Abstract
Radial sorting allows the segregation of axons by a single Schwann cell (SC) and is a prerequisite for myelination during peripheral nerve development. Radial sorting is impaired in models of human diseases, congenital muscular dystrophy (MDC) 1A, MDC1D and Fukuyama, owing to loss-of-function mutations in the genes coding for laminin α2, Large or fukutin glycosyltransferases, respectively. It is not clear which receptor(s) are activated by laminin 211, or glycosylated by Large and fukutin during sorting. Candidates are αβ1 integrins, because their absence phenocopies laminin and glycosyltransferase deficiency, but the topography of the phenotypes is different and β1 integrins are not substrates for Large and fukutin. By contrast, deletion of the Large and fukutin substrate dystroglycan does not result in radial sorting defects. Here, we show that absence of dystroglycan in a specific genetic background causes sorting defects with topography identical to that of laminin 211 mutants, and recapitulating the MDC1A, MDC1D and Fukuyama phenotypes. By epistasis studies in mice lacking one or both receptors in SCs, we show that only absence of β1 integrins impairs proliferation and survival, and arrests radial sorting at early stages, that β1 integrins and dystroglycan activate different pathways, and that the absence of both molecules is synergistic. Thus, the function of dystroglycan and β1 integrins is not redundant, but is sequential. These data identify dystroglycan as a functional laminin 211 receptor during axonal sorting and the key substrate relevant to the pathogenesis of glycosyltransferase congenital muscular dystrophies.
Keywords: Peripheral nerve development, Schwann cells, Axonal sorting, Congenital muscular dystrophy, Dystroglycan, Integrins, Mouse
INTRODUCTION
Radial sorting of axons by Schwann cells (SCs) is a multistep developmental process required for myelination. It involves segregation of large caliber axons, and depends on signals exchanged between axons and SCs. Axon signals include the amount of neuregulin type III on axons, extracellular matrix signals are initiated by laminin 211 (α2β1γ1) and 411 (α4β1γ1) via β1 integrin receptors on SCs (Feltri and Wrabetz, 2005).
Laminin 211 is missing in DyDy mutants (a model of congenital-muscular dystrophy 1A) and radial sorting is severely impaired in the proximal peripheral nervous system (i.e. spinal roots) and is mildly impaired in distal nerves in these mutants (Bradley and Jenkison, 1973; Bradley and Jenkison, 1975; Stirling, 1975). By contrast, absence of laminin 411 in mice affects proximal and distal districts only mildly (Wallquist et al., 2005), whereas absence of both laminin 211 and 411 blocks radial sorting completely in all districts (Chen and Strickland, 2003; Yang et al., 2005) (Fig. 1). The reasons for this topographical difference are unknown, but might derive from activation of different laminin receptors by different laminins.
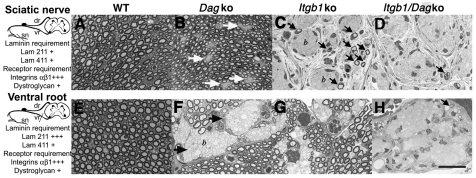
Fig. 1.
Axonal sorting requires both dystroglycan and β1 integrin in SCs. (A-H) Transverse semi-thin sections of sciatic nerves (A-D) and ventral roots (E-H) in dystroglycan (DagKO; B,F), β1 integrin (Itgβ1KO; C,G) and double SC-specific (D,H) null mice at P28. Laminins required in each district are indicated on the left (+, mild; +++, severe). In sciatic nerves, absence of dystroglycan (B) results in small bundles of unsorted axons (white arrows). The absence of β1 integrin (C) results in large bundles of unsorted axons (b) and few myelinated fibers (arrows). A complete arrest of radial sorting, with only an occasional myelinated fiber (arrow) is observed when both dystroglycan and β1 integrin are ablated (D). In ventral roots, the absence of either dystroglycan or β1 integrin causes an important arrest in radial sorting (F,G; arrows), which becomes complete when both receptors are ablated (H). dr, dorsal roots; sn, sciatic nerve; vr, ventral roots. Scale bar: 25 μm.
Deletion of receptors containing the β1 integrin subunit causes arrest in radial sorting distally, but only mild defects proximally (Feltri et al., 2002), leaving the laminin receptor involved in radial sorting in spinal roots unidentified. Radial sorting defects are also observed in models of other human muscular dystrophies (MDC1D and Fukuyama) that are the result of mutations in fukutin or LARGE glycosyltranferases (Levedakou et al., 2005; Saito et al., 2007), which have α-dystroglycan as substrate. These data suggest either that dystroglycan mediates sorting or that these glycosyltransferases act on other proteins, such as β1 integrins. However, the absence of Large or fukutin does not impair glycosylation of β1 integrin (Levedakou et al., 2005; Saito et al., 2007) and deletion of dystroglycan in SCs does not affect radial sorting, at least in distal nerves (Saito et al., 2003). Thus, the molecular mechanisms by which these mutations alter sorting are unknown.
Finally, the mechanisms by which different laminin-receptor pairs promote sorting are incompletely understood. Axonal sorting requires multiple events: the formation of `families' of SCs with multiple axons contained in a common basal lamina (Webster et al., 1973), the matching of the number of axons and SCs, the insertion of SC processes around axons to recognize and segregate large ones, and the defasciculation of single axons with their own daughter SC and basal lamina (Martin and Webster, 1973; Webster et al., 1973). It is unknown which laminin-receptor pairs contribute to each step. For example, laminins promote the formation of SC processes and the interaction with axons (Yu et al., 2009) via activation of Rac1 by a β1 integrin receptor (Benninger et al., 2007; Nodari et al., 2007), but laminins also promote SC proliferation and survival via activation of Pi3K, Fak and Cdc42 (Benninger et al., 2007; Grove et al., 2007; Yang et al., 2005; Yu et al., 2005) by an unknown laminin receptor. Thus, which laminin receptors control matching of axons and SC number is unclear.
Here, we show that deletion of dystroglycan in SCs in congenic C57BL/6 mice reveals an arrest in radial sorting that is most evident in spinal roots. When both dystroglycan and β1 integrins are deleted in SCs, proximal and distal districts are completely unsorted, replicating the absence of both laminin 211 and 411. We also show that dystroglycan and β1 integrins act at least in part through different mechanisms, as only the absence of β1 integrin impairs SC proliferation and survival, and the absence of dystroglycan arrests sorting at the later stage of defasciculation. Finally, β1 integrins and dystroglycan affect the signaling pathways that are active during radial sorting in different ways. These data show that the functions of dystroglycan and β1 integrin in radial sorting are non-redundant, identify dystroglycan as a laminin 211 receptor involved in sorting in the proximal nervous system, and provide a potential explanation for the sorting defects observed in the absence of fukutin and Large glycosyltransferases.
MATERIALS AND METHODS
Generation of transgenic mice
β1 integrin floxed (β1F/F) (Graus-Porta et al., 2001), dystroglycan floxed mice (Dag1F/F) (Moore et al., 2002; Saito et al., 2003) and mPoTOTCre transgenic mice (Feltri et al., 1999) have been described previously. Dag1F/FItgb1F/F mice were generated by crossing Itgb1F/+ and Dag1F/+ mice. Dag1F/F//P0Cre and Itgb1F/F mice were crossed to obtain Itgb1F/+Dag1F/+//P0Cre mice. These were intercrossed to obtain Itgb1F/F Dag1F/F//P0Cre mice. Offspring were analyzed by PCR on tail genomic DNA as described (Feltri et al., 1999; Saito et al., 2003). Most progeny in this study resulted from Itgb1 floxed parents that were N15 congenic for C57BL/6, and from Dag parents that were N7-N9 congenic for C57BL/6.
TUNEL and proliferation assays
TUNEL assay and BrdU incorporation and staining were performed as described (Feltri et al., 2002). Proliferation assays were performed using rabbit α-PH3 antibodies (Upstate) as described (D'Antonio at al., 2006). Nuclei were counterstained with DAPI and counted with a Leica DM5000B fluorescence microscope. For TUNEL and proliferation, three different mice per genotype and four different slides per animal were analyzed, for an approximate total number of nuclei of 85,000 for postnatal day (P) 3, 100,000 for P5, 120,000 for P15 and 140,000 for P28.
Western blot and pull-down assays
Sciatic nerves of P3 mice were lysed in 150 mM NaCl, 50 mM Tris pH 7.5, 1% Igepal, 0.1% SDS, 10% glycerol, 0.5% deoxycholate, 1 mM EDTA, 1 mM EGTA, 1 mM NaF and 1% protease inhibitor cocktail (Sigma-Aldrich) for Akt and P42-44, or 95 mM NaCl, 25 mM Tris pH 7.5, 10 mM EDTA, 2% SDS, 1 mM NaF, 1 mM NaVO4 and 1% protease inhibitor cocktail (Sigma-Aldrich) for the others, and processed by standard methods. Glutathione-s-transferase (GST)-pull down assays for Rac1, Cdc42 and RhoA were performed using 500 μg of nerve lysates as described (Nodari et al., 2007). Blots were developed with ECL or ECL plus (GE Healthcare) and band intensity was quantified using ImageJ software. The following antibodies were used: rabbit antibodies against Akt, pAktSer473, P44-42, pP44-42Thr202/Tyr204, p38, p-p38Thr180/Tyr182, Src and p-SrcTyr416(Cell Signaling Technology); rabbit anti-ErbB2 and anti-Cdc42 (Santa Cruz Biotechnology); mouse anti-Rac1 (Millipore); mouse anti-β tubulin and rabbit anti-calnexin (Sigma-Aldrich); mouse anti-glycosylated α-dystroglycan (IIH6) and goat anti-core α-dystroglycan (G20) (generous gifts from Kevin Campbell, University of Iowa, IA, USA).
