Abstract
Bag of Marbles (Bam) is a stem cell differentiation factor in the Drosophila germ line. Here, we demonstrate that Bam has a crucial function in the lymph gland, the tissue that orchestrates the second phase of Drosophila hematopoiesis. In bam mutant larvae, depletion of hematopoietic progenitors is observed, coupled with prodigious production of differentiated hemocytes. Conversely, forced expression of Bam in the lymph gland results in expansion of prohemocytes and substantial reduction of differentiated blood cells. These findings identify Bam as a regulatory protein that promotes blood cell precursor maintenance and prevents hemocyte differentiation during larval hematopoiesis. Cell-specific knockdown of bam function via RNAi expression revealed that Bam activity is required cell-autonomously in hematopoietic progenitors for their maintenance. microRNA-7 (mir-7) mutant lymph glands present with phenotypes identical to those seen in bam-null animals and mutants double-heterozygous for bam and mir-7 reveal that the two cooperate to maintain the hematopoietic progenitor population. By contrast, analysis of yan mutant lymph glands revealed that this transcriptional regulator promotes blood cell differentiation and the loss of prohemocyte maintenance. Expression of Bam or mir-7 in hematopoietic progenitors leads to a reduction of Yan protein. Together, these results demonstrate that Bam and mir-7 antagonize the differentiation-promoting function of Yan to maintain the stem-like hematopoietic progenitor state during hematopoiesis.
Keywords: Bam, Drosophila, Hematopoiesis, mir-7, Yan
INTRODUCTION
The discovery of a stem cell-like hematopoietic progenitor niche within the lymph glands of Drosophila larvae represents a significant contribution of this model system to the study of blood cell formation (Krzemien et al., 2007; Mandal et al., 2007). During the third instar, each anterior lymph gland becomes organized into three distinct regions (Jung et al., 2005). The medullary zone is populated by undifferentiated hematopoietic progenitors that express components of the Hedgehog (Hh) and JAK/STAT signaling pathways. At a peripheral position, the cortical zone is populated by differentiated plasmatocytes and crystal cells that are derived from the blood cell precursors present within the medullary zone. With certain altered genetic conditions or as a result of wasp parasitization, an additional group of hemocytes called lamellocytes appear in the cortical zone, probably generated owing to plasmatocyte plasticity (Markus et al., 2009; Tokusumi et al., 2009a; Honti et al., 2010; Stofanko et al., 2010).
The third lymph gland domain is the posterior signaling center (PSC), a region of 30-40 cells that does not give rise to differentiated blood cells (Jung et al., 2005). The PSC is formed as a result of the specification function of Antennapedia (Antp) and is maintained by the functions of Collier (Knot – FlyBase) and Wingless (Crozatier et al., 2004; Mandal et al., 2007; Sinenko et al., 2009). Cells of the PSC uniquely express the Hh and Serrate signaling proteins (Lebestky et al., 2003; Mandal et al., 2007), with Hh expression being positively regulated by the GATA factor Serpent (Tokusumi et al., 2010). Recent lineage analyses have demonstrated the existence of a fourth lymph gland region: a border zone located between the medullary and cortical zones, which harbors intermediate hematopoietic progenitors that are primed to initiate a blood cell differentiation program (Krzemien et al., 2010).
Elegant studies have demonstrated that the PSC serves as a hematopoietic progenitor niche within the lymph gland and that this functional domain is essential for preserving normal hemocyte homeostasis (Krzemien et al., 2007; Mandal et al., 2007). Specifically, communication between PSC cells and hemocyte precursors is essential for the maintenance of the progenitor population and the prevention of these cells from becoming abnormally programmed to differentiate into mature blood cells. Key aspects of this regulatory network include Hh expression in PSC cells, coupled with the non-autonomous activation of the Hh signaling pathway in hematopoietic progenitors. Additionally, the PSC plays a role in triggering the activation of the JAK/STAT pathway within prohemocytes. With the disruption of any of these events, the precursor population is lost owing to the premature differentiation of hemocytes (Krzemien et al., 2007; Mandal et al., 2007; Tokusumi et al., 2010).
