Abstract
Clinical studies on cognitive behavioral therapy (CBT) that include schizophrenia patients primarily on the basis of negative symptoms are uncommon. However, those studies are necessary to assess the efficacy of CBT on negative symptoms. This article first gives an overview of CBT on negative symptoms and discusses the methodological problems of selecting an adequate control group. Furthermore, the article describes a clinical study (the TONES-Study, ISRCTN 25455020), which aims to investigate whether CBT is specifically efficacious for the reduction of negative symptoms. This multicenter randomized clinical trial comparing CBT with cognitive remediation (CR) for control of nonspecific effects is depicted in detail. In our trial, schizophrenia patients (n = 198) participated in manualized individual outpatient treatments. Primary outcome is the negative syndrome assessed with the positive and negative syndrome scale, analyzed with multilevel linear mixed models. Patients in both groups moderately improved regarding the primary endpoint. However, against expectation, there was no difference between the groups after treatment in the intention to treat as well as in the per-protocol analysis. In conclusion, psychotherapeutic intervention may be useful for the reduction of negative symptoms. However, there is no indication for specific effects of CBT compared with CR.
Keywords: schizophrenia, cognitive behavioral therapy, cognitive remediation, negative symptoms, randomized clinical trial
Introduction
The treatment of negative symptoms is a major challenge for mental health care in schizophrenia. Even 1 year after the last episode, up to 50% of the patients suffer from negative symptoms.1 Negative symptoms have been divided into primary and secondary symptoms.2,3 Negative symptoms are viewed as secondary if they are a result of side effects of medications or are a psychological reaction to psychotic symptoms or a consequence of cooccurring depressive symptoms. In the absence of these factors, negative symptoms are thought to be primary, ie, associated with the disorder itself. Negative symptoms in general are strong predictors of a poorer prognosis, poorer social outcome, and poorer quality of life.4 Negative symptoms are present in the prodromal phase, during psychosis, and after the remission of positive symptoms.5–7 According to meta-analyses, medication has only limited effects on negative symptoms. Leucht et al8 showed that only 4 second-generation drugs were more efficacious than first-generation drugs for negative symptoms with effect sizes ranging between 0.13 and 0.32. In addition, the comparison of second-generation drugs with placebo results in a standardized mean difference of 0.39 favoring second-generation drugs.9 These findings are based on studies examining patients with predominantly positive symptoms and do not allow concluding that second-generation drugs are effective for persistent negative symptoms. On this background, the Measurement and Treatment Research to Improve Cognition in Schizophrenia initiative10 aims to develop new treatments for improving negative symptoms and cognitive impairments.4
Efficacy of Cognitive Behavioral Therapy for Negative Symptoms
According to Mäkinen’s et al review,11 the effects of family interventions or psychoeducation on negative symptoms are unsatisfying. However, several reviews and meta-analyses summarized evidence for the efficacy of cognitive behavioral therapy (CBT),12–17 which led to the recommendation of CBT for routine care (RC) in evidence-based treatment guidelines. For example, Rector and Beck14 report in their review, 3 studies18–20 analyzing change in negative symptoms. These studies that compare CBT as active treatment with RC as nonactive treatment resulted in large effect sizes between 0.88 and 1.19. When CBT was compared with control group like supportive counseling, which provides similar therapeutic attention, this resulted in lower effect sizes for CBT ranging from 0.21 to 0.47.14 This finding indicates that nonspecific control conditions have active therapeutic ingredients and are leading to unspecific effects on symptoms, contrary to the older assumption that they are therapeutically inert. Regarding negative symptoms, the meta-analysis of Wykes et al16 reported a mean-weighted effect size of 0.44 for CBT based on 23 randomized clinical trials. From these 23 studies, only 2 studies targeted negative symptoms as a primary outcome,21,22 the remaining studies with positive symptoms as primary outcome. Thus, the majority of studies are specifically tailored to address positive symptoms like delusions or hallucinations and are based