Abstract
Specification of the otic anteroposterior axis is one of the earliest patterning events during inner ear development. In zebrafish, Hedgehog signalling is necessary and sufficient to specify posterior otic identity between the 10 somite (otic placode) and 20 somite (early otic vesicle) stages. We now show that Fgf signalling is both necessary and sufficient for anterior otic specification during a similar period, a function that is completely separable from its earlier role in otic placode induction. In lia–/– (fgf3–/–) mutants, anterior otic character is reduced, but not lost altogether. Blocking all Fgf signalling at 10-20 somites, however, using the pan-Fgf inhibitor SU5402, results in the loss of anterior otic structures and a mirror image duplication of posterior regions. Conversely, overexpression of fgf3 during a similar period, using a heat-shock inducible transgenic line, results in the loss of posterior otic structures and a duplication of anterior domains. These phenotypes are opposite to those observed when Hedgehog signalling is altered. Loss of both Fgf and Hedgehog function between 10 and 20 somites results in symmetrical otic vesicles with neither anterior nor posterior identity, which, nevertheless, retain defined poles at the anterior and posterior ends of the ear. These data suggest that Fgf and Hedgehog act on a symmetrical otic pre-pattern to specify anterior and posterior otic identity, respectively. Each signalling pathway has instructive activity: neither acts simply to repress activity of the other, and, together, they appear to be key players in the specification of anteroposterior asymmetries in the zebrafish ear.
Keywords: Fgf, Hh, Axial patterning, Otic vesicle, Zebrafish
INTRODUCTION
Inner ears of jawed vertebrates are highly asymmetrical about all three body axes, particularly the anteroposterior (AP) axis, along which the main vestibular and auditory receptors, the sensory maculae, are arrayed. Asymmetrical expression of marker genes about the AP axis arises early, and is evident at placode stages in both fish and amniotes. Hmx3, for example, is expressed in a distinct anterior otic domain from as early as 16 hours post fertilisation (hpf) (14 somite stage) in zebrafish and E8.5 in the mouse (Hadrys et al., 1998; Adamska et al., 2000). Rotation and ablation experiments have shown that induction from surrounding tissues is important in establishing these asymmetries, and that at least two factors must be required to specify AP otic identity (Harrison, 1936; Wu et al., 1998; Waldman et al., 2007; Liang et al., 2010).
We have previously shown that Hedgehog (Hh) signalling from the notochord and floorplate is necessary and sufficient to specify posterior domains in the zebrafish ear: severely reduced or increased Hh signalling results in mirror image duplications of the anterior or posterior halves of the otic vesicle, respectively (Hammond et al., 2003; Hammond et al., 2010). Similar double-anterior ears have been generated in the Xenopus embryo by overexpression of the Hh inhibitor Hip (Waldman et al., 2007). Hh signalling also appears to be required for the correct development of hair cells and innervation of the zebrafish posterior (saccular) macula (Sapède and Pujades, 2010).
A good candidate for an anterior otic specification factor is Fibroblast growth factor (Fgf). In the zebrafish val–/– (mafba–/–) mutant, fgf3 expression, which is normally restricted to rhombomere (r) 4 of the hindbrain, expands posteriorly into r5 and r6. Concomitantly, expression of anterior markers expands posteriorly in the otic vesicle, whereas posterior markers are reduced (Kwak et al., 2002). Importantly, the otic phenotype can be rescued by injection of an fgf3 morpholino, which also reduces expression of anterior otic markers in wild-type embryos (Kwak et al., 2002). A more severe gain of anterior otic character is seen in hnf1ba–/– (vhnf1–/–) homozygotes, which also have expanded fgf3 expression in the hindbrain (Lecaudey et al., 2007). Previous reports also suggest that anterior otic character is disrupted in lia–/– (fgf3–/–) otic vesicles (Herzog et al., 2004; Kwak et al., 2006), which we have analysed in detail here. Consistent with an anterior otic specification role for Fgfs, Fgf receptors are expressed widely in the head, including in the otic epithelium, from placode stages (Thisse and Thisse, 2005; Thisse et al., 2008; Esterberg and Fritz, 2009; Rohner et al., 2009). Moreover, expression of pea3, spry4 and etv5b (erm), genes that are direct targets of Fgf signalling, is concentrated in anterior parts of the otic placode and vesicle (Raible and Brand, 2001; Roehl and Nüsslein-Volhard, 2001; Thisse et al., 2001; Nechiporuk et al., 2005).
Confirmation that Fgfs act as otic anteriorising factors, however, is not straightforward. The situation is complicated by their earlier essential roles in otic placode induction (Phillips et al., 2001; Léger and Brand, 2002; Maroon et al., 2002; Liu et al., 2003), which are conserved across different vertebrate species (for reviews, see Ohyama et al., 2007; Schimmang, 2007; Ladher et al., 2010). Here, using conditional approaches to manipulate Fgf signalling after otic placode induction is complete, we demonstrate that Fgf signalling is necessary and sufficient to specify anterior otic identity in the zebrafish. The critical time window for this anteriorising function is between the 10 and 20 somite stages, a time at which Hh is also acting to posteriorise the ear. Using pea3 and ptc1 (ptch2 – Zebrafish Information Network) expression as readouts of Fgf and Hh activity, respectively, we show that neither factor affects transduction of the other. In addition, when we inhibit Fgf signalling in a Hh loss-of-function background, we see an additive phenotype: both anterior and posterior otic identity are lost. Nevertheless, initial sensory differentiation proceeds, but in a pattern that is symmetrical about the AP axis. This suggests that both Fgf and Hh have independent, instructive activity, imparting anterior and posterior identity to the poles of the otic placode. Our data support a model in which Hh and Fgf act on an AP symmetrical `pre-pattern' to trigger the development of AP asymmetries in the zebrafish ear.
MATERIALS AND METHODS
Animals
Zebrafish strains were AB (wild type), liat21142 (Herzog et al., 2004), smob577 (Varga et al., 2001), Tg[hsp70:fgf3] (Lecaudey et al., 2008) and ptc1–/–;ptc2–/– (Koudijs et al., 2008). Embryonic stages are given as hours post-fertilisation (hpf) at 28.5°C or somite stages (S): 10S≅14 hpf; 20S≅19 hpf (Kimmel et al., 1995; Westerfield, 2000). All experiments with animals were performed under UK Home Office licence and conformed to UK regulatory standards.
In situ hybridisation
Whole-mount in situ hybridisation was carried out as described (Hammond et al., 2003) using probes designed against eya1, fgf8, fst1 (fsta – Zebrafish Information Network), hmx3, otx1, pax2a, pax5, ptc1 (Hammond et al., 2003), fgf3 (Kwak et al., 2002), hmx2 (Feng and Xu, 2010), hoxb1 (Prince et al., 1998), egr2b (krox20) (Oxtoby and Jowett, 1993), neurod (Blader et al., 1997), pea3 (Münchberg et al., 1999) and mafba (Moens et al., 1998).
Genotyping
ptc1–/–;ptc2–/–, 20S+ smo–/– and 72 hpf+ lia–/– embryos were identified morphologically. Other mutant embryos were genotyped. Genomic DNA was prepared as described (Westerfield, 2000). liat21142 primers were: 5′-TGTCCAGTCATGAATGTCAAAG-3′ and 5′-CCATCTCATGGTCCTTGTTG-3′. The resulting 320 bp fragment was digested with NsiI, producing 40 bp, 82 bp and 198 bp fragments from wild-type DNA and 40 bp and 280 bp fragments from liat21142 DNA. smob577 primers were: 5′-CTATACTGGCCAATTCACAG-3′ and 5′-ATGGAAAACAATGTCATAACC-3′. The resulting 325 bp PCR band was sequenced: wild-type DNA has a G at position 160, whereas smob577 DNA has a T.
FITC-phalloidin and anti-acetylated tubulin antibody staining
Staining was carried out as described (Haddon and Lewis, 1996).
Heat shock
hsp70:fgf3 transgenic and sibling embryos from a hsp70:fgf3/+ × hsp70:fgf3/+ cross were incubated at 39°C for 2 hours. Transgenic and sibling embryos were distinguished morphologically. Control embryos were kept at 28.5°C.
SU5402 treatment
Embryos were treated with 10 μM SU5402 (Merck) in 0.5% DMSO or with 0.5% DMSO alone in embryo medium from 10S and were washed after 3, 5 or 7 hours.
Microscopy
Microscopy was carried out as described (Hammond et al., 2003).
Acridine Orange staining
Embryos were treated with 5 μg/ml Acridine Orange in embryo medium from 38.5 hpf for one hour, washed in embryo medium and imaged at 40 hpf.
