This study shows that a WUSCHEL-like gene, STENOFOLIA (STF), is required for blade outgrowth, and its deletion accounts for the classical bladeless lam1 phenotype of tobacco (Nicotiana sylvestris). STF confers morphogenetic competence to leaf primordial margins and coordinates auxin/cytokinin homeostasis and hormone crosstalk with sugar metabolism, integrating metabolic and developmental signals.
Abstract
Dicot leaf primordia initiate at the flanks of the shoot apical meristem and extend laterally by cell division and cell expansion to form the flat lamina, but the molecular mechanism of lamina outgrowth remains unclear. Here, we report the identification of STENOFOLIA (STF), a WUSCHEL-like homeobox transcriptional regulator, in Medicago truncatula, which is required for blade outgrowth and leaf vascular patterning. STF belongs to the MAEWEST clade and its inactivation by the transposable element of Nicotiana tabacum cell type1 (Tnt1) retrotransposon insertion leads to abortion of blade expansion in the mediolateral axis and disruption of vein patterning. We also show that the classical lam1 mutant of Nicotiana sylvestris, which is blocked in lamina formation and stem elongation, is caused by deletion of the STF ortholog. STF is expressed at the adaxial–abaxial boundary layer of leaf primordia and governs organization and outgrowth of lamina, conferring morphogenetic competence. STF does not affect formation of lateral leaflets but is critical to their ability to generate a leaf blade. Our data suggest that STF functions by modulating phytohormone homeostasis and crosstalk directly linked to sugar metabolism, highlighting the importance of coordinating metabolic and developmental signals for leaf elaboration.
INTRODUCTION
Leaves are the principal organs for photosynthetic carbon assimilation. Independent origins of the flat lamina in different plant families provide evidence that it is an important evolutionary adaptation of land plants for efficient capture of solar energy and gaseous exchange. Dicot leaf primordia initiate at the flanks of the shoot apical meristem (SAM) and extend laterally during primary and secondary morphogenesis in which growth occurs predominantly by cell division and cell expansion, respectively (Sussex, 1955; Poethig, 1997; Scarpella et al., 2010). As the primordium extends, it asymmetrically differentiates into distinct upper (adaxial) and lower (abaxial) surfaces, forming a flattened lamina.
Leaf primordium initiation requires localized accumulation of the phytohormone auxin (Reinhardt et al., 2003; Braybrook and Kuhlemeier, 2010) and repression of Class 1 KNOTTED1-LIKE HOMEOBOX (KNOX1) gene expression by the ASSYMMETRIC LEAVES1 and 2 (AS1/AS2) complex (Long et al., 1996; Uchida et al., 2007; Guo et al., 2008; Jun et al., 2010) at the initiation site. KNOX1 genes modulate the cytokinin/gibberellin (GA) ratio in the SAM by activating cytokinin biosynthesis and repressing GA biosynthesis or activating GA catabolism (Jasinski et al., 2005; Bolduc and Hake, 2009). Shortly after primordium emergence, a distinctive band of cells along the lateral margins differentiates into the marginal blastozone (Hagemann and Gleissberg, 1996), which expands laterally to form the lamina or pinnae in compound leaves, whereas cells at the central region of the primordium differentiate to form the midrib or rachis (Poethig, 1997). The next significant insights came from investigation of mechanisms governing leaf dorsoventral polarity involving mutually antagonistic interplay of MYB, class III homeodomain Leu-zipper (HD-ZIPIII), YABBY, and KANADI class of transcription factors (Tsukaya, 2006; Husbands et al., 2009; Braybrook and Kuhlemeier, 2010; Efroni et al., 2010).
The first described dorsoventral polarity mutant in Antirrhinum, phantastica (phan), displays radialized abaxial leaves in severely affected cases and at restrictive growth temperatures (Waites and Hudson, 1995; Waites et al., 1998). PHAN encodes an MYB transcription factor required for adaxial identity and blade outgrowth (Waites et al., 1998). This was the first demonstration that the adaxial/abaxial identity sets a positional landmark for lateral outgrowth of the blade. However, loss-of-function mutants of PHAN homologs in other species have variable and less dramatic phenotypes, including crispa in pea (Pisum sativum) (Tattersall et al., 2005) and roughsheath2 in maize (Zea mays) (Timmermans et al., 1999). The Arabidopsis thaliana homolog in particular, the as1 mutant (Byrne et al., 2000), displays no obvious narrowing of lamina except occasionally in combination with the as2 mutant and in certain genetic backgrounds (Xu et al., 2003). AS2 encodes a LOB domain protein containing Leu-zipper motif and forms a complex with AS1 to promote adaxial cell fate (Lin et al., 2003; Xu et al., 2003) and KNOX1 repression (Guo et al., 2008; Jun et al., 2010).
In Nicotiana sylvestris, a unique lamina deletion mutant called lam1 has been described (McHale, 1992). Morphological and cellular studies demonstrated that mutant leaf primordia lacking LAM1 establish normal polarity, and blade founder cells are recruited in the correct position at the adaxial/abaxial boundary, but lateral outgrowth of an organized lamina fails (McHale, 1992, 1993). This demonstrated that blade formation occurs in two distinct phases. The first step involves recruitment of a loosely organized group of blade founder cells at the adaxial/abaxial boundary of the primordium, a process that does not require LAM1. The second phase, lateral outgrowth and layer organization, however, is entirely dependent on LAM1 function. Later work on periclinal chimeras revealed that wild-type LAM1 cells in the internal L3 domain could nonautonomously establish blade organization and lateral outgrowth (McHale and Marcotrigiano, 1998), and that this internal organizing influence of LAM1 is an ongoing requirement during blade expansion.
Our most recent insights on blade formation have come from analysis of genes in the WUSCHEL (WUS)-RELATED HOMEOBOX (WOX) family. WUS, the founding member of WOX, is required for stem cell maintenance in shoot and floral meristem (Laux et al., 1996; Mayer et al., 1998). WOX genes also play important roles in lateral organ development. Two mutants in maize, narrowsheath1 and 2 (ns1 and ns2) lead to a loss of leaf blade in the ns1 ns2 double mutant (Scanlon et al., 1996). NS1 and NS2 are duplicated WOX genes (Nardmann et al., 2004) related to the Arabidopsis gene PRESSED FLOWER (PRS/WOX3), the knockout of which leads to defects in lateral sepals, petals, and stipules, but not in the leaf blade (Matsumoto and Okada, 2001; Shimizu et al., 2009). Interestingly, expression of WUS using the PRS promoter rescues the prs phenotype, indicating some common mechanism in WOX gene function (Shimizu et al., 2009). A related Arabidopsis gene, wus-related homeobox 1 (wox1), has no visible mutant phenotype (Haecker et al., 2004; Vandenbussche et al., 2009), but in Petunia, mutation in WOX1-like gene, maewest (maw), has been shown to cause a narrow lamina and defective petal fusion phenotype (Vandenbussche et al., 2009). The maw mutation leads to stronger lamina reduction when combined with the chsu mutation (Vandenbussche et al., 2009), suggesting redundancy in regulating lamina expansion. Likewise, a prs wox1 double mutant in Arabidopsis has been shown to cause lamina reduction (Vandenbussche et al., 2009), although the phenotype is weaker than the maw chsu double mutant. The molecular mechanism by which the phan single mutant or ns1 ns2 or wox1 prs or maw chsu double mutants restrict(s) blade outgrowth remains unknown.
Cell proliferation and cell expansion mutants of angustifolia and rotundifolia in Arabidopsis show specific defects in mediolateral (width direction) and proximodistal (length direction) growth, respectively (Kim et al., 1998, 2002; Horiguchi et al., 2005), suggesting that these two growth patterns of the lamina may be mediated by separate mechanisms (Tsukaya, 2006). Other Arabidopsis genes, including AINTEGUMENTA (Mizukami and Fischer, 2000), ARGOS (Hu et al., 2003), JAGGED (Dinneny et al., 2004), and PEAPOD (White, 2006) also contribute to lamina size by positively regulating cell proliferation, although the mechanism remains unclear. Mutation in CINCINATA (CIN), a member of TEOSINTE BRANCHED1/CYCLOIDEA/PCF (TCP) family, leads to excessive cell proliferation; CIN may make cells sensitive to the arrest-front signal (Nath et al., 2003). TCPs are represented by a large gene family (Martín-Trillo and Cubas, 2010) and perform several vital functions in leaf development and maturation involving cell proliferation and differentiation (Nath et al., 2003; Palatnik et al., 2003; Ori et al., 2007; Koyama et al., 2010; Shleizer-Burko et al., 2011). CIN-like TCP genes negatively regulate CUP-SHAPED COTYLEDON (CUC) genes by activating miR164, AS1 and auxin response (Koyama et al., 2007; Koyama et al., 2010). CUC genes are important for organ separation at boundary regions and serration of leaf margins (Aida et al., 1997; Palatnik et al., 2003; Nikovics et al., 2006; Koyama et al., 2007; Bilsborough et al., 2011; Hasson et al., 2011). In leaf primordia, the activity of TCP genes is regulated by miR319a and this regulation is essential for proper leaf development in various leaf forms (Palatnik et al., 2003; Ori et al., 2007; Shleizer-Burko et al., 2011).
A gain-of-function mutation in blade-on-petiole (bop) or loss-of-function in bop1 bop2 double mutants leads to formation of blade-like outgrowth on the petiole (Ha et al., 2007; Jun et al., 2010). BOP1 and BOP2 encode BTB/POZ domain proteins that repress YABBY and KNOX1 genes, and activate AS2 in leaf primordia (Jun et al., 2010), suggesting that the BOP/YABBY/KNOX module is important for leaf elaboration. LYRATE, the tomato homolog of JAGGED, is shown to coordinate lateral outgrowth by interacting with KNOX1 genes and the auxin transcriptional network (David-Schwartz et al., 2009). Modulation of KNOX1 and auxin activity also appears to be a key component in dissected leaf morphogenesis in Cardamine hirsuta and other leaf forms (Barkoulas et al., 2008; Hay and Tsiantis, 2010). Together, these observations suggest a role for auxin in lamina expansion in simple- and dissected-leafed species. Although distinct aspects of leaf development, including phylotactic arrangement, margin serration, and vein patterning, are well documented to involve auxin (Reinhardt et al., 2003; Scarpella et al., 2006; Bilsborough et al., 2011), direct evidence for auxin control of lamina outgrowth and its genetic regulation at the leaf primordial margins is lacking.
Here, we report the identification and characterization of a novel leaf blade mutant in Medicago truncatula called stenofolia (stf) and a classical mutant called bladeless lam1 in N. sylvestris with very severe defects in blade outgrowth and vein patterning. We show that STF is critical for lamina outgrowth and leaf vascular patterning in simple leaf (N. sylvestris) and compound leaf (M. truncatula) species. Our data suggest that STF is a modulator of auxin and cytokinin homeostasis and hormonal crosstalk that coordinates developmental signals at the adaxial–abaxial boundary region of leaf primordia.
RESULTS
The stf Mutant Displays Multiple Defects in Leaf Lamina, Leaf Vasculature, and Flower Development
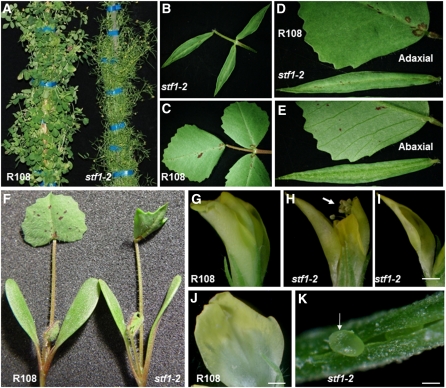
Seven narrow leaf blade mutants with identical phenotypes were identified in forward genetics screens for bladeless mutants in 5600 transposable element of Nicotiana tabacum cell type1 (Tnt1) retrotransposon tagged lines of M. truncatula genotype, R108 (Tadege et al., 2008).These mutants were named stf from Greek stenos for narrow. In all seven stf mutants, lamina growth is initiated and some blade tissue is formed, but further growth in the mediolateral axis is arrested, while growth in the proximodistal axis is virtually unaffected (Figures 1A to 1C). Mature leaves of R108 have regularly serrated margins along two-thirds of the blade distal to the petiole (Figure 1C). In stf leaves, margin serrations are absent and adaxial–abaxial differentiation is drastically reduced (Figures 1D and 1E) except in the unifoliate leaf (first leaf), which is only partially affected (Figure 1F). The lack of lateral expansion is also evident in stf flowers in which the outer petal is narrow (Figures 1I and 1J), and fails to enclose the anthers and stigma (Figures 1G and 1H), while the ovary wall fails to close leaving ovules exposed (Figure 1K), resulting in female sterility.
