Abstract
Purpose
In this study, we analyzed a cohort of children with chronic graft-versus-host disease (GvHD) according to the NIH consensus classification (NCC) in order to observe whether global assessment at diagnosis correlates with GvHD-specific endpoints. We then studied the clinical course of these patients, specifically with regards to episodes of GvHD exacerbation requiring treatment escalation.
Materials and Methods
Recipients of either allogeneic bone marrow transplantation (BMT) or peripheral blood stem cell transplantation (PBSCT) from January 2006 to August 2008 at the Department of Pediatrics, The Catholic University of Korea were evaluated for chronic GvHD, which was diagnosed according to the NCC. The course of chronic GvHD in these patients was then followed.
Results
Of 59 evaluable patients, 23 developed chronic GvHD for a cumulative incidence of 39.3%. Upon multivariate analysis, previous acute GvHD (≥grade II) had a significant impact on chronic GvHD incidence. With a median duration of systemic treatment for chronic GvHD of 501 days, no significant relationship was found between initial global severity of chronic GvHD and either duration of immunosuppressive treatment or final clinical response to treatment. Fifteen patients (65%) experienced at least one episode of chronic GvHD exacerbation during the period of follow-up, with a median of four exacerbations in the subgroup of patients who experienced such events. Lung GvHD resulted in the highest number of exacerbations per diagnosed patient, followed by oral GvHD.
Conclusion
Analysis of this small cohort indicates that global assessment as proposed by the NCC may have limited correlations with GvHD-specific endpoints, possibly due to the favorable response of children to treatment.
Keywords: Chronic GvHD, NIH consensus criteria, children
INTRODUCTION
The multitude of manifestations of chronic graft-versus-host disease (GvHD) has made its accurate diagnosis and classification a difficult task. The initial classification of chronic GvHD into limited and extensive forms1 was subsequently revised into a scheme that further elaborated on the types of organ involvement that characterize these subtypes.2 In 2005, the NIH reported a consensus criteria (NCC) for regithe diagnosis and staging of chronic GvHD, taking into account novel manifestations of chronic GvHD in the setting of post-transplantation immunomodulation,3 and a key element of this NCC diagnosis and staging is a global severity rating of chronic GvHD based on the extent of each organ's involvement. Since the announcement of this most recent diagnostic scheme, several authors have reported on a retrospective reclassification of chronic GvHD utilizing the NCC, based mostly on the adult hematopoietic stem cell transplantation (HSCT) population, with conflicting results on the prognostic utility of the NCC.4-6 Published reports on the application of the NCC in the pediatric HSCT population are lacking, yet the need for a standard and valid diagnostic scheme is urgent, considering the importance of accurate reporting of chronic GvHD severity at diagnosis and throughout treatment.
In this study, our objectives were twofold: first, we retrospectively reclassified chronic GvHD according to NCC in a cohort of pediatric recipients of either allogeneic bone marrow (BMT) or G-CSF mobilized peripheral blood stem cell transplantation (PBSCT) to observe the prognostic potential of the NCC, especially with regards to the global severity rating. Specifically, we assessed whether global severity at diagnosis correlates with GvHD-specific endpoints such as duration of systemic immunosuppressive treatment (IST), and overall clinical response to IST at the last follow-up. Correlation between global severity at diagnosis and GvHD-specific endpoints was shown in one adult-based study.5 In contrast to these findings, our hypothesis was that initial severity as measured by the NCC has limited prognostic value with regards to these GvHD-specific endpoints in children. Second, we attempted to characterize in detail the clinical course of chronic GvHD diagnosed in our cohort of pediatric patients, especially GvHD aggravation during the period of systemic IST.