Immunohistochemistry
Sciatic nerves were processed and stained as described (Nodari et al., 2008) using rabbit antibodies against neurofilament (Sigma), goat antibody against α-dystroglycan core protein, guinea-pig antibody against β1 integrin (BD Biosciences) and fluorochrome-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories) and viewed with a Leica confocal microscope.
Morphological analysis
Morphological analysis was performed as described (Wrabetz et al., 2000). Quantification of axonal sorting defects was performed on semi-thin and electron microscopy sections of roots or sciatic nerves from three animals per genotype.
Immuno-electron microscopy
P3-P4 wild-type, β1 integrin-null and dystroglycan-null mice (negative controls) were perfused with 4% paraformaldehyde (PFA)/0.05% glutaraldehyde in 0.01 M sodium periodate, 0.1 M lysine, 3% sucrose in 0.1 M phosphate buffer pH 7.4. Sciatic nerves were dissected, fixed for 2 hours, and left overnight in 3.5% sucrose in 0.1 M phosphate buffer. Tissues were processed at 4°C as described (Scherer et al., 1995) with the following modifications: Tissue was stained with 0.25% tannic acid for 1 hour, quenched in 50 mM NH4Cl, washed in 4% sucrose in 0.1 M maleate buffer (pH 6.2), incubated for 1 hour with 1% uranyl acetate in maleate buffer, dehydrated to 90% ethanol (from 70% ethanol onward; all steps at –20°C) then dipped in absolute ethanol for 15 seconds and then infiltrated first with 1:1 ratio of L.R. Gold (Polysciences)/ethanol, then with a 7:3 ratio of L.R. Gold/ethanol followed by two changes of L.R. Gold (1 hour and then overnight). Next day, the tissues were infiltrated in L.R. Gold with 0.5% benzoin methyl ether for 1 hour and then overnight, embedded in gelatin and polymerized by UV irradiation (365 nm) for 48 hours at –20°C. Sections were collected on nickel formward/carbon grids, and stained according to the methods of Li et al. (Li et al., 2005a). After two washes in PBS and block with 0.25% fish skin gelatin and 0.1% Triton X-100 in PBS for 1 hour at room temperature, sections were incubated with hamster anti-β1 integrin (BD Biosciences) or mouse anti-β-dystroglycan (Novocastra) antibodies, rinsed with PBS, incubated with secondary antibody (custom-made anti-hamster, conjugated with 5 nm gold particles or anti-mouse, conjugated with 10 nm gold particles; British BioCell). In some experiments, the hamster anti-β1 integrin antibody (BD Biosciences) was biotinylated with a EZ-Link Sulfo-NHS-biotin (Thermo Scientific) in a molecular ratio of 1:20 for 2 hours on ice in the dark, dialyzed overnight against PBS and used for immunostaining at a 1:25 dilution. A goat anti-biotin antibody (British Biocell) conjugated with 5 nm gold particles was used as secondary antibody. After staining, grids were washed on drops of PBS and distilled water and stained with saturated uranyl acetate and lead citrate.
Rac1-Pak1 binding domain localization
Organotypic neuron/SC cultures, cold jet, mouse SC cultures and immunocytochemistry for Rac1-Pak1 binding domain localization were performed as described (Nodari et al., 2007). The following antibodies were used: goat anti-GST (GE Healthcare), mouse anti-βDG (Novocastra), rabbit anti-S100 (Dako) antibody to discriminate SC from fibroblasts (not shown).
Rat SC cultures
Rat SCs were produced and expanded as described previously (Feltri et al., 1992). For β1 silencing, rat SCs were plated on poly-l-lysine and transfected the next day with 66 nM ITGB1 and control siRNA (ON-TARGET plus, Dharmacon) using Lipofectamine 2000 (Invitrogen). Next day, cells were starved for 24 hours in DMEM containing 2 μM forskolin, stimulated for 30 seconds with 1 mM of soluble NRG1-β1 (R&D Systems) or dorsal root ganglia membranes, which were prepared as described (Taveggia et al., 2005). Proteins were extracted on ice with 25 mM Tris pH 7.4, 95 mM NaCl, 10 mM EDTA, 1% SDS, 1 mM NaF, 1 mM NaVO3. Experiments were repeated at least three times.
RESULTS
Ablation of dystroglycan alone is sufficient to cause arrest in radial sorting of axons
Mutations in the genes encoding the α-dystroglycan glycosyltransferases Large and fukutin cause sorting defects, suggesting that dystroglycan might be involved (Levedakou et al., 2005; Saito et al., 2007). However, mice lacking SC dystroglycan have no sorting defects (Saito et al., 2003). These mice were generated by crossing mice carrying the Dag1 gene flanked by LoxP sites (Moore et al., 2002) with Mpz-Cre transgenic mice (mPoTOTCre) that express Cre in SCs from embryonic day (E) 13.5 (Feltri et al., 2002; Saito et al., 2003). Only the distal district (sciatic nerves) of resulting mice in a mixed background (129/Sv and C57BL/6) was analyzed (Saito et al., 2003). To determine whether dystroglycan was involved in radial sorting, we first bred dystroglycan mutants on a congenic C57BL/6 background, and analyzed proximal and distal peripheral nervous system districts. Notably, in this background the absence of SC dystroglycan caused a radial sorting defect that was mild in sciatic nerve and severe in ventral roots (Fig. 1B,F). Further breeding of dystroglycan mutants in the C57BL/6 background (N10-N13) greatly diminished the phenotype (not shown), indicating that we identified a genetic window (N5-N9) in which the role of dystroglycan in radial sorting could be unraveled. Based on the distribution of the radial sorting defect, these mice closely resemble dystrophic dy/dy or dy2J/dy2J mice, lacking laminin 211.
The function of SC dystroglycan and β1 integrin in axonal sorting is not redundant
To determine whether there is redundancy between β1 integrin and dystroglycan in sorting, we generated mice lacking both receptors in SCs. As described for Dag1 floxed mice, Itgb1 floxed mice were bred into a congenic C57BL/6 background. Immunofluorescence confirmed ablation of both proteins in SCs (see Fig. S1 in the supplementary material).
Similarly to the dystroglycan mutant, mice lacking SC β1 integrin in a pure C57BL/6 background have a more severe phenotype than those previously characterized in a mixed 129/sv-C57BL/6 background (Feltri et al., 2002). C57BL/6 β1 integrin mutants showed an almost completely arrested phenotype in sciatic nerves, which contained only few myelinated fibers and larger bundles of unsorted axons (Fig. 1C). Importantly, radial sorting defects were now apparent in spinal roots (Fig. 1G).
Double mutants were viable but, by P15, developed tremor with impaired gait, which evolved to hindlimb paralysis and severe atrophy by P60. Onset of symptoms was earlier than in single mutants, with more severe tremor and a wide-based gait. Ventral roots and sciatic nerves were completely occupied by unsorted bundles of axons (Fig. 1D,H). Dorsal roots presented defects in radial sorting, whereas small, unsorted bundles were rarely seen in single mutants (not shown). Occasional myelinated fibers were found in double-null mice (Fig. 1). The severity of double mutants is comparable to that of mice lacking both 211 and 411 laminins (Chen and Strickland, 2003; Yang et al., 2005), and suggests that dystroglycan and αβ1 integrins represent all the laminin receptors involved in radial sorting.
Although loss of β1 integrin causes a more prominent defect than loss of dystroglycan in distal nerves (compare Fig. 1B with 1C), the extent of the defect was similar between the two single mutants in ventral roots, where the area occupied by unsorted bundles was 30% in each single mutant and 93% in double mutants (Table 1).
Table 1.
Quantification of radial sorting defects in ventral roots at P28
Dystroglycan is required at a later step than β1 integrins during sorting
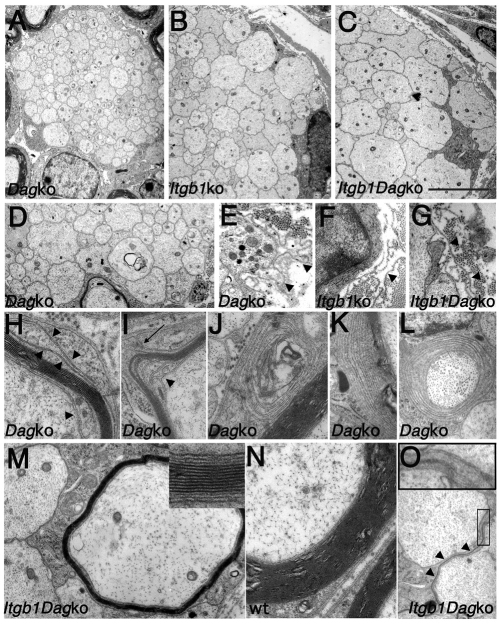
Unsorted bundles in the absence of dystroglycan were smaller than in the absence of β1 integrin. Sciatic nerves bundles in dystroglycan mutants contained fewer axons of smaller caliber (Fig. 2A and Table 2). By contrast, most bundles in β1 integrin and double mutants were large, occupied the majority of the endoneurium and contained mixed caliber axons (Fig. 2B,C and Table 2). Axons in both mutants were naked, although in certain bundles dystroglycan mutants SCs had unsheathed axons. Basal lamina was discontinuous, detached or redundant in both mutants, especially around bundles of unsorted axons (Fig. 2E-G, arrowheads). In double mutants, basal lamina defects were more severe and were present in the majority of fibers.
