Abstract
Background:
Ocular surface disorders (OSD) are challenging to treat. They can introduce serious morbidity and might even lead to visual loss. In such situations, keratoplasty remains the last option. Amniotic membrane transplantation (AMT) has been shown to be effective in the management of ocular surface pathologies. The aim of the study was to assess the efficacy of AMT for various indications of OSD.
Materials and Methods:
Experience of AMT in 65 patients with different OSD was evaluated. The aim of AMT was to achieve symptomatic relief, reduced inflammation, recurrence, and corneal haze; improve epithelization, stromal healing and visual acuity; and delay keratoplasty. Fresh amniotic membrane was used in all cases by a single surgeon. Follow-up and observations were done to evaluate success of achieving the goal.
Results:
Indications for AMT included primary and recurrent pterygium, various types of corneal ulcers (non-healing ulcer, descemetocele, corneal thinning and perforation), spheroidal degenerations, chemical burn and bullous keratopathy. The aim of AMT was different in different etiological indications. Postoperative follow-up was between 6 and 18 months. Success and complication rate were observed. Symptomatic relief (reduced pain and redness) was seen in patients with various corneal ulcers, chemical burn and bullous keratopathy. Improved epithelialization and stromal healing was noted in corneal ulcer cases. In spheroidal degenerations, keratectomy with AMT improved vision. Recurrence of pterygium was low (4.61%); graft failure in the form of graft rejection was seen in only 3.07% cases of acute keratitis. Corneal vascularization (4.61%) was present but not severe enough to hamper vision. Success in gaining intended effect was the most significant result with AMT.
Conclusion:
AMT in various ocular surface pathologies shows success in achieving the goal of symptomatic relief, improved epithelialization, stromal healing and vision. Reduction in inflammation, corneal haze and recurrence of original disease is achieved with minimum complications.
Keywords: Amniotic membrane transplantation, effective management, ocular surface disorders
Introduction
The normal ocular surface is covered by corneal, limbal and conjunctival epithelial cells. These cells, together with a stable tear film, maintain good visual acuity. Damage to these cells from various systemic or primary ocular diseases may lead to breakdown of ocular surface. This results in different ocular surface pathologies. Many times, these ocular surface disorders (OSD) are refractory to medical treatment and surgical interventions such as lamellar keratoplasty[1] and therapeutic penetrating keratoplasty (TPK), to prevent corneal scarring, thinning, perforation or extension of infection and loss of vision.[2] Although keratoplasty might eliminate residual pathology and preserve globe integrity,[2,3] it depends on the availability of good donor cornea. Recently, amniotic membrane transplantation (AMT) has been reported to be an adjunctive or alternative therapy. Though AMT was started as early as 1910 by Davis in general surgery for skin transplantation and burn dressing,[4] ocular use in published literature was reported by De Roeth in 1940 to repair conjunctival defect after symblepheron dissection.[5] This procedure was revived by Kim and Tseng in 1995 when they used amniotic membrane (AM) for ocular surface reconstruction.[6]
The aim of this case series was to study the success of AMT in various ocular surface pathologies in achieving improvement in symptoms and vision, reduction in inflammation, vascularization, graft failure and recurrence.
Materials and Methods
The study was conducted after obtaining approval from the institutional research and ethics committee for AMT. Sixtyfive patients with OSD of various etiologies, who underwent AMT, were included in this study. These patients were reviewed for age, gender, any previous surgery, pre- and postoperative visual acuity, slit-lamp examination and postoperative follow-ups. A written informed consent was obtained from each patient after explaining the surgical procedure and risk involved.
Procedure
Graft material: Patients undergoing elective cesarean section, and who were serologically negative for human immunodeficiency virus (HIV), syphilis and hepatitis B and C were counseled for donating AM. Placenta obtained at cesarean section was transported to the operation theater laboratory under aseptic conditions. It was cleaned of blood clots under laminar flow by trained personnel, followed by cleaning with sterile saline containing four antibiotics[7] (penicillin 50 µg/ml, streptomycin 50 µg/ml, neomycin 100 µg/ml, amphotericin B 2.5 µg/ml) as a safeguard against most bacterial and fungal infections.
Cleaning of placenta with these solutions was first advocated by Tseng et al. and also by Chen et al. in 1997. Amnion was separated from the chorion after cleaning was completed. Then, AM was kept in a bottle containing fresh antibiotic solution in a refrigerator at +4°C and used within a week of harvesting.
