Abstract
Objective
Bone lead is a cumulative measure of lead exposure that can also be remobilized. We examined repeated measures of bone lead over 11 years to characterize long-term changes and identify predictors of tibia and patella lead stores in an elderly male population.
Methods
Lead was measured every 3–5 years by k-x-ray fluorescence and mixed-effect models with random effects were used to evaluate change over time.
Results
554 participants provided up to 4 bone lead measurements. Final models predicted a −1.4% annual decline (95%CI: −2.2,−0.7) for tibia lead and piecewise linear model for patella with an initial decline of 5.1% per year (95%CI: −6.2,−3.9) during the first 4.6 years but no significant change thereafter (−0.4% (95% CI: −2.4, 1.7)).
Conclusions
These results suggest that bone lead half-life may be longer than previously reported.
Introduction
Bone lead (Pb) has been widely used in epidemiologic studies as a surrogate measure of long-term, chronic lead exposure. While blood lead provides an integrated measure of circulating lead, representative both of recent and chronic exposures, over 94% of lead is stored in bone where it has a half life of years to decades.1 Bone lead can be assessed using minimally invasive technology such as k-xray fluorescence (K-XRF),2 but the rate at which bone lead changes over time in individuals with historical exposure but low current exposure remains unclear. This has implications for the use of bone lead as an exposure metric in subjects with varying ages, particularly in the setting of cohort studies designed to assess chronic health effects.
Regulatory efforts beginning in the 1970s directly resulted in dramatic decreases in ambient exposures to lead. However, among older adults with higher cumulative body burden, bone lead remains a critical source of exposure through bone remodeling.3 Studies have shown that bone lead levels among older adults are associated with numerous adverse health effects including decrements in cognitive function and risk of cardiovascular disease.4 Furthermore, these harmful effects have been associated with lead levels once considered safe, and no threshold for the effect of lead has been identified.5, 6 Although biokinetic models have been developed to assess the toxicokinetics of bone lead over time,7, 8 there is little data from environmentally exposed human populations. Moreover, implementation of these models requires knowledge of the appropriate exposure and kinetic parameters. Thus, the utility of these models in predicting lead mobilization in observational settings remains limited.9
The purpose of this study was to characterize the longitudinal change in cortical and trabecular bone lead levels within a population of community exposed elderly men. Turnover is thought to occur at a higher rate in trabecular bone than in cortical bone, due to its higher rate of metabolic activity.10, 11 A previous study within this population investigated changes in bone lead after only 3 years of follow up among 70 subjects.11 We expanded upon this study to include additional measures obtained during the period at which ambient exposures had virtually ceased. We hypothesized that lead levels over an 11 year period would follow a trajectory similar to the one seen after 3 years, whereby trabecular bone would decrease slowly over years to decades and little or no change in cortical bone lead would be observed. We also sought to determine key dietary, socioeconomic and lifestyle factors which would influence changes in bone lead levels during the time of low exogenous exposures. We then reasoned that cortical bone in an older population could be a surrogate for lifetime exposure which could be utilized to assess exposure-response relationships in health effects studies.
Methods
Study population
This study utilizes data from the Normative Aging Study (NAS), a longitudinal closed-cohort study of aging established by the Veterans Administration in 1963, which enrolled men ages 21 to 80 living in the Boston metropolitan area. Participants were recruited to represent a broad range of socioeconomic characteristics in terms of education and occupation.12 Every 3 to 5 years, participants returned for follow up visits and filled out questionnaires on smoking history, education level, food intake and other risk factors for chronic disease. This study was approved by the Human Research Committees of Brigham and Women’s Hospital, the Department of Veterans Affairs Outpatient Clinic in Boston, and the Harvard School of Public Health and all participants provided written informed consent. Age, smoking, medication use, physical activity, and dietary intake were assessed at the time of each visit. The cumulative number of pack-years smoked was calculated for current and former smokers. Micro- and macronutrient information was obtained from food frequency questionnaire data, which has been shown to more accurately reflect usual dietary intake than 24 hour recall.13 Information on the reproducibility of this method in men has been published elsewhere.14
Bone lead measurement by K-XRF
Beginning in 1991, participants were invited to undergo bone and blood lead measurements at the Ambulatory Clinical Research Center of the Brigham and Women’s Hospital. K-XRF was used to assess bone lead levels for each participant at 2 sites: mid-tibia shaft and patella. These sites have been selected for bone lead measurement because they are comprised of primarily cortical and trabecular bone respectively.
