Abstract
Objective
HIV pathogenesis is characterized by destructive imbalances between virus-mediated immune damage, anti-viral immune responses, and immune activation. We characterized the effects of successful antiretroviral therapy (ART) to identify the breadth and patterns of HIV-associated gene expression.
Methods
In a prospective observational, longitudinal cohort study of 10 ART-naive Ugandans with AIDS (median 30 CD4+/μL), we measured mRNA gene profiles in peripheral blood using Affymetrix U133_Plus2.0 microarrays at 0, 2, 4, 8, and 24 weeks after ART initiation.
Results
We identified 160 mRNA transcripts that were consistently down-regulated and 48 that were up-regulated after ART at each point over 24 weeks based on linear regression modeling (adjusted-P<.05), Of these 208 transcripts, approximately half represent heretofore unrecognized ART-responsive genes and one-third have no known function. The down-regulated genes with known function encoded mediators of innate anti-viral responses, including antiviral restriction factors, pattern recognition receptors, and interferon response proteins, as well as mediators of immune activation, cellular proliferation, and apoptosis.
Conclusions
By using ART to block the viral stimulus, we identified transcripts involved in innate antiviral immunity, including antiviral restriction factors and pattern recognition receptors, that were not previously known to be induced by HIV infection.
MeSH Headings: HIV, AIDS, HAART, Gene Expression Profiling /BL, Oligonucleotide Array Sequence Analysis, Adult
INTRODUCTION
HIV infection elicits innate and adaptive host immune responses that contribute to control of the infection. However, the antiviral response is insufficient to prevent establishment of chronic infection, characterized by ongoing viral replication and viremia, destruction of lymphocytes, scarring of lymphatic tissues and immune activation.1 Although antiretroviral therapy (ART) interrupts HIV-mediated immune destruction and increases the number and function of CD4+ T-cells, the fundamental molecular mechanisms underlying HIV-associated immune compromise and subsequent ART-associated immune reconstitution remain largely unknown.
Microarray-based gene expression analyses have been used to identify pathologic pathways in cancer pathophysiology, to choose therapeutic targets, to predict treatment responses, and to identify novel pathways to categorize the pathophysiology of infectious diseases, including HIV/AIDS.2-6 Among nine in vivo studies involving HIV-1 and microarrays between 2000 and 2006,7 all but one were cross-sectional, and the sole longitudinal study followed only two subjects.8 Subsequently, Li and colleagues identified ~200 ART-responsive genes in lymph nodes obtained by serial biopsies over one month in five HIV-infected individuals receiving ART and follow on experiments in 24 subjects.2, 9 The ART-responsive genes included those encoding regulators of innate defense, cell trafficking, and tissue repair.2 More recently, Vahey and colleagues examined gene expression changes in primates infected with simian immunodeficiency virus (SIV) after two weeks of ART,3 and Jacquelin and colleagues explored the longitudinal gene expression changes in African green monkeys.10 In 2010, Rotger and colleagues reported cross-sectional genome-wide mRNA correlates of viral control.6 The initial studies of the effects of ART on gene expression in HIV-infected persons have been limited in size and duration, and none included longitudinal analyses in persons with AIDS.
We sought to use gene expression microarrays to characterize ART-induced changes in gene expression in peripheral blood of HIV-infected humans as a foundation to ultimately understand the role of ART in immune reconstitution. The use of peripheral blood in patients with successful responses to ART reveals the range and patterns of HIV-associated gene dysregulation and provides a normative framework for subsequent comparative studies in patients who fail therapy or develop ART-related complications, such as drug toxicity or immune reconstitution inflammatory syndrome (IRIS). This reverse challenge experiment can characterize the host response to HIV-infection by quantifying the intra-person changes in gene expression occurring when HIV is blocked with successful ART.
We conducted gene expression microarray analysis of whole blood samples from a prospective cohort of 10 Ugandans with AIDS before and 2, 4, 8, and 24 weeks after initiation of ART. We identified approximately 200 transcripts whose expression was consistently changed after starting ART, approximately half of which are novel ART-responsive transcripts. Concomitant with the ART-associated drop in plasma HIV RNA, we observed consistent declines in the expression of genes regulating innate antiviral responses (e.g. APOBEC3G, TRIM6, interferon (IFN)-signaling), pattern recognition receptors (DDX58), immune activation (LAMP3, NFκβ-pathway), cell apoptosis (IRF7, MX1, TREX1), cell proliferation, and numerous genes of unknown function. Our results suggest that HIV infection induces antiviral host responses, including innate antiviral responses, which are subsequently turned off when the virus is blocked with successful ART.
METHODS
Research Subjects and HIV Care
We prospectively monitored 24 ART-naive subjects with AIDS (<200 CD4+/μL) but no known active opportunistic infections, for one year after initiating ART. Exclusion criteria included anemia (hemoglobin < 8g/L), hepatitis (liver transaminases >2.5x upper limit of normal), and renal insufficiency (creatinine >1.5x upper limit of normal). Subjects were enrolled between May and July 2006 at the Infectious Disease Institute in Kampala, Uganda and seen every two weeks for 12 weeks, then monthly through 52 weeks. At each clinic visit, subjects were screened for new infections, IRIS, malignancies, treatment failure, and ART compliance via pill counts. Antiretroviral regimens were stavudine, lamivudine, and nevirapine, or zidovudine, lamivudine, and efavirenz. Plasma HIV RNA and CD4+ T-cells were measured every 12 weeks. Informed consent was obtained from subjects, and ethics approval was obtained from the University of Minnesota, Makerere University, and Uganda National Council of Science and Technology. Ten of 17 subjects with immunological recovery (CD4+ increase >50 cells/μL), viral suppression at 24 weeks (<400 copies/mL), and no new opportunistic infections or adverse events over one year of monitoring were chosen for microarray gene expression analyses.
Sample Collection and Gene Expression Analysis
We used Affymetrix microarrays for our gene expression studies performed on peripheral whole blood because this approach has been extensively validated. The correlation between Affymetrix microarrays and real-time PCR is very high (r=.87 to .92),11, 12 and the reproducibility of microarrays actually exceeds that of PCR, even for common applications such as HIV-1 viral load quantification.13, 14 We used peripheral whole blood for gene expression studies, rather than isolated peripheral blood mononuclear cells (PBMC) because in vitro artifacts can be induced during cellular isolation, ex vivo handling, and processing.15-18 We avoided these in vitro artifacts by drawing blood into PAXGene tubes, which immediately lyses cells and stabilizes RNA.15, 18
RNA for gene expression analysis was isolated from peripheral whole blood at the time of commencing ART (baseline) and at 2, 4, 8, and 24 weeks of ART. Peripheral whole blood (2.5mL) was collected into PaxGene™ tubes (PreAnalytiX, Hombrechtikon, Switzerland), incubated at room temperature for two hours, and frozen at -80°C. RNA was extracted using PreAnalytix PAXGene™ Blood RNA Kits and treated with Dnase I to remove DNA. Each subject’s RNA was processed on the same day. The quality of extracted RNA was checked with a NanoDrop spectrophotometer (Thermo Fisher Scientific, Wilmington, DE USA). cDNA, prepared from 1 μg of RNA, was used to synthesize biotinylated-labeled RNA using the MessageAmp™ II aRNA Amplification Kit (Ambion, Austin, TX). Fragmented, labeled-RNA (15 μg) was hybridized onto Human Genome U133_Plus2.0 arrays (Affymetrix, Santa Clara, CA) as specified by the manufacturer and scanned with an Affymetrix GeneChip 3000 Scanner. The U133_Plus2.0 array has 54,120 probe sets for 38,572 characterized genes.
Statistics
Feature intensities for each microarray were condensed into single intensity values for each probe set using Robust Multichip Average (RMA) normalization. RMA performs background adjustment, quantile normalization, and summarization from the perfect-match probes.19 Post-normalization, we used a linear mixed effects model to test for changes in the expression series over time.
