Abstract
We examine the effects of the artistic representation – here exemplified by Michelangelo's Expulsion from Paradise – of an action on the motor system. Using single and paired- pulse transcranial magnetic stimulation we analyze corticomotor excitability during observation of an action in the painting, during imagery of the painting, and during observation of a photograph of the same pose. We also analyze the effects of observation of two further paintings, one showing the same muscles at rest, and in the other in a more overtly emotional context. Both observation of the Expulsion and of imagery of the painting increased cortical excitability. Neither the relaxed pose of Michelangelo's Creation nor the flexed posture in the highly emotional context of Bellini's Dead Christ increased cortical excitability. Observation of a photograph of the same extended pose did not increase cortical excitability either. Moreover, intracortical inhibition was reduced during imagery of the painting. Our results offer clear motor correlates of the relationship between the esthetic quality of a work and the perception of implied movement within it.
Keywords: art, transcranial magnetic stimulation, mental imagery, mirror neurons, motor cortex
Introduction
Works of art arouse a variety of reactions in their beholders. Among the most frequently reported responses is that of a sense that beholders seem to have of imitating the actions of figures in paintings. Several philosophers of art, notably German empathy theorists such as Robert Vischer and Theodor Lipps and the French phenomenologist Maurice Merleau-Ponty, have suggested that viewers of paintings feel bodily engaged by the movements represented within them, but no empirical research has been done on this aspect of response (Vischer, 1872; Lipps, 1906; Merleau-Ponty, 1962).
A still photograph of an action conveys dynamic information. It has been suggested that observers extract dynamic information by extrapolating future position from the motion implied by the photograph (Allison et al., 2000). The processing of such information from static images with implied motion engages brain areas activated during observation of real actions and motor imagery including cortical visual motion area (V5/MT+), extrastriate body area (EBA), superior temporal sulcus (STS; BA38), and motion-related areas (Kourtzi and Kanwisher, 2000; Proverbio et al., 2009). Given the skill of 15th and 16th century artists in representing movement, we hypothesized that observation of an action by a painter such as Michelangelo would arouse the same corticomotor responses as the observation of the same action in reality.
Transcranial magnetic stimulation (TMS) can be used to investigate cortico-spinal excitability. The amplitude of the motor evoked potential (MEP), obtained with single-pulse TMS, reflects the firing of cortico-spinal neurons (Hallett, 2000). Furthermore, a TMS stimulus delivered during voluntary contraction induces a temporary pause of the target muscle contraction cortical silent period (CSP) due to activation of cortico-spinal inhibitory circuits (Cantello et al., 1992). In addition, when a test stimulus is preceded by a conditioning pulse (subthreshold) the resulting MEP can be either inhibited short-interval intracortical inhibition (SICI) or facilitated intracortical facilitation (ICF; Kujirai et al., 1993). SICI and ICF have proven to be useful parameter for probing inhibitory and facilitatory circuits within primary motor cortex (Ziemann, 2004; Fadiga et al., 2005; Battaglia et al., 2006). The technique had also been useful in elucidating the effects of observation of still photographs on motor excitability (Urgesi et al., 2006). But no study had yet been made of corticomotor excitability during the observation of an action represented in a work of art.
In this paper we use to TMS to investigate (1) whether the observation of an action in an artistic representation activates the corticomotor system; (2) whether this effect is attributable to arousal as a result of the emotional context of the actions shown in the work of art or not; (3) whether the mental rehearsal of observation of a painting induces the same degree of corticomotor activation; (4) whether there is any difference between responses to the action in the work of art and a photograph of the same pose.
Materials and Methods
Subjects
We studied 10 right-handed normal volunteer (7 men, 3 women; age range 29–37 years; mean age, 33.3 ± 2.9 years). All participants gave written informed consent and all experiments conformed to the Declaration of Helsinki. The experimental protocol was approved by the local ethics committee (NYCPM).
EMG recording
Surface EMG was recorded with disposable adhesive disk electrodes placed in a tendon–belly arrangement over the right extensor carpi radialis (ECR) muscle. The signal was amplified, filtered (bandpass 2–5 kHz), digitized (Micro 1401, Cambridge Electronics Design, Cambridge, UK), and stored in a laboratory computer for off-line analysis. During the experiments EMG activity was continuously monitored by visual (oscilloscope) and auditory (speakers) feedback to ensure either complete relaxation at rest or a constant level of EMG activity during tonic contraction.