Fig. 2.
Radial sorting and myelin defects in dystroglycan and double mutants. Electron microscopy of transverse sections of dystroglycan, β1 integrin and double mutant sciatic nerves at P28. (A-D) Nerves of all the mutants contain bundles of naked axons (A-D), but the size of those axons is smaller in dystroglycan (A) compared with β1 integrin (B) and double mutants (C). (See also Table 2.) Occasionally, large axons are found in bundles of dystroglycan mutants (D). (E-G) Detached and redundant basal lamina (arrowheads) in β1 integrin (E), dystroglycan (F) and double (G) mutants. (H-L) Myelination defects in dystroglycan mutants: disorganization of inner and outer wraps (arrowheads in H,I) and outer mesaxons (arrow in I), non-compacted lamella in the SC cytoplasm (J), non-compaction of outer myelin lamellae (K) or throughout the whole myelin thickness (L). (M-O) Hypomyelination in double mutants (M). A myelin-like process was observed separating two axon bundles (arrowheads and inset in O). Wild type (wt) is shown in N. Scale bar: in C, 5 μm for A-C; 3.2 μm for D,O; 1.6 μm for E-G; 1.25 μm for M,N; 1 μm for J-L.
Table 2.
Quantification of sorting defects in unsorted axonal bundles in sciatic nerves at P28
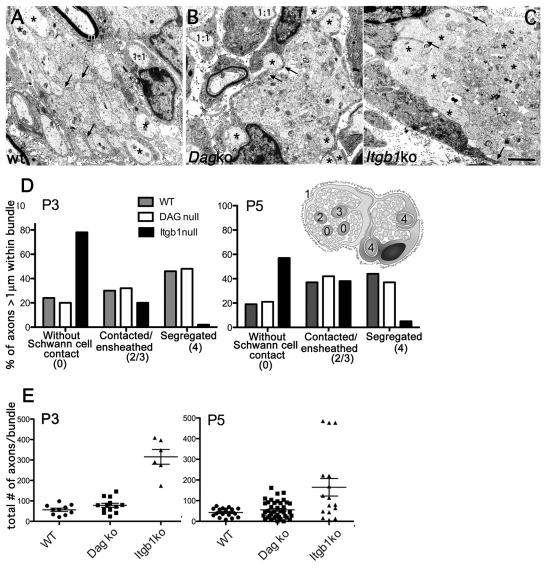
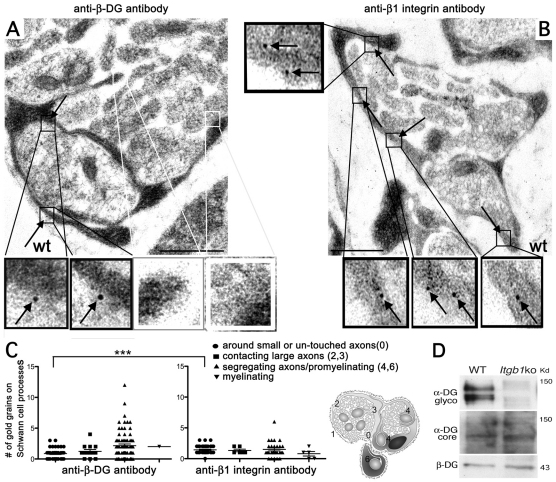
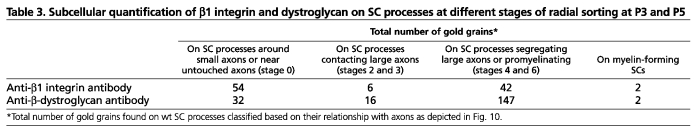
To determine at what stage radial sorting was arrested, we performed electron microscopy at P3 and P5, when radial sorting is active in sciatic nerves (Webster et al., 1973). At these ages, wild-type (wt) nerves contained small bundles with few axons (Fig. 3A), with large axons that were already segregated at the periphery (∼45%), pro-myelinating and myelinating fibers (Fig. 3A) (Webster et al., 1973). By contrast, most axons in β1 integrin nerves were trapped in large bundles (Fig. 3C-E), including large axons that were completely unsegregated (∼80% at P3 and ∼60% at P5). Less than ∼5% of large axons were segregated at the periphery (Fig. 3C, asterisks and 3D). Overall, nerves appeared more immature than previously described in mixed C57BL6/129 nerves (Feltri et al., 2002). P3 and P5 dystroglycan mutant nerves appeared delayed compared with wt, but were further along in morphogenesis than β1 mutants, as bundles contained fewer axons (Fig. 3E), and ∼40% of large axons were segregated at the periphery of the bundles (Fig. 3B, asterisks and 3D). Thus, in the absence of dystroglycan, SCs can engage axons and segregate them, but they are then arrested before defasciculating the axon into a 1:1 relationship. By immuno-electron microscopy of P3-P4 wt nerves we found more gold grains on SC processes that contacted small axons or were not engaged (stage 0) when using the anti-β1 integrin antibody than when using the dystroglycan antibody (Fig. 4C and Table 3). The number of gold grains marking β1 integrin did not increase between SC processes at stage 0 and SCs segregating or defasciculating large axons (stage 4-6). By contrast, the number of gold grains marking dystroglycan increased strikingly between the two stages (Fig. 4A,C and Table 3). Finally, α-dystroglycan glycosylation, which increases as nerves mature (Court et al., 2011), was significantly reduced in β1 integrin-null nerves. Collectively, these data suggest that dystroglycan acts after αβ1 integrin during axonal sorting.
Fig. 3.
β1 integrins act before dystroglycan during axonal sorting. (A-C) Electron microscopy of cross-sections of P3 sciatic nerves of wt, dystroglycan and β1 integrin mutants. At this stage, wt nerves (A) contained SC families around small axonal bundles, with ∼45% of large axons already segregated at the periphery (asterisks), and pro-myelinating (1:1) and myelinating (m) fibers. SC processes enwrap axons (arrows). Radial sorting in dystroglycan mutants is delayed (B): SC families have bigger bundles, but ∼40% of large axons have been properly segregated to the periphery (asterisks) and pro-myelinating SC are frequent (1:1). In addition, SC start to enwrap axons with cytoplasmic processes (arrows). By contrast, β1 integrin mutant nerves (C) at this stage are completely occupied by large axonal bundles, and most large axons are trapped within bundles (asterisks). Cytoplasmic processes of SCs do not interact with axons (arrows). (D,E) Axon bundles in β1 integrin mutants are more immature than in dystroglycan mutants. (D) Quantification of the percentage of large axons in bundles based on their relationship with SCs, as indicated in the scheme: large axons with no association with SCs are stage 0, large axons contacted by SCs are stage 2 or 3, axons segregated at the periphery are stage 4. β1 integrin mutants have significantly more axons in stage 0 and fewer axons in stage 4 than do dystroglycan mutants (P<0.0001 by χ2 test, n=3 animals per genotype). (E) β1 integrin mutants have significantly more axons in bundles than do dystroglycan mutants (by Student's t-test: β1 integrin versus dystroglycan: P=0.0001 at P3 and 0.006 at P5; wt versus dystroglycan non significant, three animals/genotype). Mean ± s.e.m. are indicated.
Fig. 4.
. β-Dystroglycan is concentrated on SC processes segregating large axons. (A,B) Immuno-electron microscopy of P3-P4 mouse sciatic nerves using anti-β-dystroglycan (A) or anti-β1 integrin (B) antibodies shows that dystroglycan is detectable on SC processes surrounding large axons (arrows in A, magnified in the black insets; white insets show absence of dystroglycan on SC processes contacting unsegregated axons). By contrast, β1 integrins are detectable in all SCs surrounding all axons (arrows in B, magnified in the insets). Scale bar: 500 nm. (C) Number of gold grains found on SC processes at different stages (0 to 6 as shown in the drawing and in Fig. 10). More gold grains are present on immature SC processes (stage 0) when using the anti-β1 integrin antibody than when using anti-β-dystroglycan antibody (P=0.004 by Student's t-test, n=35 processes for β-dystroglycan, 39 for β1 integrin from at least three different experiments). The number of gold grains marking β-dystroglycan significantly increases in SCs segregating large caliber axons and pro-myelinating SCs (stages 4,6). Mean ± s.e.m. are indicated. See also Table 3. (D) Western blot from P28 sciatic nerves shows that the α-chain of dystroglycan (DG) is hypoglycosylated in the absence of β1-integrins.
Table 3.
Subcellular quantification of β1 integrin and dystroglycan on SC processes at different stages of radial sorting at P3 and P5
Myelination defects in dystroglycan and double mutant mice
Both mutants showed myelin defects. In β1 integrin mutants, the few myelin sheaths were too thin for the caliber of the axon (data not shown). For dystroglycan mutants, in addition to an earlier onset of the redundant myelin loops previously described at 6 months of age (Saito et al., 2003) (data not shown), we observed disorganization and redundancy of inner and outer mesaxons (Fig. 2H,I, arrows), lack of compaction of myelin throughout the whole thickness of the myelin sheath (Fig. 2L) or in outer lamella (Fig. 2K) and inappropriate myelin wraps in the cytoplasm (Fig. 2J). The few myelinated fibers of double mutants were severely hypomyelinated (Fig. 2M). Myelin periodicity appeared normal, although non-compaction of the outer and/or innermost lamellae was observed (Fig. 2M, inset).