The regression tested all time points of 2, 4, 8, and 24 weeks versus baseline. The model was adjusted for the difference in log2CD4+ counts from baseline to 24 weeks (as a covariate) to assure that changes in expression were not related solely to CD4 change. The levels of other hematologic cell types (e.g. total lymphocytes, CD8+ T-cells, neutrophils, monocytes, eosinophils, basophils) did not change over time in the cohort (Minimum P=.11). A person-specific random effect allowed correlation between time points within person. The vector of coefficients for time points derived from the regression model was tested for statistical significance. For each coefficient, we test whether the difference was significantly different from zero using the appropriate partial t-test for fixed effects. In addition to tests for each time point, we also performed an overall test for a change at all time points.
Because there were a large number of tests (4 time points + one overall test × 54,675 probe sets = 273,375 statistical tests in total), p-values need to be adjusted for multiple comparisons. We employed the Benjamini-Hochberg correction to control the false discovery rate for the 273,375 tests.20 Of the 273,375 p-values, 66,212 were statistically significant with a range of adjusted p-values from 1.7×10-6 to 0.05 (unadjusted p-values of 1.1×10-10 to 0.007). All presented p-values were adjusted for multiple comparisons, unless otherwise stated.
We then filtered the list of transcripts based on i) consistent direction of expression change (up or down-regulated) at all time points, ii) genes which were statistically significant at all 4, 8, 24 week (unadjusted-P<.05) time points, and iii) statistically significant overall in the regression model (adjusted-P<.05).
Validation
To validate changes in gene transcripts, serial serum samples collected at baseline, 2,4,8,12, and 24 weeks were analyzed for changes in the levels of 27 cytokines/chemokines (Bio-Rad, Hercules, CA) per the manufacturer’s protocol via a Luminex 100 system (Austin, TX). The cytokine levels were log2 transformed and longitudinal analysis was performed mirroring the statistical analysis of gene expression using the R nlme package. We modeled changes in cytokines by linear regression versus weeks of ART with and without a covariate of absolute CD4 count and individual slope estimates for each time point after baseline. An intra-person random-effects variable was utilized for intra-person comparison of repeated-measures.
Identification of gene networks and functional pathways
Ingenuity Pathways Analysis® (www.ingenuity.com) wasused to visualize gene-regulation networks, gene-gene interactions, functional pathways, and biological processes influenced by ART. The Ingenuity Pathways Knowledge Base is the largest curated database of previously published findings on mammalian biology from the public literature. Ontology results were cross-validated with the DAVID public database.21
In order to identify transcripts related to T-cell activation, we performed a comparative analysis between transcripts that were down-regulated with ART and a previously published gene expression dataset of human T-lymphocytes stimulated in vitro with anti-CD3+/anti-CD28+ using Affymetrix U95 and U133A microarrays.22
RESULTS
We studied 10 Ugandan subjects with AIDS, randomly selected from among 17 subjects without new opportunistic infections and with appropriate immunologic recovery and viral suppression by 24 weeks of ART (Supplemental Fig. 1a). Clinical characteristics, including the low baseline CD4 counts (median 30 cells/μL; mean 72 cells/μL) are summarized in Table 1. All subjects achieved virologic suppression (<400 copies/mL) by 24 weeks of ART, and the median CD4 count increased to 102 cells/μL (IQR: 91 to 173 cells/μL).
Table 1.
HIV-related parameters in 10 Subjects selected for gene expression analysis.
| Cohort Parameters | n=10 | |||
|---|---|---|---|---|
| Age in years, Median | 36 | Range (27 – 59) | ||
|
| ||||
| Sex | 6 Men | 4 Women | ||
|
| ||||
| Body Mass Index (kg/m2) | 21.8 ± 2.8 | Range (17.1 – 28.2) | ||
|
| ||||
| HIV Parameters | Baseline | 12 week | 24 week | 48 week |
|
| ||||
| Median CD4+ (IQR) cells/μL | 30 (13,120) | 133 (105,217) | 102 (91,173) | 139 (111,180) |
|
| ||||
| Mean CD4+ (±SD) cells/μL | 72 (± 84) | 159 (± 79) | 158 (± 109) | 176 (± 110) |
|
| ||||
| CD4% | 4.6 (± 3.5) | 9.2 (± 3.8) | 10.3 (± 3.3) | 11.7 (± 4.1) |
|
| ||||
| CD8+ cells/μL | 724 (± 594) | 939 (± 330) | 765 (± 295) | 759 (± 334) |
|
| ||||
| CD4:CD8 ratio | .09 (± .07) | .17 (±.09) | .20 (± .09) | .25 (± .12) |
|
| ||||
| HIV viral load (log copies/mL) | 5.2 (± .3) | 2.7 | < 2.6 | < 2.6 |
| Undetectable <400copies/mL | 0 of 10 | 9 of 10 | 10 of 10 | 10 of 10 |
|
| ||||
| WBC ×106/L | 3.6 ± 1.5 | 5.0 ± 1.3 | 4.9 ± 2.0 | 4.2 ± 1.6 |
|
| ||||
| Neutrophils | 1.6 ± 0.7 | 1.7 ± 1.0 | 2.3 ± 1.9 | 1.8 ± 0.9 |
|
| ||||
| Lymphocytes | .95 ± .52 | 1.9 ± 0.6 | 1.6 ± 0.7 | 1.6 ± 0.6 |
|
| ||||
| Monocytes | .35 ± 0.2 | 0.5 ± 0.2 | 0.3 ± 0.1 | 0.3 ± 0.1 |
|
| ||||
| Eosinophils | 0.6 ± 1.2 | 0.8 ± 0.9 | 0.7 ± 0.6 | 0.5 ± 0.5 |
Data are expressed as mean ± SD. Past WHO clinical events were histories of: 5-10% weight loss (n=4), >10% weight loss (n=3), varicella zoster (n=2), thrush (n=1), repeated bacterial infections (n=1), pulmonary TB (n=2), and extrapulmonary TB (n=1). Pneumocystis prophylaxis had begun prior to study entry and did not change. No persons had active infections or received anti-tuberculous treatment during the study period.
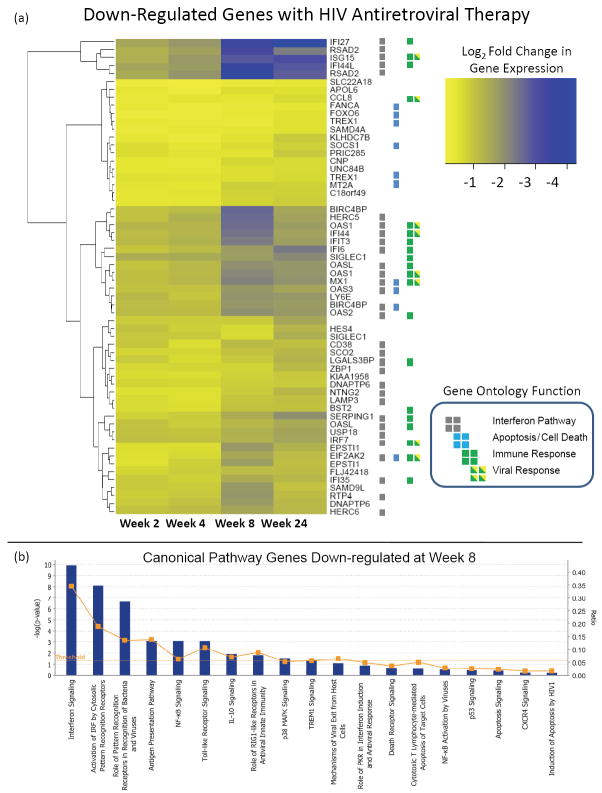
We characterized ART-induced changes in gene expression after 4, 8, or 24 weeks of ART. We found that more genes were down-regulated than up-regulated, and that the majority of genes were either not expressed or not affected by ART (Supplemental Fig 1b). We found 239 probesets with consistently changed expression at each individual time point (unadjusted-P<.05) and over the entire time course based on a linear regression model (adjusted-P<.05, adjusting for 273,375 multiple comparisons). These 239 probesets represented 208 unique genes, of which 160 were down-regulated and 48 were up-regulated. The 160 down-regulated transcripts (Table 2) showed progressive and consistent down-regulation over time during ART (Fig. 1, Supplemental Fig. 2). Even after adjusting for increases in CD4+ T-cell numbers over time, the list remained unchanged.
Table 2.