on a cognitive model of psychotic symptoms. The therapeutic strategies applied in those studies19,23 cover interventions like normalization, thought linkage, behavioral experiments, cognitive restructuring, and reattribution of voices to the self. Thus, as in drug trials, the primary outcome of the majority of CBT studies has been the positive syndrome while negative symptoms were assessed as secondary outcome measure. These studies show that patients whose positive symptoms have been treated show also improvements for negative symptoms. However, the reported effects of CBT on negative symptoms in studies mainly designed to address positive symptoms do not answer the question whether CBT is an effective intervention for persistent negative symptoms. The Wykes’ et al meta-analysis could identify only one study22 which assessed negative symptoms as a primary outcome and applied individual cognitive behavioral therapy and not group therapy or a combination of clinical interventions. The study reported a large effect on negative symptoms but has neither a randomized design nor a systematic recruitment. Further the sample size was very small. Finally, the latest National Institute for Health and Clinical Excellence (NICE) meta-analysis24 reports only small effect sizes for CBT on negative symptoms of 0.01 for posttreatment and of 0.31 for follow-up assessments. To summarize, negative symptoms constitute a key element of overall symptoms, weakening the patients’ ability to manage everyday activities and affecting their quality of life. The literature review shows that CBT might have the potential to ameliorate negative symptoms of schizophrenia disorders. However, so far there are no methodologically sound clinical trials on CBT, which address negative symptoms as a primary outcome. Based on the heterogeneous findings outlined above, there is clearly a need to further investigate CBT for the reduction of negative symptoms.
Cognitive Models of Negative Symptoms
The cognitive model25 of primary negative symptoms echoes the work of Bleuler.26 Bleuler viewed these as being the primary symptoms of schizophrenia and suggested that they represented a defensive position in relation to intolerable stress. Our own findings are in line with those interpretations. Wittorf et al27 found that the relationship between negative symptoms and specific self-concepts was consistently significant, even when the contribution of depression was partialed out. According to Kingdon and Turkington,25 negative symptoms may in fact be more widespread in people with high levels of vulnerability and a low capability to cope with stress, who often develop social phobia, agoraphobia, and tendencies toward institutionalization. In line with these assumptions, Kingdon and Turkington,25 Beck et al,28 and Rector et al29 propose several cognitive therapy explanations for negative symptoms. In their view, affective flattening may develop from demoralization, perhaps related to past traumatic events. Furthermore, avolition could be a result of the perception of being under pressure and subject to failing expectations. However, there have been only few approaches to systematically address negative symptoms by means of manualized cognitive behavioral interventions. The treatment conceptualization of Bailer et al22 focuses on symptom-management, activity scheduling, identification of stressors, problem-solving training, social skills training (SST), relaxation techniques, and cognitive remediation (CR). According to the more current literature,29 the targets of CBT in patients with negative symptoms are generalized expectations of failure and discomfort in social situations, which might be associated with a lack of motivation, avolition, and anergia. Grant et al30 found that defeatist belief endorsements were mediators in the relationship between cognitive impairment and negative symptoms. In addition, Grant et al31 found that asocial beliefs but not neurocognition and emotion perception are associated with social functioning. Reducing defeatist beliefs by means of cognitive treatment strategies could therefore enhance patients’ activity rate. A further target are social cognitive deficits regarding emotion detection, emotion expression, and social schemata which can be viewed as mediators between neurocognition, functioning, and negative symptoms like affective blunting as well as social and emotional withdrawal.32 Fostering adequate perception of social situations, improving emotion detection and expression, and training of required skills are plausible targets of intervention for the reduction of negative symptoms.33
Which Control Condition is Adequate?