Surgeries were performed under either subconjunctival or peribulbar anesthesia. Pediatric age group patients were operated under general anesthesia (GA).
Surgical procedure
The technique for AMT was decided based on the nature of the clinical condition.
In cases of non-healing corneal ulcers [Figure 1a–c], cellular debris, exudates from the base of the defect and surrounding healthy epithelium were removed using 15 number blade, plane forceps or bud swab. A single sheet of AM was sutured to cover the epithelial defect. In cases with corneal thinning, descemetocele and corneal perforation, multilayered AM [Figure 2a–d] was used to fill the depth of the defect. A larger graft with epithelial side up was then sutured to cover the de-epithelializedarea with 10-0 nylon suture. Superficial keratectomy with AMT was performed in acute chemical burn, spheroidal degeneration and bullous keratopathy. In cases of primary and recurrent pterygium, after excision of pterygium, AMT was performed and then conjunctival limbal autograft was sutured over the AMT [Figure 3a–c].
Figure 1.
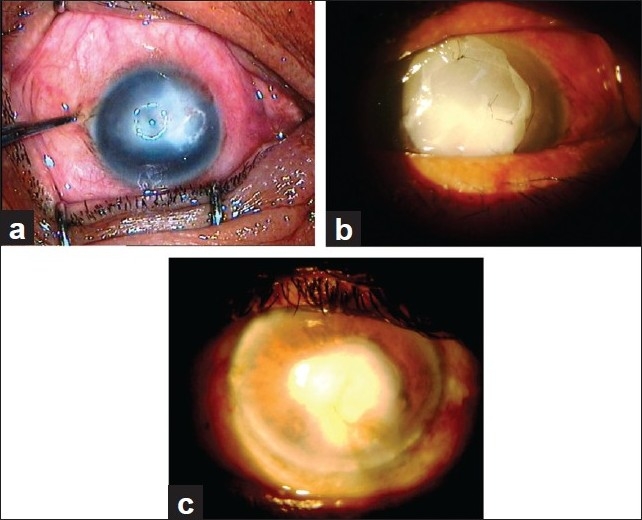
(a) Big non-healing corneal ulcer. (b) Big non-healing corneal ulcer after AMT. (c) Ulcer healed with corneal opacity
Figure 2.
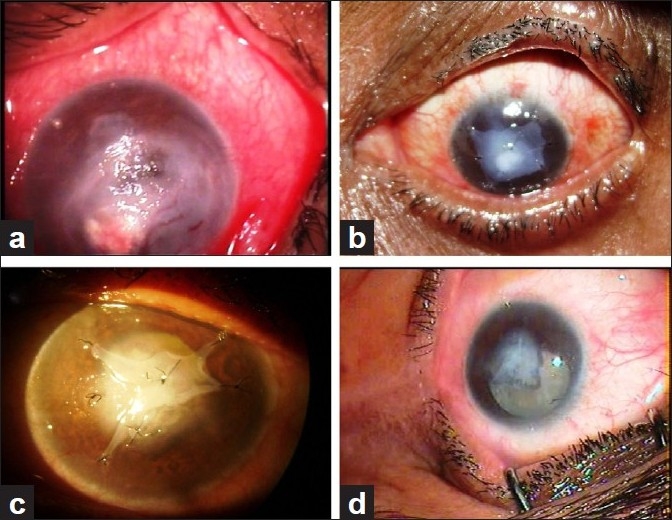
(a) Corneal thinning. (b) Corneal thinning, post multilayered AMT. (c) Multilayered AMT healing stage. (d) Multilayered AMT after healing with cataract
Figure 3.
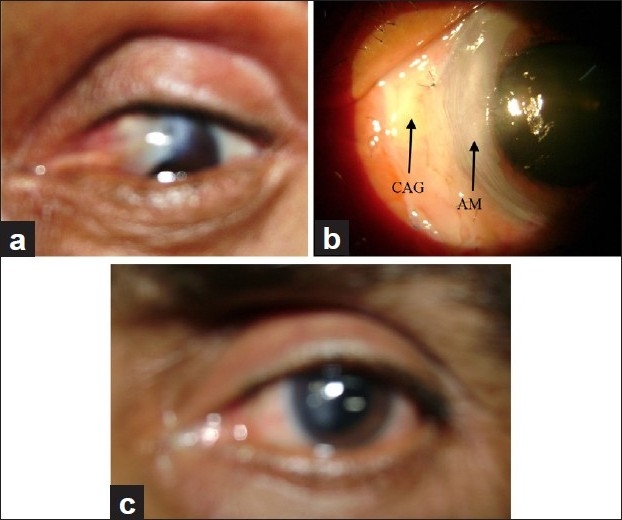
(a) Pterygium. (b) Postoperative pterygium with AMT and conjunctival autograft. (c) Pterygium follow-up
Postoperative treatment
Postoperatively, in all corneal cases, topical antibiotics were given till cornea became epithelialized. Then, steroid antibiotic drops were given in tapering dose over a period of 6 weeks along with a tear substitute.