Although important progress has been made towards an understanding of hematopoietic progenitor-niche cell interactions, there remains a paucity of information on events that control blood cell precursor maintenance versus programmed lineage differentiation. Intriguingly, we discovered that the bag of marbles (bam) gene is transcriptionally active in this hematopoietic tissue. Bam has been characterized as a key regulatory component of the germ line as its normal function is to direct germ cells towards spermatocyte or cystoblast differentiation (McKearin and Spradling, 1990; McKearin and Ohlstein, 1995). Recent studies have demonstrated that Bam and its partner Bgcn form a complex that works via a translational repression mechanism to balance stem cell self-renewal versus cystoblast differentiation (Li, Y. et al., 2009; Shen et al., 2009; Kim et al., 2010). Here, we investigate the expression and requirement of Bam in the lymph gland and demonstrate that it functions, not as a blood cell differentiation factor, but as a positive regulator of hematopoietic progenitor maintenance. Bam works with microRNA-7 (mir-7) to negatively attenuate the lymph gland function of Yan (Aop – FlyBase), a transcription factor previously shown to be a hemocyte differentiation-promoting protein (Zetervall et al., 2004). Our findings illuminate a novel mechanism for the control of hematopoietic progenitor maintenance during the final phase of Drosophila hematopoiesis.
MATERIALS AND METHODS
Drosophila strains
Lines obtained from the Bloomington Drosophila Stock Center (Indiana University, IN, USA) were: w1118, bamΔ86, yan1, UAS-2XEGFP, UAS-2XEYFP, UAS-mCD8::GFP and UAS-yan. TepIVGal4 (Avet-Rochex et al., 2010; Tokusumi et al., 2010) was obtained from the Drosophila Genetic Resource Center in Kyoto, Japan. The UAS-bam RNAi strains 10422R-1 and 10422R-2 were obtained from the National Institute of Genetics in Mishima, Japan; HMS00029 was obtained from Harvard Medical School. We also used strains provided by various colleagues: dome-lacZ (domeMESO) (Hombria et al., 2005) (N. Fossett); domeGal4 (Bourbon et al., 2002) (C. Evans); UAS-bamGFP (Chen and McKearin, 2003) (D. McKearin); mir-7Δ1, UAS-mir-7 and mir-7 enhancer-GFP (Li and Carthew, 2005; Li, X. et al., 2009) (R. Carthew); and eaterGal4, hhF4f-GFP and MSNF9mo-mCherry (Tokusumi et al., 2009a; Tokusumi et al., 2009b; Tokusumi et al., 2010).
Tissue immunostaining
Lymph glands were processed and immunostained as previously described (Tokusumi et al., 2010). The following primary antibodies were used to determine protein expression in dissected tissues: mouse anti-Yan (1:100, Developmental Studies Hybridoma Bank); rabbit anti-β-Gal (1:1000, ICN); mouse anti-β-Gal (1:500, Promega); mouse anti-Bam (McKearin and Ohlstein, 1995) (1:10, D. McKearin and Developmental Studies Hybridoma Bank); P1 antibody (Vilmos et al., 2004) (1:100, I. Ando); mouse anti-Antp (1:100, Developmental Studies Hybridoma Bank). Immunostained samples were analyzed with a Nikon A1R laser scanning confocal microscope.
Microarray analysis
RNA prepared from 300 lymph glands dissected from four-day-old wild-type larvae was analyzed on GeneChip Drosophila Genome 2.0 arrays.
RESULTS AND DISCUSSION
Bam expression and function in cells of the lymph gland hematopoietic organ
Through a microarray analysis of mRNAs present in lymph glands dissected from third-instar larvae, we determined that bam was transcriptionally active in this tissue that hosts the final phase of Drosophila hematopoiesis. The immunostaining of wild-type lymph glands with an anti-Bam antibody revealed that the protein is expressed in dome-lacZ- and TepIVGal4>UAS-mCD8::GFP-positive hematopoietic progenitors located within the medullary zone (Fig. 1A, data not shown). This finding suggested that Bam might play a key role in regulating blood cell homeostasis within the lymph gland.