Figure 1.
Morphological Phenotypes of stf Mutants.
(A) Adult M. truncatula genotype R108 (wild type) and stf1-2 mutant plant 9 weeks after transfer to soil.
(B) stf1-2 adult leaf showing trifoliate identity and normal proximodistal growth but drastically affected mediolateral growth.
(C) R108 adult leaf.
(D) Adaxial surface of R108 and stf adult leaves where margin serrations are absent in the mutant.
(E) Abaxial surface of R108 and stf adult leaves where major veins are not visible in the mutant.
(F) R108 and stf seedlings at the unifoliate (first leaf) stage where cotyledons are nearly wild type and the unifoliate leaf is partially affected.
(G) R108 flower before anthesis in which the anthers and stigma are enclosed by petal.
(H) stf flower in the same stage as in (G) but with anthers and stigma exposed (arrow) because of the narrow petal.
(I) stf outer petal showing the reduction in lateral expansion.
(J) R108 outer petal in the same stage as in (I).
(K) stf ovary wall failing to close and ovule protruding out (arrow). Scale bars in (I) and (J) = 1 mm and in (K) = 50 μM.
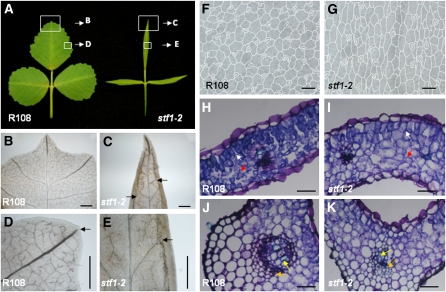
Disruption of vein patterning is another significant phenotype of stf leaves. In R108 leaves, lateral veins radiate from the midvein at regular intervals, extending laterally to the margin (Figure 2B). The tip of each lateral vein corresponds to one serration in the margins and is open ended (Figure 2D). Numerous minor veins make a complex network of branches all over the blade (Figures 2B and 2D). In stf leaves, however, the midvein is thin and less prominent and lateral veins are poorly developed, few in number, and do not extend to the margin (Figures 2C and 2E). There appears to be another major vein-like structure (marginal vein), one on either side of the midvein near the margin, extending from the base of the blade to the tip (Figures 2C and 2E). Minor veins are reduced in number (Figure 2E).
Figure 2.
The stf Mutant Is Severely Defective in Leaf Vascular Patterning.
(A) Wild-type, R108, and stf1-2 mature leaves showing the regions for close-up described in (B) to (E).
(B) to (E) Leaf material observed through a light microscope after clearing with lactic acid.
(B) Major and minor veins of R108 leaf.
(C) Disorganized and poorly developed major veins in stf. Major veins are forming near the margins (one on either side of the midvein) along the proximodistal axis (arrows).
(D) R108 major vein extends close to the margin with its tip aligned to the serration and is open ended (arrow).
(E) stf major vein poorly developed and connected to marginal vein (arrow).
(F) R108 leaf epidermal cells viewed through a light microscope.
(G) Epidermal cells of stf leaf showing narrower width.
(H) Transverse section through R108 leaf blade showing palisade mesophyll (white arrow) and spongy mesophyll (red arrow) cells. Sections were stained with Toluidine Blue.
(I) Transverse section through stf leaf blade showing the poor distinction between palisade mesophyll (white arrow) and spongy mesophyll (red arrow) cells.
(J) Transverse section through the midrib of R108 leaf showing xylem (yellow arrow) and phloem (orange arrow) vessels.
(K) Transverse section through stf midrib showing poorly differentiated xylem and phloem vessels (yellow and orange arrows) and cortical tissue. Scale bars in (B) to (E) = 500 μM, in (F) and (G) = 50 μM, and in (H) to (K) = 100 μM.
Examination of blade epidermal cells by light and scanning electron microscopy (SEM) showed that stf leaves are affected in cell division and cell expansion. Under the light microscope, epidermal cells in stf appear slightly elongated and thinner, with most cells expanding to only ~75 percent of the width of wild type (Figures 2F and 2G); this was confirmed by SEM (see Supplemental Figures 1A and 1B online). The total number of cells at the equatorial plane of the stf blade is at least threefold lower than that of wild type (see Supplemental Figures 1E and 1F online), suggesting a major defect in cell division. SEM also showed that elongated marginal cell files are shorter in stf blade margins (see Supplemental Figures 1C and 1D online).
Transverse sections through the leaf blade revealed that the mesophyll tissue is not well differentiated in stf, especially on the adaxial side. In the wild type, palisade mesophyll cells (adaxial side) are uniformly cylindrical in shape, whereas spongy mesophyll cells (abaxial side) are compact and irregular in shape (Figure 2H). In stf, although cells on either side are not identical, the contrast between palisade and spongy mesophyll cells is diminished (Figure 2I). Transverse sections through the midvein showed that the xylem and phloem cells maintain their relative adaxial and abaxial positions, respectively, but fail to differentiate further and appear difficult to distinguish in stf (Figures 2J and 2K).
Together, these observations suggest that stf retains polarity but has defects in cell division, cell expansion/differentiation, and vascular patterning that severely curtail lamina outgrowth. Unlike other lamina mutants caused by polarity defects, the stf polarity defect appears to be a consequence rather than the cause of the lamina phenotype since adaxial and abaxial cell types are correctly specified both in the mesophyll and vasculature of stf, but further differentiation of both cell types is compromised.
STF Encodes a WUS-Like Homeodomain Protein Conserved in Dicots
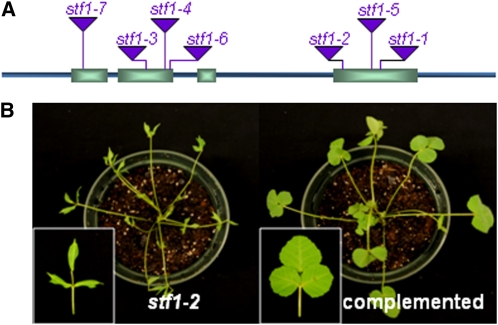
We cloned STF by PCR-based genotyping of flanking sequence tags (FST) in segregating populations (Tadege et al., 2008) and we confirmed that the seven independent lines (stf1-1 to stf1-7) are allelic, having Tnt1 insertions in exons one, two, and four (Figure 3A). This was further confirmed by complementation of the stf1-2 mutant phenotypes with a 5.3-kb genomic fragment of STF (Figure 3B). STF encodes a 358-amino acid homeodomain transcriptional regulator with 38% amino acid identity to Arabidopsis WOX1 and 45% amino acid identity to Petunia MAW. We identified STF-like (STL) sequences from alfalfa and tobacco by RT-PCR and from other dicot genomes by BLAST search (see Supplemental Figures 2 and 3 online). In most of these genomes, STF is represented by a small gene family with one or two members. In the sequenced part of the M. truncatula genome, there is a second partial STF with predicted 165 amino acids but its functionality has not yet been tested. At the sequence level, STF, MAW, and STL share conserved amino acid motifs at the N- and C-terminal regions, in addition to the homeodomain and WUS box, including a highly conserved 10-amino acid motif at the 3′ end (QFI/FEFLPLKN), which we named the STF box (see Supplemental Figure 3B online). The region corresponding to the STF box in WUS has been recognized as an EAR-like motif (Ikeda et al., 2009), but the STF box shows more similarity to the WUS box than the WUS EAR-like motif. The WUS box confers repressive functions to Arabidopsis WUS (Ikeda et al., 2009).
Figure 3.
STF Encodes a WOX Domain Protein and Complements the stf Mutant.
(A) STF gene structure showing the position of the Tnt1 insertion site in seven independent mutant lines.
(B) stf mutant complemented with 5.3-kb genomic STF.
[See online article for color version of this figure.]
Phylogenetic analysis of a subset of related WOX and STL proteins using full-length and homeodomain region amino acid sequences showed that STF and STL group together in a separate subclade that encompasses WOX1 but distinct from the closely related PRS/WOX3 and WUS subclades (see Supplemental Figure 2 online), in agreement with previous studies (Vandenbussche et al., 2009; Zhang et al., 2010). In addition to M. truncatula, tobacco, petunia, and Arabidopsis mentioned above, for which functionality has been tested, STL sequences were found in all eudicot species sequenced to date, suggesting that STF is conserved in both dicot classes: rosids and asterids. By contrast, STF homologs were not found in the genomes of rice (Oryza sativa), maize, sorghum (Sorghum bicolor), foxtail millet (Setaria italic), or purple false brome (Brachypodium distachyon), or in the wheat (Triticum aestivum) and barley (Hordeum vulgare) database of 1.5 million expressed sequence tags, as well as the banana (Musa spp) NCBI database of 89,151 expressed sequence tags. On the other hand, the related gene, PRS/WOX3, is widely conserved in angiosperms and gymnosperms (see Supplemental Figures 2B and 4 online). The molecular mechanism by which PRS or maize NS1 and NS2 orchestrate their functions is unknown, but the stf mutant phenotypes suggest that STF may not be functionally redundant with PRS/NS because the strong stf phenotype prevails in the presence of wild-type Medicago PRS (Mt WOX3). All the WUS, PRS, and MAW clades have strong conservation in the WUS box, whereas the STF box is absent from PRS/WOX3 genes and is modestly conserved in the WUS clade (see Supplemental Figure 5 online). Further investigation will be required to determine if the STF box is a functional domain and if STLs play specific roles in dicot lamina evolution.
STF Is Expressed at the Adaxial–Abaxial Boundary Layer in Leaf Primordia and Shows Developmental Regulation
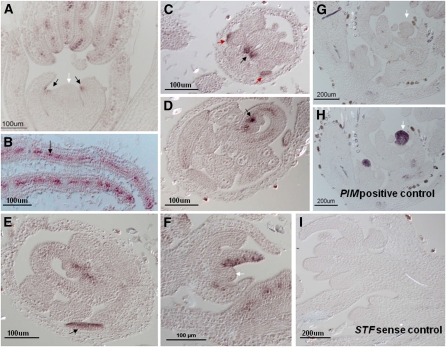
To investigate tissue-specific expression patterns of STF, we performed RNA in situ hybridization in vegetative shoot apex and flower, where the highest expression was detected by quantitative RT (qRT)-PCR. In young leaf primordia at the P1 and P2 stages, STF is adaxially expressed in few cells, but absent from the SAM (Figure 4A). In older primordia after stage P2, strong STF expression was detected in more cells and localizes in the central region of the adaxial–abaxial boundary layer extending from the distal tip to the proximal base (Figure 4B). In the flower, STF expression was detected in petal primordia and developing petals (Figures 4C and 4E); in carpels, expression was observed in developing margins (Figure 4C), in the placenta, at the base of ovules (Figure 4D) and in the central region. No expression was observed in stamens, in sepals (Figures 4D and 4E), in inflorescence meristem (Figure 4F), or in the floral meristem (Figure 4G), compared with controls (Figures 4H and 4I). Fusing a 2.6-kb region of STF promoter with a β-glucuronidase (GUS) reporter showed a developmental gradient of GUS expression in leaves. In very young trifoliate leaves, GUS staining was intense at the leaf margin in the distal half, including the leaf tip (see Supplemental Figure 6B online). As the leaf grows, expression moves to the proximal half and becomes progressively weaker, to an undetectable level, in mature leaves (see Supplemental Figures 6B to 6E online). GUS staining was also detected in the unifoliate leaf, roots, cotyledons, petals, stigma, and pods (see Supplemental Figures 6A and 6F to 6H online).
Figure 4.
STF Expression Pattern in Vegetative and Floral Apices by RNA in Situ Hybridization in R108.
(A) STF expression in 12-d-old vegetative shoot apex viewed in longitudinal sections. At very early stages, STF is adaxially expressed in few cells (black arrows), but absent from the central zone of SAM (white arrow).
(B) STF expression in older leaf primordia showing localization at the adaxial–abaxial boundary layer (arrow).
(C) STF expression in young flower showing a strong signal in petal primordia and developing petal (red arrows) and developing carpel (black arrow).
(D) STF expression in mature flower showing strong localization in the placenta at the base of the ovules (arrow).
(E) STF expression in mature flower showing expression in the petal lobe (arrow).
(F) STF expression in inflorescence apex showing activity in floral organ primordia, but no detection in the inflorescence meristem (white arrow).
(G) STF expression in inflorescence apex showing no detection in the floral meristem (white arrow).
(H) PIM (AP1) expression in inflorescence apex shown here as positive control for expression in floral meristem (arrow).
(I) RNA in situ hybridization in the inflorescence apex using STF sense probe as negative control.