MATERIALS AND METHODS
Chronic GvHD cohort
Consecutive pediatric recipients of either allogeneic BMT or PBSCT from January 2006 to August 2008 at the Department of Pediatrics, The Catholic University of Korea, Seoul, Korea were evaluated for chronic GvHD. Only patients who survived the initial 100 days after transplantation were included in this evaluation. In order to create a unified cohort for the study of chronic GvHD, recipients of additional stem cells, in the form of donor lymphocyte infusion or booster infusion, were excluded, as were recipients of G-CSF mobilized bone marrow. The study design received approval from the institutional review board of Seoul Saint Mary's Hospital, The Catholic University of Korea.
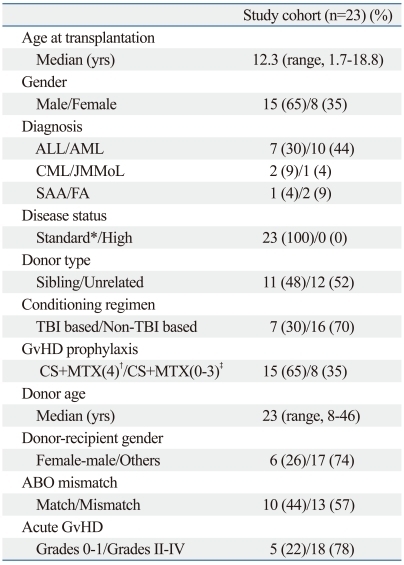
Overall, among the 59 patients included in the initial evaluation, chronic GvHD as specified by the NCC was diagnosed in 23 patients at a mean duration of 6.7 months (range: 1.8-23.8 months) after HSCT for a four-year cumulative incidence of 39.3%. The median duration of follow-up from diagnosis of chronic GvHD was 26.1 months (range: 8.1-45.6 months). The pertinent clinical characteristics of this chronic GvHD cohort are shown in Table 1.
Table 1.
Chronic GvHD Cohort
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CML, chronic myeloid leukemia; JMMoL, juvenile myelomonocytic leukemia; SAA, severe aplastic anemia; FA, Fanconi anemia; GvHD, graft-versus-host disease.
*standard risk: AML in CR1, ALL in≤CR2, CML in CP.
†cyclosporine+4 scheduled doses of mini-methotrexate.
‡cyclosporine alone, or less than 4 scheduled doses of mini-methotrexate.
Transplantation procedure and acute GvHD
Donor and recipient pairs were fully matched by HLA-A, B and DR at the molecular level, and both related and unrelated transplantations were included in the study cohort. The patients received either TBI-based conditioning regimens (total body irradiation, cytarabine, cyclophosphamide) or non-TBI-based regimens (busulfan, cyclophosphamide; busulfan, etoposide, cyclophosphamide, anti-thymocyte globulin (ATG); busulfan, fludarabine±ATG; fludarabine, cyclophosphamide, ATG). GvHD prophylaxis included either cyclosporine with 4 scheduled doses of mini-dose methotrexate or cyclosporine alone. Administration of min-dose methotrexate was withheld with the development of mucositis requiring analgesia.
The severity and extent of acute GvHD were graded according to previous consensus criteria.7 With regards to the temporal pattern of acute GvHD onset, delayed onset acute GvHD (after day 100) as characterized in the NCC was recorded in an attempt to fully classify chronic GvHD into the classic chronic GvHD and overlap syndrome subtypes.
Diagnosis and classification of chronic GvHD
Diagnosis
Retrospective diagnosis of chronic GvHD was based strictly on the NCC. For the diagnosis of chronic GvHD in organs that could prove more problematic, lung GvHD was diagnosed without a biopsy if the patient complained of symptoms such as cough and dyspnea, the radiologic findings on the chest computed tomography were consistent with air trapping without overt areas of consolidation, and the parameters on the pulmonary function test indicated an obstructive pattern of lung disease. Overall, in the diagnosis of chronic lung GvHD, efforts were made to differentiate bronchiolitis obliterans syndrome from bronchiolitis obliterans organizing pneumonia.8
Diagnostic criteria for chronic GvHD of the GI tract were limited to esophageal webs or strictures of the esophagus, and other more common symptoms such as vomiting and diarrhea did not qualify for a diagnosis of chronic GvHD.