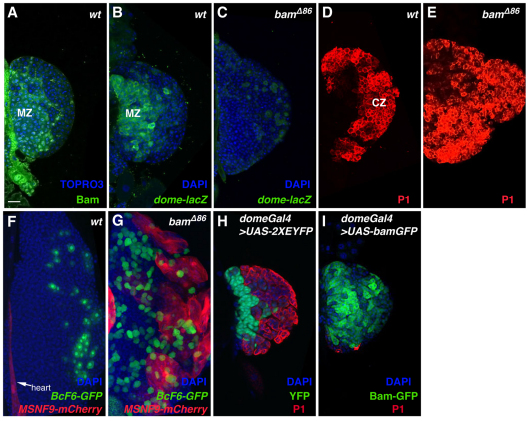
Fig. 1.
Bam expression and function during hematopoiesis. (A) Immunostaining of wild-type (wt) Drosophila lymph glands with an anti-Bam antibody. (B) dome-lacZ serves as a marker of prohemocytes in wild-type lymph glands. (C) Reduction of dome-lacZ-positive prohemocytes in lymph glands from bamΔ86 larvae. (D) P1 antibody detects plasmatocytes in lymph glands from wild-type larvae. (E) Increased numbers of plasmatocytes in lymph glands dissected from bamΔ86 larvae. (F) Expression of BcF6-GFP marks crystal cells (present), whereas activity of MSNF9-mCherry marks lamellocytes (absent) in wild-type lymph glands. (G) Increased crystal cells and induced lamellocytes in lymph glands from bamΔ86 larvae. (H) Immunostaining with P1 antibody and domeGal4-driven UAS-2XEYFP expression in control lymph glands. (I) Forced expression of the UAS-bamGFP transgene by domeGal4 results in expansion of the medullary zone and reduction of plasmatocytes. CZ, cortical zone; MZ, medullary zone.
Under normal conditions, there exists a balance between the quiescent prohemocyte population and the production of plasmatocytes and crystal cells (Fig. 1B,D,F,H). As animals homozygous null for bam are viable, we assayed lymph glands from bamΔ86/bamΔ86 larvae to monitor the status of prohemocyte maintenance versus hemocyte differentiation. bam mutant lymph glands showed a diminution of dome-lacZ-marked hematopoietic progenitors from the medullary zone (Fig. 1C), coupled with an overproduction of P1-positive plasmatocytes (Fig. 1E) and BcF6-GFP-marked crystal cells (Fig. 1G). Whereas lamellocytes are rarely observed in lymph glands of wild-type larvae, these MSNF9mCherry-expressing cells were induced in considerable numbers in the bam mutant tissue (Fig. 1G). Thus, the absence of bam function resulted in an appreciable loss of hematopoietic progenitors and the copious production of differentiated hemocytes. Conversely, forced expression of Bam culminated in an expansion of blood cell precursors throughout the lymph gland and the near complete absence of differentiated plasmatocytes (Fig. 1I).
The demonstration of Bam as a regulator of blood cell precursor maintenance implies that selective mRNA translational control is likely to be an important regulatory mechanism in the decision between hematopoietic progenitor maintenance versus lineage-specific blood cell differentiation. It is noteworthy that bgcn mutant lymph glands underwent a normal hematopoietic process (data not shown). Thus, Bam might interact functionally with a novel protein partner in regulating mRNA translation to ensure an optimal blood cell homeostasis within this hematopoietic organ.