The K-XRF instrument provides an unbiased estimate of bone lead levels normalized to bone mineral content and expressed as micrograms of lead per gram of bone mineral (µg/g).15 Measurements from 1991 through the first half of 1999 were obtained using the Abiomed technology (ABIOMED, Inc., Danvers, Massachusetts), while those performed later were obtained using a newly designed K-XRF instrument and methodology and intercalibrated with the ABIOMED through a standardized and validated set of calibration procedures. Both of these technologies provide a measure of uncertainty that is calculated using repeated measures taken over a 30-minute session and is equivalent to a single standard deviation if multiple measurements were done. Negative estimates of bone lead concentrations may occur when lead values are close to zero. Use of all point estimates without imposition of a minimum detectable limit has been identified as the most appropriate method of using these data in epidemiologic studies; therefore negative values were included in our analyses.16 A 15-parts per million (ppm) lead-doped phantom was positioned and measured 20 consecutive times a week to check the precision and accuracy of the result as a quality control. Once every 3 to 6 months, a set of lead-doped phantoms with different lead concentrations was measured to check the calibration of the system.
Statistical methods
All statistical analyses were conducted using Statistical Analysis System (SAS) software (SAS institute Inc, Cary NC, Version 9.2). Univariate and bivariate summary statistics as well as distributional plots were examined for all variables to identify potential outliers and influential points. Expected ranges for variables were designated a priori and bone lead uncertainty values greater than 10 and 15 µg/g for tibia and patella, respectively, were excluded. All statistical tests were two-sided with an alpha-level of 0.05. Nutrient variables were calorie-adjusted in order to minimize the confounding by total caloric intake. This was done by regressing a model in which total caloric intake is the independent variable and the nutrient of interest is the dependent variable and then adding the residuals from this model to a constant equal to the expected intake of the nutrient for mean total caloric intake of the study participants.
Non-linear mixed modeling was used to analyze changes in bone lead over time as a first-order decay function. Uncertainty measurements provided by XRF were utilized to weight observations using an inverse-variance approach (1/uncertainty2). In these models, change in bone lead over time was modeled using a two-level hierarchical approach such that:
Where Yit is the bone lead of subject i at time t, b0 is the mean intercept and ui is the subject-specific intercept. For the model of the change over time, b1 represents the mean effect of time and vi describes the subject-specific deviation from this slope around the mean.
Next we examined subject specific factors that modify the rate of decline of bone lead in a model that takes the form:
Where hi represents the coefficient of susceptibility factor Zi and d represents the portion of the subject specific intercept for subject i which is not explained by Zi. This model allows us to examine factors at the individual level which affect the random slopes (and similarly for the intercepts) as they vary by subject and is equivalent to testing for interaction between susceptibility factor Z and time in the model, while recognizing that this may explain all of the heterogeneity in slope.