β1 integrin receptors, but not dystroglycan, transmit survival and proliferation laminin signals
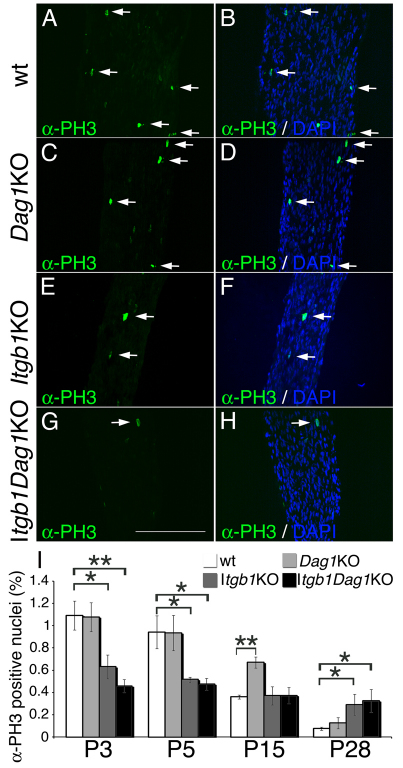
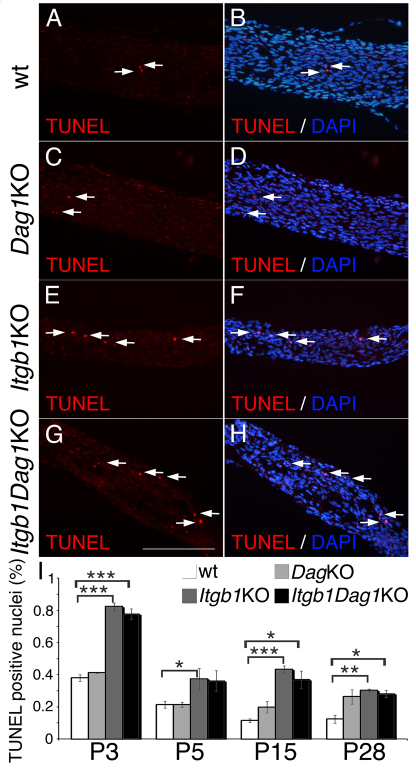
Laminins promote SC proliferation (Yang et al., 2005; Yu et al., 2005) and survival (Yu et al., 2005) and dystroglycan is required in vitro for laminin-mediated protection of SC from apoptosis (Li et al., 2005b). To determine which laminin receptor promotes proliferation and survival in vivo, we measured the rate of apoptotic and proliferating cells in mutant nerves by TUNEL and anti-histone H3 staining (D'Antonio et al., 2006). Proliferation was significantly decreased in β1 integrins and double mutants at P3 and P5 (Fig. 5). The decreased proliferating rate at P3 was confirmed using BrdU incorporation (see Fig. S2 in the supplementary material). By contrast, dystroglycan mutants showed a transient increase in proliferation at P15. Apoptosis was increased in β1 integrin and double mutants, but not dystroglycan mutants at P3 and P5 (Fig. 6). Staining with the SC marker periaxin (Gillespie et al., 1994) showed that 80% of the TUNEL positive cells were SCs (see Fig. S3 in the supplementary material). Thus, only β1 integrin receptor(s) mediate the pro-survival and proliferation signal from laminins during axonal sorting.
Fig. 5.
SC proliferation is decreased in β1-integrin mutants. (A-H) Immunostaining with anti-phospho-histone H3 (green) and staining with the nuclear dye DAPI (blue in the merged image in B,D,F,H) on longitudinal sections of control (A,B) and mutant (C-H) mice (P3 sciatic nerves) to measure the fraction of proliferating nuclei (arrows). Scale bar: 100 μm. (I) Quantification shows that the rate of proliferation is decreased in β1 integrin and double mutants at P3 and P5, but is normal by P15 and is increased later. By contrast, SC lacking dystroglycan do not show decreased proliferation. Error bars indicate s.e.m. *P≤0.05, **P≤0.005 by Student's t-test, n=3 mice per genotype.
Fig. 6.
SC survival is impaired in newborn β1 integrin mutants. (A-H) TUNEL analysis (red) on longitudinal sections of control (A,B) and mutant (C-H) mice (P3 sciatic nerves) and staining with the nuclear dye DAPI (blue in the merged image in B,D,F,H). Arrows indicate TUNEL-positive nuclei. Scale bar: 100 μm. (I) Apoptosis is increased in β1 integrin and double mutants. Error bars indicate s.e.m. *P≤0.05, **P≤0.005, ***P≤0.0005, by Student's t-test, n=3 mice per genotype.
The decreased proliferation and survival in β1 integrin mutants was surprising, as they displayed normal proliferation and survival when in a mixed 129/C57BL6 background (Feltri et al., 2002). The increased severity of the sorting defects in congenic mice could be due to a reduction in SC number in addition to the described inability to make lamellipodia (Nodari et al., 2007). Another surprise was that double mutant nerves are as severe as nerves lacking all laminins, yet the decrease in SC survival in double receptor mutants is much smaller (<1%) than that reported in mice lacking all laminins (Yu et al., 2005). To clarify these points, we confirmed our ability to detect apoptosis in nerves, and then compared β1 integrin mutants in congenic versus mixed background and compared β1 integrin mutants with laminin γ1 mutants. We performed TUNEL on P1 rat nerves after axonal transection, which is known to cause extensive SC apoptosis (Grinspan et al., 1996), and we detected a rate of apoptosis (7%), similar to that reported by Grinspan et al. (Fig. 7). When we compared the fraction of TUNEL positive SCs in P3 nerves lacking laminin γ1 with controls, we found a significant increase in apoptosis, but at a low level of <1%, similar to what we found in β1 integrin mutants (Figs 6 and 7). Finally, we re-analyzed β1 integrin mutant nerves in a mixed genetic background at P5, and we confirmed that in this situation no increase of SC apoptosis is detectable (Fig. 7), as reported (Feltri et al., 2002). We conclude that lack of β1 integrin causes a significant, but modest, decrease in SC proliferation and survival. The decrease in SC survival is similar to that observed in the absence of laminins, indicating that receptor(s) containing the β1 integrin subunit transmit a laminin survival signal in developing nerves.
Fig. 7.
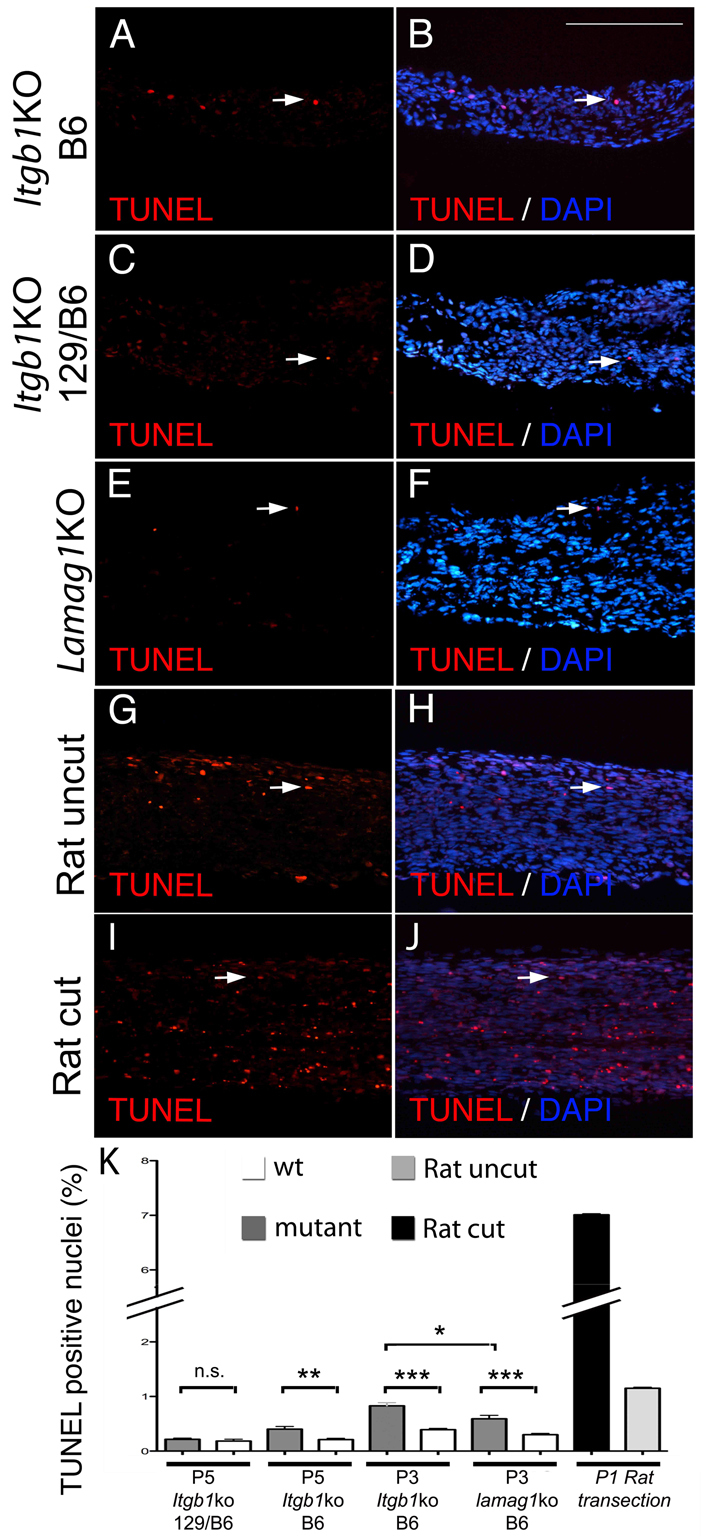
The moderate increase in apoptosis in SCs lacking β1 integrins is comparable to that observed in the absence of all laminins. (A-K) TUNEL on longitudinal sections of mutant mice at P5 (A-D), P3 (E,F) or P1 rat wt uncut and cut (G-J) sciatic nerves. (C,D,K) TUNEL on P5 β1 integrin mutant in a mixed C57BL6/129sv background (N2–N5 C57BL6) shows no difference between mutant and wt nerves, in agreement with previous data (Feltri et al., 2002). (A,B,K) By contrast, the mice used in this study, >N15 congenic in C57BL6, show a small but significant increase in apoptosis (see also Fig. 6). (E,F,K). Comparable levels of apoptosis are detected in laminin γ1 mutants (laminin γ1 KO). The increase in apoptosis (7%) detected after transection of rat sciatic nerve at P1 is significantly higher, as reported previously (Grinspan et al., 1996). *P<0.01, **P<0.001, ***P<0.0001, n.s., non significant by Student's t-test, minimum three mice per genotype. Error bars indicate s.e.m. Scale bar: 100 μm.
