Abstract
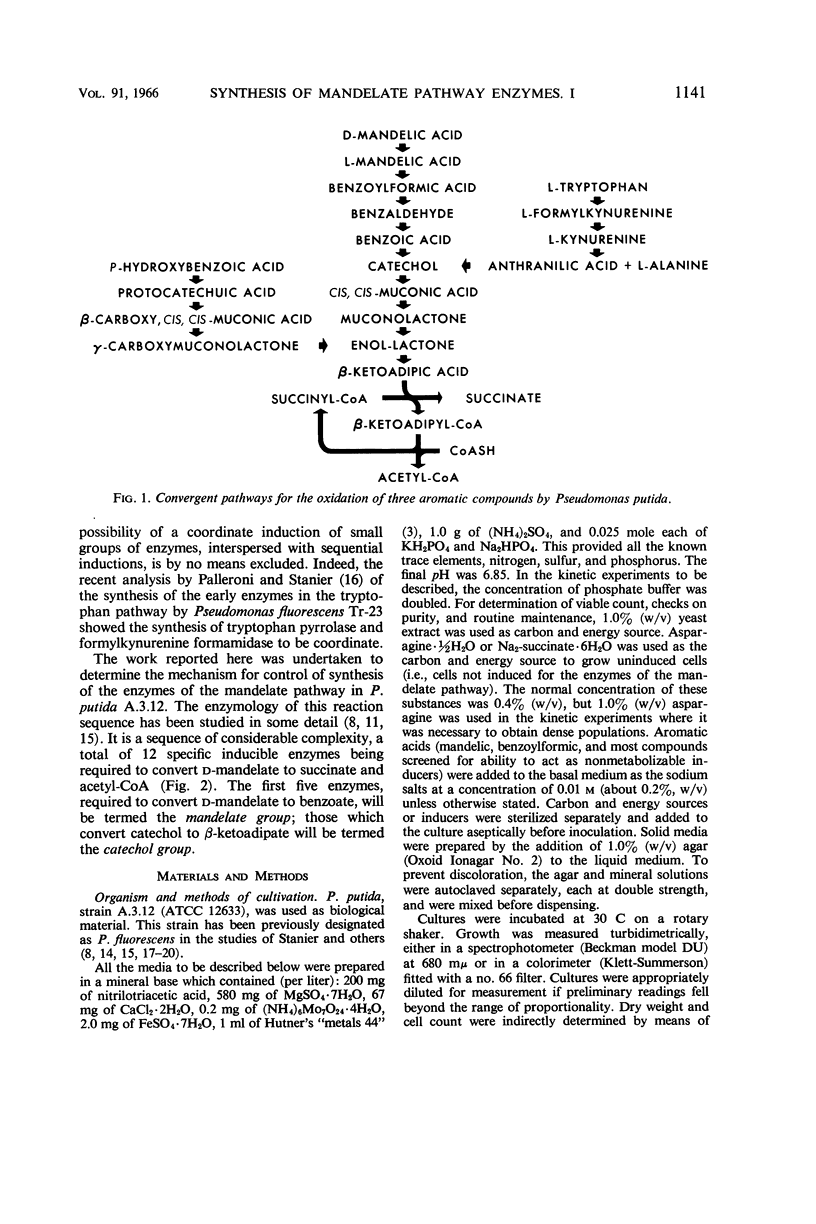
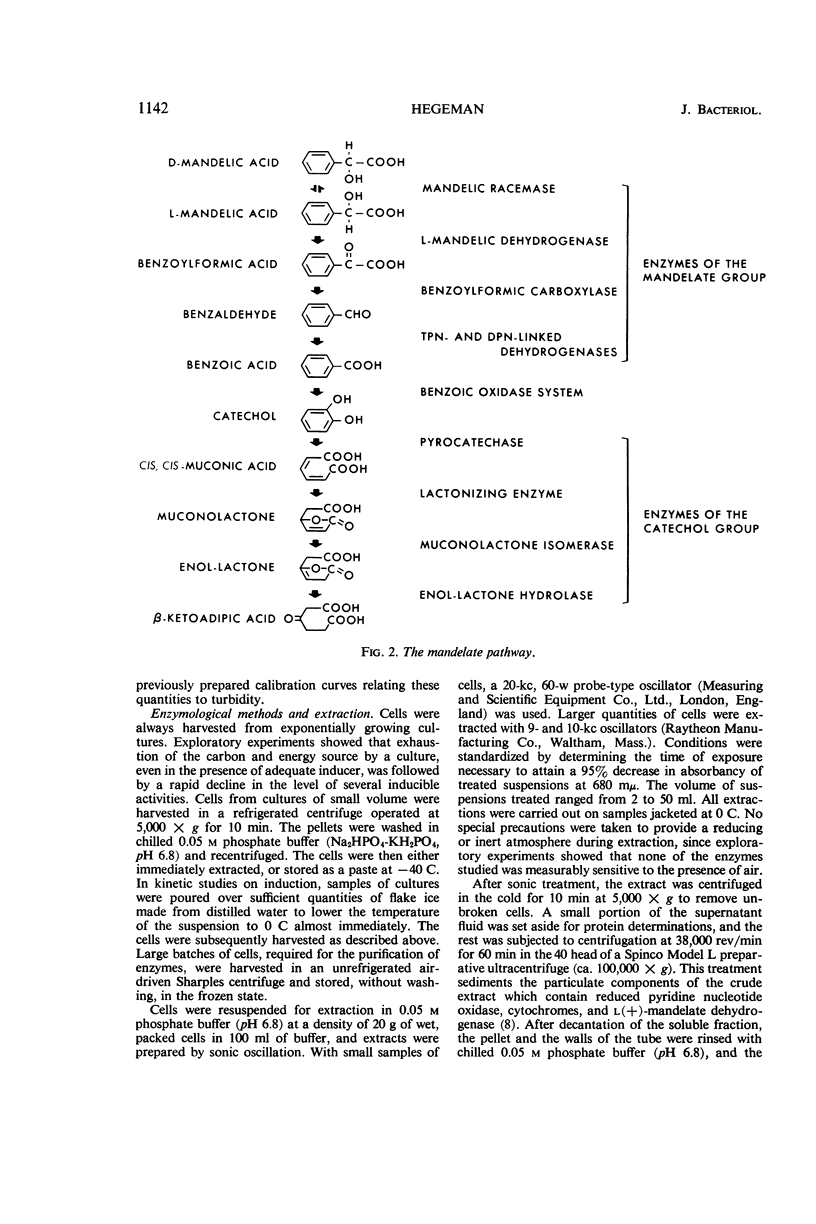
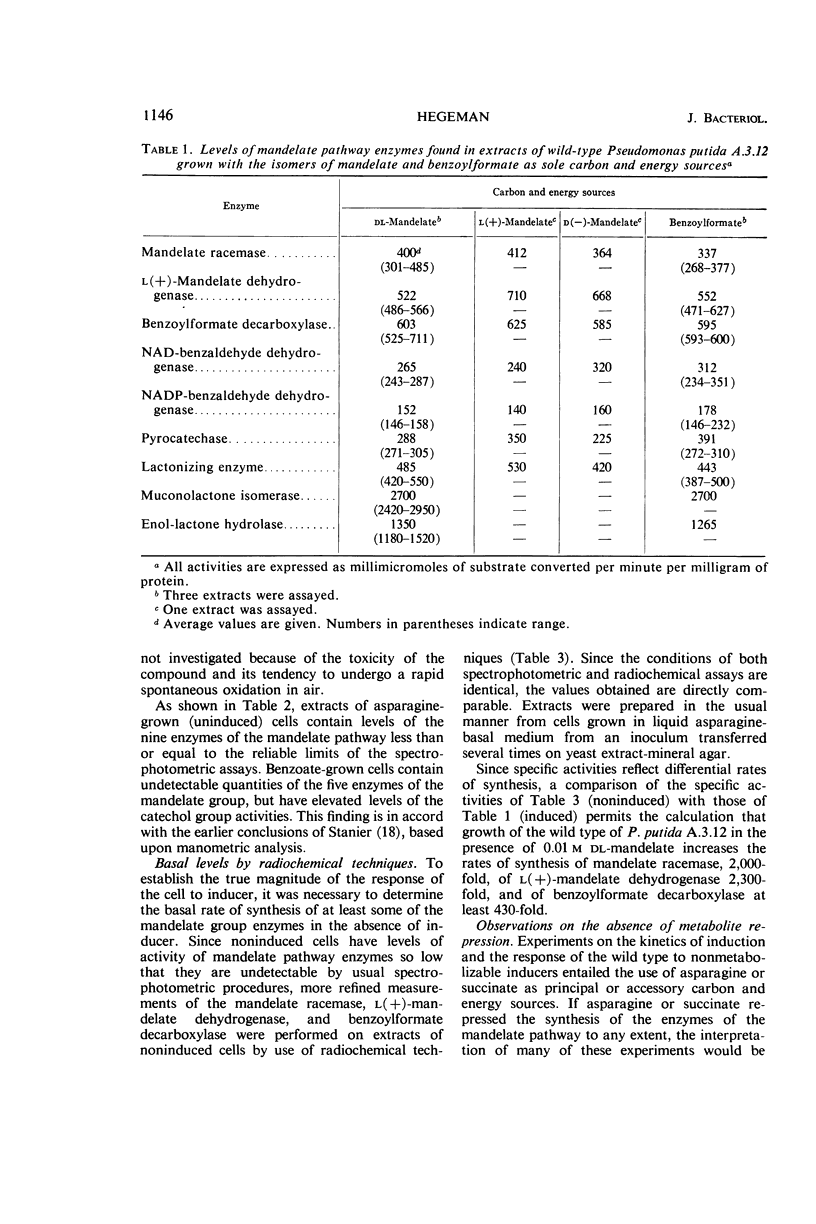
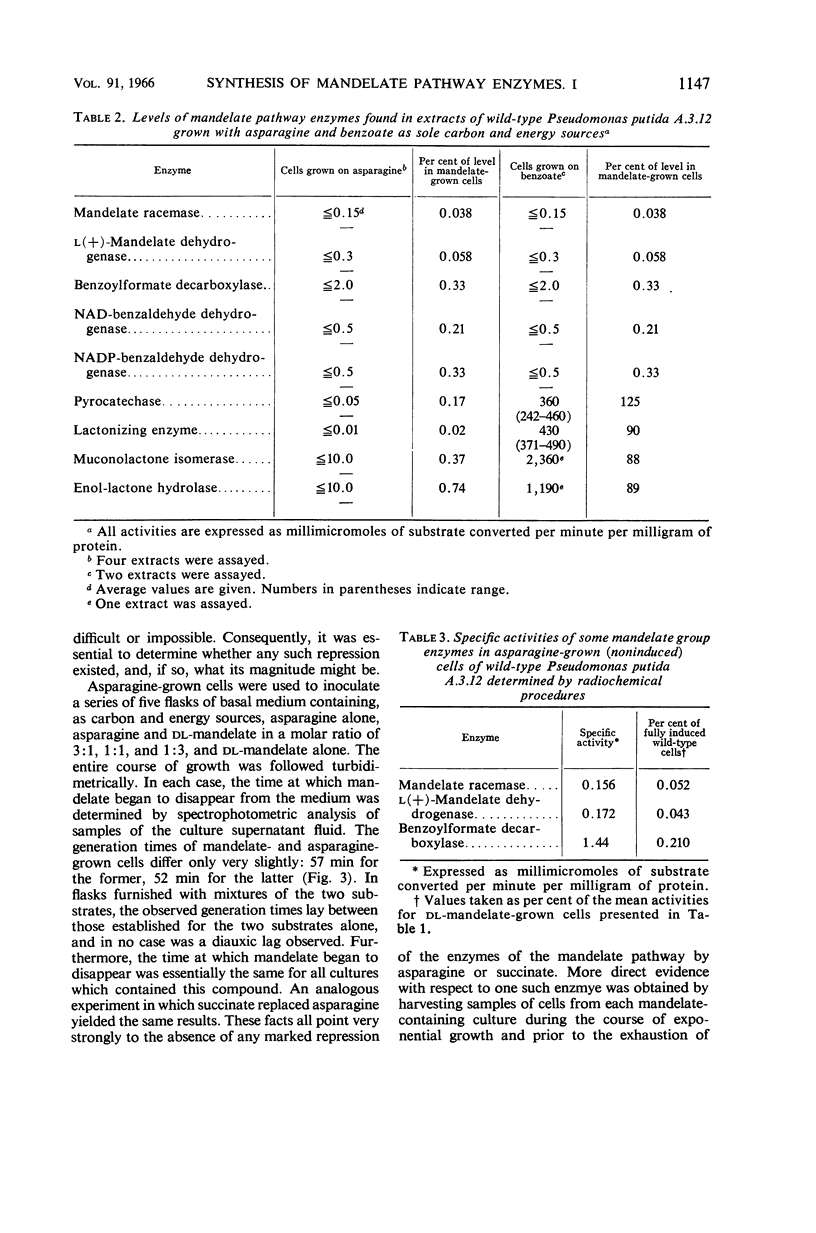
Hegeman, G. D. (University of California, Berkeley). Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. I. Synthesis of enzymes by the wild type. J. Bacteriol. 91:1140–1154. 1966.—The control of synthesis of the five enzymes responsible for the conversion of d(−)-mandelate to benzoate by Pseudomonas putida was investigated. The first three compounds occurring in the pathway, d(−)-mandelate, l(+)-mandelate, and benzoylformate, are equipotent inducers of all five enzymes. A nonmetabolizable inducer, phenoxyacetate, also induces synthesis of these enzymes; but, unlike the metabolizable inducer-substrates, it does not elicit synthesis of enzymes that mediate steps in the pathway beyond benzoate. Under conditions of semigratuity, dl-mandelate elicits immediate synthesis at a steady rate of the first two enzymes of the pathway, but two enzymes which act below the level of benzoate are synthesized only after