We considered several options for implementing a control group for the CBT, which have different implications for the interpretation of results. (1) Treatment as usual (TAU): when compared with a CBT + TAU condition, TAU is a “no treatment control group” controlling for spontaneous change of symptoms and effects of being in a trial. Effects of therapeutic commitment are not controlled by this control condition. Superiority of the CBT would imply only that any treatment can improve the course of symptoms. (2) Supportive Treatment (ST): ST controls for therapeutic commitment in addition to the factors controlled for by TAU. ST conditions are usually viewed as a kind of placebo intervention in the sense that specific factors of the test treatment are not addressed by this kind of control treatment. However, it is difficult to describe the content of ST. In particular, this is a major problem when the treatment is conducted over the long term. In addition, expectations from either the patient or the therapist whether or not this is a helpful and efficacious treatment vary to a great extent but can be responsible for different treatment effects. Finally, it is unclear whether some forms of ST might be effective treatments of negative symptoms as discussed by Penn et al.34 It is plausible to assume that structuring patients’ schedules, having a trustworthy person to talk to, and discussing day to day problems are active ingredients in the treatment of negative symptoms that should be explicitly addressed and do not represent “unspecific” factors. Thus, a more sophisticated “ST” would be a kind of active control condition enhancing the number of participants requested. (3) SST: published effects of SST for the reduction of negative symptoms indicate that SST has a moderate effect on negative symptoms (ES = 0.40, Kurtz et al35). Thus, a study investigating differences between CBT and SST would require a very large sample size, which is not available under most circumstances. (4) CR: at the time of our trial application in the year 2004, CR could have been viewed as a suitable control condition to CBT for the reduction of negative symptoms. Our search in the literature has not revealed any consistent evidence for efficacy of CR for the reduction of negative symptoms.36 Although there were studies with small samples which showed moderate effects of CR on negative symptoms,37,38 the larger studies available at that time39,40 did not find effects. In particular, the CR to be used in the present study is comparable to the study of Wölwer et al,33 which did also show no reduction of negative symptoms. The review of McGurk et al41 found a mean effect for symptom reduction of 0.28. However, no effect size for negative symptoms is reported.41 Nevertheless, the latest NICE meta-analysis24 of the effects of CR on negative symptoms demonstrated a small effect of 0.19.
The objective of our trial described below is to analyze whether CBT is efficacious for the reduction of primary negative symptoms in a randomized, controlled, single-blind multicenter study. In conclusion of the literature outlined above, we chose a CR control group. To summarize, CR controls for spontaneous change, for the effect of being in a trial, and for therapeutic commitment. CR is a plausible and acceptable treatment for this patient group as deficits regarding neuropsychological functions are common and represent a limiting factor for the reintegration.
Methods
Design
This study is a randomized clinical trial (the TONES-Study, ISRCTN 25455020) with 2 parallel groups (allocation ratio 1:1), blinded assessment, conducted in 3 study centers in Germany. After trial commencement, there were no changes to methods or outcome assessment.
Participants
A systematic recruitment strategy was applied in the catchment areas of the Departments of Psychiatry and Psychotherapy at the Universities of Düsseldorf, Frankfurt, and Tübingen between April 2006 and April 2008. All patients with psychotic disorders of participating departments were screened for eligibility for study participation. Patients were initially identified on the basis of clinical documentation systems and approached by their treatment team and a study research assistant for receiving consent for study participation. If patients gave their consent, the structured baseline assessment was conducted. The inclusion criteria were (1) Schizophrenia (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, [DSM-IV]) confirmed by a structured clinical interview (SCID-I); (2) At least one moderate negative symptom as operationalized by a modified negative syndrome (MNS) factor42 of the positive and negative syndrome scale (PANSS), ie, a PANSS-MNS score of ≥ 10, (3) Fluent speaker in German language; (4) status as outpatient; and (5) willingness to give informed consent. Exclusion criteria were (1) any PANSS positive symptom (P1–P7) ≥ 6; (2) severe depression as indicated by PANSS G6 ≥ 6; (3) any extrapyramidal symptom of at least moderate intensity as assessed with the Udvalg for Klinske Undersøgelser (UKU) side effect rating scale; (4) age < 18 or > 55; (5) verbal IQ < 80 assessed by a German multiple-choice vocabulary test; (6) organic brain disease; (7) diagnosis of substance abuse or substance dependence according to DSM-IV/SCID-I as primary clinical problem including the intention to initiate treatment of substance abuse/dependence; and (8) travel time to the study center ≥1 hour.
Patients were randomized only if they gave written informed consent to participate in the study and fulfilled all inclusion criteria. The study protocol was approved positively by the local ethics committees of each study center.
Interventions
All patients received routine psychiatric outpatient care outside of the study including antipsychotic medication and regular visits with a psychiatrist. The study intervention was either CBT or CR, each conducted as outpatient psychological intervention of 20 sessions in a period of 9 months.