Akt signaling is impaired in SCs lacking β1 integrins
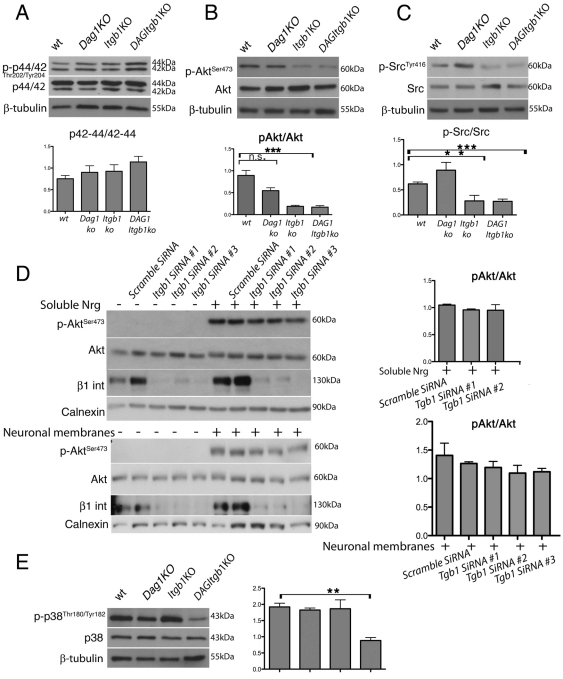
PI-3K and MAPK-Erk kinase are survival, proliferative and pro-myelinating signals in SCs (Goebbels et al., 2010; Maurel and Salzer, 2000; Newbern et al., 2011; Ogata et al., 2004). Absence of laminins causes defective activation of Akt which might explain the decreased survival and hypomyelination (Yu et al., 2005). We thus measured the levels of phospho-Akt473 and phospho-p42/44 (Erk1/2), as effectors of PI-3 and MAP kinases, in mutant nerves during sorting (P3). Similarly to nerves lacking laminins, p-Akt473 is significantly reduced in the absence of β1 integrins in SCs, whereas phospho-p42/44 (p-p42/44) is normal (Fig. 8A,B). Akt in SCs is phosphorylated in response to growth factors, such as neuregulin-type III, on axons (Taveggia et al., 2005). We therefore investigated whether the defective Akt473 phosphorylation in β1 mutants was an indirect consequence of the lack of axonal contact, or whether it represented a direct requirement of laminin/β1 integrin signaling to activate Akt. We exposed β1-deficient SCs in culture to neuregulins and examined whether they could phosphorylate Akt473. Rat SCs were transfected with three β1 integrin silencing oligonucleotides, which efficiently reduced expression (Fig. 8D), and were stimulated with either the soluble EGF domain common to all neuregulins or neuronal membranes containing neuregulin-type III (Taveggia et al., 2005). Reduction of β1 integrin did not preclude Akt phosphorylation in response to either soluble or axonal-bound neuregulins. Thus, β1 deficient SCs are competent to activate Akt in response to neuregulin provided both as a paracrine or a juxtacrine signal, suggesting that the reduced Akt473 phosphorylation observed in vivo is a consequence of the failure of mutant SCs to contact axons.
Fig. 8.
Impaired Akt, Src and p38MAPK activation during sorting. (A-C,E) Western blots of P3 sciatic nerves pooled from three to six animals from the indicated genotypes. Graphs indicate mean of at least three experiments using different pools of animals. Error bars indicate s.e.m. *P≤0.05, **P≤0.005, ***P≤0.0005, n.s., non significant, by Student's t-test. (B) Western blot using anti-p-AktSer473 antibodies and normalized for total Akt levels show a significant decrease in p-AktSer473 only in β1 mutants. (A) p-P44/42 levels are similar in mutant and wt. (C) p-SrcTyr416 is decreased in β1 integrin mutants whereas p-p38Thr180/Tyr182 (E) is decreased only when both β1 integrins and dystroglycan are deleted. (E) Western blot shows the efficient silencing of β1 integrin, and the appropriate phosphorylation of Akt on Ser473 in β1 integrin-silenced SCs. (D) Rat SCs transfected with either β1 integrin silencing or control siRNA, starved and stimulated with either the soluble EGF domain or dorsal root ganglia membranes containing neuregulin-type III (Taveggia et al., 2005).
Src activation is decreased in SCs missing β1 integrins
Binding of laminins to cultured SCs recruits dystroglycan and utrophin, and causes a dystroglycan and sulfatide-dependent phosphorylation of Src at position p-416 (Li et al., 2005b). In turn, Src phosphorylation is required for laminin-dependent protection of SCs from anoykisis (Li et al., 2005b). Src is also implicated downstream of neuregulins and the phosphatase Shp2 in SCs (Grossmann et al., 2009). To test whether the absence of dystroglycan or β1 integrin impaired Src activation during sorting, we measured Src416 phosphorylation in P3 mutant nerves. This antibody crossreacts also with activated Fyn and Lyn (Li et al., 2005b). Interestingly, β1 integrin deletion alone was sufficient to impair Src activation. These data are consistent with the fact that only β1 integrin-null SCs displayed decreased survival in vivo. To test whether dystroglycan was required for signaling after P3, we measured p-Src416, p-Akt473, p-p42/44Thr202/Tyr204 and p-p38Thr180/Tyr182 at P15 and P28, but found no significant differences between dystroglycan-null and wt nerves (see Fig. S4 in the supplementary material).
Laminin signaling to p38 MAPK requires both β1 integrins and dystroglycan
Other MAP kinases in SCs are Jnk/Sap and p38 (Cavaletti et al., 2007). Jnk/Sap is induced by NT3-TrkC in SCs (Yamauchi et al., 2003) and inhibits SC differentiation through c-Jun (Parkinson et al., 2008; Parkinson et al., 2004). By contrast, p38 is induced by laminins, and is required for myelination in neuron-SC co-cultures (Fragoso et al., 2007; Fragoso et al., 2003). To determine whether the activation of p38 MAPK by laminins requires β1-class integrins or dystroglycan, we measured phospho (p)-p38Thr180/Tyr182 in P3 sciatic nerves, and found that it was significantly impaired only when β1 integrins and dystroglycan were both lacking, but not in single mutants (Fig. 8). These data indicate that in vivo, both dystroglycan and β1 integrins are required for laminin-induced p-p38 activation during sorting and myelination.
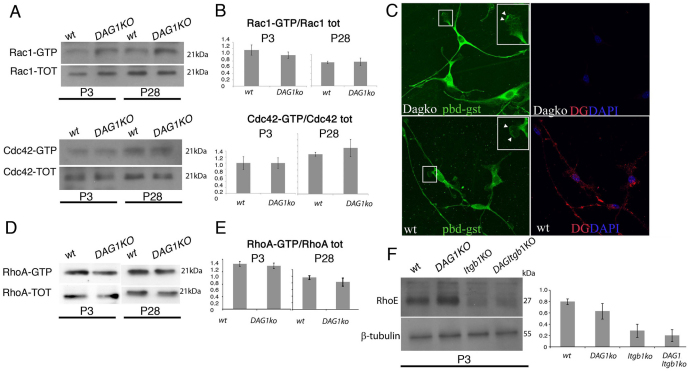
Dystroglycan does not regulate Rac1, Cdc42 or RhoA
The small Rho GTPases Rac1 and Cdc42 are required for radial sorting of axons (Benninger et al., 2007; Nodari et al., 2007), and lack of β1 integrin impairs RhoA and Rac1 activation and formation of radial lamellae during sorting (Nodari et al., 2007). Dystroglycan might also promote RhoGTPAse activation in SCs as it does in muscles (Thompson et al., 2010). The amount of active, GTP-bound Rac1, Cdc42 and RhoA in nerves from dystroglycan mutant and controls at P3 and P28 was similar (Fig. 9). When small GTPases are GTP-bound, integrins promote their targeting to the plasma membrane to interact with effectors (del Pozo et al., 2000). Indeed, β1 integrin-null SCs are unable to translocate GTP-bound Rac1 to the membrane and to produce radial lamellipodia (Nodari et al., 2007). To test whether dystroglycan has a similar role, we added purified GST-Pak-binding domain (GST-PBD) to wt or dystroglycan-deficient SCs spreading on laminin, and visualized its localization using anti-GST antibodies. Dystroglycan-null SCs were able to produce lamellipodia with PBD enrichment, suggesting that active Rac1 is properly recruited at the leading edge of SCs in the absence of dystroglycan (Fig. 9).