a considerable lag. Succinate and asparagine do not significantly repress the synthesis of the enzymes responsible for mandelate oxidation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG J. M. THE MOLAR EXTINCTION COEFFICIENT OF 2,6-DICHLOROPHENOL INDOPHENOL. Biochim Biophys Acta. 1964 Apr 4;86:194–197. doi: 10.1016/0304-4165(64)90180-1. [DOI] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- COHN M., MONOD J., POLLOCK M. R., SPIEGELMAN S., STANIER R. Y. Terminology of enzyme formation. Nature. 1953 Dec 12;172(4389):1096–1096. doi: 10.1038/1721096a0. [DOI] [PubMed] [Google Scholar]
- DAGLEY S., FEWSTER M. E., HAPPOLD F. C. The bacterial oxidation of aromatic compounds. J Gen Microbiol. 1953 Feb;8(1):1–7. doi: 10.1099/00221287-8-1-1. [DOI] [PubMed] [Google Scholar]
- GUNSALUS C. F., STANIER R. Y., GUNSALUS I. C. The enzymatic conversion of mandelic acid to benzoic acid. III. Fractionation and properties of the soluble enzymes. J Bacteriol. 1953 Nov;66(5):548–553. doi: 10.1128/jb.66.5.548-553.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegeman G. D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. II. Isolation and properties of blocked mutants. J Bacteriol. 1966 Mar;91(3):1155–1160. doi: 10.1128/jb.91.3.1155-1160.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICHIHARA A., ADACHI K., HOSOKAWA K., TAKEDA Y. The enzymatic hydroxylation of aromatic carboxylic acids; substrate specificities of anthranilate and benzoate oxidases. J Biol Chem. 1962 Jul;237:2296–2302. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MANDELSTAM J., JACOBY G. A. INDUCTION AND MULTI-SENSITIVE END-PRODUCT REPRESSION IN THE ENZYMIC PATHWAY DEGRADING MANDELATE IN PSEUDOMONAS FLUORESCENS. Biochem J. 1965 Mar;94:569–577. doi: 10.1042/bj0940569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORNSTON L. N., STANIER R. Y. MECHANISM OF BETA-KETOADIPATE FORMATION BY BACTERIA. Nature. 1964 Dec 26;204:1279–1283. doi: 10.1038/2041279a0. [DOI] [PubMed] [Google Scholar]
- PALLERONI N. J., STANIER R. Y. REGULATORY MECHANISMS GOVERNING SYNTHESIS OF THE ENZYMES FOR TRYPTOPHAN OXIDATION BY PSEUDOMONAS FLUORESCENS. J Gen Microbiol. 1964 May;35:319–334. doi: 10.1099/00221287-35-2-319. [DOI] [PubMed] [Google Scholar]
- SISTROM W. R., STANIER R. Y. The mechanism of formation of beta-ketoadipic acid by bacteria. J Biol Chem. 1954 Oct;210(2):821–836. [PubMed] [Google Scholar]
- SLEEPER B. P., TSUCHIDA M., STANIER R. Y. The bacterial oxidation of aromatic compounds; the preparation of enzymatically active dried cells and the influence thereon of prior patterns of adaptation. J Bacteriol. 1950 Jan;59(1):129–133. doi: 10.1128/jb.59.1.129-133.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANIER R. Y. Problems of bacterial oxidative metabolism. Bacteriol Rev. 1950 Sep;14(3):179–191. doi: 10.1128/br.14.3.179-191.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y. Simultaneous Adaptation: A New Technique for the Study of Metabolic Pathways. J Bacteriol. 1947 Sep;54(3):339–348. doi: 10.1128/jb.54.3.339-348.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. J., Kirby H. Paper Chromatography Using Capillary Ascent. Science. 1948 May 7;107(2784):481–483. doi: 10.1126/science.107.2784.481. [DOI] [PubMed] [Google Scholar]