Follow-up
Follow-up was done on first postoperative day, after 1 week, 2 weeks and then monthly for 6 months. Total follow-up was between 6 and 18 months.
Close follow-up was done to note reduction in pain, redness and photophobia. Slit-lamp examination with fluorescence staining of cornea, drawing and recording of ulcer size, recording visual acuity and follow-up photographs were the measures taken to observe change in size of ulcers, stromal healing, and decrease in density of corneal haze. Same measures helped to record evidence of complications like corneal vascularization, graft retraction, recurrence of primary disease and microbial infection. These observations helped to assess success of the procedure.
Results
The study group comprised 65 patients with age ranging from 7 to 70 years [Table 1]. Only two cases were under 20 years, one with chemical burn and another one with non-healing corneal ulcer. Among the patients of age between 20 and 40 years, 7 had primary pterygium, 2 had chemical burn and the rest 11 had various corneal ulcers including descemetocele and perforation. Male and adult predominance was seen in this series.
Table 1.
Age and sex ratio

The etiological groups included in the study are shown in Table 2. Most of the patients were in the pterygium and various corneal ulcer groups. Bullous keratopathy and chemical burn were less commonly opted for AMT. Severely damaged corneas which required additional procedures along with AMT, as in severe chemical burns, limbal stem cell deficiency, severe dry eye and ocular neoplasia, were excluded from the study.
Table 2.
Etiological groups included in the study

Postoperative period was specially observed for symptoms, signs and complications. Symptomatic relief in the form of reduction in pain, redness and photophobia was seen in cases with non-healing corneal ulcers, descemetocele, perforations, chemical burn, bullous keratopathy and spheroidal degenerations. There was remarkable improvement in signs. Reduction in inflammation was noted in various corneal ulcers and chemical burn. There was decreased conjunctival as well as ciliary congestion along with epithelial and stromal healing. Corneal haze in all spheroidal degeneration was dramatically reduced [Figure 4a–c]. This was appreciated very well on slit lamp and confirmed by improvement in vision. All five cases with chemical burns [Figure 5a, b] showed good and rapid re-epithelialization with no limbal ischemia or symblepheron formation [Table 3]. In this series, complications were very few. Close observation was done to note postoperative inflammation, reformation of anterior chamber in perforation, corneal vascularization, graft retraction and rejection, recurrence of primary disease and infection. One recurrent pterygium (1.53%) patient had severe inflammatory response in the form of chemosis of the graft. This reaction subsided with steroid drops. In corneal perforation cases [Figure 6a–(d)], five patients had good anterior chamber formation and only one case needed air injection to reform the collapsed anterior chamber. Corneal vascularization developed in 3 (4.61%) patients, one case of moderate chemical burn and two cases of refractive corneal ulcers. It was not in papillary area to reduce vision. Graft retraction was present in only 2 (3.07%) cases; it was very mild and did not need any repair or re-graft. Two cases (3.07%) with active keratitis had graft rejection. In these cases, therapeutic keratoplasty was necessary to prevent complications. Recurrence of pterygium was seen in 3 (4.61%) cases, 1 in primary pterygium of grade 3 and 2 in recurrent pterygium. Out of these two pterygium cases, one had been operated once and another thrice [Table 4].
Figure 4.
