A surprising common pathological processes is found between Alzheimer’s disease (AD) and type 2 diabetes mellitus (T2DM)[1]. AD and T2DM share some common pathological processes: Amyloid β (Aβ), τ hyperphosphoralation, insulin abnormality. Disturbance in insulin signalling is not only involved in blood glucose level but also in numerous degenerative processes. Glucagon-like peptide 1 (GLP-1) has attracted substantial attention for its advantage in treating T2DM. GLP-1 can reduce Aβ levels in brain. All these encourage us in a medical hypothesis: that is, that GLP-1 is a promising agent in the therapy of AD.
Commonalities Between T2DM and AD
Both AD and T2DM are the most common degenerative diseases and their prevalence increase with age. Numerous epidemiological studies have linked T2DM with an increased risk of AD[2]. An investigation of cohort of 1,301 in Stockholm, Sweden showed that T2DM increased the risk of dementia[3]. In another survey of 683 subjects cohort of persons aged 65 years and older with hyperinsulinemia in northern Manhattan is shown to be associated with a higher risk of AD and decline in memory[4]. In a prospective population-based cohort study among 6,370 elderly subjects, Ott[5] revealed that diabetes may have contributed to the clinical syndrome in a substantial proportion of all dementia patients. Peila[6] evaluated the association of diabetes alone or combined with the apolipoprotein E (ApoE) gene with incident dementia in a population-based cohort of 2,574 Japanese-American men. The result displayed that T2DM is a risk factor for AD. The association between diabetes and AD is particularly strong among carriers of the APOE epsilon4 allele.
Commonalities between T2DM and AD in epidemiology: such as degenerative change, aging diseases, fat and higher cholesterol, and cardiac risk factors, encourage us to look for the linkage of both. Research has shown an extensive expression of insulin and its receptor throughout the mammalian brain[7,8]. A fact of commonalities between neurons and β-cells launch into a reasonable explanation as to the common risk and prevention factors between T2DM and AD. Research has shown some common risk factors between T2DM and AD: higher cholesterol[9], dis-metabolism, degeneration[9], myloid β (Aβ) deposition[9], Glycogen synthesis kinase 3(GSK3), and τ protein phosphorylation[10], cardiovascular disease, oxidative stress[2], inflammation[2], ApoE4[11], apoptosis etc. A new finding shows that treatment with GLP-1 beneficially affects a number of the therapeutic targets associated with AD. This finding opens a new research area. The commonalities between T2DM and AD may contribute to the explanation of GLP-1 as a promising peptide to treat T2DM and AD.
AD is characterized by intracellular neurofibrillary tangles (NFTs), containing an abnormal hyperphosphorylated form of τ protein, and extracellular senile plaques (SPs), mainly composed of fibrillar Aβ. Both of those hallmarks are involved in T2DM[1]. Both age-related degenerative diseases, AD and T2DM, are associated with the accumulation of amyloid fibrils[12]. Another commonality, cell loss and degenerative change, is also involved in both diseases. AD is the most common neurodegenerative disease with an extensive neuron loss. T2DM is also a degenerative disease that results from the selective destruction of pancreatic β cells[13].
Aβ deposits are commonly observed in pancreatic islets of diabetic patients. These deposits consist of islet amyloid polypeptide (IAPP)[14].Considering the pathogenetic similarities and the 90% structural similarity between Aβ precursor protein and IAPP[15], it should not be surprising that AD seems to be predisposed to insulin resistance, insulin hypersecretion, and T2DM[2]. Similarly, individuals suffering from T2DM will suffer from dementia more readily[5,16]. The research has shown that a higher serum insulin level in prediabetes and early T2DM has been associated with impaired cognitive function[17]. Mechanistically this might be that elevation of Aβ levels is associated with elevated serum insulin content[18]. In other words, the evidence of the existence of links between AD and T2DM is that AD is associated with peripheral and central insulin abnormalities, and that cognitive capacities are often impaired in patients with T2DM[14]. The complex relationship between insulin, cholesterol, and AD was well described by Nelson[19]. Insulin regulates cholesterol biosynthesis by stimulating activity of 3-hydroxy-3-methylglutaryl-CoA reductase, a rate-limiting enzyme in cholesterol biosynthesis. Cholesterol is involved in AD by multifactor: ApoE4, Aβ deposition, amyloid precursor protein (APP) metabolism etc. Hypercholesterolemia is an obvious risk factor of T2DM[20]. It was shown that transgenic mice models overexpressing IAPP develop diabetes and generally subsequent to amyloid deposits[21,22]. Conversely, targeted disruption of IAPP leads to enhanced insulin secretion and improved glucose tolerance[23].
Freude[24] showed a correlation between τ phosphorylation and peripheral insulin level with a significant increase in τ phosphorylation at Ser202 in the brain within 10 min after 1-mU insulin injection and an even further increase after injection of 4 units insulin. Further, Freude demonstrated that insulin receptor signaling and τ phosphorylation were completely abolished in the brains of the mice lacking the brain insulin receptor under hyperinsulinemic conditions, indicating that the cerebral insulin receptors are a direct target of peripheral administered insulin. Further evidence of τ phosphorylation involvement in AD and T2DM is that GSK-3, a serine/threonine kinase that phosphorylates glycogen synthase in the rate-limiting step of glycogen biosynthesis, was implicated in the formation of NFTs[25,26]. GSK-3 inhibitor is an attractive target identified to be useful in the treatment of diseases such as T2DM and AD[27].
Common biological characteristics between the pancreas and the brain also include common enzymes: glutamic acid decarboxylase, tyrosine hydroxylase, and dopa decarboxylase, thyrotrophin-releasing hormone, and P75. Neuron growth factor receptors are also shared attributes of both neuronal tissues and β-cells. β-cells resemble neurons in that they are electrically excitable and respond to hormonal stimuli and glucose by depolarization and exocytosis, in a process that resembles neurotransmitter release from synaptic vesicles. All those commonalities approve a reasonable assumption that common signaling mechanisms occur in response to similar physiological responses, such as proliferation and differentiation[28]. Furthermore the impact of type 1 diabetes on brain development and function has been reviewed by Northam[29]. Either hypoinsulinemia or hyperinsulinemia are involved in the damage of brain development.