The lam1 Mutation of N. sylvestris Is Caused by Deletion of the STF Homolog
In the classical bladeless lam1 mutant of N. sylvestris, leaf blades are reduced to vestigial strips lacking mesophyll differentiation (McHale and Marcotrigiano, 1998). The lam1 mutant strongly resembles stf except that lam1 phenotypes are stronger and lack stem elongation (Figure 5A). Based on morphological characters, we hypothesized that stf and lam1 may be caused by mutations in homologous genes. To test this, we cloned an STF-like gene (Ns STF1) from wild-type N. sylvestris by RT-PCR. We also cloned the full-length Ns STF1 genomic sequence with its promoter by thermal asymmetric interlaced (TAIL)-PCR. Ns STF1 is similar to Mt STF in gene structure (see Supplemental Figure 2A online) and shares 45% amino acid identity. PCR experiments demonstrated that Ns STF1 was deleted in the lam1 mutant. Using primers specific to Ns STF1 (see Supplemental Table 1 online), including 2.65 kb of the promoter, we determined that at least a 5.67-kb region of the Ns STF1 locus was deleted in lam1 (Figure 5B). To confirm that the lam1 phenotype is due to lack of Ns STF1 function, a 5.3-kb genomic fragment of Mt STF was introduced into lam1. Mt STF fully complemented the lam1 phenotypes (Figures 5C and 5D), confirming that STF function is indeed absent in the lam1 mutant and that STF and LAM1 are functional homologs.
Figure 5.
The N. sylvestris lam1 Mutation Is Caused by Deletion of the Ns STF1 Gene.
(A) Ten-week-old adult lam1 mutant plant.
(B) Genomic PCR showing deletion of the Ns STF1 locus in the lam1 mutant. Primers F1+R2 amplify the complete CDS plus the 3′ UTR, and primers F2+R3 amplify the promoter, the 5′ UTR, plus part of the CDS, and together span 5.67 kb of the Ns STF1 region. * = 3 kb. WT, wild type.
(C) Untransformed lam1 mutant and lam1 complemented with M. truncatula 5.3-kb genomic STF regenerating in nonselective tissue culture media.
(D) Complemented lam1 in (C) 4 weeks after transfer to soil.
Transcript Profiling Identified Auxin and Multiple Hormone-Associated Changes in stf Mutant
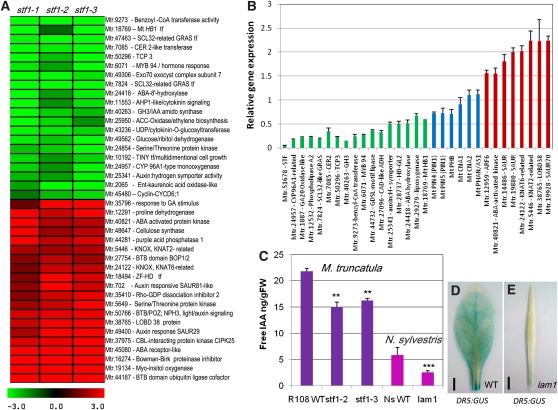
To identify potential targets of STF and to gain insight into the mechanism of its function, we performed transcript profiling analysis using the Medicago Affymetrix GeneChip containing 61,278 probe sets. We compared gene expression in three independent stf mutant lines (stf1-1/2/3) with their segregating wild types in 0.5- to 0.8-mm shoot apices of 4-week-old seedlings, and identified 241 probes differentially expressed with a twofold or more difference in the mutant; 105 probes upregulated and 136 probes downregulated. This analysis identified genes that are known to be involved in leaf development such as SCARECROW (SCR)-like, TCP3, and indole-3-acetic acid (IAA) amidosynthase (GH3) as downregulated, and BOP1/2 and KNAT2, KNAT6-related KNOX1 genes as upregulated, as well as changes in several phytohormone-associated genes, especially auxin (Figure 6A, Table 1). The PHAN and HD-ZIP III-type adaxial polarity determinants were not detected despite the presence of probes on the array. We assessed the expression level of PHAN, PHABULOSA (PHB), and CORONA together with eight upregulated and 16 downregulated genes by qRT-PCR. Consistent with the microarray data, polarity genes were found to be unaffected (PHAN and CORONA) or modestly reduced (PHB), while the other genes differentially expressed in the microarray were clearly induced or repressed in qRT-PCR (Figure 6B), confirming the notion that polarity disruption is not the primary defect in stf. The microarray data rather showed overrepresentation of genes related to phytohormones, including auxin, cytokinin, brassinosteroid, ethylene, gibberellic acid (GA), abscisic acid, and jasmonic acid (Table 1). After removing 11 redundant probes, at least 17.5% of the downregulated and 17.3% of the upregulated probes were found to be directly related to genes associated with hormones, especially auxin (Table 1). This represents a 10-fold enrichment (17.4% compared with a random 0.74%) for the seven hormones mentioned here. This enrichment is 17-fold for auxin-associated genes alone just by querying for the word “auxin.” For example, for most altered auxin genes in the array, 11 of the 48 small auxin-upregulated RNA (SAUR) probes present on the GeneChip were induced (Table 1), accounting for 4.78% of the altered genes instead of the expected 0.08% for unbiased change. Small auxin-upregulated RNA genes are known to negatively regulate auxin biosynthesis and auxin response (Kant et al., 2009). This transcriptional change in auxin-associated genes is consistent with the stf mutant phenotype and the anticipated role of auxin in lamina outgrowth because auxin has been implicated in mechanisms controlling leaf vascular patterning, leaf margin serrations, reproductive organ development, and blade outgrowth although the association with blade outgrowth is circumstantial. These observations prompted us to think that the function of STF may be connected to auxin, and the other hormone responses may have been altered as a result of hormone crosstalk. We followed this lead and evaluated the auxin connection further.
Figure 6.
Microarray Analysis and Auxin Quantification in stf and lam1 Mutants.
(A) A heat map showing differentially expressed genes in three stf mutant lines compared with wild type R108 in 4-week-old shoot apices. Representative genes that are downregulated (green) and upregulated (red) with twofold or more difference are shown. ABA, abscisic acid.
(B) Validation of relative gene expression of selected genes in the stf mutant compared with the wild type by qRT-PCR. Wild-type expression level was arbitrarily set to 1.0. Green, downregulated genes; red, upregulated genes; blue, genes not detected by the microarray. Values are the mean and se of three biological replicates.
(C) Free IAA content in 4-week-old leaves of stf and lam1 mutants compared with their wild type (WT). Values are the mean and se of five experiments (***P < 0.001, **P < 0.01).
(D) GUS staining in DR5:GUS-transformed wild-type N. sylvestris leaf.
(E) GUS staining in DR5:GUS-transformed lam1 leaf showing reduced auxin.
Table 1.
Hormone-Associated Genes Differentially Expressed in stf Shoot Apex
| Probe Sets | Putative Annotation | P Valuea | Fold Changeb | Putative Functionc |
| Mtr.18769.1.S1_at | Mt HOMEOBOX PROTEIN1 | 0 | 0.31 | Abscisic acid/auxin signaling |
| Mtr.24418.1.S1_at | Abscisic acid-8′-hydroxylase | 0 | 0.35 | Abscisic acid catabolism |
| Mtr.1887.1.S1_at | GA20 oxidase like | 0 | 0.36 | GA biosynthesis |
| Mtr.40263.1.S1_at | GH3/IAA amidosynthase | 0 | 0.36 | Auxin homeostasis |
| Mtr.43236.1.S1_at | UDP/cytokinin glucosyltransferase | 0 | 0.37 | Putative cytokinin homeostasis |
| Mtr.4206.1.S1_at | MYB 94 transcription factor | 0 | 0.31 | Multiple hormone response |
| Mtr.2065.1.S1_at | Ent-kaurenoic acid oxidase | 4.4409E-16 | 0.39 | GA biosynthesis |
| Mtr.11553.1.S1_at | AHP-like phosphotransfer protein | 2.15E-269 | 0.39 | Cytokinin signal transduction |
| Mtr.25950.1.S1_at | 1-aminocyclopropane-1-carboxylic acid oxidase | 0 | 0.41 | Ethylene biosynthesis |
| Mtr.1108.1.S1_at | MYB 77-like tf - auxin signaling | 5.266E-48 | 0.43 | Multiple hormone response |
| Mtr.10192.1.S1_at | TINY-like tf – cell growth | 6.7714E-57 | 0.44 | Ethylene response |
| Mtr.37279.1.S1_at | Xanthoxin dehydrogenase-like | 3.4884E-23 | 0.44 | Putative abscisic acid biosynthesis |
| Mtr.25341.1.S1_at | Auxin:hydrogen symporter | 4.3091E-07 | 0.45 | Auxin transport |
| Mtr.27392.1.S1_at | Auxin-induced protein 5NG4 | 2.1832E-09 | 0.47 | Auxin response |
| Mtr.42075.1.S1_at | Abscisic acid hydroxylase | 6.7002E-39 | 0.48 | Abscisic acid catabolism |
| Mtr.29279.1.S1_at | Lipoxygenase | 1.922E-33 | 0.48 | Jasmonic acid biosynthesis |
| Mtr.9203.1.S1_at | SERK1-like protein | 3.9199E-79 | 0.49 | Brassinosteroid signaling |
| Mtr.19928.1.S1_at | Auxin-responsive SAUR70 | 1.9E-19 | 2.01 | Auxin signaling |
| Mtr.19898.1.S1_x_at | Auxin-responsive SAUR76 | 3.09E-07 | 2.03 | Auxin signaling |
| Mtr.12959.1.S1_s_at | Auxin-response factor 6 | 1.22E-07 | 2.1 | Auxin signaling |
| Mtr.14486.1.S1_at | Auxin-responsive SAUR83 | 3.25E-14 | 2.11 | Auxin signaling |
| Mtr.13212.1.S1_at | Jasmonate methyltransferase | 1.0493E-12 | 2.15 | Methyl jasmonate biosynthesis |
| Mtr.19891.1.S1_s_at | Auxin-responsive SAUR81-like | 2.95E-45 | 2.18 | Auxin signaling |
| Mtr.697.1.S1_at | Auxin-responsive SAUR70-like | 9.4E-105 | 2.22 | Auxin signaling |
| Mtr.4357.1.S1_at | Auxin-responsive SAUR82-like | 0 | 2.25 | Auxin signaling |
| Mtr.33772.1.S1_at | UDP-glucuronosyltransferase | 0 | 2.27 | Putative cytokinin/auxin homeostasis |
| Mtr.19925.1.S1_x_at | Auxin-responsive SAUR69-like | 0 | 2.33 | Auxin signaling |
| Mtr.40821.1.S1_at | Abscisic acid-activated kinase | 1.6015E-07 | 2.33 | Abscisic acid signaling |
| Mtr.19880.1.S1_at | Auxin-responsive SAUR80 | 6.76E-20 | 2.33 | Auxin signaling |
| Mtr.38122.1.S1_at | EREB factor | 1.5283E-10 | 2.38 | Ethylene signaling |
| Mtr.49767.1.S1_x_at | Auxin-responsive SAUR69 | 4.62E-15 | 2.39 | Auxin signaling |
| Mtr.12648.1.S1_at | GA-induced ovary protein | 1.79E-22 | 2.44 | GA response |
| Mtr.7260.1.S1_at | Ethylene-induced esterase | 3.43E-42 | 2.53 | Ethylene response |
| Mtr.702.1.S1_at | Auxin-responsive SAUR81-like2 | 1.65E-101 | 2.56 | Auxin signaling |
| Mtr.50766.1.S1_at | BTB/POZ;NPH3 protein | 3.42E-35 | 2.66 | Light/auxin signaling |
| Mtr.38765.1.S1_at | LOB domain protein 38 | 7.6E-185 | 2.73 | Auxin signaling |
| Mtr.49400.1.S1_at | Auxin-responsive SAUR29 | 1.5983E-92 | 2.88 | Auxin signaling |
| Mtr.37975.1.S1_at | CBL-interacting protein kinase | 2.577E-125 | 3 | Abscisic acid/glucose signaling |
| Mtr.35796.1.S1_at | GAST-like gene product | 8.01E-14 | 3.23 | GA response |
| Mtr.45080.1.S1_at | Abscisic acid receptor-like kinase | 6.8378E-25 | 3.68 | Abscisic acid signaling |
P value is obtained from Associative Analysis (Dozmorov and Centola, 2003).
Relative abundance of transcript in stf shoot apex/R108 shoot apex.