Down-regulation of 53 genes at all time points with HIV therapy
| Gene Symbol | Gene Expression | Adjusted P-value1 | Entrez Gene ID | Gene Ontology Process | Ref | Gene Name | |
|---|---|---|---|---|---|---|---|
| Percentile | Avg % Decrease | ||||||
| Immune Activation Genes | |||||||
|
| |||||||
| OASL | 97.4 | 67.9 | 1.1 ×10--5 | 8638 | Im | 5, 6, 26 | 2’-5’-oligoadenylate synthetase-like |
|
| |||||||
| SIGLEC1 | 85.5 | 71.9 | 4.6 ×10-6 | 6614 | Im | 27, 28 | Sialic acid binding Ig-like lectin 1, sialoadhesin |
|
| |||||||
| OAS2 | 98.1 | 67.5 | 5.3 ×10-6 | 4939 | Im | 2, 5,6 | 2’-5’-oligoadenylate synthetase 2 |
|
| |||||||
| ZBP1 | 91.5 | 47.7 | 5.3 ×10-5 | 81030 | Im | Z-DNA binding protein 1 | |
|
| |||||||
| IFI44L | 98.6 | 82.9 | 4.9 ×10-6 | 10964 | Im | 2, 4, 6, 10 | Interferon-induced protein 44-like |
|
| |||||||
| MX1 | 98.9 | 70.4 | 6.6 ×10-6 | 4599 | Im | 2, 4, 5, 10 | Myxovirus (influenza) resistance 1, IFN-inducible protein p78 |
|
| |||||||
| OAS1 | 99.2 | 68.3 | 6.2 ×10-6 | 4938 | Im | 2, 5, 6, 10 | 2’,5’-oligoadenylate synthetase 1 |
|
| |||||||
| IRF7 | 99.1 | 60.2 | 2.1 ×10-5 | 3665 | Im | 2, 5, 6 | Interferon regulatory factor 7 |
|
| |||||||
| OAS3 | 94.6 | 63.4 | 2.2 ×10-5 | 4940 | Im | 5, 6, 29 | 2’-5’-oligoadenylate synthetase 3 |
|
| |||||||
| IFI6 | 99.1 | 69.3 | 5.1 ×10-5 | 2537 | Im | 2, 6 | Interferon, alpha-inducible protein 6 |
|
| |||||||
| RSAD2 | 98.3 | 78.9 | 1.4 ×10-5 | 91543 | Im | Radical S-adenosyl methionine domain containing 2 | |
|
| |||||||
| IFI27 | 98.8 | 85.9 | 3.7 ×10-6 | 3429 | Im | 2, 4, 6 | Interferon, α-inducible protein 27 |
|
| |||||||
| IFIT3 | 98.9 | 68.9 | 4.5 ×10-5 | 3437 | Im | 6 | Interferon-induced protein with tetratricopeptide repeats 3 |
|
| |||||||
| IFI35 | 96.7 | 55.2 | 1.2 ×10-4 | 3430 | Im | 2, 6 | Interferon-induced protein 35 |
|
| |||||||
| SERPING1 | 94.8 | 63.0 | 5.5 ×10-5 | 710 | Im | 6 | Serpin peptidase inhibitor, clade G (C1 inhibitor) |
|
| |||||||
| IFI44 | 91.0 | 71.2 | 2.1 ×10-5 | 10561 | Im | 4 | Interferon-induced protein 44 |
|
| |||||||
| LY6E | 98.5 | 64.6 | 1.3 ×10-4 | 4061 | Im | Lymphocyte antigen 6 complex, E | |
|
| |||||||
| CD38 | 90.1 | 39.9 | 4.5 ×10-3 | 952 | Im | 4, 6, 10, 30-32 | CD38, surface activation marker of T-cells |
|
| |||||||
| BST2 | 97.0 | 45.4 | 2.2 ×10-4 | 684 | Im, Sig | Bone marrow stromal cell antigen 2 | |
|
| |||||||
| APOL6 | 95.3 | 34.4 | 6.3 ×10-4 | 80830 | Im, Mito | Apolipoprotein L, 6 | |
|
| |||||||
| CCL8 | 60.0 | 29.4 | 2.8 ×10-3 | 6355 | Im, Sig | 10 | Chemokine (C-C motif) ligand 8 |
|
| |||||||
| IFIH1 | 89.3 | 61.8 | 1.3 ×10-5 | 64135 | Im | 33 | Interferon induced with helicase C domain 1 |
|
| |||||||
| MX2 | 98.4 | 53.3 | 3.4 ×10-5 | 4600 | Im | 6, 10, 34 | Myxovirus (influenza virus) resistance 2 (mouse) |
|
| |||||||
| IL1RN | 97.2 | 52.1 | 9.1 ×10-4 | 3557 | Im | 35 | Interleukin 1 receptor antagonist |
|
| |||||||
| Cell Cycle Genes | |||||||
|
| |||||||
| NTNG2 | 85.8 | 45.1 | 2.6 ×10-4 | 84628 | Cycle | Netrin G2 | |
|
| |||||||
| LAMP3 | 66.3 | 45.8 | 5.0 ×10-5 | 27074 | Cycle | 6 | Lysosomal-associated membrane protein 3 |
|
| |||||||
| FOXO6 | 51.0 | 22.8 | 1.5 ×10-3 | 100132074 | Cycle | Forkhead box protein O6 | |
|
| |||||||
| SOCS1 | 80.9 | 35.4 | 3.1 ×10-3 | 8651 | Cycle | 36 | Suppressor of cytokine signaling 1 |
|
| |||||||
| XAF1 | 94.4 | 65.4 | 2.7 ×10-5 | 54739 | Cycle | 6 | XIAP associated factor 1 |
|
| |||||||
| SHISA5 | 98.9 | 40.0 | 8.4 ×10-4 | 51246 | Cycle | Shisa homolog 5 (Xenopus laevis) | |
|
| |||||||
| Ubiquitin Cycle of Protein degradation Genes | |||||||
|
| |||||||
| ISG15 | 99.2 | 82.1 | 2.4 ×10-6 | 9636 | Ubq, Im | 4 | ISG15 ubiquitin-like modifier |
|
| |||||||
| USP18 | 87.3 | 62.6 | 6.1 ×10-6 | 11274 | Ubq | Ubiquitin specific peptidase 18 | |
|
| |||||||
| HERC6 | 89.8 | 59.3 | 1.8 ×10-5 | 55008 | Ubq | 6 | Hect domain and RLD 6 |
|
| |||||||
| HERC5 | 96.3 | 71.1 | 8.3 ×10-6 | 51191 | Ubq | 6 | Hect domain and RLD 5 |
|
| |||||||
| Transcriptional and Translation Regulation Genes | |||||||
|
| |||||||
| EIF2AK2 | 92.2 | 53.7 | 3.7 ×10-6 | 5610 | TransC | Eukaryotic translation initiation factor 2-alpha kinase 2 | |
|
| |||||||
| SAMD9L | 95.8 | 56.5 | 1.4 ×10-5 | 219285 | TransL | 6 | Sterile alpha motif domain containing 9-like |
|
| |||||||
| PRIC285 | 90.4 | 33.2 | 2.9 ×10-4 | 85441 | TransC | 6 | Peroxisomal proliferator-activated receptor A interacting cplx 285 |
|
| |||||||
| HES4 | 83.6 | 47.0 | 6.2 ×10-4 | 57801 | TransC | Hairy and enhancer of split 4 | |
|
| |||||||
| SAMD4A | 49.5 | 27.9 | 1.6 ×10-3 | 23034 | TransL | Sterile alpha motif domain containing 4A | |
|
| |||||||
| ZCCHC2 | 92.6 | 52.9 | 2.2 ×10-4 | 54877 | Zinc finger, CCHC domain containing 2 | ||
|
| |||||||
| Miscellaneous Genes | |||||||
|
| |||||||
| RTP4 | 94.2 | 58.9 | 6.3 ×10-6 | 64108 | Pr bind | Receptor (chemosensory) transporter protein 4 | |
|
| |||||||
| MT2A | 99.2 | 36.1 | 2.6 ×10-4 | 4502 | Met | 6 | Metallothionein 2A |
|
| |||||||
| TREX1 | 94.5 | 34.6 | 1.5 ×10-4 | 11277 | Repair | 10, 37 | Three prime repair exonuclease 1 |
|
| |||||||
| FANCA | 70.0 | 21.7 | 1.1 ×10-2 | 2175 | Repair | Fanconi anemia, complementation group A | |
|
| |||||||
| C18orf49 | 66.5 | 35.6 | 3.8 ×10-4 | 400653 | Sig | Zinc finger, CCHC domain containing 2 | |
|
| |||||||
| SCO2 | 98.0 | 50.5 | 8.0 ×10-4 | 9997 | Mito | SCO cytochrome oxidase deficient homolog 2 | |
|
| |||||||
| CNP | 96.0 | 32.5 | 1.6 ×10-3 | 1267 | 38 | 2’,3’-cyclic nucleotide 3’ phosphodiesterase | |
|
| |||||||
| EPSTI1 | 95.2 | 56.7 | 1.7 ×10-5 | 94240 | 6 | Epithelial stromal interaction 1 | |
|
| |||||||
| LGALS3BP | 85.3 | 49.6 | 3.0 ×10-5 | 3959 | 6 | Lectin, galactoside-binding, soluble, 3 binding protein | |
|
| |||||||
| KLHDC7B | 90.3 | 54.5 | 2.2 ×10-5 | 113730 | Kelch domain containing 7B | ||
Percentile gene expression represents the relative degree of expression in peripheral blood compared to the entire genome. The mean percent decrease in gene expression compared to baseline (0%) is presented with the adjusted P-value. The selected genes are down-regulated at 4, 8, and 24 weeks as well across all time points (by linear regression) with adjusted P<.01.