There is growing evidence that insulin is involved in cognitive decline and AD[30]. The evidence includes the insulin resistance of brain owing to alterations of insulin receptor signaling in the brain, and regulation of insulin to the metabolism of Aβ and τ protein. It is further evidence that there is widely distribution of insulin and insulin receptor (IR) in the brain, especially in the hypothalamus and the hippocampus[31]. The hippocampus- and cerebral cortex-distributed insulin/IR has also been shown to be involved in brain cognitive functions. In contrast, deterioration of insulin receptor signaling is involved in aging-related brain degeneration such as the AD and cognitive impairment in T2DM patients[31]. Moroo[32] showed a decrease of expression of IR in the brain in AD and PD patients. Frolich[33] compared the expression of insulin and its IR in neocortical brain areas of AD patients with normal controls by immunohistochemical staining. The result showed that insulin and its receptor densities decrease with aging. Brain IR densities in AD were decreased compared to middle-aged controls. The effect of insulin in AD patients includes: 1) AD may be associated with an impairment of glucose regulation. 2) AD may worsen insulin abnormalities. 3) AD patients may have a decreased cerebrospinal fluid insulin levels and/or a decreased cerebrospinal fluid-to-plasma insulin ratios. 4) Acute glucose administration can facilitate memory of AD patients and healthy older adults; however, it is abolished by suppressing endogenous insulin secretion. 5) Acute insulin administration facilitates memory of AD patients. 6) Apo E does not only involve in AD but also moderates insulin activity and affects on memory of patients with AD. Patients without an APOE ɛ4 allele have lower insulin sensitivity and occur insulin-induced memory facilitation at higher insulin doses. Reversely, Patients with at least one APOE q4 allele show insulin-induced memory facilitation at lower insulin doses and reduced insulin degrading enzyme levels. Hence, disturbances in cerebral insulin signalling pathways may be involved in AD and brain aging[34].
The relation between insulin and the metabolism of Aβ and τ is also receiving increasing attention. Insulin appears to stimulate Aβ secretion and inhibits the extracellular degradation of Aβ due to competition from insulin-degrading enzyme (IDE)[35].
Hyperglycaemia may be involved in the brain damage. Hyperglycaemic rodents, for example, express cognitive impairments and functional and structural alterations in the brain[36]. Schubert[37] hypothesized that neuronal insulin resistance contributes to defects in neuronal function and exhibited the evidence in insulin receptor knockout (NIRKO) mice. A complete loss of insulin-mediated activation of phosphatidylinositol 3-kinase and inhibition of neuronal apoptosis was shown in NIRKO mice. As a result, phosphorylation of GSK3 was markedly reduced and phosphorylation of τ protein increased. The hypothesis should be further completed because Schubert has not exhibited the alteration of neuronal proliferation survival, memory, or basal brain glucose metabolism in NIRKO mice. Involvement of other factor will be needed to develop AD based on changes in GSK3 activity and hyperphosphorylation of τ protein induced by lack of insulin signaling in the brain.
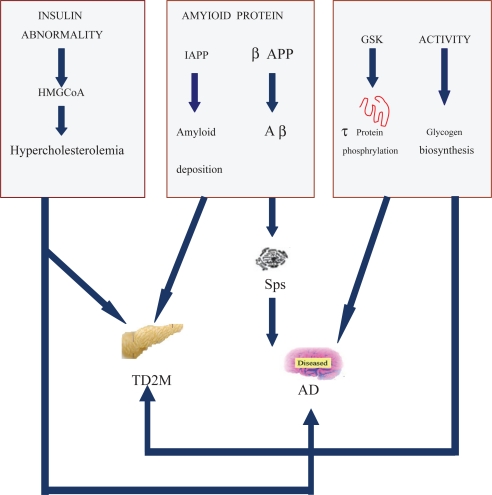
A complex relationship between diabetes and AD is shown in the Figure 1. Ninety percent structural similarity was founded in Aβ which is a hallmark pathology in AD and IAPP which is involved in T2DM. Insulin abnormality is attributed to AD by promoting Aβ deposition, and τ protein hyperphosphorylation. GSK3 is a key kinase to promote τ protein hyperphosphorylation and glycogen biosynthesis.
Figure 1.
Common pathological processes in AD and T2DM. Ninety percent homologous structure was shown between Aβ which is a hallmark pathology in AD and IAPP which is involved in T2DM. Insulin abnormality is attributed to AD by promoting Aβ deposite, and τ protein hyperphosphorylation. GSK3 is a key kinase to promoteτ protein hyperphosphorylation and glycogen biosynthesis.
Abbreviations: AD: Alzheimer’s disease; T2DM: Type 2 diabetes mellitus; IAPP: islet amyloid polypeptide; Aβ: Amyloid β; GSK3: glycogen synthase kinase.
A New Strategy Consideration to Treat AD
A cure of AD is still far off, and clinicians face the burden of caring for patients at all stages of dementia for the foreseeable future[38]. Heretofore, therapy strategies of AD, including the cholinesterase inhibitors, are only limited in heteropathy: improving cognitive impairment, decreasing complication, preventing abnormity behaviors, and avoiding psychopathic affair. Effective strategies aimed at the hallmarks of AD, and developing therapies that target Aβ production, aggregation, clearance or toxicity are likely to appear in the near future[39,40]. The therapy strategy aimed at other hallmarks of AD, neurofibrillary tangles, is also being developed[41].
Even with treatment, patients with T2DM may face some troubles: spikes in blood glucose after meals, weight gain, a loss of effectiveness of their treatments over time etc. GLP-1 draws on a better understanding of how the body responds to meals—some already available and may offer useful adjuncts to existing therapies[42].
Emerging literature, in which it is shown that hallmarks of AD can link to T2DM, and GLP-1 can reduce Aβ-peptide levels in the brain, encourage an adventurous thinking: is GLP-1 a new hope for the therapy of AD?
Synthesis, Secretion and Function of GLP-1
GLP-1 originates from expression of the glucagon gene in the L cells of the distal intestinal mucosa and contains 30 amino acids with 50% sequence homology to glucagons[43–45]. GLP-1 was found as an insulinogenic factor 20 years ago. In all candidate incretins, insulinogenic factors of the gastrointestinal mucosa, GLP-1 was regarded as the most physiological signification, possessing obvious characters of gastrone and insulinotropic substances[46]. Its effects on glucose-dependent insulin secretion and insulin gene expression have been proven[47,48]. The mechanism of GLP-1 to regulate blood glucose include 1) the stimulation of insulin secretion and of its gene expression, 2) the inhibition of glucagon secretion, 3) the inhibition of food intake, 4) the proliferation and differentiation of β cells, and 5) the protection of β cells from apoptosis[49]. 6) formation of pancreatic islet mass.