TMS measurements of cortical excitability
We used single-pulse and paired-pulse TMS to induce MEPs and to examine cortical excitability. TMS was performed with a 7-cm figure-of-eight coil and a Magstim 200 stimulator (The Magstim Company, Dyfed, UK). The coil was placed at the optimal position for eliciting MEPs from the right ECR muscle (“hot spot”). The coil was held tangentially to the skull with the handle pointing backward and laterally at an angle of 45° to the sagittal plane. Thus, the electrical current induced in the brain was approximately perpendicular to the central sulcus. This orientation of the induced electrical field is thought to produce a predominantly trans-synaptic activation of the cortico-spinal neurons (Rothwell et al., 1999). During the experiments EMG activity was continuously monitored by either visual (oscilloscope) or auditory (speakers) feedback to ensure complete relaxation.
Resting motor threshold (RMT) was determined as the minimum stimulator intensity (to the nearest 1%) to produce an MEP of 50 μV in five of 10 trials.
To assess MEP amplitude, we used a stimulus intensity of 120% of the RMT. Mean peak-to-peak MEP amplitudes were determined by averaging 10 monophasic magnetic stimuli delivered to the motor hot spot of the ECR muscle.
Cortical silent period was recorded while the subjects were performing about 50% of maximal voluntary contraction (EMG activity was monitored with an audio–video feedback). The CSP was evoked with single-pulse TMS with a stimulus intensity set at 130% of RMT. The duration of 15 CSPs was measured from the end of the MEP until the restart of a constant EMG activity of at least 50% of the pre-stimulus level and was expressed in ms. For CSP measurement, EMG traces were rectified but not averaged.
Short-interval intracortical inhibition and ICF were studied by means of the paired TMS paradigm described by Kujirai et al. (1993) with a subthreshold conditioning stimulation followed by a suprathreshold test stimulation. Inter-stimulus intervals (ISIs) of 2 ms (for SICI) and 10 ms (for ICF) were used. Each study consisted of 10 trials for each ISI, and the test stimuli alone were delivered in random order controlled by a laboratory computer (Signal software, Cambridge Electronics Design, Cambridge, UK). In all the paired-pulse studies, the test stimulus intensity was adjusted in order to evoke motor responses of a matched size (approximately 0.7 mV, peak-top-peak amplitude). TMS parameters were tested according to published guidelines for the use of TMS in clinical neurophysiology (Rossini et al., 1999; Rothwell et al., 1999).
Experimental conditions
Throughout the experiment subjects were seated comfortably in front of a computer screen (20 inches) placed approximately 50 cm in front of the participant. At the beginning of each block, subjects watched the appropriate video to obtain indication about the experimental task. Each video lasted 6 s and TMS stimuli were delivered after 3 s. Visual stimuli were administered on a Pentium IV computer, using Presentation software (Neurobehavioral Systems, Inc.) to control the presentation and timing of all stimuli. Each condition was presented 20 times, following a pre-determined random order. The order of experiments (1–4) was kept constant across participants.
Experiment 1
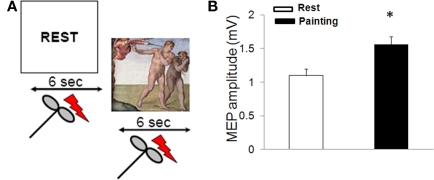
Here we examined cortico-spinal excitability during rest and during observation of a painting (Figure 1A). The selected painting was Michelangelo's Expulsion from Paradise in the Sistine Chapel (1508–1512), with its trenchant depiction of the gesture which Adam makes with his extended right hand to keep the sword-bearing angel at bay. We chose this scene for two reasons. Firstly, because Michelangelo, with his habitual skill, clearly delineates the muscles of the forearm involved in the extension of the hand; this action is unequivocally represented and easily legible. Secondly, we did so because the distal muscles involved in this action have an extensive and well defined cortical representation as tested with TMS (Chen et al., 1998). Stimulation of these cortical areas thus elicits MEPs of reliable amplitude. In addition, intracortical mechanisms for inhibition and facilitation in these muscles have been well characterized (Chen et al., 1998). The experiment consisted of eliciting MEP responses while participants observed a rest video providing a signal to relax (REST on a blank screen) or a video displaying the selected painting (the Expulsion from Paradise).