CBT applies general principles of cognitive behavior therapy (eg, case formulation based on a cognitive model, goal setting, discussion of cognitive processes, homework assignments, role-play) for the treatment of negative symptoms. Therapists have been trained to establish a supportive, nonconfrontative relationship. In the first treatment phase, CBT aims at developing a shared formulation and a shared concept of treatment. This includes discussion of symptoms, cognitive impairment, and subjective theories in a normalizing way, as well as the role of medication and the principles of early signs management. For the second treatment phase, we conceptualized 5 treatment modules addressing single negative symptoms: initiative/planning, social activity, emotional participation, emotional expression, and speech activity. These modules reflect the heterogeneity of negative symptoms and allow for flexibility in designing individual treatment plans. For each patient, 2 of these modules have been selected for the second treatment phase. This selection was guided by the symptom profile of the patient, the functional consequences of the symptoms, and the willingness of the patient to work on these symptoms and modules. Thus, the treatment manual is characterized by a high degree of flexibility to come up to the patient’s needs and requirements. According to Rector et al,29 cognitive bias contributes to negative symptoms, independent of positive symptoms. Specifically, the authors postulate a cognitive set characterized by low expectancies for pleasure, success, and acceptance, as well as the perception of limited resources, which contributes to the persistence of negative symptoms. Based on this cognitive model, our treatment aims at reducing a generalized expectancy of failure (defeatist beliefs) and improving social cognitive skills like emotion detection and expression. The treatment concept of the CBT has been described in detail elsewhere.43,44
The CR has been adapted from an earlier study.33 It applies the principles of restitution as well as compensation. The program follows the principles of errorless learning, overlearning, and immediate positive feedback (verbal), which are combined with alternative cognitive strategies such as systematic elaboration of information, verbalization, self-instruction, and structuring of information. The strategies are mostly practiced during deskwork in a first step and are then trained by computer tasks of the “cognition package/CogPack.” Based on these principles, the program comprises 3 sections of 5 sessions, which are highly structured: training of attention, memory, and executive functions. Social or emotional training aspects such as facial affect recognition are explicitly not part of the CR. An initial session is used to introduce the CR and 4 session aims at reviewing treatment goals and achievements and at motivating the participant to engage in transfer activities.
Therapists
Therapists: 5 (4 female, 1 male) specifically trained clinical psychologists (master’s level) conducted the supervised study therapies. The same therapists delivered CBT and CR. Two of the therapists were aged 20–30 years and 3 were aged 30–40 years. Three of the therapist already had completed a 3-year formal training on cognitive behavioral therapy. The 2 younger therapists were in their second year of training. Three of the therapists were classified as “experienced” in conducting cognitive behavioral therapy in psychotic patients, ie, they had completed 10-50 treatments prior to the beginning of the study. Two of the therapists were less experienced. All therapists were trained by the authors S.K. and A.W. in the application of the treatment manuals prior to the first therapy session.
Assessment of Adherence to the Manual
Therapists received regular supervision of consultant clinical psychologists. Therapists filled in structured session reports after each session. Sessions were audiotaped if patients gave written informed consent. An independent rater evaluated one randomly selected session protocol based on these audiotapes, which were available for 78 CBT patients. Regarding the categorization of sessions as fully, partially or insufficiently adherent to the manual an intraclass correlation coefficient (ICC) was computed. An ICC of 0.74 (95% CI: 0.59-0.83, P < .001) indicated sufficient reliability of the session protocols that were chosen for determining manual adherence of complete therapies. An individual study therapy was considered as having been conducted according to manual if (1) a patient has attended at least 14 treatment sessions, and 2 thirds of these sessions (2) had a duration >40 and <60 minutes, (3) had made use of manualized treatment material, and (4) showed at least an adequate cooperation of the patient. These criteria were derived from the session protocols. The therapeutic alliance was assessed at the end of the third, sixth, ninth, 12th, and 15th session of the study therapies using items from the “Bern Post Session Report 2000” (BPSR).45
Outcome Assessment
The primary endpoint is a MNS factor (PANSS-MNS, PANSS items N1, N2, N3, N4, N6, G7, G16) of the PANSS at the time of 12 months after inclusion. These items are derived from factor analysis42 and psychopathologically more homogeneous than the standard PANSS negative syndrome.