RESULTS
lia–/– (fgf3–/–) otic vesicles display a partial loss of anterior otic character
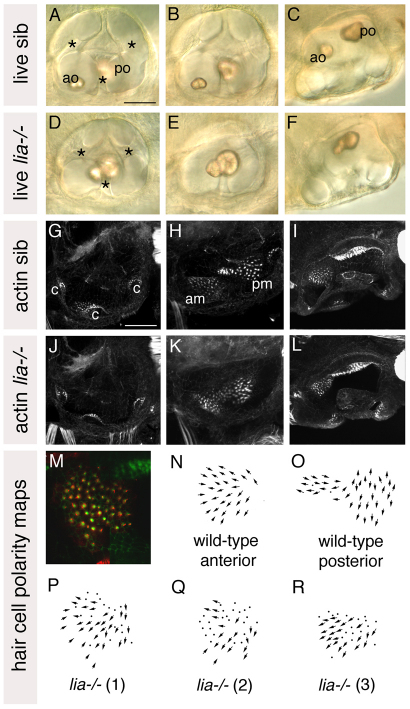
Fgf3 has a redundant role in zebrafish otic placode induction (Phillips et al., 2001; Léger and Brand, 2002; Maroon et al., 2002). Previous studies have also suggested a role in later otic development (Kwak et al., 2002; Herzog et al., 2004; Kwak et al., 2006). To clarify the role of fgf3 in otic patterning, we characterised the otic phenotype of the fgf3 mutant lim absent (liat21142–/–), which is known to have otic defects (Herzog et al., 2004). The three-dimensional structure of lia–/– otic vesicles was grossly normal at 3 days post-fertilisation (dpf): there were no obvious defects in semicircular canal pillar or crista development (Fig. 1A-G,J). However, the anterior otolith was fused to the posterior otolith and positioned medially, resembling the normal position of the posterior otolith (Fig. 1A-F) (Herzog et al., 2004). Staining with FITC-phalloidin, to reveal the actin-rich stereociliary bundles of the sensory hair cells, showed that the underlying anterior macula was positioned more medially than normal, abutting the posterior macula (Fig. 1H,I,K,L).
Fig. 1.
Anterior otic character is reduced in lia–/– (fgf3–/–) homozygotes. (A-F) Live 72 hpf lia–/– and sibling (sib) zebrafish inner ears. (G-L) Confocal z-stacks of 84 hpf ears stained with FITC-phalloidin to mark sensory hair cells. (M) The anterior macula of a 5 dpf lia–/– embryo stained with anti-acetylated tubulin antibody (kinocilia; red) and FITC-phalloidin (stereocilia; green). (N,O) Typical polarity maps for wild-type maculae. (P) Hair cell polarity map obtained from the specimen shown in M. (Q,R) Polarity maps from two further lia–/– specimens. A,B,D,E,G,H,J,K: Lateral views; anterior to left, dorsal to top. A,D,G,J: Lateral focal plane. B,E,H,K: Medial focal plane. C,F,I,L: Dorsal views; anterior to left, medial to top. am, anterior macula; ao, anterior otolith; c, cristae; pm, posterior macula; po, posterior otolith. Asterisks indicate semicircular canal pillars. Scale bars: 50 μm.
The phenotype of the lia–/– anterior macula was variable, ranging from nearly normal in size and shape, to reduced in size and resembling the posterior macula in shape (Fig. 1M-R). However, analysis of hair cell polarity patterns, by double staining for tubulin in the hair cell kinocilia and actin in the stereociliary bundles, suggested that the anterior macula retained anterior character: hair cell polarities radiated in a fan-like pattern, resembling the normal anterior macula, rather than pointing away from a central midline as in wild-type posterior maculae (Fig. 1M-R).
This suggested that some, but not all, anterior otic character was lost in lia–/– embryos. To confirm this, we performed in situ hybridisation to lia–/– and sibling embryos with a panel of anterior- and posterior-restricted otic markers (Fig. 2). Expression of pax5, hmx2, hmx3 and fgf3 itself, which were strongly expressed at the anterior of the otic vesicle in phenotypically wild-type sibling embryos at 26 hpf, were all severely reduced in lia–/– embryos (Fig. 2A-H). However, expression of neurod, which normally marks delaminating neuroblasts and nascent neurons of the statoacoustic ganglion in an anteroventral domain beneath the otic vesicle, was not significantly reduced (data not shown). Expression of two posterior markers, fst1 and otx1, was unaltered, suggesting no gain of posterior character at the anterior of the otic vesicle (Fig. 2I-L). These data suggest that Fgf3 is required to specify anterior otic domains, but that loss of Fgf3 alone is not sufficient to re-specify anterior as posterior.
Fig. 2.
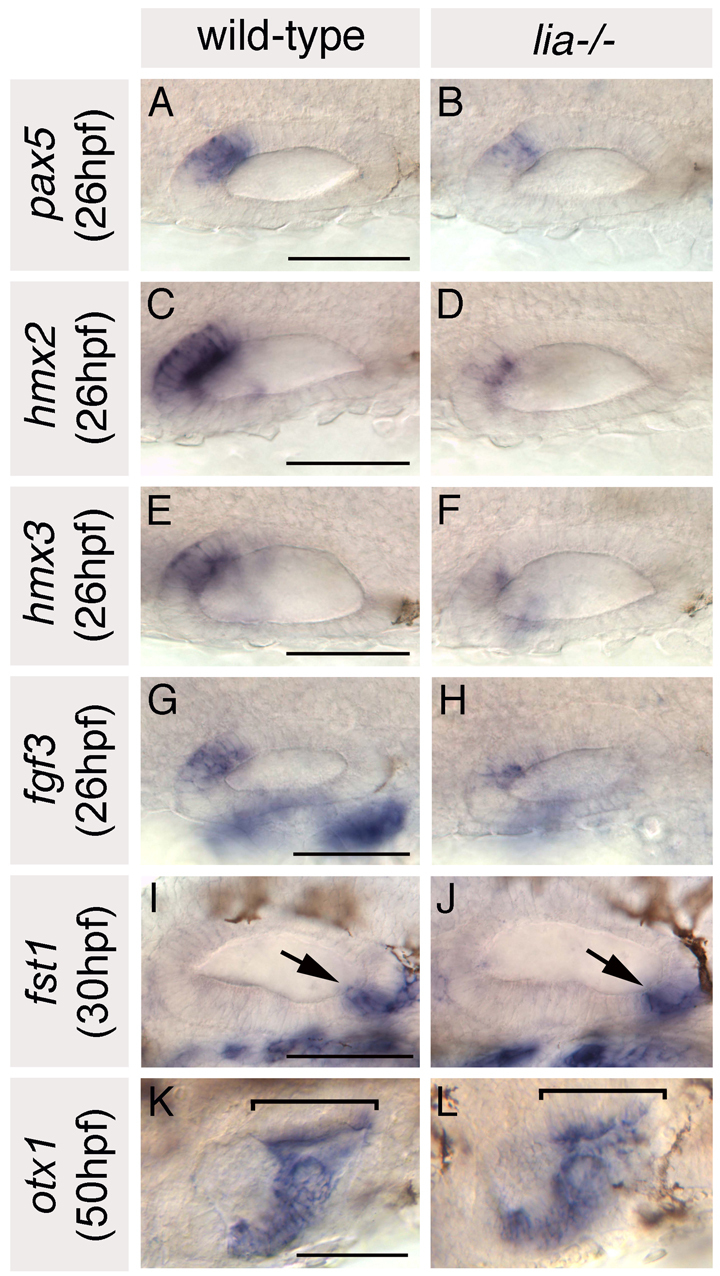
Anterior otic markers are reduced in lia–/– homozygotes; posterior markers are expressed as normal. (A-H) In situ hybridisation to anterior otic markers. (I-L) In situ hybridisation to posterior otic markers (arrows and brackets highlight the posterior otic domains). A-H,K,L: Dorsal views; anterior to left, medial to top. I,J: Lateral views; anterior to left, dorsal to top. Scale bars: 50 μm.
Inhibition of all Fgf signalling between the 10 and 20 somite stages leads to a complete loss of anterior otic character and a mirror image duplication of posterior domains
Although the liat21142 allele is thought to be a null or very strong loss-of-function allele (Herzog et al., 2004), the ears of liat21142–/– homozygotes retain some anterior character. Another factor must, therefore, be involved in specification of anterior otic identity. To test whether this is another Fgf, we used the inhibitor SU5402, which blocks all Fgf signalling (Mohammadi et al., 1997; Raible and Brand, 2001). An early block of Fgf signalling using SU5402 or lia–/–;ace–/– (fgf3–/–;fgf8–/–) double mutants, however, results in a complete absence of the otic placode, due to the essential role of Fgfs in otic induction (Phillips et al., 2001; Léger and Brand, 2002; Maroon et al., 2002; Liu et al., 2003). We therefore took a conditional approach and applied 10 μM SU5402 in 0.5% DMSO to wild-type embryos from the 10 somite stage (10S) (14 hpf) for 3, 5 or 7 hours. Longer treatments resulted in gross morphological abnormalities and early death of embryos (data not shown). Control embryos were treated with 0.5% DMSO for an equivalent time, which had no effect on ear development.
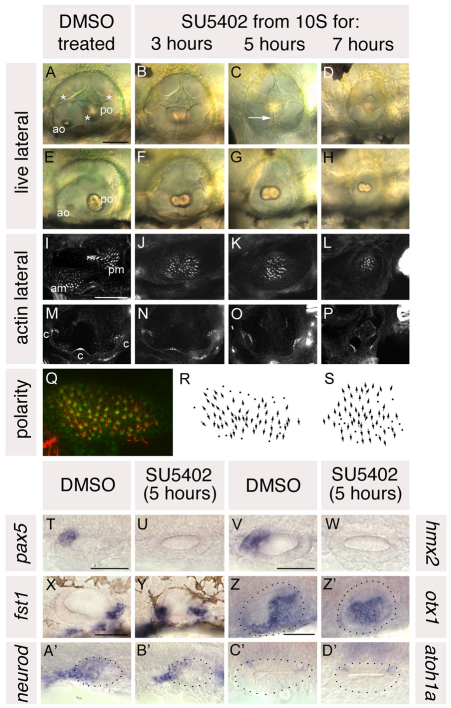
Wild-type (wt) embryos treated with SU5402 for three hours (10-16S, n=6) had well-formed ears with well-developed semicircular canal pillars. However, the otoliths were fused and positioned medially, resembling the wild-type posterior otolith (Fig. 3B,F). Phalloidin staining revealed that the anterior macula was absent; the otoliths sat over a single, medial, bow tie-shaped macula (Fig. 3J). This suggested that anterior otic structures had been lost and posterior structures duplicated in their place in a mirror image fashion. Three cristae were present, as in untreated embryos (Fig. 3N).