New discovery that GLP-1 and Exendin-4 (Ex-4), a naturally occurring stable analogue of GLP-1 that binds at the GLP-1 receptor (GLP-1R), possess neurotrophic properties and can protect neurons against glutamate-induced apoptosis and reduce level of Aβ in brain, prompt a new consideration: GLP-1 and its mimics are promising agents in therapy of AD. GLP-1 is also regarded as a promoting agent not only for T2DM but also for AD due to its extensive expression of GLP-1R in the brain[50–52].
In the human genome, the proglucagon gene is located on chromosome 17 spanning approximately 10 kb. The transcriptional unit of proglucagon contain six exons and five introns[53]. The posttranslational processing of preproglucagon differs in different tissue. They are, in the pancreas, GLP-1 (1–36 amide) and GLP-1 (1–37), while, in the ileum and hypothalamus, are GLP-1 (7–36)-amide and GLP-1 (7–37)[54]. In the pancreatic islet alpha-cells proglucagon is processed by proprotein convertase 2 to release mainly glucagon. In the intestinal L cells it is by proprotein convertase to produce mainly GLP-1, GLP2[55]. Amidation of GLP-1 has been regard as a chemical process to enhance its survival in the bloodstream. In the pancreas, the carboxyl-terminal amidation of GLP-1 is processed by the sequential enzymatic action by peptidylglycine α-monooxygenase and peptidy-lamidoglycolate lyase[56]. The significance of the amidated forms of GLP-1 in nonpancreatic targets is not clear. GLP-1 has a very short half-life (1–2 min) and is rapidly degraded in vivo by dipeptidyl peptidase-IV (DPP-IV), which cleaves GLP-1 at the penultimate N-terminal site of Ala8[57–59]. Fortunately, all GLP-1 analogues have been developed to possess a longer half-life. In vivo, several metabolites of GLP-1 are formed by enzyme digestion. They are GLP-1 (9–36), GLP-1 (7–35), and GLP-1 (7–34). GLP-1 (9–36) amide, a main metabolite of GLP-1, is present in vivo in concentrations that are up to 10-fold greater than the level of GLP-1(7–36) amide[60]. Isoforms of GLP-1 show different bioactivity; the effect of GLP-1 (7–36) amide is 100 times more potent than GLP-1 (1–37) and GLP-1 (1–36) amide in stimulating [14C]-aminopyrine accumulation[61]; GLP-1 [7–36 amide] and GLP-1 [7–37] possess an efficiency[62].GLP-1 (9–36) amide has been shown to have no effects on β cells and it is in some studies shown as an antagonist of the adenylyl cyclase activity[63]. The research also showed that GLP-1 (7–35) and GLP-1 (7–34) are an agonists. GLP-1 (7–36) and GLP-1 (7–37) are two of the main naturally occurring GLP products in vivo and the similar insulinotropic potency is proven. The structural analysis of GLP-1 shows that the first seven amino acid residues form a random coil structure followed by a first helical region (7–14), a linker region (15–17) and another helical region (18–29). Plasma levels of GLP-1 rise rapidly following nutrient ingestion. Major regulating factors of GLP-1 secretion are pancreatic hormones (insulin and glucagon)[64,65], nutrients (glucose and fatty acids), gastrointestinal hormones (gastric inhibitory polypeptide), gastrin-releasing polypeptide, gastric emptying[66,67], satiety[68], body weight[69], the vagal nerve-dependent release of acetylcholine etc[70,71]. Glucose-dependent Insulinotropic Peptide was also shown as an attractive stimulator of GLP-1 release in vitro[72]. GLP-1 secreted from intestinal L cells is inactivated by DPP-IV in intestinal capillaries. DPP-IV deactivates GLP-1 by cleavage of the N-terminal dipeptide. Thus only 25% of GLP-1 secreted enters the portal circulation in its intact form. In the liver 40% of the remaining active GLP-1 is further inactivated. As a result, only 10%–15% of GLP-1 reaches the systemic circulation and the pancreas. GLP-1 imposes on the brain by activating sensory efferent neurons from the nodose ganglion, the hepatoportal region[73,74] or the liver[75], and stimulating the neural pathway[76].
GLP-1R, a member of the seven-membrane-spanning G-protein-coupled family of receptor, is localized on chromosome 6 with 12 introns and 13 exons[49,77]. GLP-1R have been identified in brain, lung, pancreatic islets, stomach, hypothalamus, heart, intestine, and kidney[78,79]. GLP-1R consists of 463 amino acids (with a molecular weight of 65000 D) with eight hydrophobic domains. The hydrophobic segment located N-terminal is probably a signal area, whereas the others are membrane-spannin hydrophobic motifs[80]. GLP-1 receptor efficiently stimulate insulin secretion by coupling with adenyl cyclase[81].
GLP-1, an Attractive Agent to Treat T2DM and AD
Numerous researches has shown that GLP-1 is an attractive agent in therapy of T2DM[42,59,82,83]. Its pharmacological actions in glucose metabolism, including stimulation of insulin release, suppression of glucagon release, and inhibition of gastric emptying, ensure the rationale for its assessment as a therapeutic agent for T2DM[84]. The research recently focusing on intervening AD showed that GLP-1 is also a strong intervenor of AD[28,51,52].
GLP-1 regulates insulin secretion and insulin gene expression via its action on the pancreas following binding at the G-protein coupled GLP-1R. The GLP-1R signaling has been demonstrated to inhibit glucagon secretion[85]. It has been demonstrated that the commonality of expression of GLP-1R in both the rat and human brain exists[86]. Within the central nervous system (CNS), GLP-1 and several analogs that bind it to the GLP-1R possess neurotrophic properties and offer protection against glutamate-induced apoptosis and oxidative injury in cultured neuronal cells[87]. Moreover, GLP-1 can modify the processing of Aβ precursor protein in cell cultures and reduce Aβ levels in the brain in vivo[88].
The stimulus for neuronal GLP-1-transmission within the CNS is unclear. One possibility is that GLP-1-containing neurons or receptors are implicated in other neuropeptide-containing CNS pathways or down-stream from classic neurotransmitter systems such as noradrenalin, serotonin or dopamine. An alternative possibility is that peripheral GLP-1 acts on vagal afferent fibers[89], where it could influence GLP-1 neuronal transmission in the CNS.