Additional symptom measures have been assessed as secondary endpoints: negative symptoms measured with the Scale for the Assessment of Negative Symptoms (SANS) and the standard negative scale of the PANSS, positive symptoms assessed with the standard positive scale of the PANSS, depressive symptoms evaluated with the Calgary Depression Rating Scale for Schizophrenia (CDSS), clinical global impression measured with the Clinical Global Impression Scale (CGI), and symptom self ratings assessed with the Symptom Checklist (SCL-90-R).
PANSS, SANS, CDSS, CGI, type and dosage of antipsychotic medication, and medication compliance46 were documented monthly; side effects (UKU) have been assessed at baseline, 6- and 12-month follow-up. The study protocol did not regulate the medication regimen. Medication was prescribed by physicians independent of the study team. Type and dose of pharmacological interventions have been modified dependent on the patient’s needs during the course of study.
In particular in studies on severe mental illness like schizophrenia, the investigation of negative and adverse treatment effects is essential in order to establish high standards of safety. Due to the high vulnerability of these patients, psychotherapy might lead to overstimulation, increased stress level and thus to symptom exacerbation or relapse. The following events have been defined as severe adverse events (SAE): (1) death caused by suicide, (2) suicide attempt, (3) suicidal crisis which has been operationalized as rating 2 in item 8 (explicit plan for serious suicidal activity without suicide attempt) of the CDSS, and (4) severe symptom exacerbation, operationalized as CGI item 1 ≥ 6 and item 2 ≥ 6. Occurrence of SAE have been assessed and documented regularly in monthly intervals as part of the follow-up examination.
Interrater Reliability
The 4 raters were psychologist or physicians with at least 1 year of clinical experience in the treatment of patients with psychotic disorders. The raters were trained by the author A.W. prior to the beginning of the study. This training included the discussion and rating of video-based PANSS interviews. Afterward, 3 video-based PANSS interviews, which were not part of the training, were than independently assessed by the 4 raters and subjected to the reliability analysis. For the PANSS-MNS, the ICC for the interrater reliability was estimated using a SAS-Macro. The ICCs were 0.92 (95% CI: 0.56–1.00) before beginning of the study (4 raters included), 0.93 (95% CI: 0.62–1.00) after 1 year (same 4 raters included), and 0.92 (95% CI: 0.63–1.00) at the final assessment (one additional rater). In order to control for intrarater shift over time, we computed ICC for each rater over time and found an average ICC for all raters of 0.98 (95% CI: 0.86–1.00).
Randomization
A permuted block design with random blocks stratified by study center has been applied. The allocation sequence has been generated by the Institute for Medical Biometry using a computerized algorithm and was stored by CenTrial. The research assistant responsible for assessments reported inclusion of new patients by fax or email to CenTrial which returned the result of the randomization only to the therapist in order to keep the assessor blind regarding the study condition. The therapist then gives the information about treatment allocation to the patient.
Blinding
This is a single-blind study where only the assossors are not aware of the patients’ treatment condition. Therapists were not involved in assessments. The assessors instructed patients not to talk about their treatment during assessment. In case of SAE (eg, suicidality), assessors and therapists should share information about symptoms. However, even in these cases, therapists should not disclose the patient’s treatment condition as possible. The assessors filled in a blindness protocol for unintended unblindings and guessed the study condition of the patient after each assessment. In 6 CBT and in 9 CR cases, unblinding has been documented (Fisher exact test, P = .593). Among 1569 guesses (from a total of 1653 assessments), 951 (60.6%; 95% CI: 58.1–63.0%) were correct. Fifty percent of correct guesses are expected by chance.
Sample Size Calculation
We calculated the sample size based on the findings of Klingberg et al.47 Regarding negative symptoms, we found an effect size of 0.41 (posttreatment difference between a mean of 1.70 for TAU and a mean of 1.35 for CBT group with a pooled SD of 0.85) which is similar to a 0.40 effect reported by Wykes et al16 for the reduction of negative symptoms by individual CBT. As software for sample size calculation for the analysis of longitudinal data using multilevel mixed models is not available, we calculated the sample size for classical ANOVA using nQuery 4.0. For alpha .05 and beta .20, a sample size of 74 in each group will be required. In order to compensate for a loss to follow-up of 25%, we decided to include 2 × 99 patients. With a sample size of 198 individuals (99 each therapy group), 12 assessments per patient, one primary analysis variable (therapy), and one covariable (center), the power for a Mixed Models should be sufficient.