Fig. 3.
Treatment with SU5402 from 10 to 20S leads to a complete loss of anterior otic structures and a mirror image duplication of posterior otic structures. (A-H) Live ears of 84 hpf zebrafish embryos treated with 10 μM SU5402 and DMSO-treated controls. (I-P) Confocal z-stacks of 84 hpf SU5402-treated and control ears stained with FITC-phalloidin to mark sensory hair cells. (I-L) Medial focal planes showing the maculae. (M-P) Lateral focal planes showing the cristae. (Q) The macula of an 86 hpf SU5402-treated embryo stained with anti-acetylated tubulin antibody (kinocilia; red) and FITC-phalloidin (stereocilia; green). (R) Hair cell polarity map obtained from the macula shown in Q. (S) Polarity map obtained from a further SU5402-treated specimen. (T-W) In situ hybridisation to the anterior otic markers pax5 (T,U) and hmx2 (V,W). (X-Z′) In situ hybridisation to the posterior otic markers fst1 (X,Y) and otx1 (Z,Z′). (A′,B′) In situ hybridisation to neurod, which marks the statoacoustic ganglion. (C′,D′) In situ hybridisation to atoh1a, which marks the first hair cells. A-P,X,Y: Lateral views; anterior to left, dorsal to top. T-W,Z-D′: Dorsal views; anterior to left, medial to top. am, anterior macula; ao, anterior otolith; c, cristae; pm, posterior macula; po, posterior otolith. Asterisks in A indicate semicircular canal pillars; arrow in C indicates reduced lateral semicircular canal pillar. Dots in Z-D′ delineate the otic vesicle. Scale bars: 50 μm.
Ears of embryos treated for 5 hours [10-20S, n=8 (wt), n=42 (phenotypically wt smo siblings)] were similar except that the single medial macula underlying the fused otoliths was circular rather than bow tie-shaped, the lateral canal pillar was absent or reduced (arrow, Fig. 3C), the lateral crista was absent and ears were slightly reduced in size (Fig. 3C,G,K,O). This suggested a more extreme loss of anterior character than in embryos treated with SU5402 for 3 hours, with a mirror image duplication of posterior structures around a more posterior axis of duplication. The single circular macula originated as two separate domains of hair cells at the anterior and posterior poles of the vesicle, as in wild-type embryos (see Fig. S1 in the supplementary material; n=4/4). Even as early as 30 hpf, however, both groups of hair cells were positioned medially, resembling those that form the posterior macula in untreated embryos.
Ears of wild-type embryos treated for 7 hours (10-24S, n=16) were similar to those treated for 5 hours, but very reduced in size and less well formed (Fig. 3D,H,L,P). These embryos were generally very unhealthy, with large amounts of necrosis and oedema. Further analysis was therefore performed on embryos treated for 5 hours.
To confirm the loss of anterior otic character and mirror image duplication of posterior otic domains in SU5402-treated embryos, we mapped hair cell polarity patterns in the medial maculae as above (Fig. 3Q-S; see Fig. S2 in the supplementary material). Hair cells pointed away from a central midline throughout the medial maculae of SU5402-treated embryos, indicating a duplication of the posterior part of the posterior macula. We also performed in situ hybridisation with a panel of otic AP-restricted markers, as above. In SU5402-treated embryos, expression of the anterior markers hmx2 and pax5 was absent (Fig. 3U,W), whereas expression of the posterior marker fst1 was duplicated at the anterior of the ear (Fig. 3Y). Expression of otx1 became symmetrical: the posteroventral part of its normal expression domain extended into anteroventral regions of the otic vesicle, whereas the anterolateral part of its normal expression domain was lost (Fig. 3Z′). Expression of neurod was also reduced (Fig. 3A′,B′). Dorsoventral and mediolateral axes of SU5402-treated otic vesicles did not appear to be affected: pax2a, a medial marker, and eya1, a ventral marker, were expressed normally (see Fig. S3 in the supplementary material). Similarly, expression of atoh1a, a hair cell marker, was unaltered (Fig. 3C′,D′).
Taken together, these data show that Fgf signalling is absolutely required for specification of anterior otic domains during a short critical time window (between 10 and 20S) that is separable from its earlier role in otic induction. In the absence of Fgf signalling, anterior otic domains develop as posterior.
Overexpression of fgf3 during a short critical time window causes a loss of posterior otic domains and a duplication of anterior otic structures in their place
To determine whether Fgf signalling is sufficient to specify anterior otic identity, we overexpressed fgf3 throughout the embryo using a heat-shock inducible fgf3 (hsp70:fgf3) transgenic line (Lecaudey et al., 2008). In this line, fgf3 expression was severely upregulated 30-40 minutes after initiation of heat-shock; 4 hours later, fgf3 expression had declined to almost normal levels (data not shown) (Lecaudey et al., 2008).
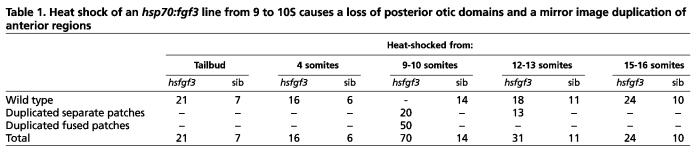
We heat-shocked hsp70:fgf3 transgenic and sibling (non-transgenic) embryos for 2 hours at tailbud stage, 4S, 9-10S, 12-13S and 15-16S. Only heat-shocking transgenic embryos at 9-10S gave a consistent otic phenotype, although this was shared with some embryos in the 12-13S batch, presumably the youngest (Table 1). Ears of other groups were largely normal. This confirmed the existence of a critical, short time window when Fgf signalling can pattern the AP axis of the ear.
Table 1.
Heat shock of an hsp70:fgf3 line from 9 to 10S causes a loss of posterior otic domains and a mirror image duplication of anterior regions
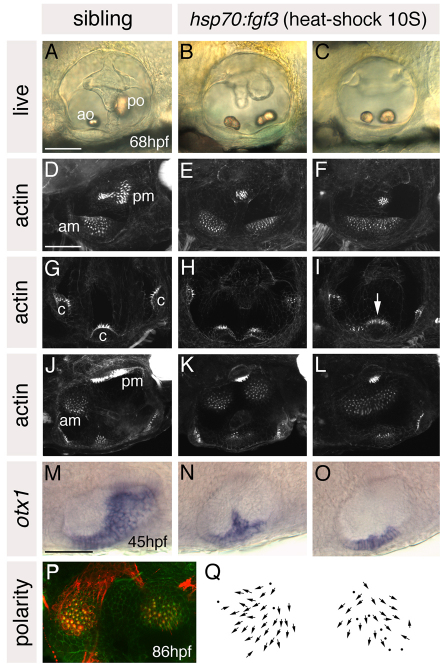
Embryos heat-shocked from 10S lost all posterior otic character, concomitant with a remarkably complete mirror image duplication of anterior otic structures at the posterior of the ear. Ears of heat-shocked embryos had two small lateral otoliths resembling the wild-type anterior otolith (Fig. 4B,C), each sitting over a ventral macula resembling the anterior macula (Fig. 4B,C,E,F,K,L). Hair cell polarities in both ventral maculae radiated in a fan-like pattern, resembling wild-type anterior maculae (Fig. 4P,Q; see Fig. S4 in the supplementary material). In some specimens, two separate anterior-like maculae were present on the ventral floor of the otic vesicle (Fig. 4E,K); in others, these were fused to form a single sensory patch (Fig. 4F,L; Table 1). There was also a remnant of the posterior macula on the medial wall of the otic vesicle (Fig. 4E,F,K,L). In one of six phalloidin-stained specimens, there were four cristae, presumed to represent duplicated anterior and lateral cristae, rather than the normal three (Fig. 4H). Where only three cristae were present (5/6 specimens), the lateral crista was positioned centrally and was enlarged, as though the axis of duplication transected this crista (Fig. 4I, arrow).
Fig. 4.
Heat shock of hsp70:fgf3 embryos at 10S leads to a complete loss of posterior otic domains and a mirror image duplication of anterior otic structures. (A-C) Ears of live 68 hpf hsp70:fgf3 and control wild-type zebrafish embryos heat-shocked at 10S. (D-L) Confocal z-stacks of 4 dpf ears stained with FITC-phalloidin to mark sensory hair cells. (D-F) Lateral focal planes showing the maculae. (G-I) Medial focal planes showing the cristae. (J-L) Dorsal views. (M-O) In situ hybridisation to otx1. (P) The macula of an 86 hpf heat-shocked hsp70:fgf3 embryo stained with anti-acetylated tubulin antibody (kinocilia; red) and FITC-phalloidin (stereocilia; green). (Q) Hair cell polarity maps obtained from the maculae shown in P. A-I: Lateral views; anterior to left, dorsal to top. J-Q: Dorsal views; anterior to left, medial to top. am, anterior macula; ao, anterior otolith; c, cristae; pm, posterior macula; po, posterior otolith. Arrow in I marks the lateral crista, which is enlarged. Scale bars: 50 μm.