Also, as outlined in the NCC, hyperbilirubinemia or elevation of AST, ALT or other liver parameters alone were not sufficient for the diagnosis of chronic GvHD.
Severity of chronic GvHD
After diagnosis of chronic GvHD, organ-specific scoring and severity assignment as "mild", "moderate", and "severe", as outlined in the NCC, were done for each patient.
For strict classification of chronic GvHD, additional rules were implemented for the scoring of GI tract GvHD and hepatic GvHD. In light of the NCC's classification of a delayed type of acute GvHD, and the potential to misdiagnose persistent acute GvHD as chronic GvHD,9 patients lacking diagnostic GI criteria and complaining of common GI symptoms such as vomiting, diarrhea and weight loss were not scored as having chronic GI GvHD unless a biopsy specimen proved otherwise.
Scoring of hepatic GvHD may also lend itself to inaccuracy as the NCC provides neither diagnostic nor distinctive criteria for chronic liver GvHD, and scoring of liver involvement is based on elevation of serum bilirubin or liver enzymes, the etiology of which may be multifactorial.10 A transient elevation of liver enzymes of greater than five times the upper normal limit is sufficient for a score of 3 and hence, classification as severe chronic GvHD in a patient, even if other physical findings that are more diagnostic for chronic GvHD are of mild severity. Yet, hepatic involvement may be common enough to have a significant impact on the severity rating of many of these patients. A liver biopsy may aid in diagnosing chronic liver GvHD with greater certainty, but such a biopsy was not undertaken in the cohort of patients. In order to resolve this problem, severity rating was done for each patient in two ways, both including and without the organ score for hepatic involvement.
Chronic GvHD study endpoints
Two endpoints were used in this study to decide upon the final outcome of chronic GvHD: duration of systemic IST, and overall clinical status of chronic GvHD at last follow-up.
The duration of systemic IST for chronic GvHD, defined from the date of chronic GvHD diagnosis to the end of systemic IST, was calculated for each patient. Then, the NCC global severity rating at chronic GvHD diagnosis was evaluated as a potential prognostic factor for predicting discontinuation of systemic IST. Attempts were made to taper and stop systemic IST within 18 months of initiation if the patient showed resolution of signs and symptoms.
The duration of systemic IST may not reflect the actual status of chronic GvHD at the last follow-up as treatment may continue despite clinical improvement of chronic GvHD signs and symptoms. For a more direct indication of chronic GvHD status, each patient's chronic GvHD at the last follow-up was classified as follows: 'resolved', indicating eradication of all signs and symptoms of chronic GvHD with systemic IST stopped; 'controlled', indicating improved condition with continued or tapered IST; and 'persistent', representing continued signs and symptoms of chronic GvHD, or aggravation at last follow-up despite treatment. Patient death that was directly related to chronic GvHD was also recorded. Again, the NCC global severity rating at chronic GvHD diagnosis was assessed as a prognostic factor for these definitions of chronic GvHD status.
Course of systemic IST for chronic GvHD
The number of organs involved and the actual sites of chronic GvHD for each patient during the course of systemic IST were recorded. Medications administered as systemic IST, first-line therapy or otherwise, were also summarized.
An important event during the course of chronic GvHD treatment is an increase in the dose of systemic IST, the addition of other immunosuppressive agents to those given previously, or the reinstitution of systemic IST after previous withdrawal due either to aggravation of chronic GvHD signs and symptoms or lengthy persistence without waning despite treatment. Such 'exacerbation' of chronic GvHD requiring strengthened systemic IST have been reported to show significant associations with survival endpoints.11,12 The number of such exacerbations, and the organs in which these exacerbations occurred were analyzed in each patient.