An initial model was developed using linear regression of the logarithm of bone lead to obtain starting values for the non-linear model of the decay function of lead in both tibia and patella. We have included a term for machine in addition to using a conversion factor for the bone lead measures obtained using the newly designed K-XRF instrument to preserve comparability between the two metrics to account for variability in the nature of the signal. The individual models included terms for years of follow up, age at baseline (1991) and K-XRF machine used. Bone lead measures made after 1999 using the XRF machine were calibrated using a correction factor of 0.87. Dietary and lifestyle covariates of interest determined from previous studies were added into the model individually to test for significance, and significant effects were included in the final model. The nutritional factors that were tested include calcium, vitamin D, vitamin C, iron, protein, total fat, as well as pack-years smoked and body mass index (BMI) based on previous studies of factors predicting lead levels.17–19 We also investigated non-linearity in the rate of decline of bone lead using piecewise linear models with knots at the 25th, 50th, and 75th percentile of follow up time since 1991, when the K-XRF measures began. Sensitivity models were also constructed in which we tested effect modification by age at baseline. We also tested whether rate of decline differed by smoking status (never versus current and former smokers). Finally, we tested effects in models excluding the participants with 4 study visits, who we hypothesized could differ from the majority of the population with only 2 or 3 repeated measures.
Results
Between June 1991 and December 2002 a total of 554 subjects completed at least 2 tibia or patella bone lead XRF measurements, 283 participants had 3 measurements and 73 had 4 measurements. The primary reason that subjects reported for not participating in the K-XRF sub-study was the inconvenience of coming back to the clinic on an additional day. Subjects with tibia and patella bone lead levels which were accompanied by measurement uncertainties greater than 10 and 15 µg/g, respectively, were not included in the analysis in order to minimize measurement error. The mean year of first visit was 1993 (standard deviation=1.3 years) and ranged from 1991 – 1999.
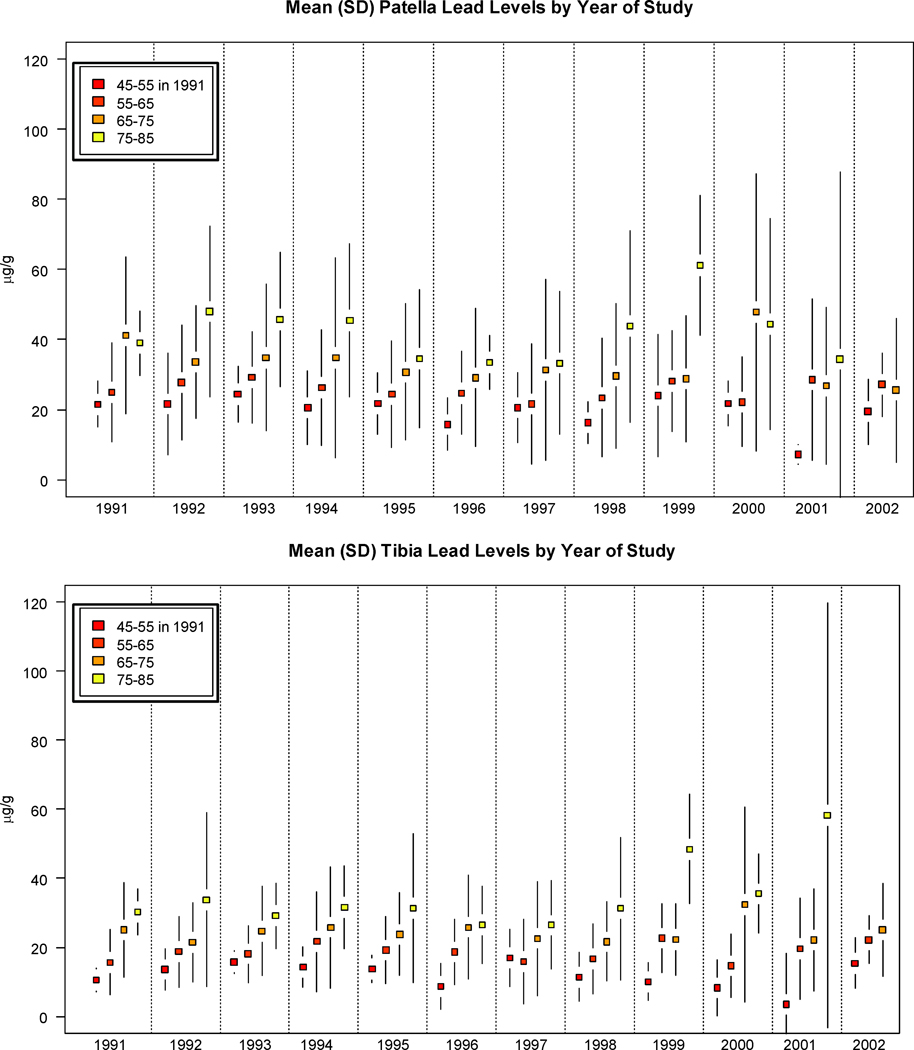