The adjusted-p-value is generated by linear regression, testing the fold change over all weeks. The range of unadjusted P-values is between 1.5×10-3 to 1.5×10-9.
Genes listed in bold have been previously reported in humans in relation to HIV infection with the literature reference (Ref).
Gene Ontology Abbreviations: Im=immune response; Cycle = cell cycle; Met=metabolism; Mito=mitochondrial; Pr Bind=protein binding; Repair=DNA repair; Sig=cell signaling; TransC=transcription regulation; TransL=translation regulation; Ubq=ubiquitin cycle
After adjustment for CD4 change, all of the above genes and seven additional genes were significantly down-regulated across all time points by linear regression. The seven additional genes were: PARP9, PARP12, PARP14, PHF11, SPTLC2, MOV10, and BIRC5.
Figure 1. ART-associated down-regulation of gene expression.
(a) This heat map presents the average fold changes in gene expression from baseline of the 53 genes which were consistently down-regulated with ART. These genes were discovered by linear regression (adjusted-P<.001) and further filtered by i) being down-regulated in direction at all time points after starting ART and ii) being testing for statistical significance for the change from baseline at individual 8 and 24 week time points by separate paired t–tests (adjusted-P<.05). Decreased gene expression is represented by the intensity of blue color. To the right of the panel, the functions and pathways are indicated for genes with known relationship to: interferon signaling, apoptosis, immune response, viral immune response, and T-cell activation. For changes by individual subject, refer to Supplemental Figure 2 online, and for representative examples of individual genes displayed via a line graph see Supplemental Figure 3 online. (b) Displays the top canonical (mechanistic) pathways down-regulated at 8-weeks of ART. The significance of the association between the data set and the canonical pathway was measured in 2 ways: First, the Fischer’s exact test was used to calculate a p-value determining the probability that the association between the genes in the dataset and the canonical pathway is explained by chance alone (Blue bars, primary y-axis). Second, a ratio of the number of genes from the data set that map to the pathway divided by the total number of genes that map to the canonical pathway is displayed as orange line (secondary y-axis).
Biological Processes
Among the 208 genes with differential regulation, 137 were mapped to a known biological process by gene ontology (108 down-regulated; 29 up-regulated) and 71 had unknown biological process. The known functions that were down-regulated included response to virus (n=16, P=10-12), response to other (foreign) organisms (n=17, P=10-9), responses to biotic stimulus (n=20, P=10-9), multi-organism processes (n=17, P=10-7), responses to stimulus (n=49, P=10-6), immune response (n=24, P=10-6), immune system process (n=26, P=10-4), and defense response (n=16, P=.007) (Table 3). Among the 160 consistently down-regulated genes, 29 (14%) were known to have HIV protein-interactions. Up-regulated genes did not map to consistent biologic processes, had largely unknown functions, and none had known HIV-protein interactions.
Table 3.
Functional categories of genes with altered expression following HIV therapy in persons with AIDS
| Categories | Genes | Fold Enrichment | Adjusted P-value |
|---|---|---|---|
| Down-regulated Gene Expression | 160 | <.05 | |
| Biologic Process | 108(mapped) | ||
| Response to virus | 16 | 23.0 | 1.2 × 10-12 |
| Response to other organism | 17 | 12.0 | 1.1 × 10-09 |
| Response to biotic stimulus | 20 | 9.1 | 1.2 × 10-09 |
| Multi-organism process | 17 | 7.6 | 8.9 × 10-07 |
| Response to stimulus | 49 | 2.3 | 4.7 × 10-06 |
| Immune response | 24 | 3.6 | 8.3 × 10-05 |
| Immune system process | 26 | 3.2 | 0.0002 |
| Defense response | 16 | 3.9 | 0.007 |
| Other – 38 processes (median) | (6) | (3.1) | >.99 |
| Unknown Biological Process | 52 | ||
| Canonical Pathways | |||
| Interferon Signaling | 6.5 × 10-9 | ||
| Role of Pattern Recognition for Viruses | 2.9 × 10-8 | ||
| Activation of Interferon Regulatory Factor by Cytosolic Pattern Recognition Receptors | 1.5 E-5 | ||
| Role of RIG1-like Receptors in Antiviral Innate immunity | 0.004 | ||
| NF-κB Signaling | 0.043 | ||
| Up-regulated Gene Expression* | 48 | <.05 | |
| Biological Process (mapped) | 24 | >.99 for all | |
| Molecular Function (mapped) | 31 | >.99 for all |
Gene ontology descriptions and pathways were obtained from the DAVID database and Ingenuity Pathway Analysis. This functional analysis identified the biological functions and canonical pathways that were most significant to the genes in the network. The network genes associated with Ingenuity, DAVID, Argonaute 2, TarBase, BIND, BIOGRID, Cognia, DIP, INTACT, Interactome studies, MINT, and MIPS databases were considered for the analysis.
Fischer’s exact test was used to calculate a P-value determining the probability that the number of gene transcripts in each biological function/pathway assigned to each network is due to chance alone with Benajamini-Hockberg adjustment.
The up-regulated genes reflect the differences observed at 8 weeks only whereas the down-regulated genes are across all time points by linear regression. No genes were up-regulated at the 2 or 4 time points when adjusting for multiple comparisons. The canonical pathways and physiologic systems of the up-regulated genes are largely unknown (99%) and not associated with any specific disease pathogenesis with the existing genomics knowledge in 2009. There are an additional 201 unmapped transcripts up-regulated for a total of 800 gene transcripts.
The annotated functions of the down-regulated genes suggest that many are involved in immune responses (both innate and anti-viral), cellular proliferation, and/or immune activation. The specific down-regulated transcripts encoded several important components of innate antiviral immune responses, including host restriction factors such as APOBEC3A, TRIM6, and TRIM38 (P<3×10-4) (Supplemental Table 2)23, 24 and chemokines such as CXCL9 and CXCL10. Paradoxically, transcripts that encode mediators of apoptosis (XAF1) as well as cell proliferation pathways were down-regulated during ART, highlighting that HIV pathogenesis is characterized by cellular activation and proliferation as well as cell death. Notably, 33% (52/160) of these down-regulated genes currently do not have known gene biologic ontology biological process assignments, and 31% (49/160) have unknown molecular function, demonstrating that numerous genes of unknown function are up-regulated by HIV-infection.