Figure 1.
MEP amplitude during observation of Expulsion from Paradise. (A) Experimental paradigm used to assess corticomotor excitability during observation of a painting. Two digitized video sequences were presented. In one sequence (REST), the participants were instructed to relax. In a second video, subjects were instructed to observe Adam's gesture in Michelangelo's Expulsion from Paradise. Each video was presented for 6 s and transcranial magnetic stimuli were delivered after 3 s. Each condition was presented 10 times. (B) Painting observation increased motor evoked potentials (MEP) size (mean ± SE)* = p < 0.05.
Experiment 2
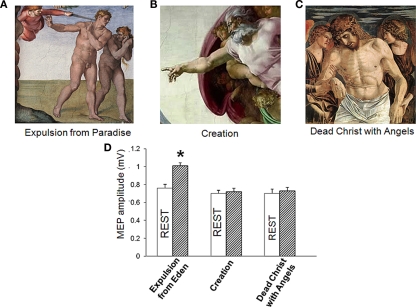
We then studied cortical-spinal excitability during observation of hand position in three paintings: Michelangelo's Expulsion from Paradise, his Creation of Adam, and Giovanni Bellini's Dead Christ with Angels (ca. 1465; Ss. Giovanni e Paolo, Venice; Figures 2A–C). In the Expulsion the ECR muscle is activated; in the Creation, it is at rest; in Bellini's Dead Christ, it is shown at rest in an overtly and highly emotional context. Subjects were asked to look at the video REST or at a video of the paintings.
Figure 2.
(A–C) Corticomotor facilitation during observation of emotionally charged paintings depicting a right hand in different postures. (D) Only observation of Adam’ gesture in Michelangelo's Expulsion from Paradise increased motor evoked potentials (MEP) size. (Mean ± SE) * = p < 0.01.
Experiment 3
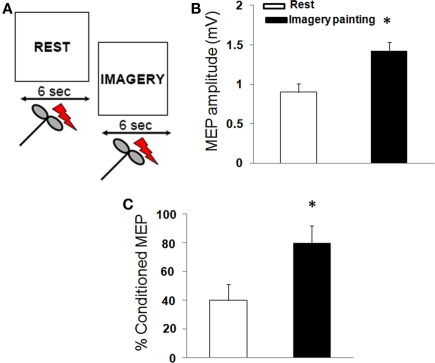
In this experiment, subjects were required to observe a REST video (signal to relax) paired with an IMAGERY video (instructing the subjects to mentally rehearse the observation of the painting; Figure 3A). Prior to TMS, all subjects underwent imagery training. This training protocol was performed under visual feedback of EMG recording of the right ECR muscle to ensure complete target muscle relaxation. The imagery of the painting was externally paced (green triangle on a computer monitor) at a rate of 1 every 10 s in blocks of 30 imagined sequences. At the end of each session, subjects were asked to describe the intensity of vividness of the imagined painting with an arbitrary scale ranging from 0 (no visual sensation) to 6 (perfectly clear sensation). The training was terminated when the subject reached vividness score of four in absence of any ECR muscle contraction.
Figure 3.
Corticomotor excitability during imagery of a painting. (A) In one sequence (REST), the participants were instructed to relax. In a second video (IMAGERY), participants were asked to imagine the painting. (B) Painting observation increased motor evoked potentials (MEP) size and (C) reduced the amount of short-interval intracortical inhibition. (Mean ± SE) * = p < 0.01.
Experiment 4
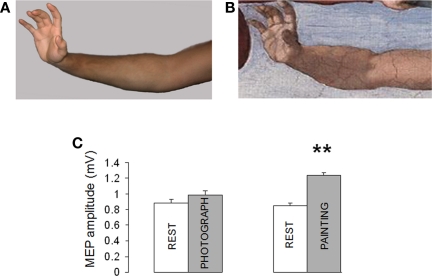
Here we investigated corticomotor responses to observation of Adam's hand action in the painting of the Expulsion and a photograph of the same action (Figure 4A).