GLP-1R positively regulates neuronal plasticity and cell survival as the stimulation of neuron[51]. It was recently reported that GLP-1 and Ex-4 possess neurotrophic properties and can protect neurons against glutamate-induced apoptosis. Perry[87] showed that GLP-1 can reduce the levels of Aβ in the brain in vivo and can reduce levels of APP in cultured neuronal cells. GLP-1 and Ex-4 protect cultured hippocampal neurons against death induced by Aβ and iron, an oxidative insult. Collectively, these data suggests that GLP-1 can modify APP processing and protect against oxidative injury. During[90] showed that intracerebroventricular exendin enhanced associative and spatial learning and prevented kainate-induced apoptosis of hippocampal neurons in rats. In recent research, Perry[91] showed that GLP-1 (Ex-4) has multiple synergistic effects on glucose-dependent insulin secretion pathways of pancreatic β-cells and on neural plasticity. Their study showed that GLP-1 (Ex-4) may offer some protection against the sensory peripheral neuropathy induced by pyridoxine. Put those data together it seems a potential role for these peptides to treat neuropathies.
Parsons[92] has made a GLP-1 minigene that can direct the secretion of active GLP-1 (amino acids 7–37) to achieve continuous GLP-1 expression to lengthen its half-life in vivo. In order to delay half-life and improve the therapeutic value of GLP-1, Youn[93] chemically modified GLP-1 with polyethylene glycol (PEG) and proved that the site-specific Lys34-PEG-GLP-1 was found to have significantly improved in vivo glucose-stabilizing efficacy than the other PEGylated GLP-1 isomers (His7- or Lys26-PEG-GLP1). Nevertheless, some of the GLP-1-derived agonists with DPP-IV resistance appear to be rapidly cleared from the plasma by renal clearance. The clinical studies of Ex-4 showed that daily administration or combination therapy with oral anti-diabetic agents was required to normalize blood glucose levels. To develop the longer-acting molecules that retain the native GLP-1 actions is a motivated effort. It is also clear that a gene therapy approach exerting long lasting effects would have advantages over parenteral protein drug delivery. A new gene therapy agent: GLP-1/IgG1-Fc fusion construct developed by Kuma[94] showed a unique advantage. Fusing active human GLP-1 and mouse IgG1 heavy chain constant regions (GLP-1/Fc) Kuma generated a plasmid encoding an IgK leader peptide-driven secretable fusion protein of the active GLP-1 and IgG1-Fc was constructed for mammalian expression. The researches in vivo and vitro showed that the bivalent GLP-1/Fc fusion protein is an effective approach for the therapy of T2DM.
In clinic treatment, GLP-1 based therapies for T2DM has made progress. Therapy strategies related to GLP-1 include injected DPPIV-resistant GLP-1 mimetics or orally active DPPIV inhibitors[75,95,96]. The GLP-1 enhancers and DPPIV inhibitors in pre-registered by FDA are Vildagliptin (LAF237), Sitagliptin (MK-0431); in clinical phase III programs are Denagliptin, SYR 322, Saxagliptin (BMS477118); in clinical phase II are Ro-0730699, PSN 9301, TA 6666. GLP-1 mimetic, Byetta-Exenatide, has been launched in U.S. (2005). GLP-1 minetics in clinical phase III programs is Liraglutide NN2211, in clinical phase II programs are ZP-10, BIM-51077, Exenatide-LAR, and CJC-113[97].
In summary, with a century of studies, the understanding of AD leads us to believe that the primary targets in AD are the Aβ and τ protein. Commonalities between T2DM and AD and the effect of GLP-1 on Aβ encourages us to forecast the possibility that GLP-1 is revealed as the highlight in further T2DM treatment and will bring forth its advantage in the therapy of AD.
References
- [1].Li L, Holscher C. Common pathological processes in Alzheimer disease and type 2 diabetes: A review. Brain Res Rev. 2007;56:384–402. doi: 10.1016/j.brainresrev.2007.09.001. [DOI] [PubMed] [Google Scholar]
- [2].Haan MN. Therapy Insight: type 2 diabetes mellitus and the risk of late-onset Alzheimer’s disease. Nature Clinical Practice Neurology. 2006;2:159–66. doi: 10.1038/ncpneuro0124. [DOI] [PubMed] [Google Scholar]
- [3].Xu WL, Qiu CX, Wahlin A, Winblad B, Fratiglioni L. Diabetes mellitus and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Neurology. 2004;63:1181–6. doi: 10.1212/01.wnl.0000140291.86406.d1. [DOI] [PubMed] [Google Scholar]
- [4].Luchsinger JA, Tang MX, Shea S, Mayeux R. Hyper-insulinemia and risk of Alzheimer disease. Neurology. 2004;63:1187–92. doi: 10.1212/01.wnl.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
- [5].Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937–42. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- [6].Peila R, Rodriguez BL, Launer LJ. Honolulu-Asia Aging Study. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–62. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- [7].Havrankova J, Roth J, Brownstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature. 1978;272:827–9. doi: 10.1038/272827a0. [DOI] [PubMed] [Google Scholar]
- [8].Havrankova J, Schmechel D, Roth J, Brownstein M. Identification of insulin in rat brain. Proc Natl Acad Sci USA. 1978;75:5737–41. doi: 10.1073/pnas.75.11.5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ristow M. Neurodegenerative disorders associated with diabetes mellitus. J Mol Med. 2004;82:510–29. doi: 10.1007/s00109-004-0552-1. [DOI] [PubMed] [Google Scholar]