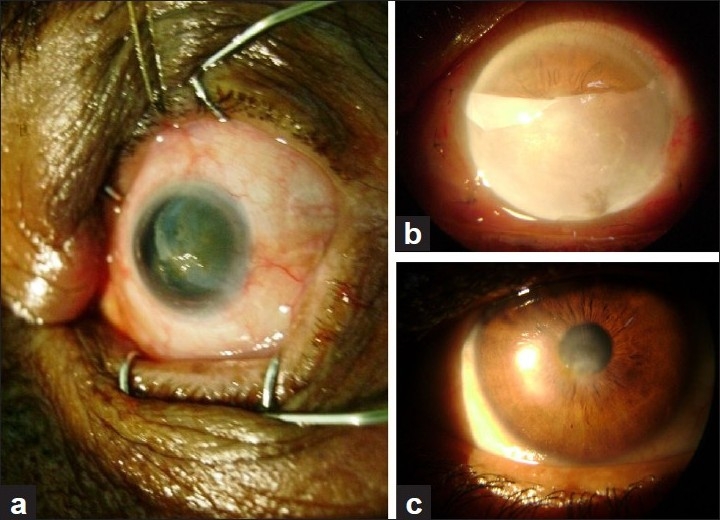
(a) Spheroidal degeneration. (b) Spheroidal degeneration after AMT. (c) Spheroidal degeneration: Follow-up; reduced corneal haze
Figure 5.
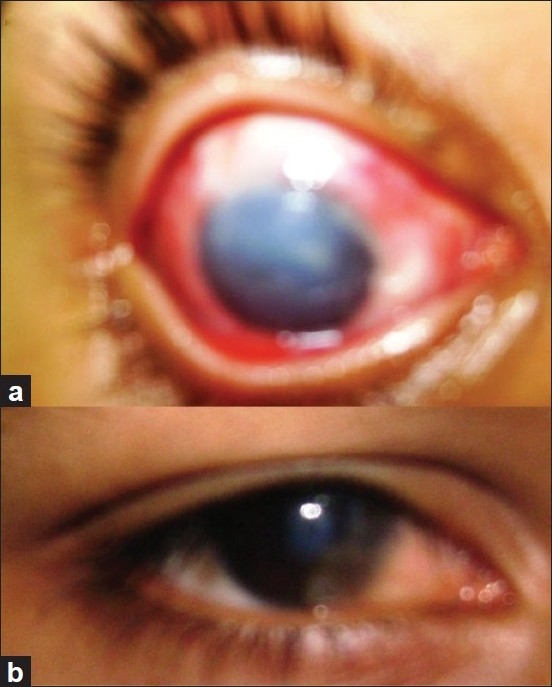
(a) Chemical burn. (b) Chemical burn after AMT
Table 3.
Vision improvement in spheroidal degeneration
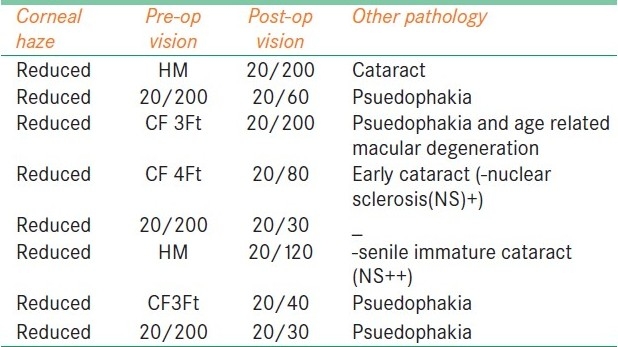
Figure 6.
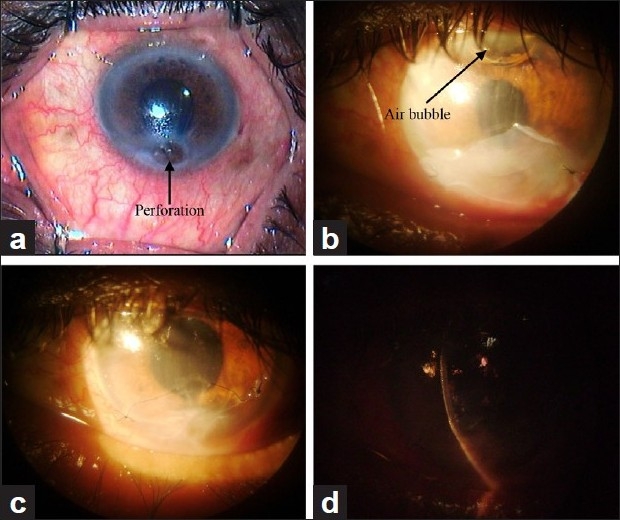
(a) Corneal perforation. (b) Postoperative corneal perforation with air bubble in anterior chamber. (c) Corneal perforation after complete healing. (d) Anterior chamber formed in postoperative corneal perforation
Table 4.