Statistical analysis
The following factors were analyzed for possibly contributingto chronic GvHD incidence using a Cox proportional hazards regression model: age of patient and donor, gender, underlying disease, donor type, cell source, donor-recipient sex mismatch, HLA-C mismatch, ABO mismatch, conditioning regimen, GvHD prophylaxis, and preceding acute GvHD of grade II or above. Risk factors for chronic GvHD incidence with a p value of <0.1 on univariate study were selected for multivariate analysis. Overall incidence of chronic GvHD and probability of systemic IST withdrawal were calculated using a cumulative incidence function with death as a competing risk event. Comparisons of the probability of systemic IST withdrawal according to the initial chronic GvHD severity were done using Gray's test. Correlations between initial chronic GvHD severity and clinical response to IST at the last follow-up were done using Fisher's exact test. The significance level was set at p<0.05. Statistical analysis was done on R package, version 2.10.1 (available at http://CRAN.R-project.org).
RESULTS
Chronic GvHD risk factors
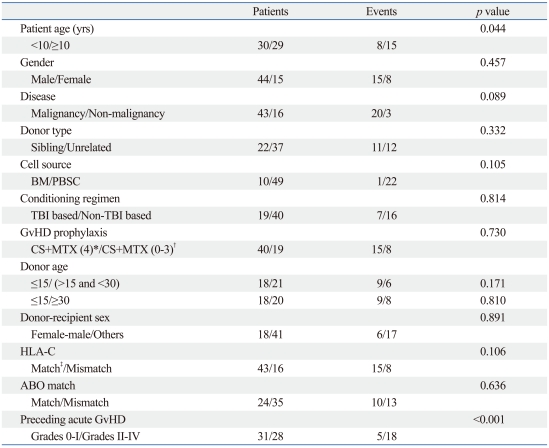
Important risk factors for chronic GvHD development on univariate study included previous acute GvHD of grade II or above (p<0.001), patient age (p=0.044), and underlying disease (p=0.089) (Table 2). However, upon multivariate analysis, only preceding acute GvHD of grade II or above proved to have a significant impact on chronic GvHD incidence (HR 5.79, 95% CI 2.06-16.24, p=0.001).
Table 2.
Univariate Study of Risk Factors for Chronic GvHD Incidence
GvHD, graft-versus-host disease; BM, bone marrow; PBSC, peripheral blood stem cell; TBI, total body irradiation; CS, cyclosporine; MTX, methotrexate.
*cyclosporine+4 scheduled doses of mini-methotrexate.
†cyclosporine alone, or less than 4 scheduled doses of mini-methotrexate.
‡Match of HLA-C at the allele level.
Diagnosis and classification of chronic GvHD
Diagnosis
The most common organ for diagnosis of chronic GvHD was the oral cavity (N=15, 65%), followed by the skin (N=3, 13%), lungs (N=3, 13%) and eyes (N=2, 9%). At diagnosis, one patient showed signs consistent with overlap syndrome (diagnosis of ocular GvHD combined with persistent acute skin GvHD), while the others were classified as classic chronic GvHD.
Global severity at diagnosis
Initial global severity was evaluated in all patients both with and without consideration of liver GvHD. With a strict application of NCC and eliminating potential liver involvement unconfirmed by biopsy, summation of initial global severity resulted in 14 patients with mild (61%), six patients with moderate (26%) and three patients with severe chronic GvHD (13%). Inclusion of liver function abnormalities at diagnosis as a manifestation of chronic GvHD led to five patients with mild chronic GvHD being reclassified as having moderate chronic GvHD, resulting in nine patients with mild (39%), 11 with moderate (48%) and three with severe chronic GvHD (13%).