The distribution of bone lead and demographic characteristics is presented in Table 1 by visit number during the study period (visits 1–4). Participants in the study had a mean patella lead measurement of 31.1 (SD=19.9) µg/g and mean tibia lead measurement of 21.6 (SD=13.6 µg/g) at the first visit at which lead was measured. The average age of participants at first visit was 65.8 (SD=6.6) years and the average BMI was 27.9 (SD=3.7) kg/m2. We also constructed plots of the mean lead levels in the study population by year stratified by age in 1991) shown in Figure 1. These plots show the trends in bone lead levels over time by age category, whereby older individuals in the study population have, on average, higher bone lead levels across the study period.
Table 1.
Population characteristics by study visit.
| first visit | second visit | third visit | fourth visit | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean/n | ±SD/(%) | N | Mean/n | ±SD/(%) | N | Mean/n | ±SD/(%) | N | Mean/n | ±SD/(%) | |
| Tibia Pb (µg/g bone) | 554 | 21.6 | ± 13.6 | 553 | 20.7 | ± 13.1 | 283 | 21.4 | ± 15.5 | 73 | 23.4 | ±17.4 |
| Patella Pb (µg/g bone) | 553 | 31.1 | ± 19.9 | 548 | 26.9 | ± 18.7 | 279 | 29.1 | ± 22.2 | 72 | 30.6 | ±24.0 |
| Age (years) | 554 | 65.8 | ± 6.6 | 554 | 69.1 | ± 6.6 | 283 | 71.9 | ± 6.5 | 73 | 73.8 | ± 6.5 |
| Systolic Blood Pressure (mmHg) | 553 | 134.9 | ± 16.8 | 549 | 138.0 | ± 18.6 | 279 | 135.0 | ± 16.7 | 72 | 130.6 | ± 17.9 |
| Diastolic Blood Pressure (mmHg) | 553 | 81.7 | ± 9.1 | 549 | 82.3 | ± 8.9 | 279 | 80.7 | ± 8.5 | 72 | 76.4 | ± 9.6 |
| Body Mass Index (kg/m2) | 552 | 27.9 | ± 3.7 | 549 | 28.0 | ± 3.8 | 281 | 27.9 | ± 4.0 | 72 | 28.7 | ±4.2 |
| Never smoker (n) | 554 | 166 | (30) | 553 | 165 | (30) | 90 | (32) | 36 | (36) | ||
| Pack-years smoked | 542 | 21.3 | ± 24.8 | 547 | 22.0 | ± 27.6 | 281 | 19.7 | ± 25.1 | 72 | 17.6 | ± 23.7 |
| Years of Education (years) | 543 | 14.8 | ± 2.9 | 544 | 14.8 | ± 2.9 | 279 | 14.9 | ± 2.9 | 72 | 14.9 | ±3.07 |
| Hypertension (n) | 553 | 288 | (52) | 549 | 381 | (69) | 281 | 202 | (71.9) | 72 | 57 | (79.2) |
| Diabetes (n) | 553 | 66 | (12) | 549 | 74 | (13.5) | 281 | 54 | (19.2) | 72 | 13 | (18.1) |
Figure 1. Age-Stratified means and Standard deviations for tibia and patella Pb levels over the study period.
Over the 11 years included in the study, older individuals in the study population maintain consistently higher tibia and patella bone lead levels.