Interferons play prominent roles in innate and antiviral responses. Indeed, annotated pathway analyses revealed that transcripts encoding multiple components of the canonical (mechanistic) pathway for type-I interferon signaling pathways were coordinately down-regulated at all points with ARV-associated viral suppression (Fig. 1b) (Fig. 2) (P<.001) Changes in the expression of transcripts encoding components of other pathways involved with innate control of viral replication were seen at later time points. For example, transcripts encoding components of the protein ubiquitination pathway (e.g. TRIM5, CUL5, and UBR2) and APOBEC3G were also coordinately down-regulated, but only after 8 weeks. In this context, decreased protein ubiquitination/degradation likely reflects decreased cell death after virologic suppression.25 Overall, we identified numerous novel ART-responsive genes that encode important components of the host response to HIV infection. That these changes occurred in the context of ART-induced suppression of HIV replication suggests that these processes were up regulated upon exposure to HIV and subsequently down-regulated when HIV was blocked with ART. Thus, ART serves as a “reverse challenge” to identify HIV-induced responses.
Figure 2. Coordinate down-regulation of genes involved in interferon signaling and innate immune responses.
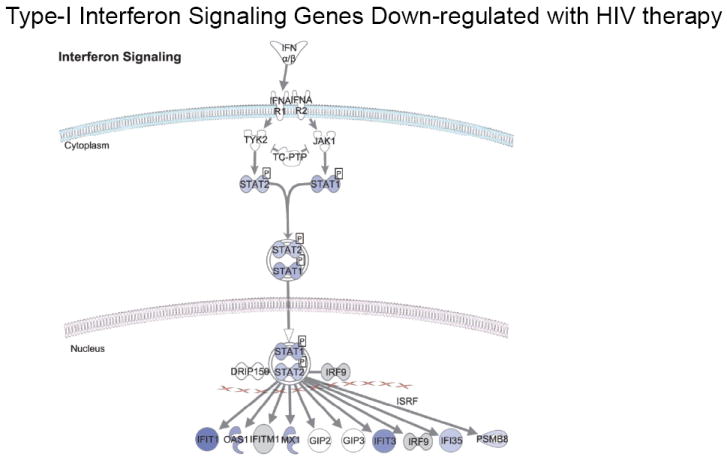
The biological network of genes within the type-I interferon-signaling pathway is shown. Genes colored in blue were down-regulated with ART, with the intensity of blue reflecting the degree of down-regulation.
Relationship with T-cell Activation
The majority (71%) of the genes down-regulated in response to ART are regulated during T cell activation. Of the 208 transcripts influenced by ART, the expression of 154 transcripts has been studied over time after ex vivo stimulation of primary human T-cells with anti-CD3 and anti-CD28 antibodies using an older generation Affymetrix microarray.22 Of the 154 transcripts, 110 showed alteration in expression compared to resting/unstimulated T-cells (P< .05) and 84 transcripts exhibited very early changes in gene expression within 30 minutes of stimulation (62 up-regulated, 22 down-regulated with a fold change of >1.5) (Supplemental_Table2). These results suggest that many of the ART-responsive genes are regulated during T-cell activation.
Protein levels of ART-responsive cytokines
For some of the ART-responsive genes encoding secreted proteins, we measured protein levels in serum to determine if changes in RNA expression correlated with changes in protein expression in order to provide validation for our results. We serially measured protein levels of 28 serum cytokines in the same 10 patients. Serum protein levels for all of the cytokines tested are provided in Supplemental Table 3. Consistent with the down-regulation in gene expression, we identified decreasing serum levels of the chemokines CXCL9 (coefficient estimates at week 8 = -1.348, week 24 = -2.752, P<.0001) and CXCL10 (coefficient estimates at week 8 = -2.327, week 24 = -2.891, P<.0001) over the first 24 weeks of ART. Thus, serum levels of CXCL10 (also called IP-10) decreased by 80% (95% CI: 33% to 118%) whereas gene expression decreased by 101%. Levels of IL-12, IL-1ra, IL-6, TNF-α, and TRAIL showed neither a statistically significant decline with ART by microarray nor by serum protein concentrations (P>0.2). Thus, in persons with AIDS in this African cohort, inflammatory serum cytokine levels did not appreciably decrease during the first 24 weeks of ART nor did the gene expression of these cytokines change. Thus ART-associated changes in protein levels of serum cytokines correlated well with changes in RNA expression.
DISCUSSION
AIDS is caused by profound T-cell loss and immunosuppression in the context of dramatic chronic immune activation. HIV infection activates antiviral immune responses that combat the virus, while at the same time, the virus promotes cell death through direct killing of infected cells and by indirect apoptotic mechanisms. We propose that the genes that were up-regulated in response to the virus during uncontrolled viremia are subsequently down-regulated when viral replication is blocked with ART. Thus, evaluating the changes in gene expression in peripheral blood in response to ART can provide insight into HIV pathogenesis by identifying gene expression pathways that were turned on in response to HIV infection.
We performed microarray gene expression analysis over time in 10 patients with AIDS from Sub-Saharan Africa who were successfully treated with ART. This study represents the largest human prospective longitudinal characterization of ART-related gene expression to date. We identified 160 genes down-regulated following ART, including 87 that are novel HIV/ART-responsive genes, not previously reported in in vitro or in vivo studies.2, 4, 6, 9, 27-29, 38 The major functions of the ART-responsive genes include: 1) innate anti-viral immune responses, including interferon responses, 2) immune activation and cellular proliferation/apoptosis, and 3) unknown function. The diversity of ART-responsive genes reveals the breadth of the host responses to HIV and the ability of ART to reverse these effects.
Many of the novel down-regulated ART-responsive genes that we identified encode regulators of innate immune responses, including innate antiviral responses. For example, transcripts encoding APOBEC3A and APOBEC3G were down-regulated after ART. APOBEC3G, which inhibits HIV replication through its cytidine deaminase activity,39 was down-regulated only at the 8 week time-point, whereas the related APOBEC3A, which has no known anti-HIV activity,40 was down-regulated at all time points. HIV has developed mechanisms to counteract effective antiviral restriction by APOBEC proteins. For example, the HIV Vif protein binds directly to APOBEC3G and inhibits its function by targeting APOBEC3G for degradation via the ubiquitin-proteasome pathway.41-43 Interestingly, we found expression of genes encoding components of this pathway,44 including CUL5, RBX1, SKP1,and UBR2 were down-regulated after 8 weeks of ART. Since the ubiquitin-proteasome pathway promotes the degradation of APOBEC3G by Vif,41-43, 45 the induction of these genes could represent an antiviral response. Our results suggest that genes controlling APOBEC and the protein degradation pathway that targets Vif are coordinately induced in response to HIV infection, since the expression of these genes was abrogated after ART. These responses likely occur as a host response to try to limit viral replication.
Expression of the TRIM5 restriction factor gene was also down-regulated at the 8 week time-point. In many species, TRIM5 prevents retroviral infection by binding to retroviruses and recruits the proteasome to degrade viral proteins.23, 24, 46 Although human TRIM5 has only weak activity against HIV, TRIM5 is effective at preventing other retroviruses from infecting human cells.23, 24, 47, 48 Another restriction factor down-regulated was TRIM22. In the context of HIV, TRIM22 is induced by interferons to disrupt viral replication by preventing the HIV structural protein Gag from trafficking to the cell surface.49 TRIM22 had high pre-ART levels of expression in peripheral blood (>99th percentile), and was down-regulated at 4 weeks, 8 weeks, and overall. The induction of viral restriction genes such as TRIM genes following HIV infection represents additional innate antiviral responses that attempt to limit viral replication, and these responses are subsequently down-regulated with ART.
Numerous other genes encoding components of innate responses were also down-regulated. For example, 16 down-regulated genes included pattern recognition receptors. ADAR, a gene up-regulated in response to poliovirus dsRNA and SIV in African green monkeys, 10, 50 which may modulate the post-transcriptional regulation of HIV-1 env gene expression,51 was down-regulated. Additional PRR genes involved in innate responses to dsRNA that were down-regulated included DDX58 (also known as RIG-1) and thereafter IRF7.5, 9, 10, 52 Although DDX58 has not been previously associated with HIV infection, DDX58 is involved in antiviral innate immunity.53, 54 We also found the transcript encoding IFIH1, a pattern recognition receptor functioning as an IFN-induced helicase, was down-regulated. IFIH1 has been associated with increased HIV viral mRNA expression in vitro,33 suggesting that HIV may usurp this antiviral response to promote viral replication. Since viral replication is virtually stopped by effective ART, understandably, many of these HIV-induced antiviral and innate defense pathways are turned off with ART-mediated viral suppression. These PRR genes are most likely induced as part of a host warning system triggered by HIV to allow other foreign material to be rapidly recognized.