In situ hybridisation with a panel of otic AP markers confirmed the loss of posterior otic identity and duplication of anterior otic regions in heat-shocked embryos. The major, posteroventral, part of the otic otx1 expression domain was lost in heat-shocked embryos, whereas the anterolateral part of the otx1 domain was duplicated at the posterior of the vesicle (Fig. 4M-O). Domains of the anterior markers hmx2 and pax5 were duplicated at the posterior of the vesicle and sometimes expanded to encompass the entire medial wall, whereas expression of the posterior marker fst1 was lost from the posterior of the ear (Fig. 5A-F). Expression of neurod was also duplicated at the posterior of the ear (Fig. 5G-J); neurod-positive cells appeared to delaminate from posteroventral as well as anteroventral regions of the otocyst. pax2a and eya1 were expressed in medial and ventral parts of the otic vesicle, respectively, as normal (see Fig. S3 in the supplementary material).
Fig. 5.
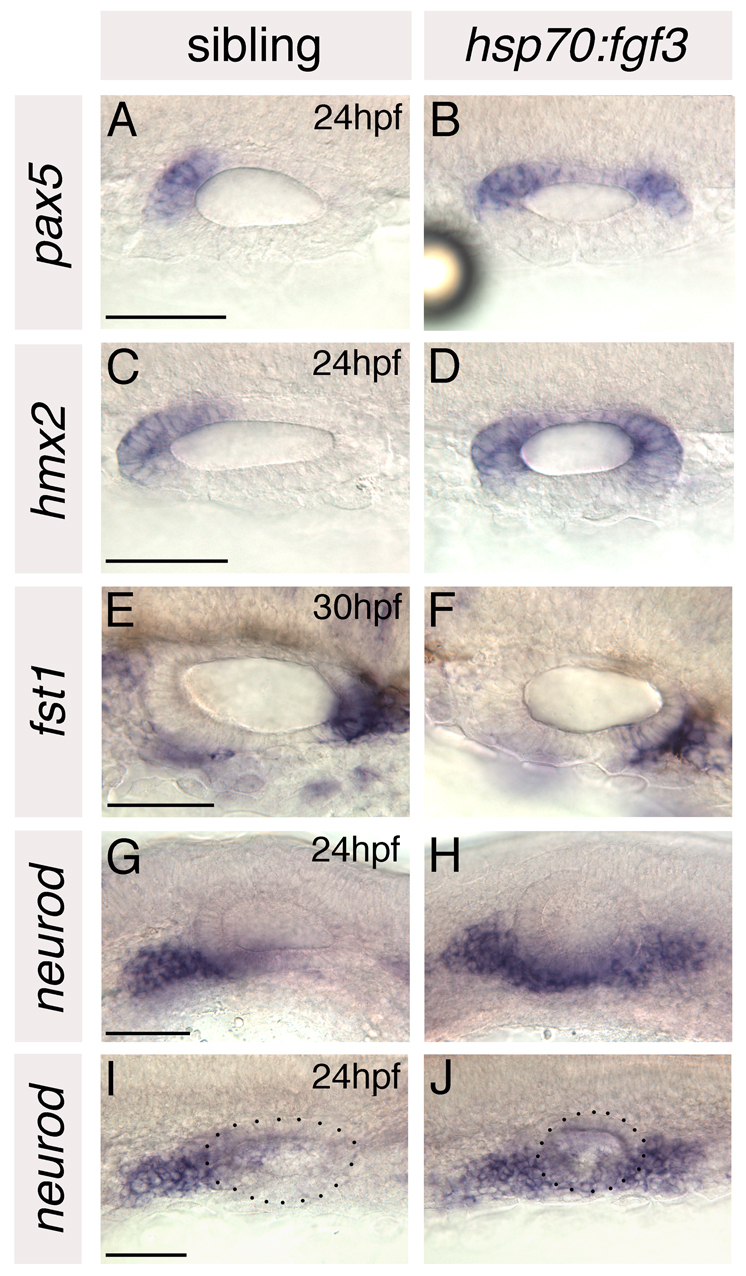
Anterior otic markers are duplicated and posterior otic markers lost in hsp70:fgf3 zebrafish embryos heat-shocked at 10S. (A-F) In situ hybridisation to anterior and posterior otic markers. (G-J) In situ hybridisation to neurod, which marks delaminating neuroblasts and nascent neurons of the statoacoustic ganglion. A-F,I,J: Dorsal views; anterior to left, medial to top. G,H: Lateral views; anterior to left, dorsal to top. Dots delineate the outline of the otic vesicle in I and J. Scale bars: 50 μm.
These data clearly demonstrate that Fgf signalling is sufficient to specify anterior otic identity: excess fgf3 prevents expression of the endogenous posterior otic specification programme and causes anterior otic structures to form at the posterior of the ear.
Altered Fgf signalling does not affect Hh signal transduction in the ear; altered Hh signalling does not affect Fgf signal transduction in the ear
The mirror image duplicated anterior and posterior otic phenotypes obtained when we alter Fgf signalling throughout the embryo are similar to those that occur when Hh signalling is altered (Hammond et al., 2003; Hammond et al., 2010): Fgf specifies anterior otic identity, whereas Hh specifies posterior otic identity. We wanted to know whether both factors are instructive, or whether one acts purely to repress activity of the other.
To test whether altering levels of Fgf signalling affects Hh transduction within the otic vesicle, we used expression of ptc1, a direct target of the Hh pathway, as a read-out of Hh signalling activity (Fig. 6). Its levels are dramatically altered in the ear in smob577–/– mutants, in which Hh signalling is severely reduced (Aanstad et al., 2009; Varga et al., 2001), and in ptc1–/–;ptc2–/– mutants, in which Hh signalling is maximal (Hammond et al., 2003; Hammond et al., 2010; Koudijs et al., 2008) (Fig. 6Q,R). We compared expression of ptc1 in embryos treated with SU5402 from 10 to 20S to remove Fgf signalling (n=6), and in hsp70:fgf3 transgenic embryos heat-shocked at 10S to increase Fgf signalling (n=7), with expression in control siblings (n=13). We found no obvious difference in ptc1 expression for either treatment at 18S or 21S, although we cannot rule out subtle changes (Fig. 6A-H). These time points were chosen to allow time for transcriptional activation or degradation of ptc1 in response to changes in Fgf signalling initiated at 10S, while still being within the critical time window when Fgf is required for AP patterning.
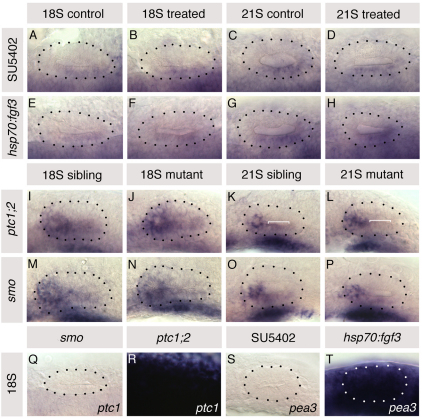
Fig. 6.
Altering Fgf and Hh signalling in the embryo does not affect otic expression of ptc1 and pea3, respectively. (A-H) ptc1 expression in hsp70:fgf3 compared with wild-type sibling zebrafish embryos heat-shocked at 10S, and in 10 μM SU5402-treated embryos compared with control DMSO-treated embryos treated from 10 to 20S. (I-P) pea3 expression in otic vesicles of ptc1–/–;ptc2–/– and smo–/– embryos compared with siblings. (Q,R) ptc1 expression in smo–/– and ptc1–/–;ptc2–/–embryos. (S,T) pea3 expression in 10 μM SU5402-treated and in hsp70:fgf3 embryos heat-shocked at 10S. A-H,Q,R: Dorsal views; anterior to left, lateral to top. I-P,S,T: Lateral views; anterior to left, dorsal to top. Dotted lines delineate otic vesicle outlines. Bracket in K marks low-level pea3 expression, lost from 21S ptc1–/–;ptc2–/– embryos (bracket, L).
To test whether altering levels of Hh signalling affects Fgf signal transduction in the ear, we used pea3 expression as a readout of Fgf signalling activity in smob577–/– embryos (n=8) and ptc1–/–;ptc2–/– double mutant embryos (n=6). Phenotypically wild-type sibling embryos (n=15) were used as controls. Expression of pea3, normally concentrated in anterior otic domains (Fig. 6I-P), was completely absent from SU5402-treated embryos and severely raised in heat-shocked hsp70:fgf3 embryos by 18S (Fig. 6S,T). We detected no obvious changes in pea3 expression in ptc1–/–;ptc2–/– embryos at 18S or in smo–/– embryos at either 18S or 21S (Fig. 6I,J,M-P). In ptc1–/–;ptc2–/– embryos at 21S, however, pea3, although still strongly expressed at the anterior of the otic vesicle, was reduced slightly along its ventral wall (bracket, Fig. 6K,L). This is likely to reflect alterations in the underlying branchial arches, which normally express fgf3, and which were reduced in ptc1–/–;ptc2–/– double homozygotes (Fig. 6K,L; see Fig. S5 in the supplementary material). Hindbrain AP patterning in ptc1–/–;ptc2–/– mutants appeared normal, as revealed by egr2b, mafb1, hoxb1 and hoxb3, as well as by r4 expression of fgf3 itself (see Fig. S5 in the supplementary material). Taken together, these data suggest that from 10 to 20 somites, the Fgf and Hh pathways act independently to pattern the otic anteroposterior axis.