Figure 4.
(A,B) Corticomotor facilitation during observation of Adam’ arm in Expulsion from Paradise and a photograph of the same pose. (C) Motor evoked potentials (MEP) increased during observation of Adam’ arm while observation of a photograph of the same pose did not induce significant changes in MEP size. (Mean ± SE) ** = p < 0.01.
The subjects attended two paired video presentations: (1) the video REST paired with the observation of Adam's hand action; (2) the video REST paired with the observation of a poser.
Analysis of variance (ANOVA) was used to assess differences between paradigms (observation of a paintings, imagery of a painting, photograph). Upon detection of significant main effects, we performed post hoc analysis to assess differences between conditions (rest vs. active observation). The statistical analysis was performed using statistical software packages (SPSS software version 13.0 for Windows® Chicago, IL, USA). The level of significance was set at p < 0.05 for all tests.
Results
Experiment 1
Regarding RMT, ANOVA did not disclose a significant difference between conditions [rest: 54.3 ± 5.1%, painting: 52.7 ± 6.3; F (1,18) = 5.4; p = 0.1]. On the contrary, observation of the painting increased MEP amplitude [F (1,18) = 15.2; p = 0.02; Figure 1B]. CSP duration was found to be no different during the two conditions (rest = 134.1 ± 11.3 ms, painting = 131.8 ± 10.5 ms; p > 0.05). Intra-cortical excitability, tested by using the paired-pulse study, revealed that observation of the painting did not induce changes in the in the amount of SICI (rest: 52 ± 9.1%, painting: 56.4 ± 12.5%; p = 0.1) and ICF (rest: 144.7 ± 10.03%, painting: 152.7 ± 11.6%; p = 0.3).
Experiment 2
Comparison of neural response to TMS during observation of the three paintings revealed a main effect of paradigms [F (1, 54) = 4.8, p = 0.01] without main effect condition [F(1,54) = 1.1, p = 0.2] with a significant paradigms x condition interaction [F(1,54) = 4.4, p = 0.01). Post hoc t-test showed that only observation of the Expulsion from Paradise increased MEP (p = 0.02) whereas the Creation of Adam and the Dead Christ with Angels did not have effect on corticomotor excitability (p > 0.05; Figure 2D). Therefore, the increased in MEP size detected during observation of the Expulsion from Paradise was due to the artistic representation of an action with a minimal contribution of emotional arousal.
Experiment 3
Mental rehearsal of the painting modulated corticomotor excitability. RMT [F(1,18) = 3.2; p = 0.4), CSP [F(1,18) = 7.1; p = 0.09], and ICF [F(1,18) = 4.4; p = 0.2] were not different between rest and imagery of the painting. Moreover, imagery of the painting increased MEP amplitude [F(1,18) = 25.1; p < 0.01] and decreased the amount of SICI [F(1,18) = 18.3; p < 0.05; Figures 3B,C).
Experiment 4
The ANOVA showed a large effect of paradigm on MEP amplitude. As shown in Figures 4B,C, observation of Adam's arm (Expulsion) increased MEP size (134.7% compared to REST). On the contrary, observation the same pose induced only a modest, non-significant, increase in MEP amplitude [111.6% compared to REST; F (1, 18) = 27, p < 0.001].
Discussion
This is the first study to investigate the effects on motor output of observation of an action in a painting. It demonstrates that observation of an action in a painting increases cortical-spinal excitability. This effect is the same as the one we found for mental rehearsal of the painting. Observation of a photograph reproducing the same action did not increase MEP amplitude.
Since observation of the photograph did not significantly affect corticomotor excitability, we assume that this effect, in the case of the painting, must be a consequence of the artist's skill in giving the illusory impression of movement. Clearly Michelangelo successfully conveyed the kinesthetic aspects of the movement he depicted in such a way as to overcome the static nature of the image. The degree to which this impression may be due to coloristic, anatomical, and lighting skills remains to be further examined.