Category of predicted gene function.
stf and lam1 Mutants Accumulate Less Free Auxin in Leaves
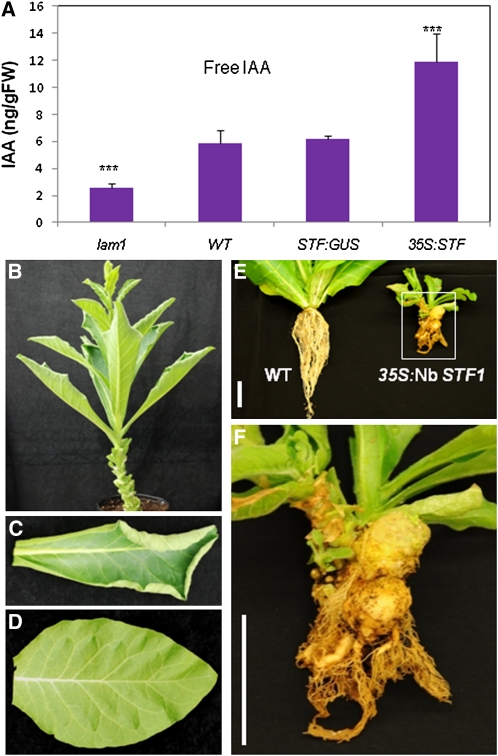
To investigate the role of auxin in stf/lam1 phenotype, we measured free auxin (IAA) directly by gas chromatography-mass spectrometry (GC-MS) in 4-week-old leaves of lam1 and two alleles of stf (stf1-2 and sft1-3). We found that stf accumulates ~68% and lam1 accumulates 50% of the respective wild-type IAA levels (Figure 6C), indicating that the mutants have an auxin deficiency. Because N. sylvestris is faster to transform and is known to be more sensitive to hormones than M. truncatula, we focused the next experiments solely on lam1.To independently confirm the reduced auxin content by a different method, we transformed lam1 and its wild type with the auxin responsive DR5:GUS construct. GUS staining in young leaves from 3- to 4-week-old plants was faintly detectable in DR5:GUS-expressing lam1 leaves, whereas strong GUS staining was observed in DR5:GUS-expressing wild-type leaves (Figures 6D and 6E), confirming that lam1 indeed accumulates reduced auxin.
The reduced steady state level of free auxin could be caused by a defect in auxin biosynthesis, signaling, transport, homeostasis, or a combination thereof. To distinguish among these possibilities, we applied exogenous auxins to lam1 shoots and roots. Foliar spraying of 2-week-old seedlings with naphthalene acetic acid (NAA) showed that mutant and wild-type leaves respond in the same way by epinastic curling and tolerated up to 100 μM NAA concentrations (see Supplemental Figures 7A and 7B online). Application of 10 mM NAA or IAA with lanolin to the shoot apex of lam1 at the 4 leaf stage or adult plants resulted in ectopic lateral bumps and branches on the leaf blade (see Supplemental Figures 7D and 7E online). Similarly, regenerating new plants from lam1 leaf explants in tissue culture via somatic organogenesis in the presence of auxin (0.53 μM NAA) and cytokinin (4.44 μM benzyl amino purine [BAP]), frequently produced variously branched and bifurcated lam1 leaves that have restricted and irregular flattening on the main leaf axis (see Supplemental Figure 7C online). These observations suggest that the lam1 mutant is not blocked in auxin response. The addition of NAA, 2,4-dichlorophenoxyacetic acid (2,4-D), IAA, or the polar auxin transport inhibitor N-1-naphthylphthalamic acid (NPA) to Murashige and Skoog (MS) medium affected lam1 and wild-type root elongation in a similar manner (see Supplemental Figure 7F online), although both roots tolerated much higher levels of the natural auxin IAA compared with the synthetic auxins. NAA and 2,4-D are known to be substrates of efflux and influx carriers, respectively (Yamada et al., 2009). The absence of a differential response to both or to NPA suggests that efflux and influx processes and transport are not significantly affected in lam1. However, growing mutant and wild-type plants on MS medium containing the auxin biosynthetic intermediate Trp, indicated that lam1 roots elongated slightly better (P < 0.05) than wild-type roots (see Supplemental Figure 7F online), suggesting that lam1 could be partly affected in auxin biosynthesis upstream of Trp.
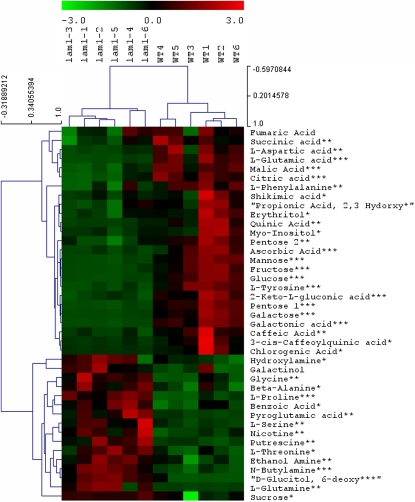
We next performed metabolite profiling in leaf extracts by GC-MS to identify any accumulated intermediates of auxin biosynthesis that would indicate a metabolic blockage point in lam1. We found that metabolites of the shikimate pathway, including shikimic acid, Tyr, and Phe, as well as five- and six-carbon sugars of sugar metabolism, including Glc and Fru were significantly reduced in the mutant (P < 0.001), indicating a defect in sugar metabolism. No significant change (P < 0.05) in 12-carbon sugars, including Suc and galactinol were observed, however, glucitol, Gln, Ser, Gly, and Pro were increased (P < 0.001) by 2- to 15-fold in lam1 (Figure 7), which also indicated a defect in sugar metabolism. Hexoses limit erythrose and pyruvate availability and could account for the downregulation of the shikimate pathway, including auxin biosynthesis. These observations suggest that the reduced auxin levels measured in lam1 leaves may be caused by a broader defect in sugar metabolism rather than a single block in the IAA biosynthetic pathway.
Figure 7.
Metabolic Profiling in 4-Week-Old Leaves of Wild-Type and lam1 Mutant N. sylvestris.
Representative common metabolites are shown. Colors indicate downregulated (green) and upregulated (red) metabolites in lam1 mutant compared with the wild type (WT). The black color shows metabolites that are unchanged. The numbers 1 through 6 at the top indicate replicates of lam1 and wild-type samples each from six individual plants. Statistical significance was calculated using Student’s t test (***P < 0.001, **P < 0.01, and *P < 0.05).
Ectopic Expression of STF Leads to Auxin and Cytokinin Overproduction Phenotypes
To evaluate if increased STF activity leads to auxin overproduction, we ectopically expressed Mt STF and Nicotiana benthamiana STF (Nb STF1) in N. sylvestris using the 35S promoter. IAA was increased by approximately twofold in transgenic lines compared with controls (Figure 8A), indicating a direct relationship between STF expression and steady state level of free auxin accumulation. Transgenic plants expressing either of the genes showed a range of auxin and cytokinin overproduction phenotypes that have been previously observed in tobacco (Eklöf et al., 2000), including leaf upward curling (Figures 8B and 8C). Eight of the strongest STF expressers among 27 independent transgenic lines displayed extreme dwarf and nonflowering phenotypes with deformed shoots, roots, and leaves (Figure 8E; see Supplemental Figure 8A online). Interestingly, these severely affected lines produced one or more tumors on the roots or at the crown (Figure 8F; see Supplemental Figure 8A online). Because tumor formation requires overproduction of auxin and cytokinin (Zambryski et al., 1989), these results suggest that STF expressors are also overproducing cytokinin. Consistent with this, exogenous application of cytokinin alone with lanolin to lam1 shoot apex frequently produced leaf branches, and rarely shoots, while application of auxin and cytokinin together partially rescued the lam1 blade phenotype (Figures 9B, 9D, and 9F), suggesting that STF may directly or indirectly affect cytokinin biosynthesis. The application of cytokinin or cytokinin and auxin together to wild-type shoots has an inhibitory effect on leaf growth (Figures 9A, 9C, and 9E), but the application of cytokinin alone rarely induces shoot regeneration on leaves similar to its effect on lam1 shoots. These data suggest that the function of STF in regulating lamina outgrowth may be connected to the auxin:cytokinin ratio.
Figure 8.
Ectopic Expression of Mt STF and Nb STF1 in N. sylvestris.
(A) Free IAA content in mature leaves of lam1 mutant, Wild type, STF:GUS transgenic control, and 35S:STF transgenic plant. Values are the mean and se of five replicates (***P < 0.001). FW, fresh weight.
(B) 35S:STF transgenic plant showing upward curling leaf phenotype.
(C) 35S:Nb-STF1 transgenic leaf showing upward curling phenotype.
(D) Wild-type leaf.
(E) Left, 4-week-old wild-type (WT) N. sylvestris; right, 17-month-old transgenic plant with highest STF overexpression showing shoot and root deformation.
(F) Close-up of the transgenic plant in (E) showing two large tumors. Scale bars = 5 cm.
Figure 9.
Exogenous Application of Auxin and Cytokinin Partially Rescues the lam1 Lamina.
(A) and (B) Wild-type N. sylvestris (A) and lam1 (B) shoots treated with 10 mM BAP. Inset shows leaf branching.
(C) and (D) Wild-type N. sylvestris (C) and lam1 (D) shoots treated with 10 mM BAP plus 10 mM IAA. Inset shows partially formed petiole and blade.
(E) and (F) Wild-type N. sylvestris (E) and lam1 (F) shoots treated with 10 mM BAP plus 1 mM IAA. Inset shows partially formed petiole and blade.
Note that lam1 leaves are uniformly thin and cannot be distinguished into petiole and lamina. Scale bars = 1 cm.
[See online article for color version of this figure.]
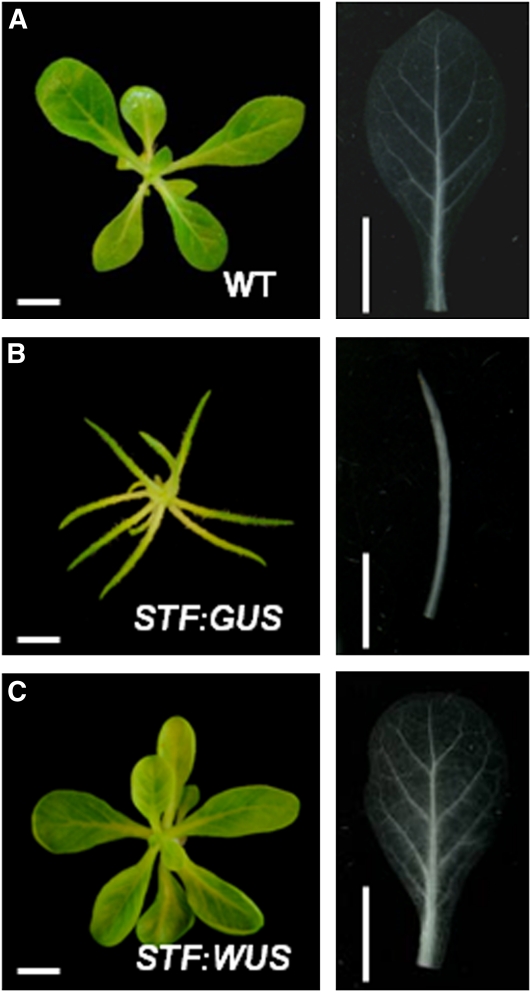
WUS activates cytokinin signaling by A-type ARR repression (Leibfried et al., 2005). To explore the possibility that cytokinin signaling is disrupted in lam1 mutant primordia, we transformed lam1 explants with STF:WUS. We observed complementation of the lamina and venation defects of lam1 (Figure 10), with this construct suggesting that cytokinin signaling is in fact a major component of LAM1 function. This is consistent with the STF overexpression phenotypes and the combined auxin and cytokinin treatments. Together, our data support a model in which auxin- and cytokinin-mediated signaling modulated by STF/LAM1 at the leaf primordial margins regulates vein patterning and blade outgrowth in the simple- and dissected-leafed eudicots.
Figure 10.
Arabidopsis WUS Complements the lam1 Mutant Phenotype.
(A) Untransformed wild-type (WT) N. sylvestris grown in tissue culture MS media. Right panel shows wild-type leaf cleared with lactic acid for looking at the venation pattern.
(B) lam1 mutant transformed with STF:GUS construct as negative control. Right panel shows lam1 leaf cleared with lactic acid.
(C) lam1 mutant complemented with STF:WUS construct. Right panel shows complemented leaf cleared with lactic acid. Note that the lamina and venation phenotypes are complemented.
[See online article for color version of this figure.]