Global severity at diagnosis and chronic GvHD prognostic parameters
Duration of systemic IST
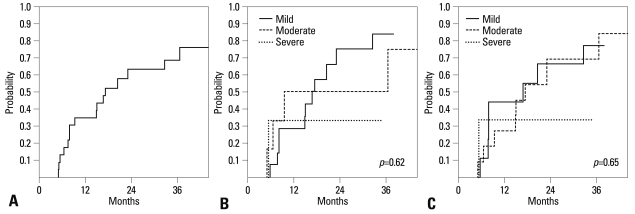
The median duration of systemic IST for the cohort was 501 days (range: 151-1,368). Sixteen of the 23 patients (70%) had systemic IST stopped at last follow-up for a probability of systemic IST withdrawal at three years of 68.7% (Fig. 1A). No significant relationship was found between initial global severity of chronic GvHD and duration of systemic IST (p=0.617) (Fig. 1B). Similarly, a second assessment of initial global severity that included possible hepatic GvHD did not alter the lack of association between initial GvHD severity and duration of systemic IST (p=0.647) (Fig. 1C).
Fig. 1.
(A) Probability of withdrawal of systemic IST. (B) Probability of withdrawal of systemic IST according to initial global severity of chronic GvHD. (C)Probability of withdrawal of systemic IST according to initial global severity of chronic GvHD (with inclusion of hepatic GvHD). GvHD, graft-versus-host disease.
Response to IST at last follow-up
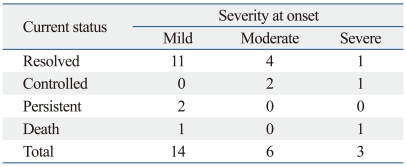
At the last follow-up, 16 patients (70%) showed resolution of chronic GvHD with discontinuation of systemic IST, and three patients (13%) had control of GvHD signs and symptoms with continued or tapered systemic IST. Only two patients (9%) had persistent chronic GvHD at last follow-up, due to skin and oral GvHD respectively, while GvHD-related mortality occurred in another two patients (9%).
No significant correlation was noted between initial severity at onset of chronic GvHD (without consideration of possible hepatic GvHD) and response to IST at the last follow-up (p=0.098) (Table 3). A similar lack of correlation was also found between the initial severity of chronic GvHD including assessment of hepatic GvHD, and final response to IST (p=0.277).
Table 3.
Response to Systemic IST at Last Follow-up According to NCC Global Severity at Onset
IST, immunosuppressive treatment.
Clinical course of chronic GvHD
Overall organ involvement
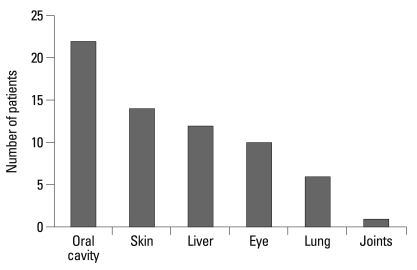
After initial diagnosis, the median number of organs involved with chronic GvHD for each patient throughout the period of follow-up was three (range: 1-6). Oral GvHD was the most common manifestation (n=22), followed by skin (n=14), liver (n=12), eye (n=10), lung (n=6), and joints (n=1) (Fig. 2).
Fig. 2.
Chronic GvHD organ involvement throughout the follow-up period. GvHD, graft-versus-host disease.
Treatment of chronic GvHD
Systemic IST included steroids±calcineurin inhibitor in 22 patients (96%). Sixteen patients were treated with steroids and cyclosporine, five were given steroids only, and one patient received steroids and cyclosporine alternate with tacrolimus. Only one patient (4%) was treated with a second-line immunosuppressive agent for chronic GvHD: steroids and cyclosporine combined with mycophenolate mofetil.
Chronic GvHD exacerbation
Overall, 57 episodes of chronic GvHD exacerbation requiring strengthened systemic IST occurred throughout the follow-up period. Of these 57 episodes, 41 (72%) were treated by increasing the dosage of the previously administered IST, four (7%) were treated by adding another immunosuppressive agent to the previous regimen, 11 (19%) were treated by reinitiating systemic IST that had been stopped, and one (2%) was treated by both increasing the dosage of the previous IST and adding a second medication to the regimen.