Crude models (adjusted for age in 1991, time since baseline and machine used only) and final adjusted models are presented in Table 2. The final models were adjusted for age at baseline, BMI, years of education, pack-years smoked, 2 or more alcoholic drinks/day, machine used (Abiomed or Harvard k-XRF) and vitamin C intake. These results from these models included a total of 538 participants with 1394 and 1383 observations in tibia and patella models respectively. There were 16 individuals missing information on years of education and they were therefore excluded.
Table 2.
Results from crudea and adjusted final b models of bone Pb over 11 years from 1991–2002 among participants in the Normative Aging Study.
| Crude Model | Final Model | ||||
|---|---|---|---|---|---|
| % change |
95%CI | % change |
95% CI | ||
| Tibia % changec | −1.5 | (−2.2, −0.8) | Tibia % changee | −1.4 | (−2.2, −0.7) |
| Patella % changed | Patella % changef | −5.1 | (−6.2, −3.9) | ||
| (0–11 years) | −3.8 | (−4.5,−3.1) | (0–4.6 years) | ||
| (4.6 –11 years) | −0.4 | (−2.4, 1.7) | |||
Crude models adjusted for: age in 1991, time since baseline and k-XRF (Abiomed or Harvard XRF) machine used.
The final models were adjusted for: age in 1991, BMI, years of education, pack-years smoked, 2 or more alcoholic drinks/day, k-XRF machine used (Abiomed or Harvard k-XRF) and vitamin C intake.
model includes 1463 observations from 554 individuals.
model 1452 observations from 554 individuals.
model includes 1394 observations from 538 indviduals.
model includes 1383 observations from 538 individuals.
The rate of change in tibia was found to be −1.4% decline per year (95% CI: −2.2,−0.7). The best model fit for patella based on Akaike’s Information Criteria (AIC) was a piecewise model with a knot at the median follow up time, 4.6 years. We observed a rate of change for patella lead of −5.1% per year initially (95% CI: −6.2,−3.9), however after 4.6 years (February 29, 1996) the piecewise model predicted no significant change (−0.4% (95% CI: −2.4, 1.7)) in patella lead level. Including a piecewise term for tibia lead did not improve model fit.
No significant age at baseline-by-time interactions were observed for either patella or tibia lead. Non-smoking participants were found to have 15.3% lower baseline patella lead levels (95% CI: −23.9,−5.4) than former smokers and had less steep rates of decline. No significant differences by smoking status were observed for tibia lead. Finally, models excluded the 73 participants with 4 study visits changed tibia and patella lead rates of decline by less than 1%.
Discussion
Over 11 years of study, we observed a slow log-linear decline for tibia and a piecewise log-linear decline with a single knot at the median follow-up time for patella bone lead. While initial declines were more than twice as fast in patella as compared to tibia lead, the patella lead did not change significantly after February 29, 1996. Although we observed higher lead levels among older individuals, we did not observe any interaction on the log scale between age at study baseline and yearly declines in our models.
Between 1976 and 2002, blood levels in the United States declined by more than 90%, in large part, due to the removal of lead from gasoline, which was complete by 1987 in the Northeast United States. Because the rate of turnover in bone lead is known to occur over a period of years, it is likely that an equilibration of bone and blood lead occurs over a similar timescale. We interpret the initial rapid rate of fall of trabecular bone lead in our population as capturing that equilibration process. The cessation of this rapid decline 10–15 years after the complete cessation of leaded gasoline use suggests that at that point a new equilibrium had been reached between trabecular bone lead, cortical bone lead, and exogenous exposure. We believe this has implications for the use of these two measures as markers of lead exposure. Specifically, these two measures of bone lead represent distinct exposure profiles. Trabecular lead, represented here by the patella measures, appears to be a reflection of a moving average of exogenous exposure in the last 10 years. In contrast, the very slow rate of decline in cortical bone lead –even during a period when environmental lead levels fell dramatically -- indicates that it is an index of lifetime exposure. In an elderly population, such as is studied for cardiovascular effects or cognitive decline, these are not the same thing, and this offers us insight into the timing of lead health effects.