MEP amplitude reflects the trans-synaptic excitability of cortico-spinal neurons and spinal motor neurons, and thus provides information about the strength of cortico-spinal connections (Boroojerdi et al., 2001). Given that observation of a movement could also have minor effects on spinal excitability (Baldissera et al., 2001), it is possible that the increase in MEP size we detected during observation of the Expulsion from Paradise might be due to both cortical and spinal effects.
Recent neuroimaging studies (Kawabata and Zeki, 2004) have found that observation of emotionally charged images induces sensory–motor activation. It is unlikely, however, that the increase in MEP size during observation of the Expulsion from Paradise can be attributed to a general, unspecific increase in arousal (Baumgartner et al., 2007) since the observation of a more overtly emotional scene (Bellini's Dead Christ with Angels) did not increase MEP size.
While a number of authors have shown that MEP size increases during motor imagery (Fourkas et al., 2006; Stinear et al., 2006), we show, for the first time that imagery of a pictorial work of art modulates cortical excitability. We speculate that since our subjects were required to imagine Adam's gesture in Michelangelo's fresco, our imagery task also involved the kind of kinesthetic information conveyed by the observation of the actual movement itself. In addition, we demonstrate that visual motor memories (imagery of the painting) exert a modulation of intracortical inhibitory circuits as demonstrated by a reduction in the amount of SICI (Di Lazzaro et al., 2007). Our results are in agreement with previous studies (Abbruzzese et al., 1999; Stinear and Byblow, 2004) that have provided evidence that SICI can be modulated in a spatially and temporally specific way during imagined motor performance. Given that our study was performed on one hemisphere we do not have information regarding the topographic specificity of motor responses to observation of a work of art. These issues need to be further investigated.
While earlier studies have suggested the involvement of a number of brain areas in esthetic judgment such as the limbic system (Di Dio et al., 2007), orbitofrontal cortex, motor (Kawabata and Zeki, 2004), visual (Zeki and Lamb, 1994), and frontal areas (Cela-Conde et al., 2004; Jacobsen et al., 2006) our results make clear that esthetic factors have a modulatory effect on motor representations in primary motor cortex. It has been demonstrated (by using both TMS and event related potentials) that static images with implied motion perception activate both motor and visual areas (Urgesi et al., 2006; Proverbio et al., 2009). Visual perception of static body parts engages the EBA (Downing et al., 2001), while ventral premotor cortex plays a critical role in the understanding of complete body postures (Urgesi et al., 2004). It is likely that the same networks are engaged during esthetic understanding (Freedberg and Gallese, 2007; Calvo-Merino et al., 2010). With regard to imagery of human body parts, it is likely that different multimodal body representations in the occipito-temporal cortex are engaged in a content-specific manner (Ishai et al., 2000; O'Craven and Kanwisher, 2000; Grossman and Blake, 2001; Costantini et al., 2011). Our results expand these findings by providing evidence of motor modulation induced by observation of an action in a work of art. It is likely that these responses are not only restricted to representational art. Recent studies have shown a similar pattern of brain activation in the case of less densely descriptive and more abstract images (Kim and Blake, 2007; Osaka et al., 2010) and during processing of human motion at a conceptual level, such as during story comprehension (Deen and McCarthy, 2010).
On the other hand, we should also consider the possibility that the motor activation we detected might be due to the specific action portrayed in the painting. In primates, electrical stimulation of the poly sensory zone in the precentral gyrus (roughly matching the dorsal part of area F4) induces a contra-lateral defensive posture consistent with the one’ portrayed by Michelangelo in the Expulsion from Paradise (Graziano et al., 2002a,b). Neurons in the poly sensory zone have bimodal, visual–tactile modalities and represent the space immediately surrounding the body through touch, and vision (Graziano et al., 2002b). Hence, in the case of Expulsion from Paradise, it is likely that the artistic nature of the action induce a stronger activation in neurons that respond to visual stimuli in both primary motor (Rizzolatti et al., 1981) and premotor cortex (Graziano et al., 2002a). The modulatory effects on motor representations consistent with defending the body against nearby threatening objects might underlie to our TMS results during perception and imagination of the painting.