Bam function is required cell-autonomously for hematopoietic progenitor maintenance
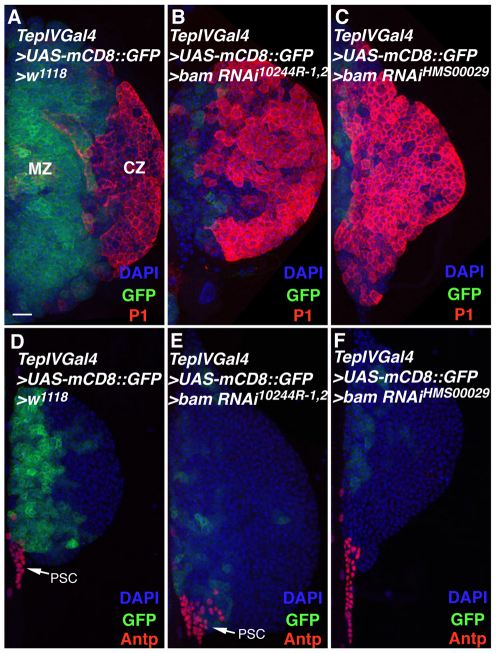
To determine whether Bam is required intrinsically in blood cell precursors to ensure their maintenance, we knocked down gene function in prohemocytes through the generation of lymph glands with the genotype TepIVGal4>UAS-bam RNAi. As TepIVGal4 is a medullary zone-specific driver (Fig. 2A,D), we were able to eliminate Bam activity precisely from the hemocyte precursor population. Expression of bam RNAi resulted in a near-complete loss of hematopoietic progenitors (Fig. 2B,C,E,F), coupled with an expansion of the P1-positive plasmatocyte population (Fig. 2B,C). We also determined that a loss of Bam activity from prohemocytes did not affect the neighboring PSC population (Fig. 2E,F). Thus, a major effect of eliminating bam function from hematopoietic progenitors is a failure to maintain the blood cell precursor state and the premature programming of precursor cells into the plasmatocyte lineage.
Fig. 2.
Bam functions cell-autonomously in hematopoietic progenitors. (A) TepIVGal4-driven UAS-mCD8::GFP marks blood cell precursors whereas P1 marks plasmatocytes within a wild-type Drosophila lymph gland. (B,C) Loss of hematopoietic progenitors and increase in plasmatocytes due to UAS-bam RNAi expression. (D) Control lymph gland stained for prohemocytes and Antp-expressing PSC cells. (E,F) Hematopoietic progenitors are decreased owing to UAS-bam RNAi expression. CZ, cortical zone; MZ, medullary zone; PSC, posterior signaling center.
Mutation of the mir-7 gene generates hematopoietic phenotypes identical to those observed in bam-null lymph glands
A recent study reported the presence of a mir-7 target site in the 3′ UTR of bam and that this sequence could be utilized to repress Bam expression during male germ line differentiation (Pek et al., 2009). To ensure proper stem cell lineage differentiation, a model was proposed in which Maelstrom represses the expression of mir-7, thus alleviating its repression of bam and allowing this differentiation factor to function normally.
Based on this report, we decided to investigate the lymph gland expression and function of mir-7. Initially, we determined that a mir-7 enhancer-GFP transgene (Li, X. et al., 2009) was expressed at low levels in cells in the proximal part of the medullary zone and more strongly in cells of the PSC (Fig. 3A). We then postulated that if mir-7 negative regulation of Bam function occurred in cells of the lymph gland, then we might discern mir-7 hematopoietic phenotypes that differed from those observed in bam mutant animals. To the contrary, mir-7 mutant lymph glands presented with altered blood cell populations identical to those found in bam mutants. mir-7-null lymph glands showed a reduction in blood cell precursors (Fig. 3B) and enhanced production of differentiated plasmatocytes and lamellocytes (Fig. 3C). Given the similar nature of the phenotypes, we assayed animals that were double-heterozygous for bam- and mir-7-null alleles and observed a significant reduction in the number of hematopoietic progenitors (Fig. 3F). When considered together, the bam and mir-7 analyses implicate a concerted function of the two in the maintenance of the stem-like blood cell precursor state.