The directionality of predictors we identified in this study were generally consistent with associations observed in previous investigations. We observed significant correlations between bone lead level with age,11, 20 whereby older individuals had higher cumulative exposures. We also observed differences in bone lead levels across measures of socioeconomic position, as indicated by lower bone lead with higher levels of education, as was previously observed in this population.21 With regards to the effects of smoking, we observed lower baseline bone lead levels among non-smokers. This is consistent with observations that cigarette smoking has been associated with higher lead levels,22 likely due to the lead content of cigarettes.23 The smaller decrement among never smokers may be due to the fact that they start at lower levels than current smokers and former smokers, who represent the largest sector of this population. Finally, vitamin C, was the only dietary factor we observed to meet significance in our final models, and has been identified as a mild chelator,24 and therefore was associated with modest decrements in bone lead levels.
Generally, the magnitude of the effects we observed in the current study corresponds to the release of bone lead through the process of bone turnover. Previously, adult tibia turnover rate has been reported as 2% per year, while trabecular lead turnover was reported to be greater than 8% per year.10 While our 5.1% decline in trabecular lead is lower than that figure, we note that it was observed at the tail end of the equilibration with the decline in exogenous lead (that is, in the 1990s, not the 1980s), and the rate of fall may have been greater in earlier years. The previous investigation within this cohort examined a limited sample size of 70 subjects and observed an association between the decreasing exogenous exposure to lead in recent years with bone lead increases with age and decreases over time.11 In this earlier study, Kim and colleagues extrapolated from 3 years of follow-up to calculate a half life for patella lead of 8 years and no change in tibia lead. Here we extended the previous investigation to include over 11 years of data from 554 individuals. In contrast to Kim and colleagues, our study predicts a significantly longer half-life in patella bone lead using a piecewise model and no net change after 4.6 years. Additionally, our findings suggest that the rate of decline in patella lead over time is not constant, as previously proposed. If we assume a constant rate of decline for tibia lead and assume that the beta coefficient in our model approximates λ, the decay constant, we estimate the tibia half life of 48.6 years. Thus, tibia lead burden does appear to remain relatively stable over time, but is reduced over the life course in the setting of low exogenous exposure.
Results of this study provide new insight into the temporal variability of tibia and patella lead levels and therefore are important to the interpretation of studies in which these measures are used to estimate long-term population lead exposures. However, there are limitations to our study. Over the course of 11 years of study, new technology was adopted to measure bone lead. Bone lead was measured using Abiomed technology initially, and later measurements were obtained using new Harvard designed in-house XRF. We investigated the comparability of these methods and developed an appropriate approach to combining data from the two machines which relies on repeated calibration of each machine against inductively coupled plasma mass-spectrometry (ICP-MS)-derived values, the gold standard for lead measurement.25
Our participants are male residents of the Boston area and were free from serious illness at the time they entered into the study. Therefore these results may not be generalizable to other populations of environmentally exposed men and women. In particular, bone turnover rates may be higher in older women, who are not included in this study. Previous studies of lead toxicity have focused primarily on exposures to men in occupational settings and have observed age effects on longitudinal lead levels as well as evidence that higher exposure is associated with longer half life.20 Here we focused on examining the changes in bone lead within an environmentally exposed population of elderly men. It is possible that men who came in for more measurements are different from the general population due to a survivorship effect in the cohort. We tested this hypothesis by performing sensitivity analyses where we excluded the participants with 4 study visits (n=73) and this did not alter the rates of decline substantially. Disentangling the effects of age and baseline lead level is more difficult within this environmentally exposed population due to the fact that age is such a strong predictor of environmental exposure.