The present results add considerably to our knowledge of the motor networks engaged in responses to works of art, and enhance our understanding of the felt imitation not just of the actions of others but also of actions in pictorial works of art. The extent to which our findings apply to sculptures remains to be seen. So does the important question of the degree to which prior acquaintance with a work of art might affect MEP size during observation and imagery. For instance, the participants were exposed several times to the painting and were required to mentally rehearse the observation of the work of art. Given that stimulus novelty has been shown to have a significant effect on esthetic perception and judgment (Oliva and Torralba, 2007; Kirk, 2008; Kirk et al., 2009) it is possible that neural responses to the work of art were shaped by previous experience. Furthermore, future studies are needed to investigate the role of cultural, social, and psychological characteristics of the observer on art perception.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank all the volunteers that participated in the study. The study was supported by a NYCPM research grant.
Abbreviations
ECR, extensor carpi radialis; MEP, motor evoked potential; RMT, resting motor threshold; TMS, transcranial magnetic stimulation.
References
- Abbruzzese G., Assini A., Buccolieri A., Marchese R., Trompetto C. (1999). Changes of intracortical inhibition during motor imagery in human subjects. Neurosci. Lett. 263, 113–116 10.1016/S0304-3940(99)00120-2 [DOI] [PubMed] [Google Scholar]
- Allison T., Puce A., McCarthy G. (2000). Social perception from visual cues: role of the STS region. Trends Cogn. Sci. (Regul. Ed.) 4, 267–278 10.1016/S1364-6613(00)01501-1 [DOI] [PubMed] [Google Scholar]
- Baldissera F., Cavallari P., Craighero L., Fadiga L. (2001). Modulation of spinal excitability during observation of hand actions in humans. Eur. J. Neurosci. 13, 190–194 10.1046/j.0953-816x.2000.01368.x [DOI] [PubMed] [Google Scholar]
- Battaglia F., Quartarone A., Ghilardi M. F., Dattola R., Bagnato S., Rizzo V., Morgante L., Girlanda P. (2006). Unilateral cerebellar stroke disrupts movement preparation and motor imagery. Clin. Neurophysiol. 117, 1009–1016 10.1016/j.clinph.2006.01.008 [DOI] [PubMed] [Google Scholar]
- Baumgartner T., Willi M., Jancke L. (2007). Modulation of corticospinal activity by strong emotions evoked by pictures and classical music: a transcranial magnetic stimulation study. Neuroreport 18, 261–265 10.1097/WNR.0b013e328012272e [DOI] [PubMed] [Google Scholar]
- Boroojerdi B., Battaglia F., Muellbacher W., Cohen L. G. (2001). Mechanisms influencing stimulus-response properties of the human corticospinal system. Clin Neurophysiol 112, 931–937 10.1016/S1388-2457(01)00523-5 [DOI] [PubMed] [Google Scholar]
- Calvo-Merino B., Urgesi C., Orgs G., Aglioti S. M., Haggard P. (2010). Extrastriate body area underlies aesthetic evaluation of body stimuli. Exp. Brain Res. 204, 447–456 10.1007/s00221-010-2283-6 [DOI] [PubMed] [Google Scholar]
- Cantello R., Gianelli M., Civardi C., Mutani R. (1992). Magnetic brain stimulation: the silent period after the motor evoked potential. Neurology 42, 1951–1959 [DOI] [PubMed] [Google Scholar]
- Cela-Conde C. J., Marty G., Maestu F., Ortiz T., Munar E., Fernandez A., Roca M., Rossello J., Quesney F. (2004). Activation of the prefrontal cortex in the human visual aesthetic perception. Proc. Natl. Acad. Sci. U.S.A. 101, 6321–6325 10.1073/pnas.0401427101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Tam A., Butefisch C., Corwell B., Ziemann U., Rothwell J. C., Cohen L. G. (1998). Intracortical inhibition and facilitation in different representations of the human motor cortex. J. Neurophysiol. 80, 2870–2881 [DOI] [PubMed] [Google Scholar]