Fig. 9.
Normal activation of small Rho GTPases in the absence of SC dystroglycan. (A,B,D,E) The GTP-bound fraction of small GTPases in P3 and P28 sciatic nerve were measured using pull-down assays. Active proteins were normalized to total Rac1, Cdc42 and RhoA. (F) Western blot for RhoE in P3 sciatic nerves. (C) SCs plated on laminin, treated with GST-PBD and saponin, and stained with anti-GST (green) and anti-dystroglycan (red) antibodies show that in the absence of dystroglycan, active Rac1 is correctly targeted to lamellipodia. The leading edge of lamellipodia is enriched with PBD (Pak binding domain) in both wt and dystroglycan-null cells (arrowheads in the enlarged insets). Graphs represent the mean of at least three different experiments, error bars indicate s.e.m.
β1 integrins regulate RhoE
Atypical Rho GTPases such as RhoE are regulated at the level of expression and might be involved in axonal sorting downstream of integrin-linked kinase (Ilk) (Feltri et al., 2008; Pereira et al., 2009). Consistent with the fact that Ilk is associated with β1 integrins, we found that the levels of RhoE were significantly reduced in nerves lacking β1 integrin (Fig. 9).
DISCUSSION
Role of dystroglycan in peripheral nerve disease
We show that dystroglycan has a previously unrecognized role in the radial sorting of axons during peripheral nervous system development. Of note, the topography of the sorting defects in the absence of dystroglycan is severe in spinal roots and mild in distal nerves, and recapitulates precisely the defect observed in the absence of laminin 211 (Bradley and Jenkison, 1973; Stirling, 1975; Yang et al., 2005). Our data nicely complement the recent finding of incomplete rescue of Dy3k/Dy3k mice using a transgenic laminin 111 lacking the Lg4-5 domain. This domain binds dystroglycan specifically, and its absence could not rescue the radial sorting defects present in roots (Gawlik et al., 2010). This, together with older data indicating that dystroglycan binds laminin 211 in SCs (Yamada et al., 1996), indicates that dystroglycan is a functional laminin 211 receptor during radial sorting. Multiple muscular dystrophies carry mutations in genes coding for both extracellular (laminin 211) and intracellular (sarcoglycans, dystrophin) components of the dystroglycan complex, as well as in the genes for at least six enzymes that mediate dystroglycan post-translational modifications, including acetylglucosaminyltransferase-like protein (Large) and fukutin. Thus, the genetic defects of multiple myopathies ultimately converge on the dystroglycan complex (reviewed by Barresi and Campbell, 2006; Michele et al., 2002; Muntoni et al., 2004). Dystroglycanopathies are often syndromic, reflecting the role of dystroglycan in many tissues. In the central nervous system, for instance, deletion of dystroglycan in mice recapitulates neuronal migration defects and other cortical abnormalities found in MDCs (Moore et al., 2002). Similarly, we show here that dystroglycan deficiency recapitulates the peripheral neural defects seen in MDC1A, MDC1C and MDC1D. These data strongly suggest that dystroglycan is the substrate of Large and fukutin during axonal sorting, explaining the puzzling observation of defective radial sorting in animal models of MDC1C and MDC1D.
Dystroglycan and αβ1 integrins cooperation in SCs
We have shown that αβ1 integrins and dystroglycan cooperate in the radial sorting of axons. Sorting is completely arrested in the absence of both β1-integrins and dystroglycan, indicating that they are the only laminin receptors required for this process, and, similar to laminins themselves, the receptors are also not redundant. Cooperation between these receptors can occur in various, non-mutually exclusive ways. αβ1 and dystroglycan might have identical roles, interacting with the same laminins and transmitting the same signals, but still be required together to achieve full activation. Our data argues against this possibility: αβ1 and dystroglycan mediate different morphogenetic steps and activate different signals during radial sorting. Alternatively, it is possible that αβ1 integrins and dystroglycan interact with different laminins, or that they interact with the same laminins but the consequences of the ligation are different. Our in vivo analysis indicates that dystroglycan acts as a laminin 211 receptor during axonal sorting. By contrast, published data in vitro suggest that αβ1 integrins are required for SC adhesion to both laminin 211 and 411 (Wallquist et al., 2005; Yang et al., 2005). Whether this means that more than one αβ1 integrin receptor in SCs binds to different laminins is not clear at the moment. Independently of how many αβ1 integrins are required, our data so far suggest that at least one αβ1 integrin is required before dystroglycan, because the β1 subunit is present in immature SCs contacting many axons, and its absence arrests radial sorting at an early stage. By contrast, dystroglycan acts at subsequent steps, as it is mostly detectable in SCs that are still in bundles, but that have committed to a large caliber axon, and its absence arrests sorting by these SCs before defasciculation of the axon. The idea that αβ1 integrins and dystroglycan act in sequence is supported by our epistatic genetic experiments in single and double-null mice and by the immature glycosylation of α-dystroglycan in β1 integrin-null nerves.
Multiple laminin receptors function in the multistep process of axonal sorting
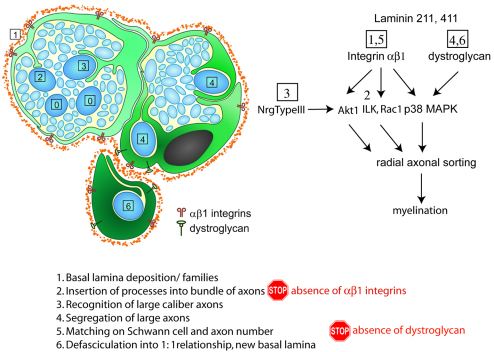
Previous experiments in mice identified roles for Cdc42, Rac1, Fak and Ilk in radial sorting (Benninger et al., 2007; Grove et al., 2007; Nodari et al., 2007; Pereira et al., 2009). Having identified the two classes of receptors involved, we searched for signaling pathways impaired by their absence. We used P3 nerves because radial sorting at this age is active in sciatic nerves of both wt and mutant nerves, so they can be compared at the molecular level. We found that multiple pathways are impaired during sorting in nerves lacking αβ1 integrins, namely PI-3K and Akt, Rac1, RhoA and RhoE, Fyn, and p38 MAPK (this paper) (Nodari et al., 2007). By contrast, the absence of dystroglycan in SCs in vivo impairs only the activation of p38 MAPK. The next step, to attribute specific signaling events to a specific mechanism, is more challenging because developing nerves contain a population of non-synchronized SCs, engaged in different aspects of radial sorting, and many of these molecules might function at one or more of the required steps. To begin to attribute receptors and their signaling to specific functions, we divide sorting into six steps, as illustrated in Fig. 10: (1) formation of SC families with a common basal lamina, (2) insertion of SC processes within axons, (3) contact and recognition of large axons, (4) segregation of large axons at the periphery, (5) matching of axons and SC number and (6) defasciculation of the axon with formation of an independent basal lamina. From our data it appears that the laminin 211-dystroglycan pair is implicated in the final steps of radial sorting, defasciculation of axons (Figs 4 and 9). By contrast, laminin 211 and 411, with β1 integrins, probably activate Fak, Ilk, Src and Rac1 at multiple steps, including early extension of radial lamellipodia to contact, recognize and ensheath axons, and matching of axons and SC number (Fig. 10, steps 2, 3 and 5). Subcellular analysis of the localization of these molecules and the timing of their activation, together with comparisons of their null phenotypes and new experimental paradigms to model each of these steps in vitro will be required to further combine molecules into pathways at the appropriate step.
Fig. 10.
Radial sorting is a multistep process. Schematic of the morphogenetic steps showing that deposition of the basal lamina and formation of `families' (step 1) requires laminins, insertion of processes into bundles (step 2) requires αβ1 integrins, whereas defasciculation of axons into pro-myelinating SCs with their own basal lamina (steps 4-6) requires dystroglycan (this paper). The presumed involvement of other signals (Nrg1 type III, Akt, Ilk, Rac1 and p38 MAPK) at various steps is also indicated.