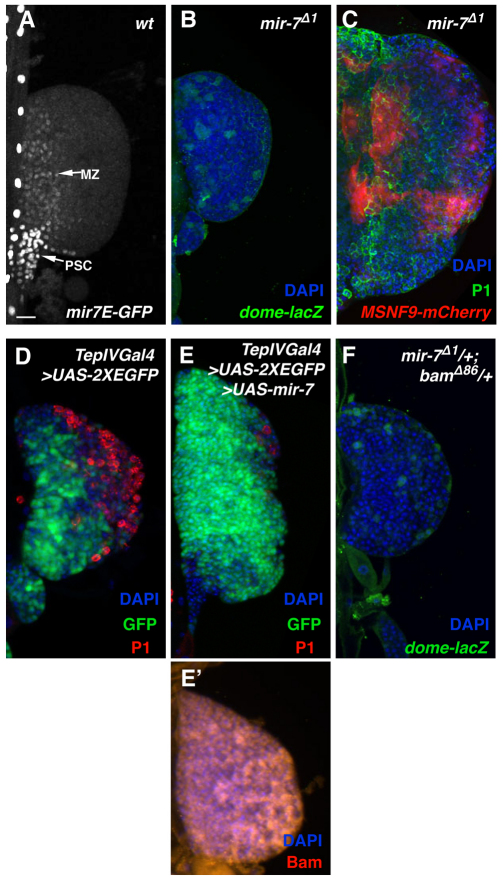
Fig. 3.
mir-7 expression and function during hematopoiesis. (A) mir-7 enhancer-GFP transgene is expressed in the medullary zone and PSC cells in control Drosophila lymph glands. (B) mir-7 mutant lymph glands possess a diminished medullary zone as marked by dome-lacZ. (C) mir-7 mutant lymph glands present with an overproduction of plasmatocytes (marked by P1) and lamellocytes (marked by MSNF9-mCherry). (D) Immunostaining with P1 antibody and expression pattern of TepIVGal4-driven GFP in a control lymph gland. (E) Forced expression of mir-7 in hematopoietic progenitors results in medullary zone expansion and plasmatocyte reduction. (E′) Expanded pattern of Bam protein accumulation in a mir-7 gain-of-function lymph gland. (F) Double-heterozygous combination of mir-7Δ1 and bamΔ86 alleles results in a lymph gland devoid of prohemocytes. MZ, medullary zone; PSC, posterior signaling center.
Forced expression of mir-7 in hematopoietic progenitors resulted in an expansion of the prohemocyte population and a decrease in the number of differentiated plasmatocytes (Fig. 3E). Notably, this mir-7 gain-of-function condition failed to repress Bam protein expression (Fig. 3E′), as would have been predicted from the prior report of mir-7 negative regulation of bam. Rather, mir-7 induction of supernumerary hematopoietic progenitors resulted in Bam expression throughout the lymph gland in the expanded prohemocyte population. The lack of mir-7 negative regulation of Bam function indicates that this regulatory RNA can function differentially based on its cell-specific context.
Yan is a blood cell differentiation factor negatively regulated by Bam and mir-7
Yan is an ETS-family protein that functions as a transcriptional repressor (Lai and Rubin, 1992). Relevant to our analysis of the genetic control of larval hematopoiesis, it was reported that forced expression of Yan in lymph glands results in a robust production of differentiated blood cells, including crystal cells and lamellocytes (Zettervall et al., 2004). Additionally, yan expression is subject to negative regulation by mir-7 during photoreceptor differentiation in the eye (Li and Carthew, 2005; Li, X. et al., 2009). Given these observations, we analyzed the expression and function of yan during hematopoiesis while considering our findings on the roles of bam and mir-7 in this developmental process.