Genes encoding multiple components of interferon-response pathways were also down-regulated after ART, suggesting that these pathways were induced by HIV infection and are components of the innate anti-viral response to HIV (Fig. 3). As circulating HIV virions decline after ART, decreased expression of type-1 interferon signaling genes, including IFI27, ISG15, ISG20 and IFI44L, occurs, likely due to lack of antigenic challenge once the viral stimulus is eliminated. ISG20 is an IFN-induced 3’→5’ exonuclease specific for ssRNA that is up-regulated with HIV-infection in vitro.55 ISG15 has known anti-viral properties in the late stage of HIV-1 assembly by inhibiting the ubiquitination of HIV Gag during endosomal trafficking.56, 57 These interferon signaling genes are known to be activated in B-lymphocytes in response to HIV-viremia.4 Once CD4+ cells are infected by HIV, interferon responses induce apoptosis of infected cells as an antiviral response facilitating the elimination of infected cells and preventing both viral replication and infection of new cells.55 The upregulation of interferon and interferon response pathways may help limit viral replication, but the associated apoptosis probably contributes to immune destruction and immunosuppression.
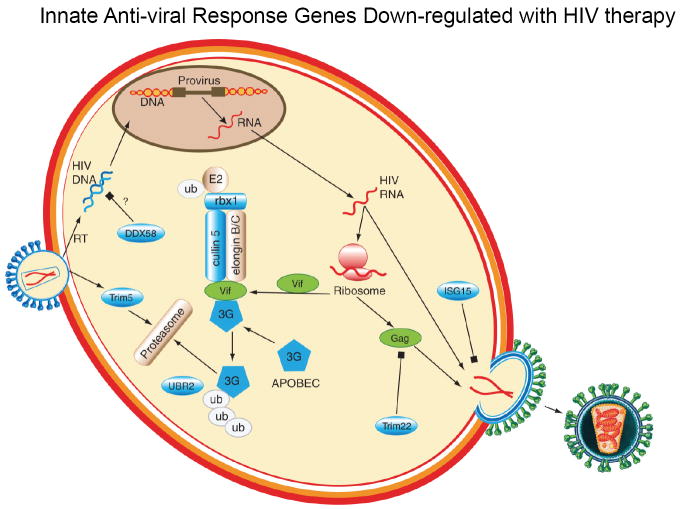
Figure 3. Innate anti-viral immune responses down-regulated with HIV therapy.
Known innate anti-viral immune response genes which are down-regulated with ART are shown in blue and HIV proteins are shown in green. After viral entry, TRIM5 binds to the viral capsule and inhibits infection, although it only a weak inhibitor of HIV infection. DDX58 primarily binds to dsRNA viruses but may also bind to dsDNA in the cytoplasm to activate downstream IRF7. Post HIV RNA-transcription, the HIV protein Vif (virion infectivity factor) binds to APOBEC3G targeting it for destruction by the ubiquitin-proteasome pathway via known interactions with CUL5 and RBX1 and recognition of the E3-ligase complex by UBR2. Additional partial innate defenses include TRIM22 which blocks the trafficking of HIV Gag protein and ISG16 which interferes with viral budding. RT=reverse transcription.
Since HIV pathogenesis is characterized by a state of chronic immune activation, it is not surprising that numerous transcripts related to immune activation were prominently down-regulated with ART. Of 160 down-regulated genes, 110 are T-cell activation genes that we have shown previously to be up-regulated after ex vivo stimulation of primary human T-cells with anti-CD3 and anti-CD28 antibodies.22 Several transcripts encoding well-known markers of cellular activation, including LAMP3 (CD63), were down-regulated over time after initiation of ART,58, 59 demonstrating that ART produces an overall decrease in immune activation. We also found that immune activation genes involved in cellular proliferation/apoptosis pathways such as the NF-κB pathway and XAF1 were down-regulated. NF-κB is a multi-subunit transcription factor that regulates the expression of numerous genes involved in immune activation, including interleukin-2 and TNF-α.60 NF-κB acts as a transcription factor activating HIV transcription and facilitating viral replication.60 In addition, NF-κB is involved in stress responses, cell death, and virus-mediated apoptosis.60 Another down-regulated apoptosis regulator was XAF1, an IFN-stimulated gene that mediates type-1 IFN-induced sensitization to TRAIL, thereafter causing apoptosis.61 Chronic immune activation and the associated activation-induced apoptosis is a major contributor to immune cellular depletion in HIV-infected persons. Our results suggest that although peripheral CD4+ T-cell counts increase following ART, the overall expression of proliferation-related and apoptosis-related transcripts in peripheral blood decreases. Thus, the contributions of cellular proliferation, activation and apoptosis to promoting viral replication and causing immunodeficiency are abrogated during ART.
A large percentage of ART-responsive genes identified (33%) have unknown biological processes and functions. The finding that ART causes down-regulation of these genes suggests that they had been induced by HIV and may therefore play roles in the host response to HIV. It is possible, however, that the expression of some of these genes may have changed as a direct consequence of exposure of human cells to antiretroviral agents, rather than as a consequence of ART-mediated viral suppression. These genes with unknown function may be targets for future studies to explore their function and role in HIV pathogenesis. A large number of genes of the genes we identified to be down-regulated following ART (and presumably up-regulated during HIV infection) are newly described in this context or have unknown function, highlighting that we are only beginning to unravel the complex nature of immune regulation during HIV infection.
Prior recognition of ART-responsive genes in human subjects has been limited and our results significantly extend our knowledge about the influence of ART on gene expression, while some of our results confirm results from previous studies. For example, of our 160 genes that were down-regulated with ART, 43 overlap with genes that up-regulated in CD4+ cells in association with increasing HIV viral load,6 suggesting that our hypothesis that these genes that were down-regulated in response to ART, had previously been up-regulated in response the HIV.
Our results also confirm the previous report from Li et al. showing that IFN-related genes, such as OAS, STAT, IRF7, CCR1, CCR5, CXCL9, CXCL10, and MX1 genes, were down-regulated in lymph node biopsy specimens after ART,2 and these results are strikingly similar to our study. In our prospective, longitudinal study, however, we identified many heretofore unrecognized ART-responsive genes in humans. This greater range of response was revealed in part because we utilized the current Affymetrix microarray which probed the entire 38,572-gene genome, whereas Li, et al., examined the expression of only 9,838 genes with the earlier generation U95A microarray. Indeed, 35% of ART-responsive genes we identified were not represented on the earlier microarray. In contrast to our results, Li et al. also found down-regulation of the transcripts encoding the cytokines IFN-γ, MIP-1β, and TRAIL in lymphatic tissue of persons with less advanced HIV;2 findings we did not observe in peripheral blood mRNA or serum cytokines. We sampled blood from 10 persons with AIDS in Sub-Saharan Africa (median CD4+ 30 cells/μL) over multiple standardized time points for 24 weeks, whereas, Li et al. tested lymphatic tissues from 5 U.S. subjects with less advanced HIV infection (mean CD4+ of 415 ±226/μL) and evaluated changes at 1 month only.2 The different results likely derive from differences in sample type, stage of infection, and timing of ART.
In conclusion, we characterized the host response to HIV through a reverse challenge approach by examining gene expression changes associated with ART-induced viral suppression in persons with AIDS. We identified numerous transcripts encoding important components of innate antiviral pathways, including antiviral restriction factors and pattern recognition receptors, which were not previously known to be regulated by HIV infection. Our results confirmed existing paradigms suggesting that chronic immune activation, cellular proliferation, and apoptosis play roles in HIV pathogenesis, and we extended knowledge in the field by defining gene expression pathways and components that regulate these responses during HIV infection. Our results also provide information about the normal response to ART in AIDS patients that may be useful for identifying abnormal responses to ART in persons who develop adverse events such as immune reconstitution inflammatory syndrome, drug reactions or new AIDS-related opportunistic infections. For example, AIDS patients with these adverse events on ART might not show the characteristic gene expression response to ART, but rather may express abnormal gene expression signatures of immune activation or inflammation that could be used to identify or diagnose adverse events.