Loss of both Fgf and Hh signalling from 10 to 20 somites results in an ear with neither anterior nor posterior otic identity
To confirm that both Hh and Fgf signalling are instructive during otic AP patterning, we treated smob577–/– embryos, in which Hh signalling is severely reduced, with 10 μM SU5402 from 10 to 20S to remove Fgf signalling (n=27). If both Hh and Fgf have instructive activity, we expected the resulting otic vesicles to have neither anterior nor posterior identity. If Hh acts merely to repress the effects of Fgf signalling, otic vesicles would resemble those seen when Fgf is absent: a mirror image double-posterior ear. If Fgf acts merely to repress the effects of Hh signalling, then the effects of a loss of Hh would be epistatic to the effects of a loss of Fgf, and the resulting otic vesicles would have two anterior poles.
As controls, we used smo+/– heterozygous and wild-type sibling embryos treated with SU5402 (n=42) and smo–/– and sibling embryos treated with 0.5% DMSO [n=32 (smo–/–), n=42 (siblings)], from 10 to 20S. Ears of all control embryos developed as expected. Sibling embryos treated with DMSO had phenotypically wild-type ears (Fig. 7). Ears of DMSO-treated smo–/– embryos displayed a loss of posterior otic domains and an incomplete duplication of anterior otic domains (data not shown), identical to the smo–/– otic phenotype (Hammond et al., 2003). SU5402-treated smo siblings developed mirror image posterior duplicated ears as described above (data not shown).
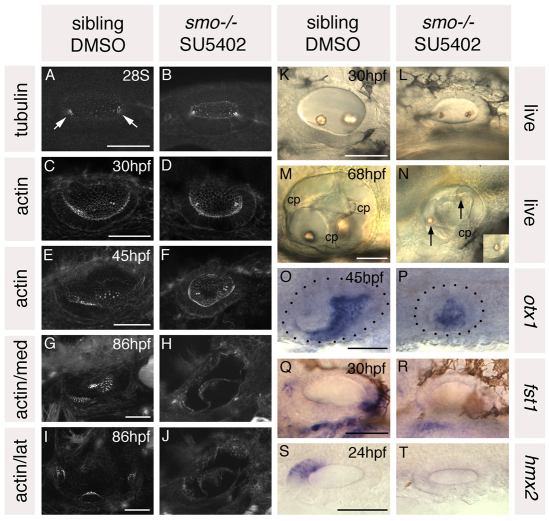
Fig. 7.
Treatment of smo–/– embryos with SU5402 from 10 to 20S leads to a loss of anterior and posterior otic character. Ears of smo–/– and sibling zebrafish embryos treated with 10 μM SU5402 and DMSO, respectively, from 10 to 20S. (A,B) Anti-acetylated tubulin antibody stain marking the kinocilia of the first-forming hair cells (arrows). (C-J) FITC-phalloidin stain marking cell outlines and stereociliary bundles of the sensory hair cells. (C-F) Views of the whole vesicle. (G,H) Medial views showing the maculae. (I,J) Lateral views showing the cristae. (K-N) Inner ears of live embryos. Arrows indicate otoliths. cp, semicircular canal projection tissue. (O-T) In situ hybridisation to anterior and posterior otic markers. Dots delineate the otic vesicle in O and P. O,P,S,T: Dorsal views; anterior to left, medial to top. A-N,Q,R: Lateral views; anterior to left, dorsal to top. Scale bars: 50 μm.
Otic vesicles of SU5402-treated smo–/– embryos initially developed relatively normally: tubulin-rich tether cells differentiated at the anterior and posterior poles of the vesicle (n=3/3 tubulin-stained specimens), and small otoliths seeded over these, as in control embryos (Fig. 7A,B; data not shown). By 30 hpf, however, otoliths remained small, granular and positioned symmetrically at the poles of the vesicle, whereas otoliths of control embryos had grown in size and were no longer located symmetrically at the anterior and posterior poles (Fig. 7K,L). In control DMSO-treated sibling embryos, anterior otoliths were now positioned ventrolaterally whereas the posterior otolith was more medial; control smo–/– DMSO-treated embryos looked like untreated smo–/– embryos with two lateral, ventral otoliths, and SU5402-treated siblings had two medial otoliths, as in SU5402-treated wild-type embryos (Fig. 7L; data not shown). At this stage there were two or three hair cells underlying each otolith in both the SU5402-treated smo–/– embryos and controls; their positions reflected the position of the overlying otoliths (n=3/3 phalloidin-stained for each category) (Fig. 7C,D; data not shown).
By 45 hpf, further macular hair cells developed in all control embryos; however, in SU5402-treated smo–/– embryos, no further hair cells were evident, even by 86 hpf (n=9/9 phalloidin-stained at 45-86 hpf) (Fig. 7E-J). Increased cell death in the otic vesicle might contribute to the lack of differentiated hair cells (see Fig. S6 in the supplementary material). Otoliths of SU5402-treated smo–/– embryos remained small, or were absent altogether (Fig. 7M,N). Development of SU5402-treated smo–/– otic vesicles was, however, not entirely arrested at an early vesicle stage: semicircular canal projection tissue formed, although it was grossly abnormal (cp, Fig. 7M,N).
Taken together, these data suggest that SU5402-treated smo–/– otic vesicles lose anterior and posterior identity but retain an initial symmetrical pattern about the AP axis, with defined poles and a centre. We confirmed this using a panel of AP-restricted otic markers as above: hmx2, an anterior marker, and fst1, a posterior marker, were both lost from SU5402-treated smo–/– otic vesicles, and expression of otx1 was reduced to a central domain, having apparently lost both anterior and posterior domains of its wild-type expression pattern (Fig. 7O-T). Expression domains of pax2a (medial) and eya1 (ventral) in SU5402-treated smo–/– otic vesicles were normal (see Fig. S3 in the supplementary material). Levels of both markers were, however, reduced. This might reflect a synergistic role for Hh and Fgf in controlling expression of these genes, but is more likely to be due to the general poor development and increased cell death in these embryos. The effects of Hh and Fgf signalling on the ear are, therefore, additive: neither is epistatic to the other, suggesting that both Fgf and Hh signalling have an instructive role in otic AP patterning, acting to impose anterior and posterior identity, respectively, on an initially symmetrical otic pre-pattern.
DISCUSSION
Fgf signalling is necessary and sufficient to specify anterior otic identity in the zebrafish
Specification of the otic anteroposterior (AP) axis is one of the earliest patterning events to take place during inner ear development. Rotation and transplantation experiments in chick and amphibian embryos have shown that the otic AP axis is fixed before the dorsoventral (DV) axis, in response to induction from surrounding tissues (Harrison, 1936; Harrison, 1945; Wu et al., 1998; Bok et al., 2005). In zebrafish, Hh signalling is necessary and sufficient to specify posterior otic identity during a time window from ∼10 to 20S, after otic placode induction is complete (Hammond et al., 2003; Hammond et al., 2010). Using conditional inhibition of Fgf signalling with SU5402, and conditional overexpression of Fgf3 using a heat-shock inducible line, we have shown that Fgf signalling is necessary and sufficient to specify anterior otic identity in zebrafish during a similar period (Fig. 8). This anteriorising function is distinct and temporally separable from the earlier role for Fgfs in otic induction.
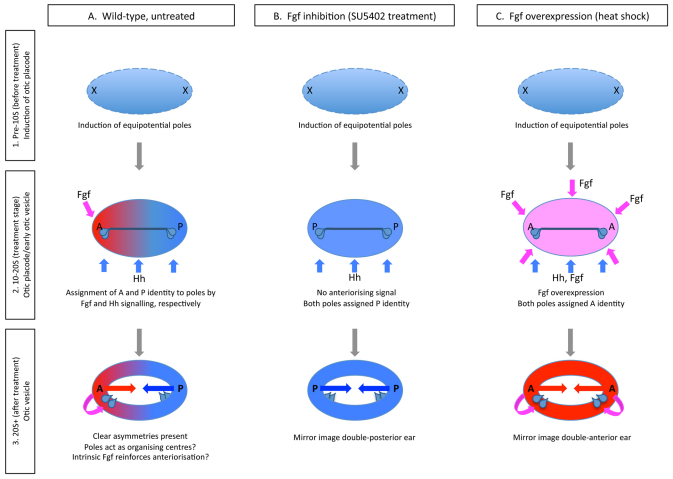
Fig. 8.
A three-step model for AP axial patterning in the zebrafish ear. (A) Step 1 (prior to 10S): Induction of a symmetrical otic placode and establishment of equipotential poles. Fgf is required as a placode-inducing factor. Step 2 (10-20S): Direct response of the poles to instructive anteriorising (Fgf, pink) and posteriorising (Hh, blue) factors, with expression of target genes in otic tissue. Step 3 (20S+): The poles of the ear act as intrinsic organising centres, with different anterior (A) and posterior (P) identities. Signalling within the otic vesicle (curved arrow) reinforces initial asymmetry. (B) In the absence of Fgf signalling from 10 to 20S, both poles acquire posterior identity, as both are under the influence of Hh signalling from midline tissues. (C) In transgenic hsp70:fgf3 embryos heat-shocked from 10 to 20S, Fgf signalling is activated in all cells, and both poles acquire anterior identity. The red (anterior) and dark blue (posterior) arrows indicate tissue polarity in each half of the otic vesicle.