DISCUSSION
STF Controls Blade Outgrowth at the Adaxial–Abaxial Boundary Layer
We have described the leaf lamina mutants, stf and lam1, representing rosids and asterids, respectively. In several adaxial polarity mutants, including phan (Waites and Hudson, 1995) and phb/phv double mutants (McConnell et al., 2001), there is not only loss of adaxial identity but also the appearance of abaxial characteristics in the adaxial domain. The abaxialization of severely affected phan mutants led Waites and Hudson (1995) to propose that the juxtaposition of adaxial and abaxial cells is a prerequisite for blade outgrowth. In the stf mutant, such complete loss of adaxial domain or appearance of abaxial characters in the adaxial surface has not been observed, and domain specification is intact. Unlike most polarity factors that exhibit domain-specific expression in leaf primordia, such as HD-ZIP III and KAN family genes (Kerstetter et al., 2001; McConnell et al., 2001; Emery et al., 2003), STF expression is not axially confined (Figure 4B). The expression domains of STF and the presence of intact dorsoventral polarity in the stf mutant are consistent with recent observations reported for maw (Vandenbussche et al., 2009). Our work on STF/LAM1, along with the studies on MAW, suggests that polarity governs only the initial phase where founders are recruited at the adaxial–abaxial boundary. Subsequent assembly of specialized cell layers and outgrowth of flattened lamina is dependent on WOX function. We propose that STF function at the adaxial–abaxial boundary is required for cell proliferation, cellular differentiation, and expansion controlling lamina elaboration in the mediolateral axis. In addition to STF, LAM1, MAW, NS1, and NS2, which are WOX genes, class I HD-ZIP genes have been reported to control tendril and bract morphogenesis without necessarily disrupting polarity. For example, the tendril-less (tl) mutation in pea revealed that tendrils are modified leaves where lamina outgrowth is inhibited by repressive function of TL (Hofer et al., 2009), and a related gene in Arabidopsis, LATE MERISTEM IDENTITY1 (LMI1), is required for suppression of bract formation (Saddic et al., 2006). It remains to be shown if class I HD-ZIP genes target or interact with WOX genes in leaf primordia.
STF Controls Blade Outgrowth and Vein Patterning
Auxin has been described as the key factor that modulates growth and pattern formation during vascular morphogenesis (Scarpella et al., 2006; Caño-Delgado et al., 2010; Scarpella et al., 2010). Recently, Scarpella et al. (2010) have suggested that auxin internalization from PIN1 convergence points in the epidermal layer induces formation of veins and that auxin maxima generated by PIN1 polarity is the major factor that orchestrates vascular patterning and blade outgrowth. However, auxin response factors that specifically and differentially regulate auxin signaling in the outer epidermal layer have not been identified, nor is there a mechanism for local auxin biosynthesis that could account for continued generation of PIN1 polarity as the leaf expands. The pattern of vein disruption and lamina growth arrest in the stf mutant fits the mechanism of blade outgrowth and venation coupling described by Scarpella et al. (2010) and suggests that the STF regulation of vascular patterning and lamina outgrowth may be connected to auxin signaling at the leaf margins.
STF May Regulate Blade Outgrowth and Vascular Patterning by Modulating Auxin and Cytokinin Homeostasis
Our microarray analysis identified a strong connection between the stf mutant and transcriptional changes in hormone-responsive genes, especially genes associated with auxin (Table 1). Consistent with this, stf and lam1 mutants accumulate less free IAA in their leaves (Figures 6C to 6E). The reduced auxin cannot be simply a consequence of the narrow leaf mutant phenotype because ectopic expression of STF in N. sylvestris results in a smaller leaf, yet there is a twofold increase in free IAA compared with controls (Figure 8A). Auxin is a multifunctional phytohormone required for cell division and cell expansion and has been associated with a plethora of developmental programs, including leaf margin serration, reproductive development, leaf vascular patterning, and blade outgrowth (Scarpella et al., 2006, 2010; Koenig et al., 2009; Pagnussat et al., 2009; Perrot-Rechenmann, 2010; Bilsborough et al., 2011), indicating that the stf phenotypes are subsets of auxin-mediated developmental programs. It has been proposed that intra- and intercellular auxin gradients are critical in conveying positional information during auxin signal transduction (Benková et al., 2003; Vanneste and Friml, 2009). It is conceivable that a twofold reduction in total free auxin content in the mutant could disrupt auxin gradients that convey developmental signals. Under the model of Scarpella et al. (2010), STF/LAM1 could function by controlling the spatial and temporal availability of auxin at the leaf margin. Normal auxin response and transport, but reduced free IAA associated with low metabolic flux in lam1 mutant, suggests that STF/LAM1 may be involved in the modulation of auxin homeostasis.
However, auxin is not the only hormone altered in stf and lam1. Abscisic acid is also lower in lam1 mutants and higher than wild type in STF-overexpressing N. sylvestris (see Supplemental Figure 8B online). Moreover, the stem elongation defect in the lam1 mutant can be rescued by GA application (McHale, 1992), which together with the downregulation of GA biosynthetic enzymes in stf microarray, suggests that STF has a pleiotropic effect on GA biosynthesis. The microarray analysis also revealed that 1-aminocyclopropane-1-carboxylic acid oxidase (a key enzyme in ethylene biosynthesis), and His-containing phosphotransmitter protein1 (a key element in two-component cytokinin signal transduction phosphorelay) are strongly downregulated (Table 1). More importantly, the highest STF overexpressor transgenic plants developed typical auxin and cytokinin overproduction phenotypes, including tumors (Figure 8). lam1 is sensitive to cytokinin application and responded by forming leaf branches similar to auxin treatment, but the application of auxin and cytokinin together partially rescued the lam1 lamina (Figure 9). Together, these data suggest the integration of multiple hormones (Jaillais and Chory, 2010) during leaf morphogenesis coordinated by auxin:cytokinin ratio in leaf primordia.
Possible Mechanisms for STF Function
We envision three scenarios for how STF may function in modulating hormone homeostasis and integration. In the first scenario, STF regulates tissue-specific hormone homeostasis by controlling one or more hormone-conjugating enzymes. In support of this hypothesis, jasmonic acid carboxyl methyltransferase and putative auxin and cytokinin glucosyltransferases are upregulated in the stf mutant (Table 1). It is known that activation of auxin-conjugating enzymes leads to auxin depletion phenotypes and impairs auxin-mediated plant growth and environmental responses (Jackson et al., 2002; Qin et al., 2005; Park et al., 2007; Tognetti et al., 2010). In this scenario, failure of STF to control IAA/cytokinin glucosyltransferase in the stf mutant could lead to the buildup of IAA/cytokinin-glucoside conjugate, which signals excess IAA/cytokinin and this feeds back to sugar metabolism to slow down biosynthesis by reducing or rechanneling hexoses (see Supplemental Figure 9 online). Decreased Glc and Fru, but increased Suc have been shown to be associated with reduced auxin biosynthesis in the maize cell wall invertase mutant mn1 (LeClere et al., 2010). This decrease in hexoses, in turn, could downregulate the entire shikimate pathway, leading to reduced auxin biosynthesis, and could also affect other hormones (see Supplemental Figure 9 online).
In the second scenario, STF modulates the auxin:cytokinin ratio by regulating cytokinin signaling through activation of a histidine-containing phosphotransmitter protein, which is strongly downregulated in stf or by repressing A-type two-component response regulators, RR9 and RR15, which are modestly upregulated in stf, analogous to repression of ARR7 and ARR15 by WUS in Arabidopsis (Leibfried et al., 2005). Complementation of lam1 with At WUS expression (Figure 10) favors this possibility. Cytokinin signaling is known to affect auxin biosynthesis, and although the interaction could be antagonistic in roots (Dello Ioio et al., 2008; Müller and Sheen, 2008; Moubayidin et al., 2009), auxin-cytokinin crosstalk in shoots is thought to be synergistic (Pernisová et al., 2009; Zhao et al., 2010). In this hypothesis, cytokinin response is the primary target of STF, whereas auxin and other hormones are altered as a result of hormone crosstalk or a change in the auxin:cytokinin ratio.
In the third scenario, STF regulates other transcription factors that may be directly or indirectly connected to auxin/cytokinin activity. Two SHORT ROOT/SCR-related GRAS family transcription factors and one D-type cyclin (CYCD6;1 homolog) are downregulated in stf. SHORT ROOT and SCR are regulators of cell proliferation in leaves (Dhondt et al., 2010) and directly target D-type cyclins to control cell cycle progression (Sozzani et al., 2010). The combined downregulation of GRAS genes and CYCD6 could lead to premature exit from the cell cycle in stf. Other possible targets include HOMEOBOX PROTEIN 1, which is abscisic acid responsive and a repressor of auxin-responsive lateral organ boundary domain protein in M. truncatula roots (Ariel et al., 2010), BOP1/2, LOBD38, TCP3, and KNAT2/KNAT6-related KNOX genes, which all have the potential to coordinate or interact with the auxin/cytokinin signaling machinery.
However, the above three scenarios are not mutually exclusive, and it remains to be shown if they act in concert. For example, STF may repress auxin/cytokinin-glucosyltransferase and also activate cytokinin by activating a His-containing phosphotransmitter protein at the same time to maintain the right auxin:cytokinin ratio and homeostasis at the leaf margins. The synergistic interaction of auxin and cytokinin could then presumably deliver the instructive spatiotemporal signal to TCPs, GRAS genes, and the cell cycle to execute morphogenic functions. In this context, STIMPY/WOX9-mediated coordination of sugar and cytokinin signaling to the cell cycle has been proposed for shoot meristem establishment in Arabidopsis (Skylar et al., 2010, 2011). Identifying the direct target(s) of STF will enlighten our understanding of the mechanism of this fundamental process.
METHODS
Mutant Screening and Cloning of STF
Insertional mutagenesis in Medicago truncatula genotype R108 using Tnt1 retrotransposon and screening conditions in the greenhouse have been previously described (Tadege et al., 2008, 2009). Forward genetics screening of 5600 Tnt1-tagged lines under standard conditions (16 h/8 h and 24°C/19°C day/night cycles) in the greenhouse for leaf blade mutants have identified seven mutants with identical phenotypes of stf designated stf1-1 to stf1-7. The cloning of STF by genotyping of FST was performed as previously described (Tadege et al., 2008).
The stf mutant phenotype segregates as a recessive mutant (95 wild types to 30 mutants in stf1-2 heterozygous) in the seven independent lines. Forty-one FST were recovered from stf1-1 by TAIL-PCR using a combination of Tnt1-specific primers (Tnt1-F1, Tntail 1, 2, 3, and LTR3 or LTR5) and arbitrary primers AD1 or AD2 (see Supplemental Table 1 online). Of 22 FST genotyped in a segregating population of stf1-1 mutants using FST-specific primers, two FST were identified with homozygous Tnt1 insertions that cosegregated with the narrow leaf mutant phenotype. Only one of the two FST (75insert1) was also found to be tagged in the other six stf mutant lines and cosegregated with the mutant phenotype. The gene corresponding to this FST was designated STF and the full-length sequence, including its promoter, was amplified from genotype R108 using primers STF1F and STF1R from the sequenced M. truncatula A17 genome. All primers are listed in Supplemental Table 1 online.
Cloning of Medicago sativa, Nicotiana benthamiana, and Nicotiana sylvestris STL Sequences
Ms STL1, Ms STL2, and partial Nb STL1 sequences were isolated by RT-PCR from shoot apex-enriched tissue of seedlings using primers STFcd1F and STFcd1R from conserved STF regions. A partial Ns STL1 sequence was amplified by RT-PCR from wild-type N. sylvestris using Nb STL1-derived primers NbSTLfd and NbSTLrs, and a full-length sequence, including its promoter and 3′ untranslated region (UTR), was isolated by TAIL-PCR (Liu et al., 1995) using primers NsSTL3′1F, NsSTL3′2F, and NsSTL5′1R, NsSTL5′2R in combination with arbitrary primers AD1 and AD2. Full-length Nb STL1 was amplified using Ns STL1-derived primers NsSTLfrd and NsSTLrvs. All other STLs were identified by BLAST search from NCBI or the respective genome databases. After functional confirmation, Nb STL1 and Ns STL1 were renamed Nb STF1 and Ns STF1, respectively. Deletion of Ns STF1 in the lam1 mutant was identified using Ns STF1-specific primers; F1 and R1 for the middle of the genomic sequence, F1 and R2 for coding sequence (CDS) extending to the 3′ UTR, and F2 and R3 for the promoter region and 5′ UTR (see Supplemental Table 1 online for primer sequences).