- [10].Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. Journal of Cell Science. 2003;116:1175–86. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Qiu WQ, Folstein MF. Insulin, insulin-degrading enzyme and amyloid-beta peptide in Alzheimer’s disease: review and hypothesis. Neurobiol Aging. 2006;27:190–8. doi: 10.1016/j.neurobiolaging.2005.01.004. [DOI] [PubMed] [Google Scholar]
- [12].Glabe CG. Common mechanisms of amyloid oligomer pathogenesis in degenerative disease. Neurobiol Aging. 2006;27:570–5. doi: 10.1016/j.neurobiolaging.2005.04.017. [DOI] [PubMed] [Google Scholar]
- [13].Roche E, Reig JA, Campos A, Paredes B, Isaac JR, Lim S, et al. Insulin-secreting cells derived from stem cells: clinical perspectives, hypes and hopes. Transpl Immunol. 2005;15:113–29. doi: 10.1016/j.trim.2005.09.008. [DOI] [PubMed] [Google Scholar]
- [14].Sun MK, Alkon DL. Links between Alzheimer’s disease and diabetes. Drugs Today (Barc) 2006;42:481–9. doi: 10.1358/dot.2006.42.7.973588. [DOI] [PubMed] [Google Scholar]
- [15].Janson J, Laedtke T, Parisi JE, O’Brien P, Petersen RC, Butler PC. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53:474–81. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- [16].Biessels GJ, Kappelle LJ. Utrecht Diabetic Encephalopathy Study Group. Increased risk of Alzheimer’s disease in Type II diabetes: insulin resistance of the brain or insulin-induced amyloid pathology? Biochem. Soc. Trans. 2005;33(Pt 5):1041–4. doi: 10.1042/BST0331041. [DOI] [PubMed] [Google Scholar]
- [17].Stolk RP, Breteler MM, Ott A, Pols HA, Lamberts SW, Grobbee DE, et al. Insulin and cognitive function in an elderly population. The Rotterdam Study. Diabetes Care. 1997;20:792–5. doi: 10.2337/diacare.20.5.792. [DOI] [PubMed] [Google Scholar]
- [18].Watson GS, Peskind ER, Asthana S, Purganan K, Wait C, Chapman D, et al. Insulin increases CSF Abeta 42 levels in normal older adults. Neurology. 2003;60:1899–903. doi: 10.1212/01.wnl.0000065916.25128.25. [DOI] [PubMed] [Google Scholar]
- [19].Nelson TJ, Alkon DL. Insulin and cholesterol pathways in neuronal function, memory and neurodegeneration. Biochem Soc Trans. 2005;33:1033–6. doi: 10.1042/BST20051033. [DOI] [PubMed] [Google Scholar]
- [20].Kompoti M, Mariolis A, Alevizos A, Kyrazis I, Protopsaltis I, Dimou E, et al. Elevated serum triglycerides is the strongest single indicator for the presence of metabolic syndrome in patients with type 2 diabetes. Cardiovasc. Diabetol. 2006;5:21. doi: 10.1186/1475-2840-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Janson J, Soeller WC, Roche PC, Nelson RT, Torchia AJ, Kreutter DK, et al. Spontaneous diabetes mellitus in transgenic mice expressing human islet amyloid polypeptide. Proc Natl Acad Sci USA. 1996;93:7283–8. doi: 10.1073/pnas.93.14.7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Verchere CB, D’Alessio DA, Palmiter RD, Weir GC, Bonner-Weir S, Baskin DG, et al. Islet amyloid formation associated with hyperglycemia in transgenic mice with pancreatic beta cell expression of human islet amyloid polypeptide. Proc Natl Acad Sci USA. 1996;93:3492–6. doi: 10.1073/pnas.93.8.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gebre-Medhin S, Mulder H, Pekny M, Westermark G, Tornell J, Westermark P, et al. Increased insulin secretion and glucose tolerance in mice lacking islet amyloid polypeptide (amylin) Biochem Biophys Res Commun. 1998;250:271–7. doi: 10.1006/bbrc.1998.9308. [DOI] [PubMed] [Google Scholar]
- [24].Freude S, Plum L, Schnitker J, Leeser U, Udelhoven M, Krone W, et al. Peripheral Hyperinsulinemia Promotes Tau Phosphorylation In Vivo. Diabetes. 2005;54:3343–8. doi: 10.2337/diabetes.54.12.3343. [DOI] [PubMed] [Google Scholar]
- [25].Phiel CJ, Wilson CA, Lee VM-Y, Klein PS. GSK-3alpha regulates production of Alzheimer’s disease amyloid-beta peptides. Nature. 2003;423:435–9. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- [26].Sivaprakasam P, Xie A, Doerksen RJ. Probing the physicochemical and structural requirements for glycogen synthase kinase-3α inhibition: 2D-QSAR. for 3-anilino-4-phenylmaleimides. Bioorganic and Medicinal Chemistry. 2006;14:8210–8. doi: 10.1016/j.bmc.2006.09.021. [DOI] [PubMed] [Google Scholar]
- [27].Qin W, Peng Y, Ksiezak-Reding H, Ho L, Stetka B, Lovati E, et al. Inhibition of cyclooxygenase as potential novel therapeutic strategy in N.141I presenilin-2 familial Alzheimer’s disease. Mol Psychiatry. 2006;11:172–81. doi: 10.1038/sj.mp.4001773. [DOI] [PubMed] [Google Scholar]
- [28].Perry TA, Greig NH. The glucagon-like peptides: a new genre in therapeutic targets for intervention in Alzheimer’s disease. J Alzheimer Dis. 2002;4:487–96. doi: 10.3233/jad-2002-4605. [DOI] [PubMed] [Google Scholar]
- [29].Northam EA, Rankins D, Cameron FJ. Therapy Insight: the impact of type 1 diabetes on brain development and function. Nature Clinical Practice Neurology. 2006;2:78–86. doi: 10.1038/ncpneuro0097. [DOI] [PubMed] [Google Scholar]
- [30].de la Monte SM, Wands JR. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer’s disease. J Alzheimers Dis. 2005;7:45–61. doi: 10.3233/jad-2005-7106. [DOI] [PubMed] [Google Scholar]
- [31].Zhao WQ, Chen H, Quon MJ, Alkon DL. Insulin and the insulin receptor in experimental models of learning and memory. Eur J Pharmacol. 2004;490:71–81. doi: 10.1016/j.ejphar.2004.02.045. [DOI] [PubMed] [Google Scholar]