Control of cell number during radial sorting
The absence of laminins 211 and 411, β1 integrin (this paper), Fak, and Cdc42 in SCs causes an arrest in axonal sorting accompanied by a decrease in proliferation. In the absence of Cdc42 and γ1 laminins, cell death is also increased, in the latter case at high levels, in contrast with similar studies conducted in the absence of laminins α2 and α4 (Benninger et al., 2007; Grove et al., 2007; Yang et al., 2005; Yu et al., 2005). Thus, whether laminins promote SC survival in vivo remains controversial. In our hands, γ1 laminin and β1 integrin mutants showed a modest increase in apoptosis. We found that the genetic background of the mutants influences SC survival (Fig. 7), possibly explaining the conflicting findings. Still, having compared all the mutants with the same method we conclude that in laminin and integrin mutants apoptosis rates are only modestly increased (below 1%). Although minimal, this increase in apoptosis with concomitant decrease in SC proliferation could contribute to diminishing the number of SCs available to ensheath axons during radial sorting. Owing to the altered shape of developmentally arrested SCs in these mutants, unbiased stereology with cell-specific marking will be required to determine whether this is the case. At least for αβ1 integrin, the decreased proliferation and survival is, at least in part, an indirect consequence of the failed access of mutant SCs to neuregulins on axons, as providing neuregulins directly to β1 integrin-null SCs in culture rescues phosphorylation of Akt473.
Surprisingly, dystroglycan mutants showed instead a transient increased in proliferation at P15. Because dystroglycan-null SCs form short internodes evident after P10 (Court et al., 2009), it is possible that mutant SCs require a burst of proliferation at this time to cover up all the axolemma. Alternatively, the incomplete radial sorting could cause the SCs around bundles to proliferate because they are not proceeding to myelinate. This transient proliferation was not due to decreased expression of p21Kip1 and p27Cip1 (cyclin-dependent kinase inhibitors p21 and p27) (data not shown).
Laminin receptors and myelination
It is unclear whether laminins and receptors are required for myelination after radial sorting is complete. Direct in vivo evidence is lacking, owing to the redundancy and compensation of the laminin isoforms when only one is missing (Patton et al., 1997; Previtali et al., 2003) and the arrest before myelination when all are lacking (Yu et al., 2005). Our data show that the few SCs that escape arrest in sorting in the absence of β1 integrins or β1 integrins and dystroglycan form thin myelin sheaths (Figs 1, 2), suggesting a role for laminin receptors after radial sorting. In support of this, laminins are strictly required for myelination in vitro (Podratz et al., 2001). It is thus possible that laminins and laminin receptors would modulate signaling pathways required for myelination. A possible effector is p38MAPK, which is activated by laminins and is required for myelination in vitro by both SCs and oligodendrocytes (Bhat et al., 2007; Chew et al., 2010; Fragoso et al., 2007; Fragoso et al., 2003). Interestingly, we show that the absence of either αβ1 integrins or dystroglycan is insufficient to impair the activation of p38MAPK, but when both are missing phosphorylation of p38MAPK is reduced. Thus, p38 MAPK might act downstream of laminins to promote myelination in vivo.
Supplementary Material
Acknowledgments
We thank Kevin Campbell (HHMI, University of Iowa) and Fumiaki Saito (Teikyo University, Tokyo) for dystroglycan floxed mice and antibodies; Ulrich Mueller (Scripps Institute, LaJolla) for Itgb1 floxed mice; Peter Brophy (University of Edinburgh) for antibodies; Diane Sherman (University of Edinburgh) and Xinghua Yin (Cleveland Clinic) for suggestions with immuno-electron microscopy; and Yannick Poitelon for artwork. This work was supported by NINDS R01NS045630 (to M.L.F.) and R01-NS055256 (to L.W.), Telethon Italia (GGP08021 and GPP1007A to M.L.F. and GGP071100 to L.W.) and the European Community's Seventh Framework Programme (FP7/2007-2013) HEALTH-F2-2008-201535, UE-FP7 (NGIDD). Deposited in PMC for release after 12 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.065490/-/DC1
References
- Barresi R., Campbell K. P. (2006). Dystroglycan: from biosynthesis to pathogenesis of human disease. J. Cell Sci. 119, 199-207 [DOI] [PubMed] [Google Scholar]
- Benninger Y., Thurnherr T., Pereira J. A., Krause S., Wu X., Chrostek-Grashoff A., Herzog D., Nave K. A., Franklin R. J., Meijer D., et al. (2007). Essential and distinct roles for cdc42 and rac1 in the regulation of Schwann cell biology during peripheral nervous system development. J. Cell Biol. 177, 1051-1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat N. R., Zhang P., Mohanty S. B. (2007). p38 MAP kinase regulation of oligodendrocyte differentiation with CREB as a potential target. Neurochem.Res. 32, 293-302 [DOI] [PubMed] [Google Scholar]
- Bradley W. G., Jenkison M. (1973). Abnormalities of peripheral nerves in murine muscular dystrophy. J. Neurol. Sci. 18, 227-247 [DOI] [PubMed] [Google Scholar]
- Bradley W. G., Jenkison M. (1975). Neural abnormalities in the dystrophic mouse. J. Neurol. Sci. 25, 249-255 [DOI] [PubMed] [Google Scholar]
- Cavaletti G., Miloso M., Nicolini G., Scuteri A., Tredici G. (2007). Emerging role of mitogen-activated protein kinases in peripheral neuropathies. J. Peripher. Nerv. Syst. 12, 175-194 [DOI] [PubMed] [Google Scholar]
- Chen Z. L., Strickland S. (2003). Laminin gamma1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve. J. Cell Biol. 163, 889-899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew L. J., Coley W., Cheng Y., Gallo V. (2010). Mechanisms of regulation of oligodendrocyte development by p38 mitogen-activated protein kinase. J. Neurosci. 30, 11011-11027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court F. A., Hewitt J. E., Davies K., Patton B. L., Uncini A., Wrabetz L., Feltri M. L. (2009). A laminin-2, dystroglycan, utrophin axis is required for compartmentalization and elongation of myelin segments. J. Neurosci. 29, 3908-3919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court F. A., Zambroni D., Pavoni E., Colombelli C., Baragli C., Figlia G., Sorokyn L., Ching W., Salzer J., Wrabetz L., et al. (2011). MMP2-9 cleavage of dystroglycan alters the size and molecular composition of Schwann cells domains. J. Neurosci. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Antonio M., Droggiti A., Feltri M. L., Roes J., Wrabetz L., Mirsky R., Jessen K. R. (2006). TGFbeta type II receptor signaling controls Schwann cell death and proliferation in developing nerves. J. Neurosci. 26, 8417-8427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo M. A., Price L. S., Alderson N. B., Ren X. D., Schwartz M. A. (2000). Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO J. 19, 2008-2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltri M. L., Wrabetz L. (2005). Laminins and their receptors in Schwann cells and hereditary neuropathies. J. Peripher. Nerv. Syst. 10, 128-143 [DOI] [PubMed] [Google Scholar]
- Feltri M. L., Scherer S. S., Wrabetz L., Kamholz J., Shy M. E. (1992). Mitogen-expanded Schwann cells retain the capacity to myelinate regenerating axons after transplantation into rat sciatic nerve. Proc. Natl. Acad. Sci. USA 89, 8827-8831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltri M. L., D'Antonio M., Previtali S., Fasolini M., Messing A., Wrabetz L. (1999). P0-Cre transgenic mice for inactivation of adhesion molecules in Schwann cells. Ann. New York Acad. Sci. 883, 116-123 [PubMed] [Google Scholar]
- Feltri M. L., Graus Porta D., Previtali S. C., Nodari A., Migliavacca B., Cassetti A., Littlewood-Evans A., Reichardt L. F., Messing A., Quattrini A., et al. (2002). Conditional disruption of beta 1 integrin in Schwann cells impedes interactions with axons. J. Cell Biol. 156, 199-209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltri M. L., Suter U., Relvas J. B. (2008). The function of RhoGTPases in axon ensheathment and myelination. Glia 56, 1508-1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragoso G., Robertson J., Athlan E., Tam E., Almazan G., Mushynski W. E. (2003). Inhibition of p38 mitogen-activated protein kinase interferes with cell shape changes and gene expression associated with Schwann cell myelination. Exp. Neurol. 183, 34-46 [DOI] [PubMed] [Google Scholar]
- Fragoso G., Haines J. D., Roberston J., Pedraza L., Mushynski W. E., Almazan G. (2007). p38 mitogen-activated protein kinase is required for central nervous system myelination. Glia 55, 1531-1541 [DOI] [PubMed] [Google Scholar]
- Gawlik K. I., Akerlund M., Carmignac V., Elamaa H., Durbeej M. (2010). Distinct roles for laminin globular domains in laminin alpha1 chain mediated rescue of murine laminin alpha2 chain deficiency. PLoS One 5, e11549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie C. S., Sherman D. L., Blair G. E., Brophy P. J. (1994). Periaxin, a novel protein of myelinating Schwann cells with a possible role in axonal ensheathment. Neuron 12, 497-508 [DOI] [PubMed] [Google Scholar]