- Costantini M., Urgesi C., Galati G., Romani G. L., Aglioti S. M. (2011). Haptic perception and body representation in lateral and medial occipito-temporal cortices. Neuropsychologia 49, 821–829 10.1016/j.neuropsychologia.2011.01.034 [DOI] [PubMed] [Google Scholar]
- Deen B., McCarthy G. (2010). Reading about the actions of others: biological motion imagery and action congruency influence brain activity. Neuropsychologia 48, 1607–1615 10.1016/j.neuropsychologia.2010.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Dio C., Macaluso E., Rizzolatti G. (2007). The golden beauty: brain response to classical and renaissance sculptures. PLoS ONE 2, e1201. 10.1371/journal.pone.0001201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V., Pilato F., Dileone M., Profice P., Ranieri F., Ricci V., Bria P., Tonali P. A., Ziemann U. (2007). Segregating two inhibitory circuits in human motor cortex at the level of GABAA receptor subtypes: a TMS study. Clin. Neurophysiol. 118, 2207–2214 10.1016/j.clinph.2007.07.005 [DOI] [PubMed] [Google Scholar]
- Downing P. E., Jiang Y., Shuman M., Kanwisher N. (2001). A cortical area selective for visual processing of the human body. Science 293, 2470–2473 10.1126/science.1063414 [DOI] [PubMed] [Google Scholar]
- Fadiga L., Craighero L., Olivier E. (2005). Human motor cortex excitability during the perception of others’ action. Curr. Opin. Neurobiol. 15, 213–218 10.1016/j.conb.2005.03.013 [DOI] [PubMed] [Google Scholar]
- Fourkas A. D., Avenanti A., Urgesi C., Aglioti S. M. (2006). Corticospinal facilitation during first and third person imagery. Exp. Brain Res. 168, 143–151 10.1007/s00221-005-0076-0 [DOI] [PubMed] [Google Scholar]
- Freedberg D., Gallese V. (2007). Motion, emotion and empathy in esthetic experience. Trends Cogn. Sci. (Regul. Ed.) 11, 197–203 10.1016/j.tics.2007.02.003 [DOI] [PubMed] [Google Scholar]
- Graziano M. S., Taylor C. S., Moore T. (2002a). Probing cortical function with electrical stimulation. Nat. Neurosci. 5, 921. 10.1038/nn1002-921 [DOI] [PubMed] [Google Scholar]
- Graziano M. S., Taylor C. S., Moore T., Cooke D. F. (2002b). The cortical control of movement revisited. Neuron 36, 349–362 10.1016/S0896-6273(02)01003-6 [DOI] [PubMed] [Google Scholar]
- Grossman E. D., Blake R. (2001). Brain activity evoked by inverted and imagined biological motion. Vision Res. 41, 1475–1482 10.1016/S0042-6989(00)00317-5 [DOI] [PubMed] [Google Scholar]
- Hallett M. (2000). Transcranial magnetic stimulation and the human brain. Nature 406, 147–150 10.1038/35018000 [DOI] [PubMed] [Google Scholar]
- Ishai A., Ungerleider L. G., Haxby J. V. (2000). Distributed neural systems for the generation of visual images. Neuron 28, 979–990 10.1016/S0896-6273(00)00168-9 [DOI] [PubMed] [Google Scholar]
- Jacobsen T., Schubotz R. I., Hofel L., Cramon D. Y. (2006). Brain correlates of aesthetic judgment of beauty. Neuroimage 29, 276–285 10.1016/j.neuroimage.2005.07.010 [DOI] [PubMed] [Google Scholar]
- Kawabata H., Zeki S. (2004). Neural correlates of beauty. J. Neurophysiol. 91, 1699–1705 10.1152/jn.00696.2003 [DOI] [PubMed] [Google Scholar]
- Kim C. Y., Blake R. (2007). Brain activity accompanying perception of implied motion in abstract paintings. Spat. Vis. 20, 545–560 10.1163/156856807782758395 [DOI] [PubMed] [Google Scholar]
- Kirk U. (2008). The neural basis of object-context relationships on aesthetic judgment. PLoS ONE 3, e3754. 10.1371/journal.pone.0003754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk U., Skov M., Christensen M. S., Nygaard N. (2009). Brain correlates of aesthetic expertise: a parametric fMRI study. Brain Cogn. 69, 306–315 10.1016/j.bandc.2008.08.004 [DOI] [PubMed] [Google Scholar]
