poor metabolizers in their study populations. Considering the higher frequency of poor metabolizers in the Asian population, as compared to the Caucasian and Black African populations, different antiplatelet activities between intermediate and poor metabolizers should be confirmed in clinical outcome studies in the Asian population.18
We found a possible role of CCBs in the inhibition of clopidogrel antiplatelet activity. Dihydropyridine CCBs are known to be metabolized by hepatic CYP3A4 and to act as competitive inhibitors of clopidogrel metabolism via CYP3A4. Studies have reported clopidogrel resistance in patients treated with CCBs.11,19 Our results showed a non-significant trend of clopidogrel resistance in patients treated with CCBs and when we analyzed the effects of CCBs in the extensive metabolizer group, CCBs were clearly related to clopidogrel resistance. We speculate that the effect of CYP2C19 polymorphism on clopidogrel resistance was large enough to mask the CCB's contribution to clopidogrel resistance in the intermediate and poor metabolizer groups. However, the small number of extensive metabolizer group with CCBs merit careful interpretation of the effects of CCBs on clopidogrel resistance. Hypertension is a major risk factor for coronary artery and cerebrovascular disease. CCBs and clopidogrel are commonly co-administered for secondary prevention. Therefore, the effects of CCBs on clopidogrel resistance should be confirmed by a large prospective clinical study.
This study is limited by the absence of clinical outcome measurements. We showed only the correlation of CYP2C19 polymorphism genotyping and the VerifyNow P2Y12 assay in cerebrovascular disease, which has already been demonstrated in coronary artery disease. Considering the different mechanisms of ischemic stroke from coronary artery disease and the high risk of coronary stent thrombosis in previous studies of clopidogrel resistance, stroke recurrence in ischemic stroke patient with clopidogrel resistance may have different results. In addition, we did not analyze CYP2C19*17, which is known to be an ultra-metabolizer phenotype. It is possible that the presence of CYP2C19*17 in our intermediate metabolizer group and in such cases may yield unexpected results on the VerifyNow P2Y12 assay. However, the frequency of CYP2C19*17 has been reported to be very low in Asian population.20
Our results showed a good correlation between CYP2C19 polymorphism genotyping and the VerifyNow P2Y12 assay. Both diagnostic methods may play complimentary roles in defining clopidogrel resistance in clinical practice. Further prospective studies to prove the effects of clopidogrel resistance on clinical outcomes and the efficacy of various methods of overcoming clopidogrel resistance, such as increased clopidogrel dose or other antiplatelet agents that are independent of CYP2C19 metabolism, are needed.
ACKNOWLEDGEMENTS
This work was supported by a Faculty Research Grant from Yonsei University College of Medicine (6-2009-0172).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Järemo P, Lindahl TL, Fransson SG, Richter A. Individual variations of platelet inhibition after loading doses of clopidogrel. J Intern Med. 2002;252:233–238. doi: 10.1046/j.1365-2796.2002.01027.x. [DOI] [PubMed] [Google Scholar]
- 2.Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol. 2005;45:246–251. doi: 10.1016/j.jacc.2004.09.067. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen TA, Diodati JG, Pharand C. Resistance to clopidogrel: a review of the evidence. J Am Coll Cardiol. 2005;45:1157–1164. doi: 10.1016/j.jacc.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 4.Klages B, Brandt U, Simon MI, Schultz G, Offermanns S. Activation of G12/G13 results in shape change and Rho/Rho-kinase-mediated myosin light chain phosphorylation in mouse platelets. J Cell Biol. 1999;144:745–754. doi: 10.1083/jcb.144.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohlmann P, Eckly A, Freund M, Cazenave JP, Offermanns S, Gachet C. ADP induces partial platelet aggregation without shape change and potentiates collagen-induced aggregation in the absence of Galphaq. Blood. 2000;96:2134–2139. [PubMed] [Google Scholar]