Fifteen patients (65%) experienced at least one episode of chronic GvHD exacerbation during the period of follow-up. In these patients, the median number of chronic GvHD exacerbations was four episodes (range: 1-10). With regards to the organ in which chronic GvHD exacerbation occurred, the oral cavity was the most common organ of exacerbation with 35 episodes, followed by the lung with 13 episodes, the skin with nine episodes, and the eye with three. However, when this crude number of total exacerbation episodes per organ was adjusted for the number of patients who experienced chronic GvHD in that specific organ, lung GvHD showed the highest frequency of exacerbation, with 2.2 exacerbations per patient, followed by oral GvHD with 1.6.
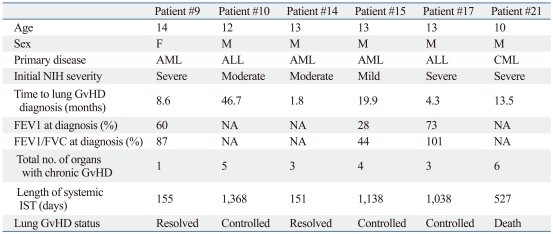
Clinical course of patients with chronic lung GvHD
The course of lung GvHD, which resulted in the highest number of exacerbations per patient, was analyzed as a subset (Table 4). All six patients with lung GvHD were 10 years old or older at the time of HSCT, had underlying malignancy, were recipients of PBSCT, and had experienced prior acute GvHD. At the last follow-up, two patients had resolution of lung GvHD (withdrawal of systemic IST), three patients had control of lung GvHD, and one patient, who had chronic GvHD involvement in five other organs, died. Systemic IST was comprised of steroids+cyclosporine in five patients, while one patient also received mycophenolate mofetil for 10 months. Other supportive systemic agents included macrolide antibiotics as anti-inflammatory agents, xanthine derivatives, and β2-agonists, while inhaled steroids and bronchodilators were also administered.
Table 4.
Clinical Course of Patients with Lung GvHD*
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CML, chronic myeloid leukemia; FEV1, forced expiratory volume in 1s; FVC, forced vital capacity; NA, not available; IST, immunosuppressive treatment; GvHD, graft-versus-host disease.
*Mean follow-up duration since diagnosis of lung GvHD: 23.0 months (range: 6.2-36.3).
DISCUSSION
Although undertaken with a small cohort of patients, this study has significance because it adds to the limited body of research on the application of the NCC to children with minichronic GvHD, as well as outlining in detail the clinical course and response to systemic IST in children, especially with regards to GvHD exacerbation requiring IST incrementation.
With regards to risk factors for chronic GvHD incidence, only antecedent acute GvHD of grade II or above proved to have a significant impact on multivariate analysis. Previous studies have shown that variables such as older patient age and malignant disease also act as significant risk factors,13,14 and our results emphasize the pivotal role of previous acute GvHD in subsequent chronic GvHD incidence. Also, upon diagnosis of chronic GvHD, overlap syndrome as proposed by the NCC was observed only in one patient, in contrast to studies including adult patients in which this GvHD entity accounted for a much greater proportion of post-day 100 GvHD.4,5
One important classification proposed by the NCC is a global assessment of GvHD based on the severity of involvement in each organ. Our study shows that such a global assessment at initial diagnosis of chronic GvHD has prognostic correlations with neither duration of systemic IST, which is a commonly used endpoint in the study of chronic GvHD, nor with the overall response to systemic IST at the end of follow-up, a parameter that was defined specifically in this study. In the pediatric HSCT population, the prognostic utility of the boundaries of global assessment may be blurred by the more favorable outcome of chronic GvHD in children as compared to adults, which was confirmed in our study in which 83% of patients had either resolution of chronic GvHD with discontinuation of systemic IST, or improved symptoms with continued systemic IST. In all but one patient, treatment consisted of first line IST (steroid±calcineurin inhibitor), further emphasizing the relatively positive response to treatment of chronic GvHD in this cohort of children.