The immunostaining of hematopoietic tissues obtained from wild-type animals with an anti-Yan antibody revealed that the protein is expressed in cells present in a border zone located between the medullary and cortical zones (Fig. 4A). As Yan-expressing cells did not express the hematopoietic progenitor marker TepIVGal4>UAS-mCD8::GFP or the plasmatocyte marker eaterGal4>UAS-GFP (Fig. 4B,C), the Yan-positive cells probably correspond to intermediate hematopoietic progenitors that are primed to initiate hemocyte differentiation (Krzemien et al., 2010). The absence of Yan in cells of the cortical zone suggested that the protein functions transiently in blood cell precursors and is downregulated during the differentiation of mature hemocytes. The analysis of yan mutant lymph glands revealed an enlargement of the dome-lacZ-expressing hematopoietic progenitor pool (Fig. 4D). By contrast, forced expression of Yan resulted in the loss of blood cell precursors coupled with an expansion of P1-positive plasmatocytes (Fig. 4F). These findings strengthened the notion that Yan functions as a hemocyte differentiation factor during larval hematopoiesis.
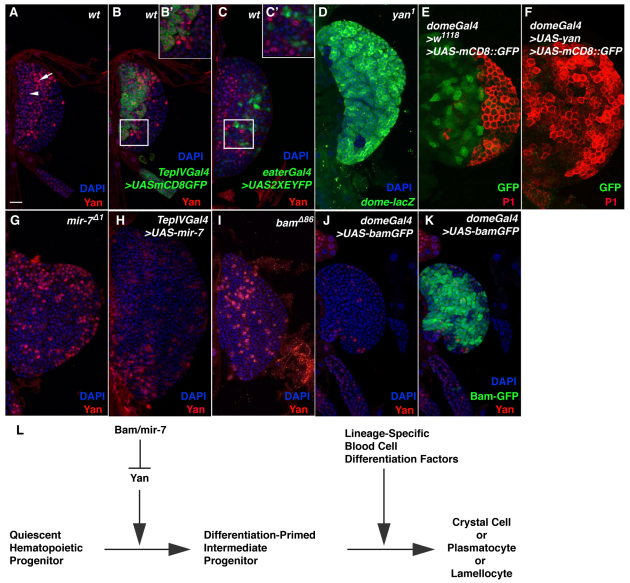
Fig. 4.
Yan expression and function during hematopoiesis. (A) Immunostaining of wild-type lymph glands with an anti-Yan antibody. Yan-positive (arrow) and Yan-negative (arrowhead) cells are indicated. (B,B′) Yan-positive cells do not express a quiescent hematopoietic progenitor marker. (C,C′) Yan-positive cells do not express a plasmatocyte marker. (D) Overproduction of prohemocytes in lymph glands from yan1 mutant larvae. (E) Lymph gland expression of domeGal4>UAS-mCD8::GFP and immunostaining with P1 antibody (which marks plasmatocytes) in a control genetic background. (F) Forced expression of yan results in a loss of prohemocytes and increase of plasmatocytes in lymph glands. (G) In mir-7Δ1 lymph glands, an increase in Yan protein level is observed in a dispersed population of progenitors. (H) Forced expression of mir-7 in medullary zone cells results in reduced Yan expression. (I) In bamΔ86 lymph glands, an increase in Yan protein level is observed in a dispersed population of progenitors. (J,K) Forced expression of a bamGFP fusion gene results in medullary zone expansion and a loss of Yan-expressing cells. (L) Model for the regulatory interactions between Bam, mir-7 and Yan during lymph gland hematopoiesis.
yan loss- and gain-of-function animals show completely opposite phenotypes to those observed for comparably altered mir-7 and bam larvae. We thus tested the possibility that mir-7 and Bam could function as negative regulators of Yan expression in hematopoietic cells. In mir-7- or bam-null lymph glands, we detected an increased level of Yan protein in a dispersed population of progenitor cells (Fig. 4G,I). Conversely, forced expression of mir-7 or bam in blood cell precursors resulted in a reduction of Yan protein from the expanded prohemocyte population (Fig. 4H,J,K).