The requirement for Fgf in zebrafish anterior otic patterning was revealed by the partial loss of anterior character in lia–/– (fgf3–/–) mutants and a complete loss of anterior character in embryos treated with SU5402 from 10 to 20S. This differs from the situation in the mouse, in which a loss of Fgf3 function results in dorsal (endolymphatic duct) defects and partially penetrant posterior otic defects for some Fgf3–/– alleles (Hatch et al., 2007; Mansour et al., 1993). These phenotypic differences might reflect differences in the spatiotemporal expression of hindbrain Fgf relative to the otic vesicle between zebrafish and mouse. In the zebrafish, fgf3 is expressed in r4, anterior to the developing otic placode and vesicle (Maves et al., 2002; Walshe et al., 2002). By contrast, in the mouse, Fgf3 is expressed in r4-6 at otic placode stages, becoming restricted to r5/6 at otic cup stages and eventually to r6 (i.e. posterior to the ear) at otic vesicle stages (Wilkinson et al., 1988; Mahmood et al., 1996; McKay et al., 1996; Alvarez et al., 2003). In keeping with this, work in both mouse and chick has suggested that hindbrain-derived signals do not specify the AP axis of otic vestibular structures in these species (Bok et al., 2005), and that mesodermal and surface ectodermal signals are more important (Liang et al., 2010; Bok et al., 2011).
Sufficiency of Fgf for anterior otic patterning was revealed by the mirror image duplicated anterior otic phenotypes that resulted from heat shock of hsp70:fgf3 embryos at 10S. This phenotype is stronger than the otic phenotypes of two zebrafish mutants that show posteriorly expanded fgf3 expression in the hindbrain: val–/– (mafba–/–) and hnf1ba–/– (Kwak et al., 2002; Hernandez et al., 2004; Lecaudey et al., 2007). In val–/– embryos, anterior otic domains are expanded, but not duplicated (Kwak et al., 2002; Whitfield and Hammond, 2007). This might be because hindbrain fgf3 expression in val–/– does not extend far enough to influence the posterior pole of the otic vesicle, which retains fst1 expression (Lecaudey et al., 2007) (K.L.H., unpublished). In hnf1ba–/– mutants, expansion of fgf3 expression in the hindbrain is more extensive than in val–/– (Hernandez et al., 2004), and duplication of anterior otic markers is seen in 25-50% of hnf1ba–/– mutants, with expansion in others (Lecaudey et al., 2007). In our heat-shocked embryos, fgf3 expression was activated in all cells, resulting in anterior duplications that were complete and fully penetrant. Additional hindbrain patterning defects in val–/– and hnf1ba–/– embryos, including the reduction of wnt expression domains, and prolonged disruption of Fgf expression, might account for the presence of additional otic defects in these lines (Wiellette and Sive, 2003; Hernandez et al., 2004; Riley et al., 2004; Hans and Westerfield, 2007; Lecaudey et al., 2007).
The most likely source of Fgf for anterior otic patterning in the zebrafish is r4 of the hindbrain, although there might also be a contribution from the developing branchial arches. The anterior position of r4 relative to the otic placode from 10S, and the parallel expansion of hindbrain fgf3 expression and anterior otic identity in val–/– and hnf1ba–/– mutants, support the notion that r4-derived Fgf acts as an anteriorising signal for patterning the zebrafish ear. Note also that the positioning of the otic rudiment with respect to the hindbrain rhombomeres alters over time in the zebrafish embryo, consistent with the proposed sequential roles of r4-derived Fgf signalling in otic induction and AP patterning. At the tailbud/1S stage, when otic induction is occurring, the otic rudiment (marked by pax8 expression) develops in close association with r4 (Phillips et al., 2001), which at this stage expresses both fgf3 and fgf8 (Maves et al., 2002; Walshe et al., 2002). By 10S, when Fgf-mediated otic AP patterning begins, the otic placode is positioned next to r5, with its anterior end opposite r4 (which retains fgf3 expression) and its posterior end opposite r6 (Maves et al., 2002).
The action of Fgfs on the otic placode is likely to be direct: several Fgf target genes are expressed at the anterior of the zebrafish ear from early placode stages (Raible and Brand, 2001; Roehl and Nüsslein-Volhard, 2001; Thisse et al., 2001; Nechiporuk et al., 2005). It is unlikely that a secondary signal, resulting from AP hindbrain patterning defects, accounts for the otic anterior and posterior duplications that we see when Fgf signalling is lost or increased. There were no hindbrain AP patterning defects in 10-20S SU5402-treated embryos, as assayed by mafba, hoxb1 and egr2b expression (see Fig. S7 in the supplementary material), although there was a slight expansion of r5 markers into r6 in 10S heat-shocked hsp70:fgf3 embryos (see Fig. S7 in the supplementary material).
At least three Fgf genes, fgf3, fgf8 and fgf10a, are also expressed at the anterior of the developing zebrafish inner ear by otic vesicle stages (fgf8 and fgf10a by 18S; fgf3 by 21S) (Kudoh et al., 2001; Léger and Brand, 2002; Walshe and Mason, 2003; Nechiporuk and Raible, 2008). Our data, however, show that Fgf is required for otic AP patterning prior to this, suggesting that otic Fgf expression might reinforce, rather than specify, anterior otic identity. Consistent with this idea, when Fgf signalling is inhibited from 18S in zebrafish, anterior otic pax5 expression is lost (Léger and Brand, 2002). Similarly, otic Fgf8 expression has been proposed to regulate Sox3 expression and neurogenesis in an anterior otic territory in the chicken embryo (Abelló et al., 2010).
Fgf and Hh act in an opposite, but independent, manner to pattern the otic AP axis
The enantiomorphic twinned ear phenotypes that we have obtained through Fgf manipulation are very similar, but opposite with respect to the AP axis, to those seen when Hh signalling is altered in zebrafish and Xenopus. Increased Fgf or a loss of Hh leads to double-anterior ears, whereas a loss of Fgf or increased Hh leads to double-posterior ears (Hammond et al., 2003; Waldman et al., 2007; Hammond et al., 2010; Sapède and Pujades, 2010).
The Hh- and Fgf-induced otic mirror image duplication phenotypes are not, however, identical. The otic duplications seen when Fgf is increased or absent were remarkably complete, whereas the duplications are only partial in Hh pathway loss-of-function mutants (Hammond et al., 2003; Sapède and Pujades, 2010). This might be explained as follows. In the absence of Fgf signalling, Hh can effectively re-specify anterior otic domains as posterior because it emanates from the ventral midline, which lies adjacent to anterior as well as posterior parts of the otic placode/vesicle. In the absence of Hh signalling, Fgf does not fully re-specify posterior otic domains as anterior because the Fgf source is located at the anterior of the ear. Posterior otic domains are thus substantially removed from its influence. Overexpression of either Fgf or Hh throughout the embryo is sufficient to re-specify the otic poles accordingly. Note, however, that neither factor affects transduction of the other in the ear, as assayed by expression of direct pathway target genes (ptc1, pea3). Integration of the effects of the two signalling pathways must occur at the level of shared targets further downstream, which are likely to include the earliest markers of asymmetry, such as hmx2 and hmx3.
Additional otic DV and mediolateral (ML) patterning defects are seen in the posterior duplicated ears of ptc1–/–;ptc2–/– embryos, which have severely increased levels of Hh signalling (Koudijs et al., 2008; Hammond et al., 2010). This might reflect an additional patterning role for Hh not shared with Fgf, or might be because Hh signalling remains upregulated throughout development in ptc1–/–;ptc2–/– embryos, whereas we only inhibited Fgf for a short time. Interestingly, even when Fgf was upregulated throughout the embryo, expression domains of hmx2 and pax5 remained medial. This suggests that correct otic mediolateral patterning depends on mechanisms that are independent and separable from the anteriorising function of Fgf.
Hh and Fgf act on an initially symmetrical otic placode with equipotential poles
The ability to generate mirror symmetrical double-anterior and -posterior ears by altering Fgf or Hh signalling demonstrates that the anterior and posterior halves of the otic rudiment are initially equipotential. This was first shown in the 1930s when Harrison produced double-anterior and -posterior ears by rotating the salamander otic rudiment about its AP axis (Harrison, 1936; Harrison, 1945). There have since been other reports of physical and genetic manipulations resulting in duplicated ears (Hammond et al., 2003; Waldman et al., 2007; Bok et al., 2011). This suggests that prior to the action of axial patterning signals, the otic placode is symmetrical about its AP axis (Fig. 8). Indeed, when we inhibited both Fgf and Hh signalling pathways from 10 to 20S, an AP symmetrical otic pre-pattern, with neither anterior nor posterior identity, was revealed.
Several genes, including those of the delta and atoh families, are expressed symmetrically at the anterior and posterior poles of the otic placode by 10S, prior to the AP patterning activity of Fgf and Hh (Haddon et al., 1998; Millimaki et al., 2007). Their expression prefigures the symmetrical appearance of tether cells, which differentiate into the first functioning hair cells at either end of the ear; later hair cells are added asymmetrically (Haddon and Lewis, 1996; Riley et al., 1997; Millimaki et al., 2007; Sapède and Pujades, 2010; Tanimoto et al., 2011). Tether cells are not only the first hair cells to develop in the ear, but also have different genetic requirements: they are dependent on the function of atoh1b, whereas later hair cells require the function of atoh1a (Millimaki et al., 2007). In our experiments, tether cells still differentiated when Fgf and Hh were inhibited from 10 to 20S, implying that tether cells need neither anterior nor posterior identity to differentiate into hair cells. However, further hair cell addition did not occur and development of the sensory patches did not progress beyond a symmetrical stage.