Supplementary Material
Acknowledgments
We wish to thank Ashley Haase, Quinsheng Li, and Anne Marie Weber-Main for critically reading this manuscript This work was supported in part by the University of Minnesota Center for Infectious Diseases and Microbiology Translational Research (PRB) and Academic Health Center (PRB,DBM), Tibotec REACH Initiative (PRB, DRB, DBM, AK), National Institutes of Health (R03AI078750 PRB; T32AI055433; L30AI066779; K23AI073192: DRB; R01HD059527 ENJ), Accordia Global Health Foundation (AK, DBM), Minnesota Medical Foundation through its grants program and through the Robert and Mabel Bohjanen Immune Reconstitution Research Fund (PRB,DRB,DBM), Gilead Infectious Disease Scholarship Program (DBM), the Mucosal and Vaccine Research Program Colorado (ENJ) and the Veterans Affairs Research Service (ENJ).
Footnotes
Conflicts of Interest: No conflicts of interest by any author.
Presentation: This was presented as an abstract at the 2008 International AIDS Conference in Mexico City.
References
- 1.Schacker TW, Nguyen PL, Beilman GJ, et al. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J Clin Invest. 2002;110(8):1133–1139. doi: 10.1172/JCI16413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Q, Schacker T, Carlis J, Beilman G, Nguyen P, Haase AT. Functional genomic analysis of the response of HIV-1-infected lymphatic tissue to antiretroviral therapy. J Infect Dis. 2004;189(4):572–582. doi: 10.1086/381396. [DOI] [PubMed] [Google Scholar]
- 3.Vahey MT, Ockenhouse CF, Wang Z, et al. Impact of antiretroviral treatment on gene expression in peripheral blood mononuclear cells from SIVmac251-infected macaques. J Infect Dis. 2007;196(3):384–393. doi: 10.1086/519388. [DOI] [PubMed] [Google Scholar]
- 4.Moir S, Malaspina A, Pickeral OK, et al. Decreased survival of B cells of HIV-viremic patients mediated by altered expression of receptors of the TNF superfamily. J Exp Med. 2004;200(5):587–599. doi: 10.1084/jem.20032236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montano M, Rarick M, Sebastiani P, et al. Gene-expression profiling of HIV-1 infection and perinatal transmission in Botswana. Genes Immun. 2006;7(4):298–309. doi: 10.1038/sj.gene.6364297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rotger M, Dang KK, Fellay J, et al. Genome-Wide mRNA Expression Correlates of Viral Control in CD4+ T-Cells from HIV-1-Infected Individuals. PLoS Pathog. 2010;6(2):e1000781. doi: 10.1371/journal.ppat.1000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giri MS, Nebozhyn M, Showe L, Montaner LJ. Microarray data on gene modulation by HIV-1 in immune cells: 2000-2006. J Leukoc Biol. 2006;80(5):1031–1043. doi: 10.1189/jlb.0306157. [DOI] [PubMed] [Google Scholar]
- 8.Guadalupe M, Reay E, Sankaran S, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77(21):11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Q, Smith AJ, Schacker TW, et al. Microarray analysis of lymphatic tissue reveals stage-specific, gene expression signatures in HIV-1 infection. J Immunol. 2009;183(3):1975–1982. doi: 10.4049/jimmunol.0803222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacquelin B, Mayau V, Targat B, et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009;119(12):3544–3555. doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Consortium M, Shi L, Reid LH, et al. The MicroArray Quality Control (MAQC) project shows inter- and intraplatform reproducibility of gene expression measurements. Nat Biotechnol. 2006;24(9):1151–1161. doi: 10.1038/nbt1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen JJ, Hsueh HM, Delongchamp RR, Lin CJ, Tsai CA. Reproducibility of microarray data: a further analysis of microarray quality control (MAQC) data. BMC Bioinformatics. 2007;8:412. doi: 10.1186/1471-2105-8-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spanish Society of Infectious D. Clinical M, Orta Mira N, et al. Analysis of the results of the SEIMC External Quality Control Program for HIV-1 and HCV viral loads, 2007. Enferm Infecc Microbiol Clin. 2008;26(Suppl 13):8–13. [PubMed] [Google Scholar]
- 14.Brambilla D, Jennings C, Aldrovandi G, et al. Multicenter evaluation of use of dried blood and plasma spot specimens in quantitative assays for human immunodeficiency virus RNA: measurement, precision, and RNA stability. J Clin Microbiol. 2003;41(5):1888–1893. doi: 10.1128/JCM.41.5.1888-1893.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baechler EC, Batliwalla FM, Karypis G, et al. Expression levels for many genes in human peripheral blood cells are highly sensitive to ex vivo incubation. Genes Immun. 2004;5(5):347–353. doi: 10.1038/sj.gene.6364098. [DOI] [PubMed] [Google Scholar]
- 16.Debey S, Schoenbeck U, Hellmich M, et al. Comparison of different isolation techniques prior gene expression profiling of blood derived cells: impact on physiological responses, on overall expression and the role of different cell types. Pharmacogenomics J. 2004;4(3):193–207. doi: 10.1038/sj.tpj.6500240. [DOI] [PubMed] [Google Scholar]
- 17.Feezor RJ, Baker HV, Mindrinos M, et al. Whole blood and leukocyte RNA isolation for gene expression analyses. Physiol Genomics. 2004;19(3):247–254. doi: 10.1152/physiolgenomics.00020.2004. [DOI] [PubMed] [Google Scholar]
- 18.Chai V, Vassilakos A, Lee Y, Wright JA, Young AH. Optimization of the PAXgene blood RNA extraction system for gene expression analysis of clinical samples. J Clin Lab Anal. 2005;19(5):182–188. doi: 10.1002/jcla.20075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19(2):185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 20.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B. 1995;57(1):289–300. [Google Scholar]
- 21.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 22.Raghavan A, Dhalla M, Bakheet T, et al. Patterns of coordinate down-regulation of ARE-containing transcripts following immune cell activation. Genomics. 2004;84(6):1002–1013. doi: 10.1016/j.ygeno.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Huthoff H, Towers GJ. Restriction of retroviral replication by APOBEC3G/F and TRIM5alpha. Trends Microbiol. 2008;16(12):612–619. doi: 10.1016/j.tim.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langelier CR, Sandrin V, Eckert DM, et al. Biochemical characterization of a recombinant TRIM5alpha protein that restricts human immunodeficiency virus type 1 replication. J Virol. 2008;82(23):11682–11694. doi: 10.1128/JVI.01562-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82(2):373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 26.Imbeault M, Ouellet M, Tremblay MJ. Microarray study reveals that HIV-1 induces rapid type-I interferon-dependent p53 mRNA up-regulation in human primary CD4+ T cells. Retrovirology. 2009;6:5. doi: 10.1186/1742-4690-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rempel H, Calosing C, Sun B, Pulliam L. Sialoadhesin expressed on IFN-induced monocytes binds HIV-1 and enhances infectivity. PLoS ONE. 2008;3(4):e1967. doi: 10.1371/journal.pone.0001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Kuyl AC, van den Burg R, Zorgdrager F, Groot F, Berkhout B, Cornelissen M. Sialoadhesin (CD169) expression in CD14+ cells is upregulated early after HIV-1 infection and increases during disease progression. PLoS ONE. 2007;2(2):e257. doi: 10.1371/journal.pone.0000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silverman RH, Sengupta DN. Translational regulation by HIV leader RNA, TAT, and interferon-inducible enzymes. J Exp Pathol. 1990;5(2):69–77. [PubMed] [Google Scholar]
- 30.Vollbrecht T, Brackmann H, Henrich N, et al. Impact of changes in antigen level on CD38/PD-1 co-expression on HIV-specific CD8 T cells in chronic, untreated HIV-1 infection. J Med Virol. 2010;82(3):358–370. doi: 10.1002/jmv.21723. [DOI] [PubMed] [Google Scholar]