Conclusion
Our findings on Bam, mir-7 and Yan suggest a mechanism for the interaction of these regulators in the control of blood cell homeostasis (Fig. 4L). The role of Yan is to direct a quiescent hematopoietic progenitor, through a primed intermediate progenitor state, towards a blood cell differentiation fate as crystal cell, plasmatocyte or lamellocyte. The final differentiation status of these cells would be subject to the function of distinct lineage-determining transcription factors. By contrast, Bam and mir-7 cooperate to negatively modulate yan mRNA translation in the quiescent hematopoietic progenitor, thus maintaining the initial prohemocyte state. Although details of this possible translational repression remain to be parsed out, it is likely to include an as yet unidentified protein partner of Bam that would facilitate mir-7 and yan mRNA packaging within an inhibitory RNA-induced silencing complex in prohemocytes (Bartel, 2004).
Acknowledgments
We thank I. Ando, R. Carthew, C. Evans, N. Fossett, D. McKearin and various stock centers for antibodies and Drosophila strains. This work was supported by grants to R.A.S. from the NIH (HL071540) and the Notre Dame Initiative in Adult Stem Cell Research. Deposited in PMC for release after 12 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- Avet-Rochex A., Boyer K., Polesello C., Gobert V., Osman D., Roch F., Augé B., Zanet J., Haenlin M., Waltzer L. (2010). An in vivo RNA interference screen identifies gene networks controlling Drosophila melanogaster blood cell homeostasis. BMC Dev. Biol. 10, 65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel D. P. (2004). MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281-297 [DOI] [PubMed] [Google Scholar]
- Bourbon H. M., Gonzy-Treboul G., Peronnet F., Alin M. F., Ardourel C., Benassayag C., Cribbs D., Deutsch J., Ferrer P., Haenlin M., et al. (2002). A P-insertion screen identifying novel X-linked essential genes in Drosophila. Mech. Dev. 110, 71-83 [DOI] [PubMed] [Google Scholar]
- Chen D., McKearin D. M. (2003). A discrete transcriptional silencer in the bam gene determines asymmetric division of the Drosophila germline stem cell. Development 130, 1159-1170 [DOI] [PubMed] [Google Scholar]
- Crozatier M., Ubeda J. M., Vincent A., Meister M. (2004). Cellular immune response to parasitization in Drosophila requires the EBF orthologue Collier. PLoS Biol. 2, e196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombria J. C., Brown S., Hader S., Zeidler M. P. (2005). Characterization of Upd2, a Drosophila JAK/STAT pathway ligand. Dev. Biol. 288, 420-433 [DOI] [PubMed] [Google Scholar]
- Honti V., Csordas G., Markus R., Kurucz E., Jankovics F., Ando I. (2010). Cell lineage tracing reveals the plasticity of the hemocyte lineages and of the hematopoietic compartments in Drosophila melanogaster. Mol. Immunol. 47, 1997-2004 [DOI] [PubMed] [Google Scholar]
- Jung S. H., Evans C. J., Uemura C., Banerjee U. (2005). The Drosophila lymph gland as a developmental model of hematopoiesis. Development 132, 2521-2533 [DOI] [PubMed] [Google Scholar]
- Kim J. Y., Lee Y. C., Kim C. (2010). Direct inhibition of Pumilio activity by Bam and Bgcn in Drosophila germ line stem cell differentiation. J. Biol. Chem. 285, 4741-4746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzemien J., Dubois L., Makki R., Meister M., Vincent A., Crozatier M. (2007). Control of blood cell homeostasis in Drosophila larvae by the posterior signalling centre. Nature 446, 325-328 [DOI] [PubMed] [Google Scholar]
- Krzemien J., Oyallon J., Crozatier M., Vincent A. (2010). Hematopoietic progenitors and hemocyte lineages in the Drosophila lymph gland. Dev. Biol. 346, 310-319 [DOI] [PubMed] [Google Scholar]
- Lai Z. C., Rubin G. M. (1992). Negative control of photoreceptor development in Drosophila by the product of the yan gene, an ETS domain protein. Cell 70, 609-620 [DOI] [PubMed] [Google Scholar]