Transgene Construction and Plant Transformation
A 5.3-kb genomic fragment of the M. truncatula STF gene was amplified using primers STFgattB1 and STFgattB2 and was cloned in gateway vector pMDC99 for plant transformation (see Supplemental Table 2 online). A genomic DNA fragment of STF containing a 2663-bp region immediately upstream of the translation start was cloned into pMDC162 vector for the STF:GUS construct using primers pSTF1F and pSTF1R. The DR5 promoter was cloned into pMDC162 vector for the DR5:GUS construct using primers DR5gusattB1p and DR5gusattB2p. For overexpression constructs, the Mt STF CDS and Nb STF1 CDS were cloned into pMDC32 using primers STFcdattB1 and STFcdattB2 for STF and NbSTF1attB1 and NbSTF1attB2 for Nb STF1. For the STF:WUS construct, the At WUS1 CDS was amplified from PRS:WUS plasmid using WUSgattB1 and WUSgattB2 primers and was cloned in front of the STF promoter in pMDC162 vector (see Supplemental Table 2 online). Constructs were introduced into Agrobacterium tumefaciens by electroporation. A. tumefaciens strain AGL1 was used for M. truncatula transformation as described (Cosson et al., 2006) and strain GV2260 was used for N. sylvestris transformation.
Phylogenetic Analysis
Phylogenetic analysis was performed using full-length and homeodomain region amino acid sequences (see Supplemental Data Set 1 online). Sequences were aligned using Clustal W, and a neighbor-joining phylogenetic tree was constructed using MEGA 4 software. The most parsimonious trees with bootstrap values from 1000 trials were shown.
Tissue Fixation and Embedding
Leaf samples from 4-week-old seedlings of M. truncatula were cut into small pieces and collected directly into 4% formaldehyde made in PHEM buffer (60 mM Pipes, 25 mM HEPES, 2 mM MgCl, and 10 mM EGTA, pH 6.9). Samples were vacuum infiltrated for 30 min and then left in the fixative solution for an additional 2 h. After fixation, samples were washed three times in PHEM buffer and dehydrated by passing through a graded ethanol series of 25%, 50%, 75%, 95%, and 100% ETOH, each step lasting for 1 h at room temperature and repeating three times for the 100% ETOH step. Ethanol was then replaced with a graded series of low-melting-temperature Steedman’s wax in ethanol (WAX:ETOH). The Steedman’s wax was prepared by melting 900 g of polyethylene glycol 400 distearate (Sigma-Aldrich) and 100 g of 1-hexadecanol (Sigma-Aldrich) and stirring at 65°C, aliquoted in 50-mL volumes and stored at −20°C. The wax was melted at 38°C before use. Samples were treated with 1ETOH:1WAX once for 3 h, 1ETOH:3WAX once for 3 h, and 100% WAX three times for 3 h. Tissues were then embedded in plastic molds and after hardening at room temperature, blocks were stored at 4°C prior to sectioning. Embedded tissues were sectioned at 15 to 20 μm using a Leica RM 2235 rotary microtome (Leica Microsystems). The wax was removed by treating sections with absolute ethanol. After air drying, sections were stained with Toluidine Blue (Sigma-Aldrich) for visibility and were viewed under an Olympus BX-51 compound microscope (Hitschfel Instruments).
Tissue Preparation for Light Microscopy
Equivalent leaves from 4-week-old R108 and stf mutant plants were fixed in 6:1 ethanol:glacial acetic acid overnight at room temperature and were washed with 95% ethanol twice for 10 min. Samples were then cleared with 85% lactic acid over 24 h until the tissue became transparent. Cleared tissues were mounted with 30% glycerol on glass slide and were viewed under anOlympus BX-51 compound microscope equipped with a digital camera.
SEM
Leaf tissue from 4-week-old R108 and stf1-2 plants were vacuum infiltrated in fixative solution (3% glutaraldehyde in 25 mM phosphate buffer, pH 7.0) for 1 h. Samples were further fixed with 1.0% osmium tetroxide overnight, dehydrated in graded ethanol series, critical point dried, coated with Gold as previously described (Wang et al., 2008), and viewed using a ZEISS DSM-960A scanning electron microscope (Carl Zeiss MicroImaging).
RNA in Situ Hybridization
RNA in situ hybridization with digoxigenin-labeled STF-specific probe was performed on shoot apices of M. truncatula plants grown for 12 d after germination or on inflorescence apices as described (Ferrándiz et al., 2000). RNA antisense and sense probes were generated with the T7 and SP6 polymerases, respectively, using a 780-bp STF cDNA template, which included the last 431 bp of CDS plus 355 bp of the 3′ UTR.
Histochemical GUS Staining
GUS staining assay was performed as described (Zhao et al., 2001) and images of GUS staining patterns of tissues were collected with a digital camera mounted on an Olympus BX-51 compound microscope (Hitschfel Instruments) or an Olympus SZX-16 Stereoscope (Hitschfel Instruments).
Microarray Analysis
Microarray was performed on RNA extracted from 0.5- to 0.8-mm shoot apices of 4-week-old seedlings. Three independent stf alleles (stf1-1, stf1-2, and stf1-3) and their corresponding wild type in segregating F2 populations were used. The three lines were treated as three biological replicates, and for each replicate, a pooled tissue collected from 20 plants was used to make RNA preparation. Total RNA was extracted using RNeasy Plant Mini Kit (Qiagen). The microarray analysis was performed using Medicago Affymetrix GeneChip. Probe labeling, hybridization, and scanning were conducted according to the manufacturer’s instructions (Affymetrix). For each microarray sample, the .CEL file was exported from GeneChip Operating System software (Affymetrix) and imported into robust multi-chip average for normalization. The presence/absence call for each probe set was obtained from dCHIP. Differentially expressed genes between the stf mutant and the wild type were selected based on “associative analysis” (Dozmorov and Centola, 2003) using Matlab (MathWorks). In this method, the background noise presented between replicates and technical noise during microarray experiments was measured by the residual presented among a group of genes whose residuals are homoscedastic. Genes whose residuals between the compared sample pairs that are significantly higher than the measured background noise level were considered to be differentially expressed. A selection threshold of 2 for transcript ratios and a Bonferroni-corrected P value threshold of 8.15954E-07 were used. The Bonferroni-corrected P value threshold was derived from 0.05/N in these analyses, where N is the number of probe sets (61,278) on the chip.
Quantitative RT-PCR
Total RNA from 0.5- to 0.8-mm shoot apices of 4-week-old plants was extracted using RNeasy Plant Mini Kit (Qiagen) and the RNA was treated with Turbo DNase (Ambion) to remove contaminating genomic DNA. Two micrograms of total RNA was used for cDNA synthesis using the Omniscript Kit (Qiagen). Primers were designed to anneal near the 3′ end or at the 3′ UTR (see Supplemental Table 4 online). Three stf mutants (stf1-1, stf1-2, and stf1-3) and their segregating wild type were used as biological replicates for the analysis and three technical replicates were run for each. The qRT-PCR analysis was performed as described in Pfaffl (2001) using Power SYBR Green PCR master mix (Applied Biosystems) in an ABI Prism 7900 HT sequence detection system (Applied Biosystems). Gene expression was normalized using the expression of the EF1α and with UBQ5 used as housekeeping genes. Relative gene expression for each gene in the mutant plants was compared with that obtained for wild type, which was arbitrarily set to 1.0.
Quantification of Auxin
Quantification of free IAA was performed using GC–selective ion monitoring–MS essentially as described (Chen et al., 1988) using fresh tissue from equivalent leaves of 4-week-old wild-type, mutant, and transgenic plants with the following modifications. 0.5 g of fresh tissue was ground and extracted with 2 mL of 65% isopropanol in 200 mM imidazole buffer, pH 7.0. d7-IAA (100 pM) (CDN) was added as an internal standard and was equilibrated in the extract for 1 h at 4°C. The extract was centrifuged and the supernatant was diluted to 12.5 mL using water. The diluted extract was then applied to a conditioned amino anion exchange column (BAKER-10 SPE 3 mL). After the diluted extract passed through the column, aspiration was continued for 30 s and the column was washed sequentially with 2.0 mL of each of hexane, ethyl acetate, acetonitrile, and methanol. The IAA was eluted from the amino column using 3.0 mL of 2% acetic acid in methanol. The acidic methanol eluent was evaporated to near dryness and the residue was redissolved in 10% aqueous methanol and applied to a C18 SPE column. The column was washed with 10% aqueous methanol containing 1% acetic acid, and IAA was eluted from the column with 2% acetic acid in methanol. The acidic methanol was evaporated to dryness, and the residue was redissolved in 50 μL of methanol. IAA was methylated by the addition of 2 μL of 2.0 M trimethylsilyldiazomethane (Sigma-Aldrich), the reaction was allowed to go for 30 min at room temperature and excess trimethylsilyldiazomethane was quenched by the addition of 2 μL of 2.0 M acetic acid in hexane. One microliter of methylated IAA solution was injected in an Agilent 6890 GC connected to 5973 MS detector with electron ionization source (Agilent Technologies). The injector was at 280°C and in splitless mode. The oven temperature was initially at 70°C for 2 min and then ramped to 315°C at 5°C/min. The monitored ions were mass-to-charge ratio 130 and 137, which correspond to the quinolinium ions from IAA and d7-IAA, respectively, as well as the mass-to-charge ratio 189 and 196 for the corresponding molecular ions. Dwell times were 100 ms for each ion. Abscisic acid was quantified by reanalyzing the same samples using abscisic acid internal standard.
Metabolite Profiling
Leaves from equivalent positions of lam1 and wild-type plants were collected from 4-week-old plants grown in growth cabinets. Metabolite analysis by GC-MS was performed as described (Broeckling et al., 2005). The GC system used was an Agilent 6890 GC coupled to a 5973 mass spectrometry detector. One microliter of samples was injected at a 15:1 split ratio and the injector was held at 280°C. Separation was achieved on DB-5MS column (J & W Scientific; 60 m, 0.25 mm i.d., and 0.25-μm film). Helium was used as carrier gas at constant flow of 1 mL/min. The temperature program was 2 min at 80°C followed by a 5°C/min ramp to 315°C and this was held at 315°C for 12 min. Mass spectra were scanned from m/z 50 to 650 with the acquisition rate of 2 spectra/s. Acquired mass spectra were deconvoluted using AMDIS software, and metabolite identifications were achieved by mass spectral matching to the Noble Foundation’s in-house EIMS spectral library of authentic compounds, the publicly available GOLM library (http://csbdb.mpimpgolm.mpg.de/csbdb/dbma/msri.html), and the NIST08 library. Peak selection and alignment were performed using MET-IDEA software (Broeckling et al., 2006). The area of each peak was normalized against the area of the internal standard, and absolute quantification for selected metabolites was achieved using authentic standard calibration curves.
Application of Auxin and Cytokinin to Shoot Apex
For foliar spray of auxin, 10 mL of 1, 10, 50, or 100 μM NAA was sprayed per plant to shoots of 2-week-old seedlings daily for 10 d. For local treatments of apices with hormones, 10 mM IAA, 10 mM NAA, 10 mM IAA plus 10 mM BAP, or 1 mM IAA plus 10 mM BAP were dissolved in a prewarmed (50°C) lanolin paste. The paste was manually administered to shoot apices of 2-week-old N. sylvestris and lam1 seedlings or 6-week-old lam1 plants with pipette tips.
Root Elongation Assay
To measure root elongation in the presence of auxin, auxin polar transport inhibitor, or auxin biosynthetic intermediates, seeds were germinated on 0.5 MS plates and 9-d-old seedlings were transferred to 0.5 MS plates containing different concentrations of IAA, NAA, 2,4-D, Trp, or NPA. Root length was measured 4 or 5 d after transfer. Auxins and all other chemicals used in this assays were obtained from Sigma-Aldrich.
Accession Numbers
Sequence data from this article can be found in the EMBL/GenBank data libraries under accession numbers STF, JF276252; Ns STF1, JF276252; Ms STL1, JF276252 and Ms STL2, JF276252 (see also Supplemental Table 3 online). Microarray data from this manuscript can be found in ArrayExpress under accession number E-MEXP-3187.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Scanning Electron Micrograph of R108 and stf Leaf Surfaces and Measurement of Leaf Size.
Supplemental Figure 2. Phylogenetic Analysis of Closely Related WOX Proteins using Full-Length and Homeodomain Regions.
Supplemental Figure 3. Gene Structure and Multiple Amino Acid Sequence Alignment of STF and STL Proteins.
Supplemental Figure 4. Multiple Amino Acid Sequence Alignment of the Homeodomain Region of WUS-, PRS-, and STF-Related Proteins.
Supplemental Figure 5. Multiple Amino Acid Sequence Alignment of the C-terminal Region of WUS-, PRS-, and STF-Related Proteins.
Supplemental Figure 6. STF:GUS Expression in M. truncatula.
Supplemental Figure 7. Treatment of lam1 Shoots and Roots with Auxins, Trp, and NPA.