- [32].Moroo I, Yamada T, Makino H, Tooyama I, McGeer PL, McGeer EG, et al. Loss of insulin receptor immunoreactivity from the substantia nigra pars compacta neurons in Parkinson’s disease. Acta Neuropathol. 1994;87:343–8. doi: 10.1007/BF00313602. [DOI] [PubMed] [Google Scholar]
- [33].Frolich L, Blum-Degen D, Bernstein HG, Engelsberger S, Humrich J, Laufer S, et al. Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J Neural Transm. 1998;105:423–38. doi: 10.1007/s007020050068. [DOI] [PubMed] [Google Scholar]
- [34].Watson GS, Craft S. Modulation of memory by insulin and glucose: neuropsychological obserbations in Alzheimer’s disease. Eur J Pharamcol. 2004;490:97–113. doi: 10.1016/j.ejphar.2004.02.048. [DOI] [PubMed] [Google Scholar]
- [35].Gasparini L, Xu H. Potential roles of insulin and IGF-1 in Alzheimer’s disease. Trends in neurosciences. 2003;26:404–6. doi: 10.1016/S0166-2236(03)00163-2. [DOI] [PubMed] [Google Scholar]
- [36].Gispen WH, Biessels GJ. Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci. 2000;23:542–9. doi: 10.1016/s0166-2236(00)01656-8. [DOI] [PubMed] [Google Scholar]
- [37].Schubert M, Gautam D, Surjo D, Ueki K, Baudler S, Schubert D, et al. Role for neuronal insulin resistance in neurodegenerative diseases. PNAS. 2004;101:3100–3. doi: 10.1073/pnas.0308724101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ghosh R. Current Status: Alzheimer’s Disease. Kathmandu Uni Med J. 2003;1:205–21. [PubMed] [Google Scholar]
- [39].Small DH, Alois RC. Alzheimer and Alzheimer’s disease: a centennial perspective. J Neurochem. 2006;99:708–10. doi: 10.1111/j.1471-4159.2006.04212.x. [DOI] [PubMed] [Google Scholar]
- [40].Golde TE. Disease modifying therapy for AD. J Neurochem. 2006;99:689–707. doi: 10.1111/j.1471-4159.2006.04211.x. [DOI] [PubMed] [Google Scholar]
- [41].Lee VM, Trojanowski JQ. Progress from Alzheimer’s tangles to pathological tau points towards more effective therapies now. J. Alzheimers Dis. 2006;9(Suppl):257–62. doi: 10.3233/jad-2006-9s328. [DOI] [PubMed] [Google Scholar]
- [42].Kuehn BM. New diabetes drugs target gut hormones. JAMA. 2006;296:380–1. doi: 10.1001/jama.296.4.380. [DOI] [PubMed] [Google Scholar]
- [43].Holst JJ. Glucagonlike peptide 1: a newly discovered gastrointestinal hormone. Gastroenterology. 1994;107:1848–55. doi: 10.1016/0016-5085(94)90831-1. [DOI] [PubMed] [Google Scholar]
- [44].Knudscn LB. Glucagon-like peptide-1: the basis of a new class of treatment for type 2 diabetes. J Med Chem. 2004;47:4128–34. doi: 10.1021/jm030630m. [DOI] [PubMed] [Google Scholar]
- [45].Gutniak M, Orskov C, Holst JJ, Ahren B, Efendic S. Antidiabetogenic effect of glucagon-like peptide-1 (7–36) in normal subjects and patients with diabetes mellitus. N Engl J Med. 1992;326:1316–22. doi: 10.1056/NEJM199205143262003. [DOI] [PubMed] [Google Scholar]
- [46].Creutzfeldt W. The incretin concept today. Diabetologia. 1979;16:75–85. doi: 10.1007/BF01225454. [DOI] [PubMed] [Google Scholar]
- [47].Flatt PR, Green BD. Nutrient regulation of pancreatic beta-cell function in diabetes: problems and potential solutions. Biochem. Soc. Trans. 2006;34(Pt 5):774–8. doi: 10.1042/BST0340774. [DOI] [PubMed] [Google Scholar]
- [48].Perfetti R, Zhou J, Doyle ME, Egan JM. Glucagon-like peptide-1 induces cell proliferation and pancreatic—duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology. 2000;141:4600–5. doi: 10.1210/endo.141.12.7806. [DOI] [PubMed] [Google Scholar]
- [49].Hui H, Zhao X, Perfetti R. Structure and function studies of glucagon-like peptide-1 (GLP-1): the designing of a novel pharmacological agent for the treatment of diabetes. Diabetes. Metab Res Rev. 2005;21:313–31. doi: 10.1002/dmrr.553. [DOI] [PubMed] [Google Scholar]
- [50].Alvarez E, Martinez MD, Roncero I, Chowen JA, Garcia-Cuartero B, Gispert JD, et al. The expression of GLP-1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J Neurochem. 2005;92:798–806. doi: 10.1111/j.1471-4159.2004.02914.x. [DOI] [PubMed] [Google Scholar]
- [51].Perry T, Greig NH. A new alzheimer’s disease interventive strategy: GLP-1. Current Drug Targets. 2004;5:565–71. doi: 10.2174/1389450043345245. [DOI] [PubMed] [Google Scholar]
- [52].Perry T, Greig NH. Enhancing central nervous system endogenous GLP-1 receptor pathway for intervention in Alzheimer’s disease. Curr Alzheimer Res. 2005;2:377–85. doi: 10.2174/1567205054367892. [DOI] [PubMed] [Google Scholar]
- [53].Bell GI. The glucagon superfamily: precursor structure and gene organization. Peptides. 1986;7(Suppl):27–36. doi: 10.1016/0196-9781(86)90160-9. [DOI] [PubMed] [Google Scholar]
- [54].Suda K, Takahashi H, Fukase N, Manaka H, Tominaga M, Sasaki H. Distribution and molecular forms of glucagon-like peptide in the dog. Life Sci. 1989;45:1793–8. doi: 10.1016/0024-3205(89)90519-5. [DOI] [PubMed] [Google Scholar]
- [55].Steiner DF, Rouille Y, Gong Q, Martin S, Carroll R, Chan SJ. The role of prohormone convertases in insulin biosynthesis: evidence for inherited defects in their action in man and experimental animals. Diabetes Metab. 1996;22:94–104. [PubMed] [Google Scholar]
- [56].Wettergren A, Pridal L, Wojdemann M, Holst JJ. Amidated and non-amidated glucagon-likepeptide-1(GLP-1): non-pancreatic effects (cephalic phase acid secretion) and stability in plasma in humans. Regul Pept. 1998;77:83–7. doi: 10.1016/s0167-0115(98)00044-5. [DOI] [PubMed] [Google Scholar]