Post AMT follow-up observations

Discussion
The use of AM in ocular surface has been extensively reported.[8–10]
AM has special properties which promote its use in OSD:[7]
AM stimulates re-epithelialization; therefore, it is used to treat persistent and recurrent epithelial and stromal defects.
It has anti-cicatricial activity. This activity reduces the tendency for scar tissue proliferation. It also acts as a barrier against fibroblast multiplication by creating obstacle to growth factors.
It also prevents neovascularization by antiprotease activity. Therefore, once transplanted, previous tendency for blood vessel proliferation is significantly diminished.
It has anti-inflammatory quality due to suppression of certain inflammatory cytokines. Therefore, it is effective in acute chemical burn to reduce inflammation.
It does not trigger immunological rejection as it does not express HLA-A, -B or -DR antigens. Immune rejection does not occur after the transplantation. This is an advantageous property as postoperatively immunosuppressants are not required.
The basement has high tensile strength. This property is utilized to cover larger ocular surface area, especially in cases of fornix reconstruction.
It allows the surrounding epithelial cells to migrate over it. It also differentiates these epithelial cells into conjunctival and corneal epithelial cells.
Its acceptance by ocular surface is an important factor because it takes nourishment by simple diffusion from the surrounding host tissue.
It creates resistance for infection and provides protective sheet covering for the affected tissue. This explains its use in corneal ulcers.
It facilitates nerve re-growth as it contains large amount of nerve growth factor. Thus, it is used effectively in corneal surface reconstruction for regaining sensitivity.
It has nonspecific, mild antimicrobial effects, both antiviral and antibacterial, and therefore reduces the risk of postoperative infections.
These effective features of AM make its use successful in various OSD. This study comprises various etiological groups with indication of AMT. Results are observed in the form of success rate of AMT [Table 5].
Table 5.
Indication for amniotic membrane transplantation and success achieved
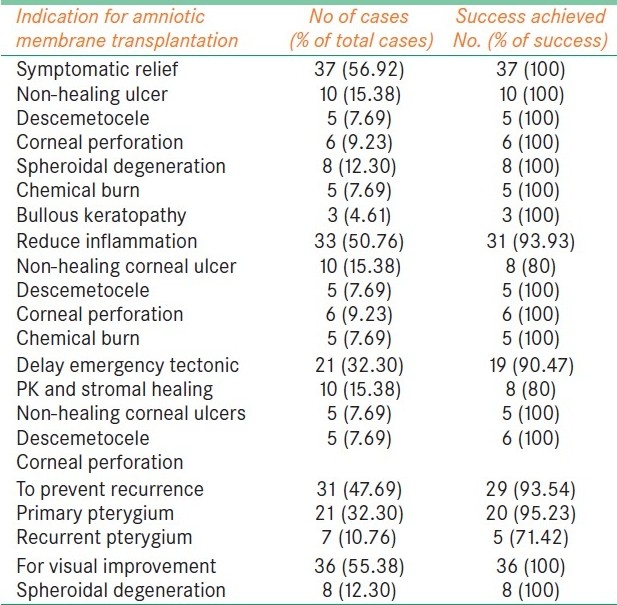
In this series, in primary pterygium cases, only one recurrence was observed (4.76%); but in recurrent pterygium cases, out of seven, two had recurrence (28.5%). They required repeat extensive surgery. Recurrence in these eyes was probably due to greater subconjunctival fibroblastic response as a result of number of previous surgeries. Prabhasawat et al. reported the recurrence rate in primary pterygium to be 10.9% with conjunctival limbal auto graft (CLAG) and AMT. They also observed the recurrence rate in recurrent pterygium with simple excision along with AMT to be 33.3%. When CLAG was also done with AMT, the recurrence rate was only 17.3%.[11]
Solomon et al. found that double-layered AMT with intraoperative subconjunctival triamcinolone injection reduces recurrence rate to 3% for primary and 9.5% for recurred pterygium cases.[12]
Cases with corneal pathology showed improvement in healing process and vision, reduction in inflammation and stromal haze, and good restoration of corneal surface. This healing process prevented further complications and thus delayed therapeutic keratoplasty. Later on, in healed corneal pathology, keratoplasty was performed successfully. Corneal vascularization was seen in 2 (3.07%) cases of refractive corneal ulcers, which was correlated as a part of healing. Graft melt was observed in 2 (3.07%) cases of active infective keratitis.