The absence of otic AP asymmetries when both Fgf and Hh signalling were inhibited from 10 to 20S suggests that, in zebrafish, Fgf and Hh are the major early otic AP patterning signals. However, manipulations of retinoic acid (RA) signalling in the chick have recently also been shown to result in AP mirror image duplications of the whole inner ear (Bok et al., 2011). High RA levels appear to specify posterior otic development (in particular Tbx1 expression), and low levels are required for anterior gene expression (including markers of neurogenesis) (Bok et al., 2011). A similar positive regulation of otic tbx1 expression by RA (and negative regulation of neurogenesis) has recently been reported in the zebrafish, although mirror image duplications were not observed (Radosevic et al., 2011). Manipulation of RA signalling is also known to affect earlier stages of zebrafish otic development, but only indirectly; notably, early RA inhibition delays the onset of hindbrain fgf3 expression, and so interferes with otic induction (Hans and Westerfield, 2007). Any interaction of RA with the Fgf and Hh signalling pathways between the 10 and 20S stages in the zebrafish remains to be determined.
Although Hh and Fgf appear to act instructively to specify zebrafish anterior and posterior otic identity, extrinsic sources of Fgf and Hh are unlikely to provide the polarity information for subsequent patterning of the ear. This is because when Hh or Fgf signalling is activated throughout the embryo (with no focal source or gradation of signal), the result is a mirror image duplication of the ear, with two correctly patterned mirror image halves, rather than a single enlarged anterior or posterior domain. It is thus likely that organising centres exist within the ear to impart polarity to the sensory patches. Ablation experiments indicate that these centres must reside at or very near the poles of the otic placode (Waldman et al., 2007). It is thus tempting to speculate that the tether cells themselves might play an active role in signalling to organise polarity in the ear (Fig. 8).
In conclusion, we have shown that in zebrafish, between 10 and 20S, Fgf and Hh act on a symmetrical pre-pattern to specify otic anterior and posterior identity, respectively. Both factors appear to have instructive activity to assign identity to the otic poles, but act permissively to allow polarised patterning of each half of the ear, which is likely to rely on additional intrinsic patterning mechanisms.
Supplementary Material
Acknowledgments
This work was funded by the BBSRC (BB/E015875/1) and the Wellcome Trust (092401). We are grateful to G. Cakan-Akdogan and D. Gilmour for providing the hsp70:fgf3 line, and to H. Roehl for making this available to us in Sheffield. We thank N. Monk for helpful discussion, members of the zebrafish community for providing probes, and the aquarium staff for expert care of the zebrafish. The MRC CDBG zebrafish aquaria and imaging facilities were supported by the MRC (G0700091), with additional support from the EU FP6 (ZF-MODELS) and the Wellcome Trust (GR077544AIA). Deposited in PMC for release after 6 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.066639/-/DC1
References
- Aanstad P., Santos N., Corbit K. C., Scherz P. J., Trinh L. A., Salvenmoser W., Huisken J., Reiter J. F., Stainier D. Y. (2009). The extracellular domain of Smoothened regulates ciliary localization and is required for high-level Hh signaling. Curr. Biol. 19, 1034-1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abelló G., Khatri S., Radosevic M., Scotting P. J., Giráldez F., Alsina B. (2010). Independent regulation of Sox3 and Lmx1b by FGF and BMP signaling influences the neurogenic and non-neurogenic domains in the chick otic placode. Dev. Biol. 339, 166-178 [DOI] [PubMed] [Google Scholar]
- Adamska M., Léger S., Brand M., Hadrys T., Braun T., Bober E. (2000). Inner ear and lateral line expression of a zebrafish Nkx5-1 gene and its downregulation in the ears of FGF8 mutant, ace. Mech. Dev. 97, 161-165 [DOI] [PubMed] [Google Scholar]
- Alvarez Y., Alonso M. T., Vendrell V., Zelarayan L. C., Chamero P., Theil T., Bösl M. R., Kato S., Maconochie M., Riethmacher D., et al. (2003). Requirements for FGF3 and FGF10 during inner ear formation. Development 130, 6329-6338 [DOI] [PubMed] [Google Scholar]
- Blader P., Fischer N., Gradwohl G., Guillemot F., Strähle U. (1997). The activity of Neurogenin1 is controlled by local cues in the zebrafish embryo. Development 124, 4557-4569 [DOI] [PubMed] [Google Scholar]
- Bok J., Bronner-Fraser M., Wu D. K. (2005). Role of the hindbrain in dorsoventral but not anteroposterior axial specification of the inner ear. Development 132, 2115-2124 [DOI] [PubMed] [Google Scholar]
- Bok J., Raft S., Kong K. A., Koo S. K., Dräger U. C., Wu D. K. (2011). Transient retinoic acid signaling confers anterior-posterior polarity to the inner ear. Proc. Natl. Acad. Sci. USA 108, 161-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterberg R., Fritz A. (2009). dlx3b/4b are required for the formation of the preplacodal region and otic placode through local modulation of BMP activity. Dev. Biol. 325, 189-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Xu Q. (2010). Pivotal role of hmx2 and hmx3 in zebrafish inner ear and lateral line development. Dev. Biol. 339, 507-518 [DOI] [PubMed] [Google Scholar]
- Haddon C., Lewis J. (1996). Early ear development in the embryo of the zebrafish, Danio rerio. J. Comp. Neurol. 365, 113-123 [DOI] [PubMed] [Google Scholar]
- Haddon C., Jiang Y.-J., Smithers L., Lewis J. (1998). Delta-Notch signalling and the patterning of sensory cell differentiation in the zebrafish ear: evidence from the mind bomb mutant. Development 125, 4637-4644 [DOI] [PubMed] [Google Scholar]
- Hadrys T., Braun T., Rinkwitz-Brandt S., Arnold H.-H., Bober E. (1998). Nkx5-1 controls semicircular canal formation in the mouse inner ear. Development 125, 33-39 [DOI] [PubMed] [Google Scholar]
- Hammond K. L., Loynes H. E., Folarin A. A., Smith J., Whitfield T. T. (2003). Hedgehog signalling is required for correct anteroposterior patterning of the zebrafish otic vesicle. Development 130, 1403-1417 [DOI] [PubMed] [Google Scholar]
- Hammond K. L., van Eeden F. J., Whitfield T. T. (2010). Repression of Hedgehog signalling is required for the acquisition of dorsolateral cell fates in the zebrafish otic vesicle. Development 137, 1361-1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans S., Westerfield M. (2007). Changes in retinoic acid signaling alter otic patterning. Development 134, 2449-2458 [DOI] [PubMed] [Google Scholar]
- Harrison R. G. (1936). Relations of symmetry in the developing ear of Amblystoma punctatum. Proc. Natl. Acad. Sci. USA 22, 238-247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R. G. (1945). Relations of symmetry in the developing embryo. Trans. Conn. Acad. Arts Sci. USA 36, 277-330 [Google Scholar]
- Hatch E. P., Noyes C. A., Wang X., Wright T. J., Mansour S. L. (2007). Fgf3 is required for dorsal patterning and morphogenesis of the inner ear epithelium. Development 134, 3615-3625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez R. E., Rikhof H. A., Bachmann R., Moens C. B. (2004). vhnf1 integrates global RA patterning and local FGF signals to direct posterior hindbrain development in zebrafish. Development 131, 4511-4520 [DOI] [PubMed] [Google Scholar]
- Herzog W., Sonntag C., von der Hardt S., Roehl H. H., Varga Z. M., Hammerschmidt M. (2004). Fgf3 signaling from the ventral diencephalon is required for early specification and subsequent survival of the zebrafish adenohypophysis. Development 131, 3681-3692 [DOI] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253-310 [DOI] [PubMed] [Google Scholar]
- Koudijs M. J., den Broeder M. J., Groot E., van Eeden F. J. (2008). Genetic analysis of the two zebrafish patched homologues identifies novel roles for the hedgehog signaling pathway. BMC Dev. Biol. 8, 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudoh T., Tsang M., Hukriede N. A., Chen X., Dedekian M., Clarke C. J., Kiang A., Schultz S., Epstein J. A., Toyama R., et al. (2001). A gene expression screen in zebrafish embryogenesis. ZFIN Direct Data Submission (http://zfin.org/cgi-bin/webdriver?MIval=aa-pubview2.apg&OID=ZDB-PUB-050309-6) [DOI] [PubMed]
- Kwak S.-J., Phillips B. T., Heck R., Riley B. B. (2002). An expanded domain of fgf3 expression in the hindbrain of zebrafish valentino mutants results in mis-patterning of the otic vesicle. Development 129, 5279-5287 [DOI] [PubMed] [Google Scholar]
- Kwak S. J., Vemaraju S., Moorman S. J., Zeddies D., Popper A. N., Riley B. B. (2006). Zebrafish pax5 regulates development of the utricular macula and vestibular function. Dev. Dyn. 235, 3026-3038 [DOI] [PubMed] [Google Scholar]