Data Management and Quality Assurance
A good clinical practice (GCP) compliant electronic case report form with remote data entry was used (www.koordobas.de; Institute of Medical Biometry, University of Tübingen). CenTrial (a trial supporting company of the Medical Faculty, University of Tübingen) took responsibility for on site monitoring. Each study center was visited 8 times. The monitoring focused on the process of obtaining informed consent, on diagnostic procedures, the completeness of assessments, and on appropriate documentation of adverse events. Whenever possible, source data verification was applied. Assessments were regarded as valid only if visits have been conducted within a time frame of ±1 week of the scheduled point in time. The database was closed for the final analysis on June 23, 2009.
Statistical Methods
The statistical analysis was carried out according to a preestablished analysis plan. The intention-to-treat sample includes all randomized patients. The per-protocol population includes only those randomized patients who have still been available at the 11- or 12-month assessment and who where regarded as treatment completer.
The primary endpoint analysis was conducted with linear mixed models (LMMs) using the intercept and assessments as random effects. The final models were optimized by defining the covariance structure via assessments as repeated effects. SAS “Proc-Mixed” with 2-sided significance tests were used for all analyses. Hedge’s g were estimated from the LMM according to Feingold.48 Only the primary endpoint was subjected to confirmatory analysis. Secondary endpoints were analyzed using standard ANCOVAs and will be interpreted as exploratory.
Results
Participant Flow
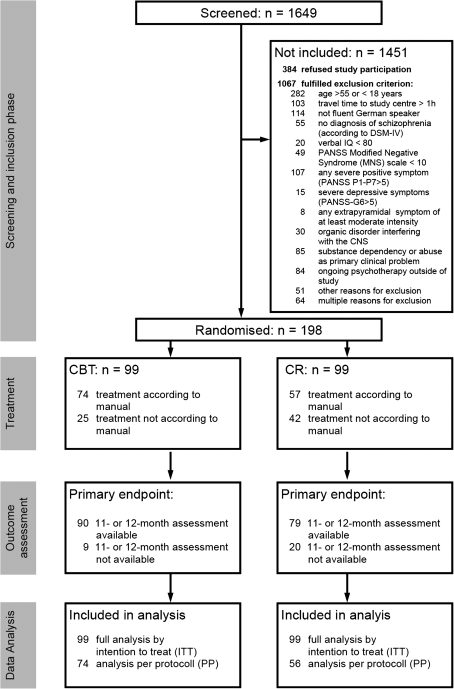
One thousand six hundred and forty-nine patients with a potential diagnosis of schizophrenia were identified by the systematic recruitment strategy and assessed with regard to the inclusion criteria (figure 1). 198 (12% of the screened population) gave written informed consent and fulfilled all inclusion criteria.
Fig. 1.
CONSORT flow chart.
Baseline Data
Table 1 shows the sample description at randomization. In order to control for the success of the randomization, we analyzed the differences between the study conditions. There were no significant differences between the groups.
Table 1.