Pain and photophobia were reduced in bullous keratopathy. In spheroidal degeneration, the aim of AMT was to improve vision, which was achieved in all spheroidal degeneration cases. All these results are similar to those of previously reported studies.[13–16]
Cases selected for AMT in chemical burn had only mild to moderate grade of burn with or without limbal stem cell deficiency. These patients showed remarkable symptomatic relief and rapid epithelialization. There was reduction in inflammation and cicatricial sequelae postoperatively, similar to that reported by Josef et al.,[17] Meller et al.,[18] and Tandon.[19] One case (1.53%) developed corneal vascularization on follow-up.
Postoperative complications in this series have not been very significant. Although fresh AM was used, postoperative microbial infection was not seen. It was observed in other series that postoperative microbial infection is very low with cryopreserved AM (1.6%),[20] while with fresh AM it is as high as 8%.[21]
The complication in the form of hematoma was not seen in this series. Incidence of graft rejection (3.07%) and recurrence of primary disease (4.61%) was very less as compared to other studies,[11,12,22–24] which may be as a result of meticulous selection of donor and recipient wherein severely damaged ocular surface requiring additional procedure was excluded from the study.
In summary, AM has improved our ability to treat OSD. It has unique properties such as promoting conjunctival and corneal epithelialization, reducing inflammation and fibrosis, faster healing, no immunological reaction, easy availability, cost-effectiveness and very low rate of complications.
AMT is found to be effective in relieving pain in chemical burn and bullous keratopathy. It achieves healing and prevents complications in various corneal ulcers and pterygium. Visual improvement is remarkable, especially in spheroidal degenerations. The success rate of AMT is quite high in mild to moderate cases with OSD. Severely damaged cornea and cases requiring additional surgeries need to be evaluated further for the effect of AMT.
Acknowledgments
I am highly obliged to our institute, Sri Aurobindo Institute of Medical Sciences, especially the Chairman, Dr. Vinod Bhandari, who has always been encouraging. Institutional Ethics and Research committee members gave permission to conduct this study and also provided norms to avoid any legal problems. I am thankful to them for helping and guiding me. I am also grateful to the staff members of Burn unit who were always ready to provide the required amount of amniotic membrane. It is only because of Burn unit in charge, Dr Lunawat, and its staff that I could think of starting this project. Of course, this project was a team work. The cooperation and help of departmental staff, OT staff, obstetrics staff and all colleagues are gratefully acknowledged.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Xie L, Shi W, Liu Z, Li S. Lamellar keratoplasty for the treatment of fungal keratitis. Cornea. 2002;21:33–7. doi: 10.1097/00003226-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Sanitato JJ, Kelley CG, Kaufman HE. Surgical management of peripheral fungal keratitis (keratomycosis) Arch Ophthalmol. 1984;102:1506–9. doi: 10.1001/archopht.1984.01040031226023. [DOI] [PubMed] [Google Scholar]
- 3.Singh G, Malik SR. Therapeutic keratoplasty in fungal corneal ulcers. Br J Ophthalmol. 1972;56:41–5. doi: 10.1136/bjo.56.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis JW. Skin transplantation of 550 cases of John hopkin Hospital. Johns Hopkins Med Journal. 1910;15:307. [Google Scholar]
- 5.De Roeth A. Plastic repair of conjunctival defects with membranes. Arch Ophthalmol. 1940;23:522–5. [Google Scholar]
- 6.Kim JC, Tseng SC. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995;14:473–84. [PubMed] [Google Scholar]
- 7.Sangwan VS, Burman S, Tejwani S, Mahesh SP, Murthy R. Amniotic membrane transplantation, A review of current indications and management of ophthalmic conditions. Indian J Ophthalmol. 2007;55:251–60. doi: 10.4103/0301-4738.33036. [DOI] [PubMed] [Google Scholar]