In conclusion, we observed slower rates of decline in bone lead levels than have been previously reported, indicating that lead exposure due to mobilization from bone continues for decades after exogenous exposures decline. The repeated measures and completeness of the data in an environmentally exposed population is unique and having detailed information on covariates of interest allowed us to efficiently address the relationship between endogenous lead stores and effects of time. Because adverse health effects associated with bone lead have been observed at levels once considered safe, it is particularly important to clarify the time period of exposure reflected in this exposure measure to better understand the dose-response relation of chronic lead exposure with prevalent chronic disease in the elderly. Most importantly, our findings suggest that, among older adults, exposure to lead from release of endogenous bone stores may continue for decades after exogenous exposure sources have been mitigated.
Acknowledgments
Disclosure of Funding & sources of support: NIEHS R01- ES005257, R01-ES007821, and 000002, T32-ES07069 and T32-HL007374
Test subjects were evaluated for measurement of bone lead levels in the outpatient Clinical Research Center of the Brigham and Women’s Hospital. The K-XRF instrument used in some of this work was developed by ABIOMED, Inc., of Danvers, Massachusetts, with support from NIH grant no. SBIR 2R44 ES03918-02. The Normative Aging Study is supported by the Research Services and the Cooperative Studies Program/ERIC of the US Department of Veterans Affairs, and is a research component of the Massachusetts Veterans Epidemiology Research and Information Center (MAVERIC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barry PS. A comparison of concentrations of lead in human tissues. Br J Ind Med. 1975;32:119–139. doi: 10.1136/oem.32.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu H, Rabinowitz M, Smith D. Bone lead as a biological marker in epidemiologic studies of chronic toxicity: conceptual paradigms. Environ Health Perspect. 1998;106:1–8. doi: 10.1289/ehp.981061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu H, Shih R, Rothenberg S, Schwartz BS. The epidemiology of lead toxicity in adults: measuring dose and consideration of other methodologic issues. Environ Health Perspect. 2007;115:455–462. doi: 10.1289/ehp.9783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin D, Glass TA, Bandeen-Roche K, Todd AC, Shi W, Schwartz BS. Association of blood lead and tibia lead with blood pressure and hypertension in a community sample of older adults. Am J Epidemiol. 2006;163:467–478. doi: 10.1093/aje/kwj060. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz BS, Hu H. Adult lead exposure: time for change. Environ Health Perspect. 2007;115:451–454. doi: 10.1289/ehp.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grandjean P. Even low-dose lead exposure is hazardous. Lancet. 2010;376:855–856. doi: 10.1016/S0140-6736(10)60745-3. [DOI] [PubMed] [Google Scholar]
- 7.Leggett RW. An age-specific kinetic model of lead metabolism in humans. Environ Health Perspect. 1993;101:598–616. doi: 10.1289/ehp.93101598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Flaherty EJ. A physiologically based kinetic model for lead in children and adults. Environ Health Perspect. 1998;106 Suppl 6:1495–1503. doi: 10.1289/ehp.98106s61495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nie H, Chettle DR, Webber CE, Brito JA, O'Meara JM, McNeill FE. The study of age influence on human bone lead metabolism by using a simplified model and X-ray fluorescence data. J Environ Monit. 2005;7:1069–1073. doi: 10.1039/b507749d. [DOI] [PubMed] [Google Scholar]