- Ladher R. K., O'Neill P., Begbie J. (2010). From shared lineage to distinct functions: the development of the inner ear and epibranchial placodes. Development 137, 1777-1785 [DOI] [PubMed] [Google Scholar]
- Lecaudey V., Ulloa E., Anselme I., Stedman A., Schneider-Maunoury S., Pujades C. (2007). Role of the hindbrain in patterning the otic vesicle: A study of the zebrafish vhnf1 mutant. Dev. Biol. 303, 134-143 [DOI] [PubMed] [Google Scholar]
- Lecaudey V., Cakan-Akdogan G., Norton W. H., Gilmour D. (2008). Dynamic Fgf signaling couples morphogenesis and migration in the zebrafish lateral line primordium. Development 135, 2695-2705 [DOI] [PubMed] [Google Scholar]
- Léger S., Brand M. (2002). Fgf8 and Fgf3 are required for zebrafish ear placode induction, maintenance and inner ear patterning. Mech. Dev. 119, 91-108 [DOI] [PubMed] [Google Scholar]
- Liang J. K., Bok J., Wu D. K. (2010). Distinct contributions from the hindbrain and mesenchyme to inner ear morphogenesis. Dev. Biol. 337, 324-334 [DOI] [PubMed] [Google Scholar]
- Liu D., Chu H., Maves L., Yan Y.-L., Morcos P. A., Postlethwait P., Westerfield M. (2003). Fgf3 and Fgf8 dependent and independent transcription factors are required for otic placode specification. Development 130, 2213-2224 [DOI] [PubMed] [Google Scholar]
- Mahmood R., Mason I. J., Morriss-Kay G. M. (1996). Expression of Fgf-3 in relation to hindbrain segmentation, otic pit position and pharyngeal arch morphology in normal and retinoic acid-exposed mouse embryos. Anat. Embryol. 194, 13-22 [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Goddard J. M., Capecchi M. R. (1993). Mice homozygous for a targeted disruption of the proto-oncogene int-2 have developmental defects in the tail and inner ear. Development 117, 13-28 [DOI] [PubMed] [Google Scholar]
- Maroon H., Walshe J., Mahmood R., Kiefer P., Dickson C., Mason I. (2002). Fgf3 and Fgf8 are required together for formation of the otic placode and vesicle. Development 129, 2099-2108 [DOI] [PubMed] [Google Scholar]
- Maves L., Jackman W., Kimmel C. B. (2002). FGF3 and FGF8 mediate a rhombomere 4 signaling activity in the zebrafish hindbrain. Development 129, 3825-3837 [DOI] [PubMed] [Google Scholar]
- McKay I. J., Lewis J., Lumsden A. (1996). The role of FGF-3 in early inner ear development: An analysis in normal and kreisler mutant mice. Dev. Biol. 174, 370-378 [DOI] [PubMed] [Google Scholar]
- Millimaki B. B., Sweet E. M., Dhason M. S., Riley B. B. (2007). Zebrafish atoh1 genes: classic proneural activity in the inner ear and regulation by Fgf and Notch. Development 134, 295-305 [DOI] [PubMed] [Google Scholar]
- Moens C. B., Cordes S. P., Giorgianni M. W., Barsh G. S., Kimmel C. B. (1998). Equivalence in the genetic control of hindbrain segmentation in fish and mouse. Development 125, 381-391 [DOI] [PubMed] [Google Scholar]
- Mohammadi M., McMahon G., Sun L., Tang C., Hirth P., Yeh B. K., Hubbard S. R., Schlessinger J. (1997). Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science 276, 955-960 [DOI] [PubMed] [Google Scholar]
- Münchberg S. R., Ober E. A., Steinbeisser H. (1999). Expression of the Ets transcription factors erm and pea3 in early zebrafish development. Mech. Dev. 88, 233-236 [DOI] [PubMed] [Google Scholar]
- Nechiporuk A., Raible D. W. (2008). FGF-dependent mechanosensory organ patterning in zebrafish. Science 320, 1774-1777 [DOI] [PubMed] [Google Scholar]
- Nechiporuk A., Linbo T., Raible D. W. (2005). Endoderm-derived Fgf3 is necessary and sufficient for inducing neurogenesis in the epibranchial placodes in zebrafish. Development 132, 3717-3730 [DOI] [PubMed] [Google Scholar]
- Ohyama T., Groves A. K., Martin K. (2007). The first steps towards hearing: mechanisms of otic placode induction. Int. J. Dev. Biol. 51, 463-472 [DOI] [PubMed] [Google Scholar]
- Oxtoby E., Jowett T. (1993). Cloning of the zebrafish krox-20 gene (krx-20) and its expression during hindbrain development. Nucleic Acids Res. 21, 1087-1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips B. T., Bolding K., Riley B. B. (2001). Zebrafish fgf3 and fgf8 encode redundant functions required for otic placode induction. Dev. Biol. 235, 351-365 [DOI] [PubMed] [Google Scholar]
- Prince V. E., Moens C. B., Kimmel C. B., Ho R. K. (1998). Zebrafish hox genes: expression in the hindbrain region of wild-type and mutants of the segmentation gene, valentino. Development 125, 393-406 [DOI] [PubMed] [Google Scholar]
- Radosevic M., Robert-Moreno A., Coolen M., Bally-Cuif L., Alsina B. (2011). Her9 represses neurogenic fate downstream of Tbx1 and retinoic acid signaling in the inner ear. Development 138, 397-408 [DOI] [PubMed] [Google Scholar]
- Raible F., Brand M. (2001). Tight transcriptional control of the ETS domain factors Erm and Pea3 by Fgf signaling during early zebrafish development. Mech. Dev. 107, 105-117 [DOI] [PubMed] [Google Scholar]
- Riley B. B., Zhu C., Janetopoulos C., Aufderheide K. J. (1997). A critical period of ear development controlled by distinct populations of ciliated cells in the zebrafish. Dev. Biol. 191, 191-201 [DOI] [PubMed] [Google Scholar]
- Riley B. B., Chiang M. Y., Storch E. M., Heck R., Buckles G. R., Lekven A. C. (2004). Rhombomere boundaries are Wnt signaling centers that regulate metameric patterning in the zebrafish hindbrain. Dev. Dyn. 231, 278-291 [DOI] [PubMed] [Google Scholar]
- Roehl H., Nüsslein-Volhard C. (2001). Zebrafish pea3 and erm are general targets of FGF8 signalling. Curr. Biol. 11, 503-507 [DOI] [PubMed] [Google Scholar]
- Rohner N., Bercsényi M., Orbán L., Kolanczyk M. E., Linke D., Brand M., Nüsslein-Volhard C., Harris M. P. (2009). Duplication of fgfr1 permits Fgf signaling to serve as a target for selection during domestication. Curr. Biol. 19, 1642-1647 [DOI] [PubMed] [Google Scholar]
- Sapède D., Pujades C. (2010). Hedgehog signaling governs the development of otic sensory epithelium and its associated innervation in zebrafish. J. Neurosci. 30, 3612-3623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmang T. (2007). Expression and functions of FGF ligands during early otic development. Int. J. Dev. Biol. 51, 473-481 [DOI] [PubMed] [Google Scholar]
- Tanimoto M, Ota Y., Inoue M., Oda Y. (2011). Origin of inner ear hair cells: morphological and functional differentiation from ciliary cells into hair cells in zebrafish inner ear. J. Neurosci. 31, 3784-3794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse B., Pflumio S., Fürthauer M., Loppin B., Heyer V., Degrave A., Woehl R., Lux A., Steffan T., Charbonnier X. Q., et al. (2001). Expression of the zebrafish genome during embryogenesis. ZFIN Direct Data Submission (http://zfin.org/cgi-bin/webdriver?MIval=aa-pubview2.apg&OID=ZDB-PUB-010810-1)
- Thisse B., Wright G. J., Thisse C. (2008). Embryonic and larval expression patterns from a large scale screening for novel low affinity extracellular protein interactions. ZFIN Direct Data Submission (http://zfin.org/cgi-bin/webdriver?MIval=aa-pubview2.apg&OID=ZDB-PUB-080227-22) [DOI] [PMC free article] [PubMed]
- Thisse C., Thisse B. (2005). High throughput expression analysis of ZF-models consortium clones. ZFIN Direct Data Submission (http://zfin.org/cgi-bin/webdriver?MIval=aa-pubview2.apg&OID=ZDB-PUB-051025-1)
- Varga Z. M., Amores A., Lewis K. E., Yan Y. L., Postlethwait J. H., Eisen J. S., Westerfield M. (2001). Zebrafish smoothened functions in ventral neural tube specification and axon tract formation. Development 128, 3497-3509 [DOI] [PubMed] [Google Scholar]
- Waldman E. H., Castillo A., Collazo A. (2007). Ablation studies on the developing inner ear reveal a propensity for mirror duplications. Dev. Dyn. 236, 1237-1248 [DOI] [PubMed] [Google Scholar]
- Walshe J., Mason I. (2003). Fgf signalling is required for formation of cartilage in the head. Dev. Biol. 264, 522-536 [DOI] [PubMed] [Google Scholar]
- Walshe J., Maroon H., McGonnell I. M., Dickson C., Mason I. (2002). Establishment of hindbrain segmental identity requires signaling by FGF3 and FGF8. Curr. Biol. 12, 1117-1123 [DOI] [PubMed] [Google Scholar]
- Westerfield M. (2000). The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio). Eugene, OR: University of Oregon Press; [Google Scholar]
- Whitfield T. T., Hammond K. L. (2007). Axial patterning in the developing vertebrate inner ear. Int. J. Dev. Biol. 51, 507-520 [DOI] [PubMed] [Google Scholar]
- Wiellette E. L., Sive H. (2003). vhnf1 and Fgf signals synergize to specify rhombomere identity in the zebrafish hindbrain. Development 130, 3821-3829 [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Peters G., Dickson C., McMahon A. P. (1988). Expression of the FGF-related proto-oncogene int-2 during gastrulation and neurulation in the mouse. EMBO J. 7, 691-695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D. K., Nunes F. D., Choo D. (1998). Axial specificiation for sensory organs versus non-sensory structures of the chicken inner ear. Development 125, 11-20 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.