The median duration of systemic IST in our cohort was 501 days. Two previous studies on a cohort that included adult HSCT recipients reported median systemic IST durations of 272 days15 and 23 months,16 but an accurate comparison with our study may not be possible due to the different lengths of follow-up after diagnosis of chronic GvHD.
Notably, 65% of patients experienced at least one exacerbation of chronic GvHD necessitating systemic IST escalation, with a median of four exacerbations in the subgroup of patients with such an event. Considering that a maximum of three such "flare-ups" per patient were documented in one adult patient-based study,11 the number of exacerbations per child is considerable and may represent attempts to minimize steroid administration. Consistent with its poor prognosis, lung GvHD resulted in the largest number of such exacerbations when adjusted for the number of patients with site-specific involvement. However, in our cohort, despite the high frequency of exacerbations, all but one patient with lung GvHD had either resolved or controlled symptoms at the last follow-up, with first line IST as the mainstay of treatment.
After lung GvHD, oral GvHD resulted in the most exacerbations per diagnosed patient. This result is perhaps not surprising considering that the oral cavity was responsible for the greatest number of initial chronic GvHD diagnoses, as well as being the most commonly involved organ in overall chronic GvHD. However, the frequency with which oral GvHD was encountered also emphasizes the need to minimize systemic IST escalation as a response to GvHD aggravation, possibly through local therapeutic measures such as topical steroids and calcineurin inhibitors.17
Although more than 80% of the chronic GvHD cohort had either resolution or control of symptoms at the last follow-up, complications resulting from systemic IST remain an important issue. Twelve patients (52%) were diagnosed with either osteopenia or osteoporosis on post-transplantation bone densitometry. Other endocrine problems such as growth hormone deficiency and gonadal deficiency were also diagnosed. Long term evaluation of such problems remains a critical part of chronic GvHD care, especially in the pediatric setting.
One significant limitation of our data is the diagnostic uncertainty of chronic hepatic GvHD due to lack of biopsy corroboration. However, the NCC does not outline criteria for the diagnosis of chronic GvHD through hepatic involvement alone, and a diagnosis requires distinction from acute GvHD. Hence, evaluation of global severity was done both with and without incorporation of possible chronic hepatic GvHD for a strict application of the NCC. Diagnosis of lung GvHD also proved problematic, as none of our patients deemed to have pulmonary involvement had undergone lung biopsy.
Another important limitation of our study was the small number of children that formed the chronic GvHD cohort; inclusion of more patients may have led to greater statistical significance than can be currently extrapolated. The NCC should, therefore, be given further evaluation in a larger cohort of children with chronic GvHD.
In conclusion, our evaluation of the NCC found the global assessment to have limited prognostic correlation with valid chronic GvHD endpoints such as duration of systemic IST, possibly due to the comparably favorable clinical course of pediatric chronic GvHD. Although the majority of patients in the cohort had chronic GvHD controlled at last follow-up, multiple exacerbations were frequent in the course of treatment. Trials of localized therapy, especially for oral GvHD, are warranted as a first-line response to symptom aggravation in order to minimize treatment-related complications.
Footnotes
A minor and non-updated part of this document was presented in poster form at the 36th Annual Meeting of the European Group for Blood and Marrow Transplantation (EBMT), March 21st - 24th, 2010, Vienna, Austria.
The authors have no financial conflicts of interest.