- Kourtzi Z., Kanwisher N. (2000). Implied motion activates extrastriate motion-processing areas response to David and Senior (2000). Trends Cogn. Sci. (Regul. Ed.) 4, 295–296 10.1016/S1364-6613(00)01512-6 [DOI] [PubMed] [Google Scholar]
- Kujirai T., Caramia M. D., Rothwell J. C., Day B. L., Thompson P. D., Ferbert A., Wroe S., Asselman P., Marsden C. D. (1993). Corticocortical inhibition in human motor cortex. J. Physiol. (Lond.) 471, 501–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipps T. (1906). Die ästhetische Betrachtung und die bildende Kunst. Hamburg and Leipzig: Voss [Google Scholar]
- Merleau-Ponty M. (1962). Phenomenology of Perception. Colin Smith. (Translator). New York: Humanities Press [Google Scholar]
- O'Craven K. M., Kanwisher N. (2000). Mental imagery of faces and places activates corresponding stimulus-specific brain regions. J. Cogn. Neurosci. 12, 1013–1023 10.1162/08989290051137549 [DOI] [PubMed] [Google Scholar]
- Oliva A., Torralba A. (2007). The role of context in object recognition. Trends Cogn. Sci. (Regul. Ed.) 11, 520–527 10.1016/j.tics.2007.09.009 [DOI] [PubMed] [Google Scholar]
- Osaka N., Matsuyoshi D., Ikeda T., Osaka M. (2010). Implied motion because of instability in Hokusai Manga activates the human motion-sensitive extrastriate visual cortex: an fMRI study of the impact of visual art. Neuroreport 21, 264–267 10.1097/WNR.0b013e328335b371 [DOI] [PubMed] [Google Scholar]
- Proverbio A. M., Riva F., Zani A. (2009). Observation of static pictures of dynamic actions enhances the activity of movement-related brain areas. PLoS ONE 4, e5389. 10.1371/journal.pone.0005389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G., Scandolara C., Matelli M., Gentilucci M. (1981). Afferent properties of periarcuate neurons in macaque monkeys. II. Visual responses. Behav. Brain Res. 2, 147–163 10.1016/0166-4328(81)90053-X [DOI] [PubMed] [Google Scholar]
- Rossini P. M., Berardelli A., Deuschl G., Hallett M., Maertens de Noordhout A. M., Paulus W., Pauri F. (1999). Applications of magnetic cortical stimulation. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 52, 171–185 [PubMed] [Google Scholar]
- Rothwell J. C., Hallett M., Berardelli A., Eisen A., Rossini P., Paulus W. (1999). Magnetic stimulation: motor evoked potentials. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 52, 97–103 [PubMed] [Google Scholar]
- Stinear C. M., Byblow W. D. (2004). Modulation of corticospinal excitability and intracortical inhibition during motor imagery is task-dependent. Exp. Brain Res. 157, 351–358 10.1007/s00221-004-1851-z [DOI] [PubMed] [Google Scholar]
- Stinear C. M., Fleming M. K., Byblow W. D. (2006). Lateralization of unimanual and bimanual motor imagery. Brain Res. 1095, 139–147 10.1016/j.brainres.2006.04.008 [DOI] [PubMed] [Google Scholar]
- Urgesi C., Berlucchi G., Aglioti S. M. (2004). Magnetic stimulation of extrastriate body area impairs visual processing of nonfacial body parts. Curr. Biol. 14, 2130–2134 10.1016/j.cub.2004.11.031 [DOI] [PubMed] [Google Scholar]
- Urgesi C., Moro V., Candidi M., Aglioti S. M. (2006). Mapping implied body actions in the human motor system. J. Neurosci. 26, 7942–7949 10.1523/JNEUROSCI.1289-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vischer R. (1872). Über das optische FormgefÜhl: ein Beitrag zur Aesthetik. Dissertation, Tubingen [Google Scholar]
- Zeki S., Lamb M. (1994). The neurology of kinetic art. Brain 117(Pt 3), 607–636 [DOI] [PubMed] [Google Scholar]
- Ziemann U. (2004). TMS and drugs. Clin Neurophysiol 115, 1717–1729 10.1016/j.clinph.2004.03.006 [DOI] [PubMed] [Google Scholar]