- [57].Scharpe S, De Meester I. Peptide truncation by dipeptidyl peptidase IV: a new pathway for drug discovery? Verh K Acad Geneeskd Belg. 2001;63:5–32. [PubMed] [Google Scholar]
- [58].Demuth HU, McIntosch CHS, Pederson RA. Type 2 diabetes-therapy with dipeptidyl peptidase IV inhibitors. Biochim Biophys Acta. 2005;1751:33–45. doi: 10.1016/j.bbapap.2005.05.010. [DOI] [PubMed] [Google Scholar]
- [59].Chia CW, Egan JM. Biology and therapeutic potential of GLP-1 in the treatment of diabetes, Drug Discov Today. Dis Mechan. 2005;2:295–301. [Google Scholar]
- [60].Knudsen LB, Pridal L. Glucagon-like peptide-1-(9–36) amide is a major metabolite of glucagon-like peptide-1-(7–36) amide after in vivo administration to dogs, and it acts as anantagonist on the pancreatic receptor. Eur J Pharmacol. 1996;318:429–35. doi: 10.1016/s0014-2999(96)00795-9. [DOI] [PubMed] [Google Scholar]
- [61].Schmidtler J, Schepp W, Janczewska I, Weigert N, Furlinger C, Schusdziarra V, et al. GLP-1-(7–36) amide, (1–), and—(1–36) amide: potent cAMP-dependent stimuli of rat parietal cell function. Am J Physiol. 1991;60:G940–G50. doi: 10.1152/ajpgi.1991.260.6.G940. [DOI] [PubMed] [Google Scholar]
- [62].Nauck MA, Weber I, Bach I, Richter S, Orskov C, Holst JJ, et al. Normalization of fasting glycaemia by intravenous GLP-1 (7–36 amide) or (7–37) in type 2 diabetes patients. Diabet Med. 1988;15:937–45. doi: 10.1002/(SICI)1096-9136(1998110)15:11<937::AID-DIA701>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- [63].Deacon CF, Plamboeck A, Moller S, Holst JJ. GLP-1- (9–36) amide reduces blood glucose in anesthetized pigs by a mechanism that does not involve insulin secretion. Am J Physiol Endocrinol Metab. 2002;282:E873–9. doi: 10.1152/ajpendo.00452.2001. [DOI] [PubMed] [Google Scholar]
- [64].Rachman J, Gribble FM, Barrow BA, Levy JC, Buchanan KD, Turner RC. Normalization of insulin response to glucose by overnight infusion of glucagon-like peptide 1 (7–36) amide in patients with NIDDM. Diabetes. 1996;45:1524–30. doi: 10.2337/diab.45.11.1524. [DOI] [PubMed] [Google Scholar]
- [65].Salehi M, D’Alessio DA. New therapies for type 2 diabetes based on glucagon-like peptide 1. Cleve Clin J Med. 2006;73:382–9. doi: 10.3949/ccjm.73.4.382. [DOI] [PubMed] [Google Scholar]
- [66].D’Alessio DA, Vahl TP. Glucagon-like peptide 1: evolution of an incretin into a treatment for diabetes. Am J Physiol Endocrinol Metab. 2004;286:E882–90. doi: 10.1152/ajpendo.00014.2004. [DOI] [PubMed] [Google Scholar]
- [67].Willms B, Werner J, Holst JJ, Orskov C, Creutzfeldt W, Nauck MA. Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: effects of exogenous glucagon-like peptide-1 (GLP-1)-(7–36) amide in type 2 (noninsulin-dependent) diabetic patients. J Clin Endocrinol Metab. 1996;81:327–32. doi: 10.1210/jcem.81.1.8550773. [DOI] [PubMed] [Google Scholar]
- [68].Toft-Nielsen MB, Madsbad S, Holst JJ. Continuous subcutaneous infusion of glucagon-like peptide 1 lowers plasma glucose and reduces appetite in type 2 diabetic patients. Diabetes Care. 1999;22:1137–43. doi: 10.2337/diacare.22.7.1137. [DOI] [PubMed] [Google Scholar]
- [69].Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type-2 Diabetes: a parallel-group study. Lancet. 2002;359:824–830. doi: 10.1016/S0140-6736(02)07952-7. [DOI] [PubMed] [Google Scholar]
- [70].Drucker DJ. Biological actions and therapeutic potential of the glucagon-like peptides. Gastroenterology. 2002;122:531–44. doi: 10.1053/gast.2002.31068. [DOI] [PubMed] [Google Scholar]
- [71].Ahren B. Gut peptides and type 2 diabetes mellitus treatment. Curr Diab Rep. 2003;3:365–72. doi: 10.1007/s11892-003-0079-9. [DOI] [PubMed] [Google Scholar]
- [72].Huang THJ, Brubaker PL. Synthesis and secretion of glucagon-like peptide-1 by fetal rat intestinal cells in culture. Endocrine. 1995;3:499–503. doi: 10.1007/BF02738824. [DOI] [PubMed] [Google Scholar]
- [73].Burcelin R, Da Costa A, Drucker D, Thorens B. Glucose competence of the hepatoportal vein sensor requires the presence of an activated glucagon-like peptide-1 receptor. Diabetes. 2001;50:1720–8. doi: 10.2337/diabetes.50.8.1720. [DOI] [PubMed] [Google Scholar]
- [74].Dardevet D, Moore MC, DiCostanzo CA, Farmer B, Neal DW, Snead W, et al. Insulin secretion-independent effects of GLP-1 on canine liver glucose metabolism do not involve portal vein GLP-1 receptors. Am J Physiol Gastrointest Liver Physiol. 2005;289:G806–14. doi: 10.1152/ajpgi.00121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Holst JJ. Glucagon-like peptide-1: from extract to agent. The Claude Bernard Lecture, 2005. Diabetologi. 2006;49:253–60. doi: 10.1007/s00125-005-0107-1. [DOI] [PubMed] [Google Scholar]
- [76].Ionut V, Hucking K, Liberty IF, Bergmann RN. Synergistic effect of portal glucose and glucagon-like peptide-1 to lower systemic glucose and stimulate counter-regulatory hormones. Diabetologia. 2005;48:967–75. doi: 10.1007/s00125-005-1709-3. [DOI] [PubMed] [Google Scholar]
- [77].Stoffel M, Espinosa R, 3rd, Le Beau MM, Bell GI. Human glucagon-like peptide-1 receptor gene. Localization to chromosome band 6p21 by fluorescence in situ hybridization and linkage of a highly polymorphic simple tandem repeat DNA polymorphism to other markers on chromosome 6. Diabetes. 1993;42:1215–8. doi: 10.2337/diab.42.8.1215. [DOI] [PubMed] [Google Scholar]