References
- 1.Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 2.Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9:215–233. doi: 10.1053/bbmt.2003.50026. [DOI] [PubMed] [Google Scholar]
- 3.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Jagasia M, Giglia J, Chinratanalab W, Dixon S, Chen H, Frangoul H, et al. Incidence and outcome of chronic graft-versus-host disease using National Institutes of Health consensus criteria. Biol Blood Marrow Transplant. 2007;13:1207–1215. doi: 10.1016/j.bbmt.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Cho BS, Min CK, Eom KS, Kim YJ, Kim HJ, Lee S, et al. Feasibility of NIH consensus criteria for chronic graft-versus-host disease. Leukemia. 2009;23:78–84. doi: 10.1038/leu.2008.276. [DOI] [PubMed] [Google Scholar]
- 6.Vigorito AC, Campregher PV, Storer BE, Carpenter PA, Moravec CK, Kiem HP, et al. Evaluation of NIH consensus criteria for classification of late acute and chronic GVHD. Blood. 2009;114:702–708. doi: 10.1182/blood-2009-03-208983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 8.Yoshihara S, Yanik G, Cooke KR, Mineishi S. Bronchiolitis obliterans syndrome (BOS), bronchiolitis obliterans organizing pneumonia (BOOP), and other late-onset noninfectious pulmonary complications following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:749–759. doi: 10.1016/j.bbmt.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Akpek G, Chinratanalab W, Lee LA, Torbenson M, Hallick JP, Anders V, et al. Gastrointestinal involvement in chronic graft-versus-host disease: a clinicopathologic study. Biol Blood Marrow Transplant. 2003;9:46–51. doi: 10.1053/bbmt.2003.49999. [DOI] [PubMed] [Google Scholar]
- 10.Tomas JF, Pinilla I, Garcia-Buey ML, Garcia A, Figuera A, Gomez-Garcia de Soria VGG, et al. Long-term liver dysfunction after allogeneic bone marrow transplantation: clinical features and course in 61 patients. Bone Marrow Transplant. 2000;26:649–655. doi: 10.1038/sj.bmt.1702532. [DOI] [PubMed] [Google Scholar]
- 11.Kim DH, Sohn SK, Baek JH, Lee KH, Lee JH, Choi SJ, et al. Time to first flare-up episode of GVHD can stratify patients according to their prognosis during clinical course of progressive- or quiescent-type chronic GVHD. Bone Marrow Transplant. 2007;40:779–784. doi: 10.1038/sj.bmt.1705806. [DOI] [PubMed] [Google Scholar]
- 12.Flowers ME, Storer B, Carpenter P, Rezvani AR, Vigorito AC, Campregher PV, et al. Treatment change as a predictor of outcome among patients with classic chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2008;14:1380–1384. doi: 10.1016/j.bbmt.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zecca M, Prete A, Rondelli R, Lanino E, Balduzzi A, Messina C, et al. Chronic graft-versus-host disease in children: incidence, risk factors, and impact on outcome. Blood. 2002;100:1192–1200. doi: 10.1182/blood-2001-11-0059. [DOI] [PubMed] [Google Scholar]
- 14.Kondo M, Kojima S, Horibe K, Kato K, Matsuyama T. Risk factors for chronic graft-versus-host disease after allogeneic stem cell transplantation in children. Bone Marrow Transplant. 2001;27:727–730. doi: 10.1038/sj.bmt.1702868. [DOI] [PubMed] [Google Scholar]
- 15.Lee JH, Lee JH, Choi SJ, Kim S, Seol M, Lee YS, et al. Graft-versus-host disease (GVHD)-specific survival and duration of systemic immunosuppressive treatment in patients who developed chronic GVHD following allogeneic haematopoietic cell transplantation. Br J Haematol. 2003;122:637–644. doi: 10.1046/j.1365-2141.2003.04472.x. [DOI] [PubMed] [Google Scholar]
- 16.Stewart BL, Storer B, Storek J, Deeg HJ, Storb R, Hansen JA, et al. Duration of immunosuppressive treatment for chronic graft-versus-host disease. Blood. 2004;104:3501–3506. doi: 10.1182/blood-2004-01-0200. [DOI] [PubMed] [Google Scholar]
- 17.Treister NS, Woo SB, O'Holleran EW, Lehmann LE, Parsons SK, Guinan EC. Oral chronic graft-versus-host disease in pediatric patients after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:721–731. doi: 10.1016/j.bbmt.2005.06.002. [DOI] [PubMed] [Google Scholar]