Supplemental Figure 8. Extreme Phenotypes of Nb STF1 Overexpression in N. sylvestris and Measurements of Abscisic Acid in lam1 and Transgenic Lines.
Supplemental Figure 9. Putative Model for the Mechanism of STF Function.
Supplemental Table 1. Primers Used for Recovering FST, Genotyping FST, and Cloning of STLs.
Supplemental Table 2. Primers Used for Plasmid Construction.
Supplemental Table 3. Primers Used for qRT-PCR.
Supplemental Table 4. Accession Numbers or Gene Identifiers of Sequences Used for Multiple Sequence Alignment and Phylogenetic Tree Construction.
Supplemental Data Set 1. Text File of Alignment Corresponding to Supplemental Figure 2 Online.
Supplementary Material
Acknowledgments
We thank David Meinke for critical reading of the manuscript; Stacy Allen, Keri Wang, Preston Larson, Tamding Wangdi, Vagner Benedito, and Elison Blancaflor for technical assistance; Jiri Friml for providing the DR5:GUS plasmid; Michael Scanlon for PRS:WUS plasmid; and Hee-Kyung Lee and Janie Gallaway for taking care of tissue culture and greenhouse plants. This material is based on work supported by the National Science Foundation under Grant EPS-0814361 and DBI 0703285, and in part by the Samuel Roberts Noble Foundation. Work by A.B. and F.M. was supported by the Spanish Ministerio de Ciencia e Innovación (Grant BIO2009-10876) and the Generalitat Valenciana.
AUTHOR CONTRIBUTIONS
M.T. designed the research, performed research, analyzed data, and wrote the paper; H.L., M.B., Y.T, and F.M. performed research and analyzed data; A.B., J.W., C.M.R., and L.N. performed research; P.R. and L.S. contributed analytical tools; N.A.M. analyzed data and edited the paper; and K.S.M. designed the research, analyzed data, and edited the paper.
References
- Aida M., Ishida T., Fukaki H., Fujisawa H., Tasaka M. (1997). Genes involved in organ separation in Arabidopsis: An analysis of the cup-shaped cotyledon mutant. Plant Cell 9: 841–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariel F., Diet A., Verdenaud M., Gruber V., Frugier F., Chan R., Crespi M. (2010). Environmental regulation of lateral root emergence in Medicago truncatula requires the HD-Zip I transcription factor HB1. Plant Cell 22: 2171–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkoulas M., Hay A., Kougioumoutzi E., Tsiantis M. (2008). A developmental framework for dissected leaf formation in the Arabidopsis relative Cardamine hirsuta. Nat. Genet. 40: 1136–1141 [DOI] [PubMed] [Google Scholar]
- Benková E., Michniewicz M., Sauer M., Teichmann T., Seifertová D., Jürgens G., Friml J. (2003). Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 [DOI] [PubMed] [Google Scholar]
- Bilsborough G.D., Runions A., Barkoulas M., Jenkins H.W., Hasson A., Galinha C., Laufs P., Hay A., Prusinkiewicz P., Tsiantis M. (2011). Model for the regulation of Arabidopsis thaliana leaf margin development. Proc. Natl. Acad. Sci. USA 108: 3424–3429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolduc N., Hake S. (2009). The maize transcription factor KNOTTED1 directly regulates the gibberellin catabolism gene ga2ox1. Plant Cell 21: 1647–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braybrook S.A., Kuhlemeier C. (2010). How a plant builds leaves. Plant Cell 22: 1006–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeckling C.D., Huhman D.V., Farag M.A., Smith J.T., May G.D., Mendes P., Dixon R.A., Sumner L.W. (2005). Metabolic profiling of Medicago truncatula cell cultures reveals the effects of biotic and abiotic elicitors on metabolism. J. Exp. Bot. 56: 323–336 [DOI] [PubMed] [Google Scholar]
- Broeckling C.D., Reddy I.R., Duran A.L., Zhao X., Sumner L.W. (2006). MET-IDEA: Data extraction tool for mass spectrometry-based metabolomics. Anal. Chem. 78: 4334–4341 [DOI] [PubMed] [Google Scholar]
- Byrne M.E., Barley R., Curtis M., Arroyo J.M., Dunham M., Hudson A., Martienssen R.A. (2000). Asymmetric leaves1 mediates leaf patterning and stem cell function in Arabidopsis. Nature 408: 967–971 [DOI] [PubMed] [Google Scholar]
- Caño-Delgado A., Lee J.Y., Demura T. (2010). Regulatory mechanisms for specification and patterning of plant vascular tissues. Annu. Rev. Cell Dev. Biol. 26: 605–637 [DOI] [PubMed] [Google Scholar]
- Chen K.H., Miller A.N., Patterson G.W., Cohen J.D. (1988). A rapid and simple procedure for purification of indole-3-acetic acid prior to GC-SIM-MS analysis. Plant Physiol. 86: 822–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosson V., Durand P., d’Erfurth I., Kondorosi A., Ratet P. (2006). Medicago truncatula transformation using leaf explants. Methods Mol. Biol. 343: 115–127 [DOI] [PubMed] [Google Scholar]
- David-Schwartz R., Koenig D., Sinha N.R. (2009). LYRATE is a key regulator of leaflet initiation and lamina outgrowth in tomato. Plant Cell 21: 3093–3104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dello Ioio R., Nakamura K., Moubayidin L., Perilli S., Taniguchi M., Morita M.T., Aoyama T., Costantino P., Sabatini S. (2008). A genetic framework for the control of cell division and differentiation in the root meristem. Science 322: 1380–1384 [DOI] [PubMed] [Google Scholar]
- Dhondt S., Coppens F., De Winter F., Swarup K., Merks R.M.H., Inzé D., Bennett M.J., Beemster G.T.S. (2010). SHORT-ROOT and SCARECROW regulate leaf growth in Arabidopsis by stimulating S-phase progression of the cell cycle. Plant Physiol. 154: 1183–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinneny J.R., Yadegari R., Fischer R.L., Yanofsky M.F., Weigel D. (2004). The role of JAGGED in shaping lateral organs. Development 131: 1101–1110 [DOI] [PubMed] [Google Scholar]
- Dozmorov I., Centola M. (2003). An associative analysis of gene expression array data. Bioinformatics 19: 204–211 [DOI] [PubMed] [Google Scholar]
- Efroni I., Eshed Y., Lifschitz E. (2010). Morphogenesis of simple and compound leaves: A critical review. Plant Cell 22: 1019–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklöf S., Astot C., Sitbon F., Moritz T., Olsson O., Sandberg G. (2000). Transgenic tobacco plants co-expressing Agrobacterium IAA and ipt genes have wild-type hormone levels but display both auxin- and cytokinin-overproducing phenotypes. Plant J. 23: 279–284 [DOI] [PubMed] [Google Scholar]
- Emery J.F., Floyd S.K., Alvarez J., Eshed Y., Hawker N.P., Izhaki A., Baum S.F., Bowman J.L. (2003). Radial patterning of Arabidopsis shoots by class III HD-ZIP and KANADI genes. Curr. Biol. 13: 1768–1774 [DOI] [PubMed] [Google Scholar]
- Ferrándiz C., Gu Q., Martienssen R., Yanofsky M.F. (2000). Redundant regulation of meristem identity and plant architecture by FRUITFULL, APETALA1 and CAULIFLOWER. Development 127: 725–734 [DOI] [PubMed] [Google Scholar]
- Guo M., Thomas J., Collins G., Timmermans M.C.P. (2008). Direct repression of KNOX loci by the ASYMMETRIC LEAVES1 complex of Arabidopsis. Plant Cell 20: 48–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha C.M., Jun J.H., Nam H.G., Fletcher J.C. (2007). BLADE-ON-PETIOLE 1 and 2 control Arabidopsis lateral organ fate through regulation of LOB domain and adaxial-abaxial polarity genes. Plant Cell 19: 1809–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haecker A., Gross-Hardt R., Geiges B., Sarkar A., Breuninger H., Herrmann M., Laux T. (2004). Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development 131: 657–668 [DOI] [PubMed] [Google Scholar]
- Hagemann W., Gleissberg S. (1996). Organogenetic capacity of leaves: The significance of marginal blastozones in angiosperms. Plant Syst. Evol. 199: 121–152 [Google Scholar]
- Hasson A., Plessis A., Blein T., Adroher B., Grigg S., Tsiantis M., Boudaoud A., Damerval C., Laufs P. (2011). Evolution and diverse roles of the CUP-SHAPED COTYLEDON genes in Arabidopsis leaf development. Plant Cell 23: 54–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay A., Tsiantis M. (2010). KNOX genes: Versatile regulators of plant development and diversity. Development 137: 3153–3165 [DOI] [PubMed] [Google Scholar]
- Hofer J., Turner L., Moreau C., Ambrose M., Isaac P., Butcher S., Weller J., Dupin A., Dalmais M., Le Signor C., Bendahmane A., Ellis N. (2009). Tendril-less regulates tendril formation in pea leaves. Plant Cell 21: 420–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi G., Kim G.T., Tsukaya H. (2005). The transcription factor AtGRF5 and the transcription coactivator AN3 regulate cell proliferation in leaf primordia of Arabidopsis thaliana. Plant J. 43: 68–78 [DOI] [PubMed] [Google Scholar]
- Hu Y., Xie Q., Chua N.H. (2003). The Arabidopsis auxin-inducible gene ARGOS controls lateral organ size. Plant Cell 15: 1951–1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husbands A.Y., Chitwood D.H., Plavskin Y., Timmermans M.C. (2009). Signals and prepatterns: New insights into organ polarity in plants. Genes Dev. 23: 1986–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M., Mitsuda N., Ohme-Takagi M. (2009). Arabidopsis WUSCHEL is a bifunctional transcription factor that acts as a repressor in stem cell regulation and as an activator in floral patterning. Plant Cell 21: 3493–3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R.G., Kowalczyk M., Li Y., Higgins G., Ross J., Sandberg G., Bowles D.J. (2002). Over-expression of an Arabidopsis gene encoding a glucosyltransferase of indole-3-acetic acid: Phenotypic characterisation of transgenic lines. Plant J. 32: 573–583 [DOI] [PubMed] [Google Scholar]
- Jaillais Y., Chory J. (2010). Unraveling the paradoxes of plant hormone signaling integration. Nat. Struct. Mol. Biol. 17: 642–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski S., Piazza P., Craft J., Hay A., Woolley L., Rieu I., Phillips A., Hedden P., Tsiantis M. (2005). KNOX action in Arabidopsis is mediated by coordinate regulation of cytokinin and gibberellin activities. Curr. Biol. 15: 1560–1565 [DOI] [PubMed] [Google Scholar]
- Jun J.H., Ha C.M., Fletcher J.C. (2010). BLADE-ON-PETIOLE1 coordinates organ determinacy and axial polarity in Arabidopsis by directly activating ASYMMETRIC LEAVES2. Plant Cell 22: 62–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant S., Bi Y.M., Zhu T., Rothstein S.J. (2009). SAUR39, a small auxin-up RNA gene, acts as a negative regulator of auxin synthesis and transport in rice. Plant Physiol. 151: 691–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter R.A., Bollman K., Taylor R.A., Bomblies K., Poethig R.S. (2001). KANADI regulates organ polarity in Arabidopsis. Nature 411: 706–709 [DOI] [PubMed] [Google Scholar]
- Kim G.T., Shoda K., Tsuge T., Cho K.H., Uchimiya H., Yokoyama R., Nishitani K., Tsukaya H. (2002). The ANGUSTIFOLIA gene of Arabidopsis, a plant CtBP gene, regulates leaf-cell expansion, the arrangement of cortical microtubules in leaf cells and expression of a gene involved in cell-wall formation. EMBO J. 21: 1267–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G.T., Tsukaya H., Uchimiya H. (1998). The ROTUNDIFOLIA3 gene of Arabidopsis thaliana encodes a new member of the cytochrome P-450 family that is required for the regulated polar elongation of leaf cells. Genes Dev. 12: 2381–2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig D., Bayer E., Kang J., Kuhlemeier C., Sinha N. (2009). Auxin patterns Solanum lycopersicum leaf morphogenesis. Development 136: 2997–3006 [DOI] [PubMed] [Google Scholar]
- Koyama T., Furutani M., Tasaka M., Ohme-Takagi M. (2007). TCP transcription factors control the morphology of shoot lateral organs via negative regulation of the expression of boundary-specific genes in Arabidopsis. Plant Cell 19: 473–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T., Mitsuda N., Seki M., Shinozaki K., Ohme-Takagi M. (2010). TCP transcription factors regulate the activities of ASYMMETRIC LEAVES1 and miR164, as well as the auxin response, during differentiation of leaves in Arabidopsis. Plant Cell 22: 3574–3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux T., Mayer K.F., Berger J., Jürgens G. (1996). The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 122: 87–96 [DOI] [PubMed] [Google Scholar]