- 8.Chen HJ, Pires RT, Tseng SC. Amniotic membrane transplantation for severe neurotrophic corneal ulcers. Br J Ophthalmol. 2000;84:826–33. doi: 10.1136/bjo.84.8.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimazaki J, Yang HY, Tsubota K. Amniotic membrane transplantation for ocular surface reconstruction in patients with chemical and thermal burns. Ophthalmology. 1997;104:2068–76. doi: 10.1016/s0161-6420(97)30057-8. [DOI] [PubMed] [Google Scholar]
- 10.Ma DH, See LC, Liau SB, Tsai RJ. Amniotic membrane graft for primary pterygium: Comparison with conjunctival autograft and topical mitomycin C treatment. Br J Ophthalmol. 2000;84:973–8. doi: 10.1136/bjo.84.9.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prabhasawat P, Barton K, Burkett G, Tseng SC. Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmolgy. 1997;104:974–85. doi: 10.1016/s0161-6420(97)30197-3. [DOI] [PubMed] [Google Scholar]
- 12.Solomon A, Pires RT, Tseng SC. Amniotic membrane transplantation after extensive removal of primary and recurrent pterygium. Ophthalmology. 2001;108:449–60. doi: 10.1016/s0161-6420(00)00567-4. [DOI] [PubMed] [Google Scholar]
- 13.Solomon A, Meller D, Prabhasawat P, John T, Espana EM, Steuhl KP, Tseng SC. Amniotic membrane grafts for nontraumatic corneal perforations, descemetoceles, and deep ulcers. Ophthalmology. 2002;109:694–703. doi: 10.1016/s0161-6420(01)01032-6. [DOI] [PubMed] [Google Scholar]
- 14.Chen JH, Ma DH, Tsai RJ. Amniotic membrane transplantation for psuedomonal keratitis with impending perforation. Chang gung Med J. 2002;25:144–52. [PubMed] [Google Scholar]
- 15.Nubile M, Carpineto P, Lanzini M, Ciancaglini M, Zuppardi E, Mastropasqua L. Multilayer amniotic membrane transplantation for bacterial keratitis with corneal perforation after hyperopic photorefractive keratectomy: Case report and literature review. J Cataract Refract Surg. 2007;33:1636–40. doi: 10.1016/j.jcrs.2007.04.040. [DOI] [PubMed] [Google Scholar]
- 16.Espana EM, Grueterich M, Sandoval H, Solomon A, Alfonso E, Karp CL, et al. Amniotic membrane transplantation for bullous keratopathy in eyes with poor visual potential. J Cataract Refract Surg. 2003;29:279–84. doi: 10.1016/s0886-3350(02)01525-0. [DOI] [PubMed] [Google Scholar]
- 17.Joseph A, Dua HS, King AJ. Failure of Amniotic membrane transplantation in treatment of acute burns. Br J Ophthalmol. 2001;85:1065–9. doi: 10.1136/bjo.85.9.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meller D, Pires RT, Mack RJ, Figueiredo F, Heiligenhaus A, Park WC, et al. Amniotic membrane transplantation for acute chemical or thermal burns. Ophthalmology. 2000;107:980–90. doi: 10.1016/s0161-6420(00)00024-5. [DOI] [PubMed] [Google Scholar]
- 19.Tandon R, Gupta N, Kalaivani M, Sharma N, Titiyal JS, Vajpayee RB. Amniotic membrane transplantation as an adjuct to medical therapy in acute ocular burns. Br J Ophthalmol. 2011;95:199–204. doi: 10.1136/bjo.2009.173716. [DOI] [PubMed] [Google Scholar]
- 20.Marangon FB, Alfonso EC, Miller D, Remonda NM, Muallem MS, Tseng SC. Incidence of microbial infections after amniotic membrane transplantation. Cornea. 2004;23:264–9. doi: 10.1097/00003226-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Khokhar S, Sharma N, Kumar H, Soni A. Infection after use of non-preserved human amniotic membrane for the reconstruction of ocular surface. Cornea. 2001;20:773–4. doi: 10.1097/00003226-200110000-00023. [DOI] [PubMed] [Google Scholar]
- 22.Hanada K, Shimazaki J, Shimmura S, Tsubota K. Multilayered amniotic membrane transplantation for severe ulceration of cornea and sclera. Am J Ophthalmol. 2001;131:324–31. doi: 10.1016/s0002-9394(00)00825-4. [DOI] [PubMed] [Google Scholar]
- 23.Azura-Blanco A, Pillai CT, Dua HS. Amniotic membrane transplantation for ocular surface reconstruction. Br J Ophthalmol. 1999;83:399–402. doi: 10.1136/bjo.83.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prabhasawat P, Tesavibul N, Komolsuradej W. Single and multilayer amniotic membrane transplantation for persistent corneal epithelial defect with and without stromal thinning and perforation. Br J Ophthalmol. 2001;85:1455–63. doi: 10.1136/bjo.85.12.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]