- [78].Campos RV, Lee YC, Drucker DJ. Divergent tissue-specific and developmental expression of receptors for glucagon and glucagon-like peptide-1 in the mouse. Endocrinology. 1994;134:2156–64. doi: 10.1210/endo.134.5.8156917. [DOI] [PubMed] [Google Scholar]
- [79].Wheeler MB, Lu M, Dillon JS, Leng XH, Chen C, Boyd AE. Functional expression of the rat glucagon-like peptide-I receptor, evidence for coupling to both adenylyl cyclase and phospholipase-C. Endocrinology. 1993;133:57–62. doi: 10.1210/endo.133.1.8391428. [DOI] [PubMed] [Google Scholar]
- [80].Wilmen A, Van Eyll B, Goke B, Goke R. Five out of six tryptophan residues in the N.-terminal extracellular domain of the rat GLP-1 receptor are essential for its ability to bind GLP-1. Peptides. 1997;18:301–5. doi: 10.1016/s0196-9781(96)00321-x. [DOI] [PubMed] [Google Scholar]
- [81].Salapatek AM, MacDonald PE, Gaisano HY, Wheeler MB. Mutations to the third cytoplasmic domain of the glucagon-like peptide 1 (GLP-1) receptor can functionally uncouple GLP-1 stimulated insulin secretion in HIT-T15 cells. Mol Endocrino. 1999;13:1305–17. doi: 10.1210/mend.13.8.0321. [DOI] [PubMed] [Google Scholar]
- [82].Deacon CF. Therapeutic strategies based on glucagon-like peptide 1. Diabetes. 2004;53:2181–9. doi: 10.2337/diabetes.53.9.2181. [DOI] [PubMed] [Google Scholar]
- [83].Drucker DJ. Glucagon-like peptides. Diabetes. 1998;47:159–169. doi: 10.2337/diab.47.2.159. [DOI] [PubMed] [Google Scholar]
- [84].Behme MT, Dupré J, McDonald TJ. Glucagon-like peptide 1 improved glycemic control in type 1 diabetes. BMC Endocr Disord. 2003;3:3–12. doi: 10.1186/1472-6823-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Gromada J, Holst JJ, Rorsman P. Cellular regulation of islet hormone secretion by the incretin hormone glucagon-like peptide 1. Pflugers Arch. 1998;435:583–94. doi: 10.1007/s004240050558. [DOI] [PubMed] [Google Scholar]
- [86].Satoh F, Beak SA, Small CJ, Falzon M, Ghatei MA, Bloom SR, et al. Characterization of human and rat glucagon-like peptide-1 receptors in the neurointermediate lobe, lack of coupling to either stimulation or inhibition of adenyl cyclase. Endocrinology. 2000;141:1301–9. doi: 10.1210/endo.141.4.7420. [DOI] [PubMed] [Google Scholar]
- [87].Perry T, Lahiri DK, Sambamurti K, Chen D, Mattson MP, Egan JM, Greig NH. Glucagon-like peptide-1 decreases endogenous amyloid β peptide (Aβ) levels and protects hippocampal neurons from death induced by Aβ and iron. J Neuroscience Res. 2003;72:603–12. doi: 10.1002/jnr.10611. [DOI] [PubMed] [Google Scholar]
- [88].Mattson MP, Lovell MA, Furukawa K, Markesbery WR. Neurotrophic factors attenuate glutamate-induced accumulation of peroxides, elevation of intracellular Ca2+ concentration and neurotoxicity and increase antioxidant enzyme activities in hippocampal neurons. J Neurochem. 1995;65:1740–51. doi: 10.1046/j.1471-4159.1995.65041740.x. [DOI] [PubMed] [Google Scholar]
- [89].Imeryuz N, Yegen BC, Bozkurt A, Coskun T, Villanueva-Penacarrillo ML, Ulusoy NB. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am J Physiol. 1997;273:G920–7. doi: 10.1152/ajpgi.1997.273.4.G920. [DOI] [PubMed] [Google Scholar]
- [90].During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, et al. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med. 2003;9:1173–9. doi: 10.1038/nm919. [DOI] [PubMed] [Google Scholar]
- [91].Perry T, Holloway HW, Weerasuriya A, Mouton PR, Duffy K, Mattison JA, et al. Evidence of GLP-1-mediated neuroprotection in an animal model of pyridoxine-induced peripheral sensory neuropathy. Exp Neurol. 2007;203:293–301. doi: 10.1016/j.expneurol.2006.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Parsons GB, Souza DW, Wu H, Yu D, Wadsworth SG, Gregory RJ, et al. Ectopic expression of glucagon-like peptide 1 for gene therapy of type II diabetes. Gene Ther. 2007;14:38–48. doi: 10.1038/sj.gt.3302842. [DOI] [PubMed] [Google Scholar]
- [93].Youn Yk, Chae SY, Lee S, Jeon JE, Shin HG, Lee KC. Evaluation of therapeutic potentials of site-specific PEGylated glucagon-like peptide-1 isomers as a type 2 anti-diabetic treatment: Insulinotropic activity, glucose-stabilizing capability, and proteolytic stability. Biochemical Pharmacology. 2007;73:84–93. doi: 10.1016/j.bcp.2006.09.013. [DOI] [PubMed] [Google Scholar]
- [94].Kuma M, Hunag Y, Glinka Y, Prud’Homme GJ, Wang Q. Gene therapy of diabetes using a novel GLP-1/IgG1-Fc fusion construct normalizes glucose levels in db/db mice. Gene Therapy. 2007;14:162–72. doi: 10.1038/sj.gt.3302836. [DOI] [PubMed] [Google Scholar]
- [95].Sinclair EM, Drucker DJ. Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase IV inhibitors: new therapeutic agents for the treatment of type 2 diabetes. Curr Opin Endocrinol Diabet. 2005;12:146–51. [Google Scholar]
- [96].Gautier JF, Fetita S, Sobngwi E, Salaun-Martin C. Biological actions of the incretins GIP and GLP-1 and therapeutic perspectives in patients with type 2 diabetes. Diabetes Metab. 2005;31:233–42. doi: 10.1016/s1262-3636(07)70190-8. [DOI] [PubMed] [Google Scholar]
- [97].Combettes MM. GLP-1 and type 2 diabetes: physiology and new clinical advances. Current Opinion in Pharmacology. 2006;6:598–605. doi: 10.1016/j.coph.2006.08.003. [DOI] [PubMed] [Google Scholar]