- 6.Kazui M, Nishiya Y, Ishizuka T, Hagihara K, Farid NA, Okazaki O, et al. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos. 2010;38:92–99. doi: 10.1124/dmd.109.029132. [DOI] [PubMed] [Google Scholar]
- 7.Lee JM, Park S, Shin DJ, Choi D, Shim CY, Ko YG, et al. Relation of genetic polymorphisms in the cytochrome P450 gene with clopidogrel resistance after drug-eluting stent implantation in Koreans. Am J Cardiol. 2009;104:46–51. doi: 10.1016/j.amjcard.2009.02.045. [DOI] [PubMed] [Google Scholar]
- 8.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 9.Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Méneveau N, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363–375. doi: 10.1056/NEJMoa0808227. [DOI] [PubMed] [Google Scholar]
- 10.Collet JP, Hulot JS, Pena A, Villard E, Esteve JB, Silvain J, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet. 2009;373:309–317. doi: 10.1016/S0140-6736(08)61845-0. [DOI] [PubMed] [Google Scholar]
- 11.Gremmel T, Steiner S, Seidinger D, Koppensteiner R, Panzer S, Kopp CW. Calcium-channel blockers decrease clopidogrel-mediated platelet inhibition. Heart. 2010;96:186–189. doi: 10.1136/hrt.2009.171488. [DOI] [PubMed] [Google Scholar]
- 12.Norgard NB, Mathews KD, Wall GC. Drug-drug interaction between clopidogrel and the proton pump inhibitors. Ann Pharmacother. 2009;43:1266–1274. doi: 10.1345/aph.1M051. [DOI] [PubMed] [Google Scholar]
- 13.Neuvonen PJ, Niemi M, Backman JT. Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther. 2006;80:565–581. doi: 10.1016/j.clpt.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Malinin A, Pokov A, Spergling M, Defranco A, Schwartz K, Schwartz D, et al. Monitoring platelet inhibition after clopidogrel with the VerifyNow-P2Y12(R) rapid analyzer: the VERIfy Thrombosis risk ASsessment (VERITAS) study. Thromb Res. 2007;119:277–284. doi: 10.1016/j.thromres.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 15.Godino C, Mendolicchio L, Figini F, Latib A, Sharp AS, Cosgrave J, et al. Comparison of VerifyNow-P2Y12 test and Flow Cytometry for monitoring individual platelet response to clopidogrel. What is the cut-off value for identifying patients who are low responders to clopidogrel therapy? Thromb J. 2009;7:4. doi: 10.1186/1477-9560-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patti G, Nusca A, Mangiacapra F, Gatto L, D'Ambrosio A, Di Sciascio G. Point-of-care measurement of clopidogrel responsiveness predicts clinical outcome in patients undergoing percutaneous coronary intervention results of the ARMYDA-PRO (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty-Platelet Reactivity Predicts Outcome) study. J Am Coll Cardiol. 2008;52:1128–1133. doi: 10.1016/j.jacc.2008.06.038. [DOI] [PubMed] [Google Scholar]
- 17.Kim IS, Choi BR, Jeong YH, Kwak CH, Kim S. The CYP2C19*2 and CYP2C19*3 polymorphisms are associated with high post-treatment platelet reactivity in Asian patients with acute coronary syndrome. J Thromb Haemost. 2009;7:897–899. doi: 10.1111/j.1538-7836.2009.03319.x. [DOI] [PubMed] [Google Scholar]
- 18.Desta Z, Zhao X, Shin JG, Flockhart DA. Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin Pharmacokinet. 2002;41:913–958. doi: 10.2165/00003088-200241120-00002. [DOI] [PubMed] [Google Scholar]
- 19.Siller-Matula JM, Lang I, Christ G, Jilma B. Calcium-channel blockers reduce the antiplatelet effect of clopidogrel. J Am Coll Cardiol. 2008;52:1557–1563. doi: 10.1016/j.jacc.2008.07.055. [DOI] [PubMed] [Google Scholar]
- 20.Kim KA, Song WK, Kim KR, Park JY. Assessment of CYP2C19 genetic polymorphisms in a Korean population using a simultaneous multiplex pyrosequencing method to simultaneously detect the CYP2C19*2, CYP2C19*3, and CYP2C191*7 alleles. J Clin Pharm Ther. 2010;35:697–703. doi: 10.1111/j.1365-2710.2009.01069.x. [DOI] [PubMed] [Google Scholar]