- Lebestky T., Jung S. H., Banerjee U. (2003). A Serrate-expressing signaling center controls Drosophila hematopoiesis. Genes Dev. 17, 348-353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Carthew R. W. (2005). A microRNA mediates EGF receptor signaling and promotes photoreceptor differentiation in the Drosophila eye. Cell 123, 1267-1277 [DOI] [PubMed] [Google Scholar]
- Li X., Cassidy J. J., Reinke C. A., Fischboeck S., Carthew R. W. (2009). A microRNA imparts robustness against environmental fluctuation during development. Cell 137, 273-282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Minor N., Park J. K., McKearin D. M., Maines J. Z. (2009). Bam and Bgcn antagonize Nanos-dependent germ-line stem cell maintenance. Proc. Natl. Acad. Sci. USA 106, 9304-9309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal L., Martinez-Agosto J. A., Evans C. J., Hartenstein V., Banerjee U. (2007). A Hedgehog- and Antennapedia-dependent niche maintains Drosophila haematopoietic precursors. Nature 446, 320-324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus R., Laurinyecz B., Kurucz E., Honti V., Bajusz I., Sipos B., Somogyi K., Kronhamn J., Hultmark D., Ando I. (2009). Sessile hemocytes as a hematopoietic compartment in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 106, 4805-4809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKearin D. M., Spradling A. C. (1990). bag-of-marbles: a Drosophila gene required to initiate both male and female gametogenesis. Genes Dev. 4, 2242-2251 [DOI] [PubMed] [Google Scholar]
- McKearin D., Ohlstein B. (1995). A role for the Drosophila bag-of-marbles protein in the differentiation of cystoblasts from germline stem cells. Development 121, 2937-2947 [DOI] [PubMed] [Google Scholar]
- Pek J. W., Lim A. K., Kai T. (2009). Drosophila maelstrom ensures proper germline stem cell lineage differentiation by repressing microRNA-7. Dev. Cell 17, 417-424 [DOI] [PubMed] [Google Scholar]
- Shen R., Weng C., Yu J., Xie T. (2009). eIF4A controls germline stem cell self-renewal by directly inhibiting Bam function in the Drosophila ovary. Proc. Natl. Acad. Sci. USA 106, 11623-11628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinenko S. A., Mandal L., Martinez-Agosto J. A., Banerjee U. (2009). Dual role of Wingless signaling in stem-like hematopoietic precursor maintenance in Drosophila. Dev. Cell 16, 756-763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stofanko M., Kwon S. Y., Badenhorst P. (2010). Lineage tracing of lamellocytes demonstrates Drosophila macrophage plasticity. PLoS One 5, e14051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokusumi T., Sorrentino R. P., Russell M., Ferrarese R., Govind S., Schulz R. A. (2009a). Characterization of a lamellocyte transcriptional enhancer located within the misshapen gene of Drosophila melanogaster. PLoS One 4, e6429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokusumi T., Shoue D. A., Tokusumi Y., Stoller J. R., Schulz R. A. (2009b). New hemocyte-specific enhancer-reporter transgenes for the analysis of hematopoiesis in Drosophila. Genesis 47, 771-774 [DOI] [PubMed] [Google Scholar]
- Tokusumi Y., Tokusumi T., Stoller-Conrad J., Schulz R. A. (2010). Serpent, Suppressor of Hairless and U-shaped are crucial regulators of hedgehog niche expression and prohemocyte maintenance during Drosophila larval hematopoiesis. Development 137, 3561-3568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilmos P., Nagy I., Kurucz E., Hultmatk D., Gateff E., Ando I. (2004). A rapid rosetting method for separation of hemocyte sub-populations of Drosophila melanogaster. Dev. Comp. Immunol. 28, 555-556 [DOI] [PubMed] [Google Scholar]
- Zettervall C. J., Anderl I., Williams M. J., Palmer R., Kurucz E., Ando I., Hultmark D. (2004). A directed screen for genes involved in Drosophila blood cell activation. Proc. Natl. Acad. Sci. USA 101, 14192-14197 [DOI] [PMC free article] [PubMed] [Google Scholar]