- 10.Rabinowitz MB. Toxicokinetics of bone lead. Environ Health Perspect. 1991;91:33–37. doi: 10.1289/ehp.919133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim R, Landrigan C, Mossmann P, Sparrow D, Hu H. Age and Secular Trends in Bone Lead Levels in Middle-aged and Elderly Men: Three-Year Longitudinal Follow-up in the Normative Aging Study. Am J Epidemiol. 1997;146:586–591. doi: 10.1093/oxfordjournals.aje.a009318. [DOI] [PubMed] [Google Scholar]
- 12.Bell B, Rose C, Damon A. The Normative Aging Study: an interdisciplinary and longitudinal study of health and aging. Aging Human Develop. 1972;3:4–17. [Google Scholar]
- 13.Beaton GH, Milner J, McGuire V, Feather TE, Little JA. Source of variance in 24-hour dietary recall data: implications for nutrition study design and interpretation. Carbohydrate sources, vitamins, and minerals. Am J Clin Nutr. 1983;37:986–995. doi: 10.1093/ajcn/37.6.986. [DOI] [PubMed] [Google Scholar]
- 14.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. discussion 1127–1136. [DOI] [PubMed] [Google Scholar]
- 15.Hu H, Milder M, Burger D. X-Ray Fluorescence: Issues Surrounding the Application of a New Tool for Measuring Burden of Lead. Environ Res. 1989;49:295–317. doi: 10.1016/s0013-9351(89)80074-x. [DOI] [PubMed] [Google Scholar]
- 16.Kim R, Aro A, Rotnitzky A, Amarasiriwardena C, Hu H. K X-ray fluorescence measurements of bone lead concentration: the analysis of low-level data. Phys Med Biol. 1995;40:1475–1485. doi: 10.1088/0031-9155/40/9/007. [DOI] [PubMed] [Google Scholar]
- 17.Cheng Y, Willett WC, Schwartz J, Sparrow D, Weiss S, Hu H. Relation of nutrition to bone lead and blood lead levels in middle-aged to elderly men. The Normative Aging Study. Am J Epidemiol. 1998;147:1162–1174. doi: 10.1093/oxfordjournals.aje.a009415. [DOI] [PubMed] [Google Scholar]
- 18.DeLuca J, Hardy CA, Burright RG, Donovick PJ, Tuggy RL. The effects of dietary fat and lead ingestion on blood lead levels in mice. J Toxicol Environ Health. 1982;10:441–447. doi: 10.1080/15287398209530266. [DOI] [PubMed] [Google Scholar]
- 19.Korrick SA, Schwartz J, Tsaih SW, Hunter DJ, Aro A, Rosner B, et al. Correlates of bone and blood lead levels among middle-aged and elderly women. Am J Epidemiol. 2002;156:335–343. doi: 10.1093/aje/kwf042. [DOI] [PubMed] [Google Scholar]
- 20.Brito JA, McNeill FE, Stronach I, Webber CE, Wells S, Richard N, et al. Longitudinal changes in bone lead concentration: implications for modelling of human bone lead metabolism. J Environ Monit. 2001;3:343–351. doi: 10.1039/b101493p. [DOI] [PubMed] [Google Scholar]
- 21.Elreedy S, Krieger N, Ryan PB, Sparrow D, Weiss ST, Hu H. Relations between individual and neighborhood-based measures of socioeconomic position and bone lead concentrations among community-exposed men: the Normative Aging Study. Am J Epidemiol. 1999;150:129–141. doi: 10.1093/oxfordjournals.aje.a009972. [DOI] [PubMed] [Google Scholar]
- 22.Kosnett MJ, Becker CE, Osterloh JD, Kelly TJ, Pasta DJ. Factors influencing bone lead concentration in a suburban community assessed by noninvasive K x-ray fluorescence. JAMA. 1994;271:197–203. [PubMed] [Google Scholar]
- 23.Shaper AG, Pocock SJ, Walker M, Wale CJ, Clayton B, Delves HT, et al. Effects of alcohol and smoking on blood lead in middle-aged British men. Br Med J (Clin Res Ed) 1982;284:299–302. doi: 10.1136/bmj.284.6312.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goyer RA, Cherian MG. Ascorbic acid and EDTA treatment of lead toxicity in rats. Life Sci. 1979;24:433–438. doi: 10.1016/0024-3205(79)90215-7. [DOI] [PubMed] [Google Scholar]
- 25.Aro AC, Todd AC, Amarasiriwardena C, Hu H. Improvements in the calibration of 109Cd K x-ray fluorescence systems for measuring bone lead in vivo. Phys Med Biol. 1994;39:2263–2271. doi: 10.1088/0031-9155/39/12/009. [DOI] [PubMed] [Google Scholar]