- Goebbels S., Oltrogge J. H., Kemper R., Heilmann I., Bormuth I., Wolfer S., Wichert S. P., Mobius W., Liu X., Lappe-Siefke C., et al. (2010). Elevated phosphatidylinositol 3,4,5-trisphosphate in glia triggers cell-autonomous membrane wrapping and myelination. J. Neurosci. 30, 8953-8964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus-Porta D., Blaess S., Senften M., Littlewood-Evans A., Damsky C., Huang Z., Orban P., Klein R., Schittny J. C., Muller U. (2001). Beta1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron 31, 367-379 [DOI] [PubMed] [Google Scholar]
- Grinspan J. B., Marchionni M. A., Reeves M., Coulaloglou M., Scherer S. S. (1996). Axonal interactions regulate Schwann cell apoptosis in developing peripheral nerve: neuregulin receptors and the role of neuregulins. J. Neurosci. 16, 6107-6118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann K. S., Wende H., Paul F. E., Cheret C., Garratt A. N., Zurborg S., Feinberg K., Besser D., Schulz H., Peles E., et al. (2009). The tyrosine phosphatase Shp2 (PTPN11) directs Neuregulin-1/ErbB signaling throughout Schwann cell development. Proc. Natl. Acad. Sci. USA 106, 16704-16709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove M., Komiyama N. H., Nave K. A., Grant S. G., Sherman D. L., Brophy P. J. (2007). FAK is required for axonal sorting by Schwann cells. J. Cell Biol. 176, 277-282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levedakou E. N., Chen X. J., Soliven B., Popko B. (2005). Disruption of the mouse Large gene in the enr and myd mutants results in nerve, muscle, and neuromuscular junction defects. Mol. Cell Neurosci. 28, 757-769 [DOI] [PubMed] [Google Scholar]
- Li J., Bai Y., Ghandour K., Qin P., Grandis M., Trostinskaia A., Ianakova E., Wu X., Schenone A., Vallat J. M., et al. (2005a). Skin biopsies in myelin-related neuropathies: bringing molecular pathology to the bedside. Brain 128, 1168-1177 [DOI] [PubMed] [Google Scholar]
- Li S., Liquari P., McKee K. K., Harrison D., Patel R., Lee S., Yurchenco P. D. (2005b). Laminin-sulfatide binding initiates basement membrane assembly and enables receptor signaling in Schwann cells and fibroblasts. J. Cell Biol. 169, 179-189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. R., Webster H. D. (1973). Mitotic Schwann cells in developing nerve: their changes in shape, fine structure, and axon relationships. Dev. Biol. 32, 417-431 [DOI] [PubMed] [Google Scholar]
- Maurel P., Salzer J. L. (2000). Axonal regulation of Schwann cell proliferation and survival and the initial events of myelination requires PI 3-kinase activity. J. Neurosci. 20, 4635-4645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michele D. E., Barresi R., Kanagawa M., Saito F., Cohn R. D., Satz J. S., Dollar J., Nishino I., Kelley R. I., Somer H., et al. (2002). Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature 418, 417-422 [DOI] [PubMed] [Google Scholar]
- Moore S. A., Saito F., Chen J., Michele D. E., Henry M. D., Messing A., Cohn R. D., Ross-Barta S. E., Westra S., Williamson R. A., et al. (2002). Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature 418, 422-425 [DOI] [PubMed] [Google Scholar]
- Muntoni F., Brockington M., Torelli S., Brown S. C. (2004). Defective glycosylation in congenital muscular dystrophies. Curr. Opin. Neurol. 17, 205-209 [DOI] [PubMed] [Google Scholar]
- Newbern J. M., Li X., Shoemaker S. E., Zhou J., Zhong J., Wu Y., Bonder D., Hollenback S., Coppola G., Geschwind D. H., et al. (2011). Specific functions for ERK/MAPK signaling during PNS development. Neuron 69, 91-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodari A., Zambroni D., Quattrini A., Court F. A., D'Urso A., Recchia A., Tybulewicz V. L., Wrabetz L., Feltri M. L. (2007). Beta1 integrin activates Rac1 in Schwann cells to generate radial lamellae during axonal sorting and myelination. J. Cell Biol. 177, 1063-1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodari A., Previtali S. C., Dati G., Occhi S., Court F. A., Colombelli C., Zambroni D., Dina G., Del Carro U., Campbell K. P., et al. (2008). Alpha6beta4 integrin and dystroglycan cooperate to stabilize the myelin sheath. J. Neurosci. 28, 6714-6719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata T., Iijima S., Hoshikawa S., Miura T., Yamamoto S., Oda H., Nakamura K., Tanaka S. (2004). Opposing extracellular signal-regulated kinase and Akt pathways control Schwann cell myelination. J. Neurosci. 24, 6724-6732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson D. B., Bhaskaran A., Droggiti A., Dickinson S., D'Antonio M., Mirsky R., Jessen K. R. (2004). Krox-20 inhibits Jun-NH2-terminal kinase/c-Jun to control Schwann cell proliferation and death. J. Cell Biol. 164, 385-394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson D. B., Bhaskaran A., Arthur-Farraj P., Noon L. A., Woodhoo A., Lloyd A. C., Feltri M. L., Wrabetz L., Behrens A., Mirsky R., et al. (2008). c-Jun is a negative regulator of myelination. J. Cell Biol. 181, 625-637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton B. L., Miner J. H., Chiu A. Y., Sanes J. R. (1997). Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice. J. Cell Biol. 139, 1507-1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira J. A., Benninger Y., Baumann R., Goncalves A. F., Ozcelik M., Thurnherr T., Tricaud N., Meijer D., Fassler R., Suter U., et al. (2009). Integrin-linked kinase is required for radial sorting of axons and Schwann cell remyelination in the peripheral nervous system. J. Cell Biol. 185, 147-161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podratz J. L., Rodriguez E., Windebank A. J. (2001). Role of the extracellular matrix in myelination of peripheral nerve. Glia 35, 35-40 [DOI] [PubMed] [Google Scholar]
- Previtali S. C., Nodari A., Taveggia C., Pardini C., Dina G., Villa A., Wrabetz L., Quattrini A., Feltri M. L. (2003). Expression of laminin receptors in schwann cell differentiation: evidence for distinct roles. J. Neurosci. 23, 5520-5530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito F., Moore S. A., Barresi R., Henry M. D., Messing A., Ross-Barta S. E., Cohn R. D., Williamson R. A., Sluka K. A., Sherman D. L., et al. (2003). Unique role of dystroglycan in peripheral nerve myelination, nodal structure, and sodium channel stabilization. Neuron 38, 747-758 [DOI] [PubMed] [Google Scholar]
- Saito F., Masaki T., Saito Y., Nakamura A., Takeda S., Shimizu T., Toda T., Matsumura K. (2007). Defective peripheral nerve myelination and neuromuscular junction formation in fukutin-deficient chimeric mice. J. Neurochem. 101, 1712-1722 [DOI] [PubMed] [Google Scholar]
- Scherer S. S., Xu Y. T., Bannerman P. G., Sherman D. L., Brophy P. J. (1995). Periaxin expression in myelinating Schwann cells: modulation by axon-glial interactions and polarized localization during development. Development 121, 4265-4273 [DOI] [PubMed] [Google Scholar]
- Stirling C. A. (1975). Abnormalities in Schwann cell sheaths in spinal nerve roots of dystrophic mice. J. Anat. 119, 169-180 [PMC free article] [PubMed] [Google Scholar]
- Taveggia C., Zanazzi G., Petrylak A., Yano H., Rosenbluth J., Einheber S., Xu X., Esper R. M., Loeb J. A., Shrager P., et al. (2005). Neuregulin-1 type III determines the ensheathment fate of axons. Neuron 47, 681-694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson O., Moore C. J., Hussain S. A., Kleino I., Peckham M., Hohenester E., Ayscough K. R., Saksela K., Winder S. J. (2010). Modulation of cell spreading and cell-substrate adhesion dynamics by dystroglycan. J. Cell Sci. 123, 118-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallquist W., Plantman S., Thams S., Thyboll J., Kortesmaa J., Lannergren J., Domogatskaya A., Ogren S. O., Risling M., Hammarberg H., et al. (2005). Impeded interaction between Schwann cells and axons in the absence of laminin alpha4. J. Neurosci. 25, 3692-3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster H. D., Martin R., O'Connell M. F. (1973). The relationships between interphase Schwann cells and axons before myelination: a quantitative electron microscopic study. Dev. Biol. 32, 401-416 [DOI] [PubMed] [Google Scholar]
- Wrabetz L., Feltri M. L., Quattrini A., Imperiale D., Previtali S., D'Antonio M., Martini R., Yin X., Trapp B. D., Zhou L., et al. (2000). P(0) glycoprotein overexpression causes congenital hypomyelination of peripheral nerves. J. Cell Biol. 148, 1021-1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Denzer A. J., Hori H., Tanaka T., Anderson L. V., Fujita S., Fukuta-Ohi H., Shimizu T., Ruegg M. A., Matsumura K. (1996). Dystroglycan is a dual receptor for agrin and laminin-2 in Schwann cell membrane. J. Biol. Chem. 271, 23418-23423 [DOI] [PubMed] [Google Scholar]
- Yamauchi J., Chan J. R., Shooter E. M. (2003). Neurotrophin 3 activation of TrkC induces Schwann cell migration through the c-Jun N-terminal kinase pathway. Proc. Natl. Acad. Sci. USA 100, 14421-14426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Bierman J., Tarumi Y. S., Zhong Y. P., Rangwala R., Proctor T. M., Miyagoe-Suzuki Y., Takeda S., Miner J. H., Sherman L. S., et al. (2005). Coordinate control of axon defasciculation and myelination by laminin-2 and -8. J. Cell Biol. 168, 655-666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W. M., Feltri M. L., Wrabetz L., Strickland S., Chen Z. L. (2005). Schwann cell-specific ablation of laminin gamma1 causes apoptosis and prevents proliferation. J. Neurosci. 25, 4463-4472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W. M., Chen Z. L., North A. J., Strickland S. (2009). Laminin is required for Schwann cell morphogenesis. J. Cell Sci. 122, 929-936 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.