- 31.Coetzee LM, Tay SS, Lawrie D, Janossy G, Glencross DK. From research tool to routine test: CD38 monitoring in HIV patients. Cytometry B Clin Cytom. 2009;76(6):375–384. doi: 10.1002/cyto.b.20478. [DOI] [PubMed] [Google Scholar]
- 32.Tuaillon E, Al Tabaa Y, Baillat V, et al. Close association of CD8+/CD38 bright with HIV-1 replication and complex relationship with CD4+ T-cell count. Cytometry B Clin Cytom. 2009;76B(4):249–260. doi: 10.1002/cyto.b.20467. [DOI] [PubMed] [Google Scholar]
- 33.Cocude C, Truong MJ, Billaut-Mulot O, et al. A novel cellular RNA helicase, RH116, differentially regulates cell growth, programmed cell death and human immunodeficiency virus type 1 replication. J Gen Virol. 2003;84(Pt 12):3215–3225. doi: 10.1099/vir.0.19300-0. [DOI] [PubMed] [Google Scholar]
- 34.Kalia V, Sarkar S, Gupta P, Montelaro RC. Rational site-directed mutations of the LLP-1 and LLP-2 lentivirus lytic peptide domains in the intracytoplasmic tail of human immunodeficiency virus type 1 gp41 indicate common functions in cell-cell fusion but distinct roles in virion envelope incorporation. J Virol. 2003;77(6):3634–3646. doi: 10.1128/JVI.77.6.3634-3646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kreuzer KA, Dayer JM, Rockstroh JK, Sauerbruch T, Spengler U. The IL-1 system in HIV infection: peripheral concentrations of IL-1beta, IL-1 receptor antagonist and soluble IL-1 receptor type II. Clin Exp Immunol. 1997;109(1):54–58. doi: 10.1046/j.1365-2249.1997.4181315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yadav A, Fitzgerald P, Sajadi MM, et al. Increased expression of suppressor of cytokine signaling-1 (SOCS-1): A mechanism for dysregulated T helper-1 responses in HIV-1 disease. Virology. 2009;385(1):126–133. doi: 10.1016/j.virol.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan N, Cherepanov P, Daigle JE, Engelman A, Lieberman J. The SET complex acts as a barrier to autointegration of HIV-1. PLoS Pathog. 2009;5(3):e1000327. doi: 10.1371/journal.ppat.1000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zauli G, Milani D, Mirandola P, et al. HIV-1 Tat protein down-regulates CREB transcription factor expression in PC12 neuronal cells through a phosphatidylinositol 3-kinase/AKT/cyclic nucleoside phosphodiesterase pathway. FASEB J. 2001;15(2):483–491. doi: 10.1096/fj.00-0354com. [DOI] [PubMed] [Google Scholar]
- 39.Mangeat B, Turelli P, Caron G, Friedli M, Perrin L, Trono D. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature. 2003;424(6944):99–103. doi: 10.1038/nature01709. [DOI] [PubMed] [Google Scholar]
- 40.Goila-Gaur R, Khan M, Miyagi E, Kao S, Strebel K. Targeting APOBEC3A to the viral nucleoprotein complex confers antiviral activity. Retrovirology. 2007;4(1):61. doi: 10.1186/1742-4690-4-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu X, Yu Y, Liu B, et al. Induction of APOBEC3G Ubiquitination and Degradation by an HIV-1 Vif-Cul5-SCF Complex. Science. 2003;302(5647):1056–1060. doi: 10.1126/science.1089591. [DOI] [PubMed] [Google Scholar]
- 42.Mehle A, Goncalves J, Santa-Marta M, McPike M, Gabuzda D. Phosphorylation of a novel SOCS-box regulates assembly of the HIV-1 Vif-Cul5 complex that promotes APOBEC3G degradation. Genes & Development. 2004;18(23):2861–2866. doi: 10.1101/gad.1249904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao Z, Ehrlich E, Yu Y, et al. Assembly of HIV-1 Vif-Cul5 E3 ubiquitin ligase through a novel zinc-binding domain-stabilized hydrophobic interface in Vif. Virology. 2006;349(2):290–299. doi: 10.1016/j.virol.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Ockenhouse CF, Bernstein WB, Wang Z, Vahey MT. Functional genomic relationships in HIV-1 disease revealed by gene-expression profiling of primary human peripheral blood mononuclear cells. J Infect Dis. 2005;191(12):2064–2074. doi: 10.1086/430321. [DOI] [PubMed] [Google Scholar]
- 45.Zhang W, Chen G, Niewiadomska AM, Xu R, Yu X-F. Distinct Determinants in HIV-1 Vif and Human APOBEC3 Proteins Are Required for the Suppression of Diverse Host Anti-Viral Proteins. PLoS ONE. 2008;3(12):e3963. doi: 10.1371/journal.pone.0003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sawyer SL, Emerman M, Malik HS. Discordant evolution of the adjacent antiretroviral genes TRIM22 and TRIM5 in mammals. PLoS Pathog. 2007;3(12):e197. doi: 10.1371/journal.ppat.0030197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaiser SM, Malik HS, Emerman M. Restriction of an extinct retrovirus by the human TRIM5alpha antiviral protein. Science. 2007;316(5832):1756–1758. doi: 10.1126/science.1140579. [DOI] [PubMed] [Google Scholar]
- 48.Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427(6977):848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- 49.Barr SD, Smiley JR, Bushman FD. The Interferon Response Inhibits HIV Particle Production by Induction of TRIM22. PLoS Pathog. 2008;4(2):e1000007. doi: 10.1371/journal.ppat.1000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morrissey LM, Kirkegaard K. Regulation of a double-stranded RNA modification activity in human cells. Mol Cell Biol. 1991;11(7):3719–3725. doi: 10.1128/mcb.11.7.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phuphuakrat A, Kraiwong R, Boonarkart C, Lauhakirti D, Lee TH, Auewarakul P. Double-stranded RNA adenosine deaminases enhance expression of human immunodeficiency virus type 1 proteins. J Virol. 2008;82(21):10864–10872. doi: 10.1128/JVI.00238-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harenberg A, Guillaume F, Ryan EJ, Burdin N, Spada F. Gene profiling analysis of ALVAC infected human monocyte derived dendritic cells. Vaccine. 2008;26(39):5004–5013. doi: 10.1016/j.vaccine.2008.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gack MU, Shin YC, Joo C-H, et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446(7138):916–920. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- 54.Biacchesi S, LeBerre M, Lamoureux A, et al. MAVS Plays a Major Role in Induction of the Fish Innate Immune Response against RNA and DNA Viruses. J Virol. 2009:JVI.00404–00409. doi: 10.1128/JVI.00404-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Espert L, Degols G, Lin YL, Vincent T, Benkirane M, Mechti N. Interferon-induced exonuclease ISG20 exhibits an antiviral activity against human immunodeficiency virus type 1. J Gen Virol. 2005;86(Pt 8):2221–2229. doi: 10.1099/vir.0.81074-0. [DOI] [PubMed] [Google Scholar]
- 56.Okumura A, Lu G, Pitha-Rowe I, Pitha PM. Innate antiviral response targets HIV-1 release by the induction of ubiquitin-like protein ISG15. Proc Natl Acad Sci U S A. 2006;103(5):1440–1445. doi: 10.1073/pnas.0510518103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harty RN, Pitha PM, Okumura A. Antiviral Activity of Innate Immune Protein ISG15. J Innate Immun. 2009;1(5):397–404. doi: 10.1159/000226245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen H, Dziuba N, Friedrich B, et al. A critical role for CD63 in HIV replication and infection of macrophages and cell lines. Virology. 2008;379(2):191–196. doi: 10.1016/j.virol.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaufmann GR, Zaunders JJ, Cunningham P, et al. Rapid restoration of CD4 T cell subsets in subjects receiving antiretroviral therapy during primary HIV-1 infection. AIDS. 2000;14(17):2643–2651. doi: 10.1097/00002030-200012010-00003. [DOI] [PubMed] [Google Scholar]
- 60.Bren GD, Trushin SA, Whitman J, Shepard B, Badley AD. HIV gp120 induces, NF-kappaB dependent, HIV replication that requires procaspase 8. PLoS ONE. 2009;4(3):e4875. doi: 10.1371/journal.pone.0004875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Micali OC, Cheung HH, Plenchette S, et al. Silencing of the XAF1 gene by promoter hypermethylation in cancer cells and reactivation to TRAIL-sensitization by IFN-beta. BMC Cancer. 2007;7:52. doi: 10.1186/1471-2407-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.