- LeClere S., Schmelz E.A., Chourey P.S. (2010). Sugar levels regulate tryptophan-dependent auxin biosynthesis in developing maize kernels. Plant Physiol. 153: 306–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibfried A., To J.P., Busch W., Stehling S., Kehle A., Demar M., Kieber J.J., Lohmann J.U. (2005). WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature 438: 1172–1175 [DOI] [PubMed] [Google Scholar]
- Lin W.C., Shuai B., Springer P.S. (2003). The Arabidopsis LATERAL ORGAN BOUNDARIES-domain gene ASYMMETRIC LEAVES2 functions in the repression of KNOX gene expression and in adaxial-abaxial patterning. Plant Cell 15: 2241–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.G., Mitsukawa N., Oosumi T., Whittier R.F. (1995). Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J. 8: 457–463 [DOI] [PubMed] [Google Scholar]
- Long J.A., Moan E.I., Medford J.I., Barton M.K. (1996). A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379: 66–69 [DOI] [PubMed] [Google Scholar]
- Martín-Trillo M., Cubas P. (2010). TCP genes: A family snapshot ten years later. Trends Plant Sci. 15: 31–39 [DOI] [PubMed] [Google Scholar]
- Matsumoto N., Okada K. (2001). A homeobox gene, PRESSED FLOWER, regulates lateral axis-dependent development of Arabidopsis flowers. Genes Dev. 15: 3355–3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer K.F., Schoof H., Haecker A., Lenhard M., Jürgens G., Laux T. (1998). Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95: 805–815 [DOI] [PubMed] [Google Scholar]
- McConnell J.R., Emery J., Eshed Y., Bao N., Bowman J., Barton M.K. (2001). Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature 411: 709–713 [DOI] [PubMed] [Google Scholar]
- McHale N.A. (1992). A nuclear mutation blocking initiation of the lamina in leaves of Nicotiana sylvestris. Planta 186: 355–360 [DOI] [PubMed] [Google Scholar]
- McHale N.A. (1993). LAM-1 and FAT genes control development of the leaf blade in Nicotiana sylvestris. Plant Cell 5: 1029–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHale N.A., Marcotrigiano M. (1998). LAM1 is required for dorsoventrality and lateral growth of the leaf blade in Nicotiana. Development 125: 4235–4243 [DOI] [PubMed] [Google Scholar]
- Mizukami Y., Fischer R.L. (2000). Plant organ size control: AINTEGUMENTA regulates growth and cell numbers during organogenesis. Proc. Natl. Acad. Sci. USA 97: 942–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moubayidin L., Di Mambro R., Sabatini S. (2009). Cytokinin-auxin crosstalk. Trends Plant Sci. 14: 557–562 [DOI] [PubMed] [Google Scholar]
- Müller B., Sheen J. (2008). Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature 453: 1094–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardmann J., Ji J., Werr W., Scanlon M.J. (2004). The maize duplicate genes narrow sheath1 and narrow sheath2 encode a conserved homeobox gene function in a lateral domain of shoot apical meristems. Development 131: 2827–2839 [DOI] [PubMed] [Google Scholar]
- Nath U., Crawford B.C., Carpenter R., Coen E. (2003). Genetic control of surface curvature. Science 299: 1404–1407 [DOI] [PubMed] [Google Scholar]
- Nikovics K., Blein T., Peaucelle A., Ishida T., Morin H., Aida M., Laufs P. (2006). The balance between the MIR164A and CUC2 genes controls leaf margin serration in Arabidopsis. Plant Cell 18: 2929–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori N., et al. (2007). Regulation of LANCEOLATE by miR319 is required for compound-leaf development in tomato. Nat. Genet. 39: 787–791 [DOI] [PubMed] [Google Scholar]
- Pagnussat G.C., Alandete-Saez M., Bowman J.L., Sundaresan V. (2009). Auxin-dependent patterning and gamete specification in the Arabidopsis female gametophyte. Science 324: 1684–1689 [DOI] [PubMed] [Google Scholar]
- Palatnik J.F., Allen E., Wu X., Schommer C., Schwab R., Carrington J.C., Weigel D. (2003). Control of leaf morphogenesis by microRNAs. Nature 425: 257–263 [DOI] [PubMed] [Google Scholar]
- Park J.-E., Park J.-Y., Kim Y.-S., Staswick P.E., Jeon J., Yun J., Kim S.-Y., Kim J., Lee Y.-H., Park C.-M. (2007). GH3-mediated auxin homeostasis links growth regulation with stress adaptation response in Arabidopsis. J. Biol. Chem. 282: 10036–10046 [DOI] [PubMed] [Google Scholar]
- Pernisová M., Klíma P., Horák J., Válková M., Malbeck J., Soucek P., Reichman P., Hoyerová K., Dubová J., Friml J., Zazímalová E., Hejátko J. (2009). Cytokinins modulate auxin-induced organogenesis in plants via regulation of the auxin efflux. Proc. Natl. Acad. Sci. USA 106: 3609–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot-Rechenmann C. (2010). Cellular responses to auxin: Division versus expansion. Cold Spring Harb. Perspect. Biol. 2: a001446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M.W. (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poethig R.S. (1997). Leaf morphogenesis in flowering plants. Plant Cell 9: 1077–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin G., Gu H., Zhao Y., Ma Z., Shi G., Yang Y., Pichersky E., Chen H., Liu M., Chen Z., Qu L.J. (2005). An indole-3-acetic acid carboxyl methyltransferase regulates Arabidopsis leaf development. Plant Cell 17: 2693–2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D., Pesce E.R., Stieger P., Mandel T., Baltensperger K., Bennett M., Traas J., Friml J., Kuhlemeier C. (2003). Regulation of phyllotaxis by polar auxin transport. Nature 426: 255–260 [DOI] [PubMed] [Google Scholar]
- Saddic L.A., Huvermann B., Bezhani S., Su Y., Winter C.M., Kwon C.S., Collum R.P., Wagner D. (2006). The LEAFY target LMI1 is a meristem identity regulator and acts together with LEAFY to regulate expression of CAULIFLOWER. Development 133: 1673–1682 [DOI] [PubMed] [Google Scholar]
- Scanlon M.J., Schneeberger R.G., Freeling M. (1996). The maize mutant narrow sheath fails to establish leaf margin identity in a meristematic domain. Development 122: 1683–1691 [DOI] [PubMed] [Google Scholar]
- Scarpella E., Barkoulas M., Tsiantis M. (2010). Control of leaf and vein development by auxin. Cold Spring Harb. Perspect. Biol. 2: a001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpella E., Marcos D., Friml J., Berleth T. (2006). Control of leaf vascular patterning by polar auxin transport. Genes Dev. 20: 1015–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu R., Ji J., Kelsey E., Ohtsu K., Schnable P.S., Scanlon M.J. (2009). Tissue specificity and evolution of meristematic WOX3 function. Plant Physiol. 149: 841–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shleizer-Burko S., Burko Y., Ben-Herzel O., Ori N. (2011). Dynamic growth program regulated by LANCEOLATE enables flexible leaf patterning. Development 138: 695–704 [DOI] [PubMed] [Google Scholar]
- Skylar A., Hong F., Chory J., Weigel D., Wu X. (2010). STIMPY mediates cytokinin signaling during shoot meristem establishment in Arabidopsis seedlings. Development 137: 541–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skylar A., Sung F., Hong F., Chory J., Wu X. (2011). Metabolic sugar signal promotes Arabidopsis meristematic proliferation via G2. Dev. Biol. 351: 82–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozzani R., Cui H., Moreno-Risueno M.A., Busch W., Van Norman J.M., Vernoux T., Brady S.M., Dewitte W., Murray J.A., Benfey P.N. (2010). Spatiotemporal regulation of cell-cycle genes by SHORTROOT links patterning and growth. Nature 466: 128–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussex I.M. (1955). Morphogenesis in Solanum tuberosum L.: Experimental investigation of leaf dorsoventrality and orientation in the juvenile shoot. Phytomorphology 5: 286–300 [Google Scholar]
- Tadege M., Wang T.L., Wen J., Ratet P., Mysore K.S. (2009). Mutagenesis and beyond! Tools for understanding legume biology. Plant Physiol. 151: 978–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadege M., Wen J., He J., Tu H., Kwak Y., Eschstruth A., Cayrel A., Endre G., Zhao P.X., Chabaud M., Ratet P., Mysore K.S. (2008). Large-scale insertional mutagenesis using the Tnt1 retrotransposon in the model legume Medicago truncatula. Plant J. 54: 335–347 [DOI] [PubMed] [Google Scholar]
- Tattersall A.D., Turner L., Knox M.R., Ambrose M.J., Ellis T.H., Hofer J.M. (2005). The mutant crispa reveals multiple roles for PHANTASTICA in pea compound leaf development. Plant Cell 17: 1046–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans M.C., Hudson A., Becraft P.W., Nelson T. (1999). ROUGH SHEATH2: A Myb protein that represses knox homeobox genes in maize lateral organ primordia. Science 284: 151–153 [DOI] [PubMed] [Google Scholar]
- Tognetti V.B., et al. (2010). Perturbation of indole-3-butyric acid homeostasis by the UDP-glucosyltransferase UGT74E2 modulates Arabidopsis architecture and water stress tolerance. Plant Cell 22: 2660–2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukaya H. (2006). Mechanism of leaf-shape determination. Annu. Rev. Plant Biol. 57: 477–496 [DOI] [PubMed] [Google Scholar]
- Uchida N., Townsley B., Chung K.H., Sinha N. (2007). Regulation of SHOOT MERISTEMLESS genes via an upstream-conserved noncoding sequence coordinates leaf development. Proc. Natl. Acad. Sci. USA 104: 15953–15958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche M., Horstman A., Zethof J., Koes R., Rijpkema A.S., Gerats T. (2009). Differential recruitment of WOX transcription factors for lateral development and organ fusion in Petunia and Arabidopsis. Plant Cell 21: 2269–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S., Friml J. (2009). Auxin: A trigger for change in plant development. Cell 136: 1005–1016 [DOI] [PubMed] [Google Scholar]
- Waites R., Hudson A. (1995). Phantastica: A gene required for dorsoventrality of leaves in Antirrhinum majus. Development 121: 2143–2154 [Google Scholar]
- Waites R., Selvadurai H.R.N., Oliver I.R., Hudson A. (1998). The PHANTASTICA gene encodes a MYB transcription factor involved in growth and dorsoventrality of lateral organs in Antirrhinum. Cell 93: 779–789 [DOI] [PubMed] [Google Scholar]
- Wang H., Chen J., Wen J., Tadege M., Li G., Liu Y., Mysore K.S., Ratet P., Chen R. (2008). Control of compound leaf development by FLORICAULA/LEAFY ortholog SINGLE LEAFLET1 in Medicago truncatula. Plant Physiol. 146: 1759–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D.W.R. (2006). PEAPOD regulates lamina size and curvature in Arabidopsis. Proc. Natl. Acad. Sci. USA 103: 13238–13243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Xu Y., Dong A., Sun Y., Pi L., Xu Y., Huang H. (2003). Novel as1 and as2 defects in leaf adaxial-abaxial polarity reveal the requirement for ASYMMETRIC LEAVES1 and 2 and ERECTA functions in specifying leaf adaxial identity. Development 130: 4097–4107 [DOI] [PubMed] [Google Scholar]
- Yamada M., Greenham K., Prigge M.J., Jensen P.J., Estelle M. (2009). The TRANSPORT INHIBITOR RESPONSE2 gene is required for auxin synthesis and diverse aspects of plant development. Plant Physiol. 151: 168–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambryski P., Tempe J., Schell J. (1989). Transfer and function of T-DNA genes from agrobacterium Ti and Ri plasmids in plants. Cell 56: 193–201 [DOI] [PubMed] [Google Scholar]
- Zhang X., Zong J., Liu J., Yin J., Zhang D. (2010). Genome-wide analysis of WOX gene family in rice, sorghum, maize, Arabidopsis and poplar. J. Integr. Plant Biol. 52: 1016–1026 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Christensen S.K., Fankhauser C., Cashman J.R., Cohen J.D., Weigel D., Chory J. (2001). A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 291: 306–309 [DOI] [PubMed] [Google Scholar]
- Zhao Z., Andersen S.U., Ljung K., Dolezal K., Miotk A., Schultheiss S.J., Lohmann J.U. (2010). Hormonal control of the shoot stem-cell niche. Nature 465: 1089–1092 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.