Sample Description
| Statistic | Total Sample | CBT | CR | Test (2-Tailed) | P | |
| N | 198 | 99 | 99 | |||
| Anamnestic information | ||||||
| Age (y) at study inclusion | Mean (SD) | 36.9 (9.9) | 37.6 (9.8) | 36.2 (10.0) | t test | .32 |
| Age (y) at first hospitalizationa | Mean (SD) | 24.4 (8.9) | 24.5 (8.7) | 24.3 (9.1) | t test | .87 |
| Gender (female) | % | 43.9 | 41.4 | 46.5 | Fishers exact test | .57 |
| First hospitalization | % | 4.5 | 4.0 | 5.1 | Fishers exact test | 1.00 |
| Number of previous hospitalizations | Median (range) | 3 (0-48) | 3 (0-48) | 3 (0-47) | U testb | .65 |
| Mother and/or father with schizophreniaa | % | 8.1 | 9.1 | 7.1 | Fishers exact test | .79 |
| Diagnosis (according to DSM-IV/SCID I) | ||||||
| Schizophrenia, paranoid type | % | 60.1 | 58.6 | 61.6 | Fishers exact test | .77 |
| Schizophrenia, residual type | % | 31.8 | 33.3 | 30.3 | Fishers exact test | .76 |
| Schizophrenia, other types | % | 8.1 | 8.1 | 8.1 | Fishers exact test | 1.00 |
| Comorbidity Axis I (according to DSM-IV/SCID-I) | % | 15.7 | 16.7 | 15.2 | Fishers exact test | 1.00 |
| Symptoms | ||||||
| Global assessment of functioning scale (GAF) | Mean (SD) | 59.2 (8.8) | 59.3 (9.6) | 59.2 (7.9) | t test | .96 |
| Positive and negative syndrome scale (PANSS) | ||||||
| Modified negative syndrome score (PANSS-MNS) | Mean (SD) | 3.0 (0.8) | 3.1 (0.8) | 3.0 (0.7) | t-test | .50 |
| Positive syndrome (standard scale P1–P7) | Mean (SD) | 1.5 (0.4) | 1.5 (0.4) | 1.5 (0.4) | t test | .92 |
| Negative syndrome (standard scale N1–N7) | Mean (SD) | 2.6 (0.7) | 2.7 (0.7) | 2.6 (0.7) | t test | .82 |
| General psychopathology (standard scale G1–G16) | Mean (SD) | 1.9 (0.5) | 1.9 (0.4) | 1.9 (0.5) | t test | .66 |
| Persistence of negative symptoms >6 mo | % | 84.9 | 86.9 | 82.8 | Fishers exact test | .82 |
| Symptom checklist SCL-90-R, GSI | Mean (SD) | 0.9 (0.6) | 1.0 (0.7) | 0.9 (0.5) | t test | .25 |
| Verbal-IQ (MWT-B) | Mean (SD) | 107.6 (15.8) | 106.1 (15.8) | 109.2 (16.2) | t test | .18 |
| Medication | ||||||
| Dose of antipsychotic medication (converted to mg chlorpromazine) | Mean (SD) | 543 (400) | 525 (333) | 561 (460) | U test | .74 |
| Medication-compliance (% favorable) | % | 88.7 | 91.8 | 85.6. | Fishers exact test | .18 |
| Social situation | ||||||
| Occupation | ||||||
| Employed, without support | % | 24.2 | 24.2 | 24.2 | Fishers exact test | 1.00 |
| Supported employment/unemployed/housewife | % | 75.8 | 75.8 | 75.8 | ||
| Financial support | ||||||
| None | % | 21.2 | 24.2 | 18.2 | Fishers exact test | .44 |
| Supported by family or government | % | 78.3 | 74.8 | 81.8 | ||
| Living condition | ||||||
| Unsupported | % | 68.2 | 67.7 | 68.7 | Fishers exact test | .72 |
| Supported (by family/supported housing) | % | 22.7 | 20.2 | 25.3 | ||
| Social contact | ||||||
| Frequent contacts (once a week or more) | % | 44.4 | 42.4 | 46.5 | Fishers exact test | .22 |
| Rare contacts (less than once a week) | % | 55.6 | 57.6 | 53.5 | ||
| Relatives | ||||||
| Rare contacts (less than weekly/no relatives) | % | 9.1 | 9.1 | 9.1 | Fishers exact test | .88 |
| With contact (once a week or more) | % | 90.9 | 90.9 | 90.9 |
Note: CBT, Cognitive behavioral therapy; CR, Cognitive remediation; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; SCID, Structured clinical interview for DSM-IV; GSI, Global severity index; MWT-B, Multiple-choice vocabulary test.
More than 10% missing values.
U test has been computed because of not normal distribution.
Study Treatment
In the CBT, a mean of 16.6 sessions have been conducted with mean duration of 51.8 minutes; in the CR, 13.7 sessions with 47.5 minutes duration. 1329 CBT sessions and 1044 CR sessions were audiotaped. In the CBT, 74 patients received treatment according to the manual; in the CR, 57 (fisher exact test, P = .024, see figure 1).
The mean alliance scores across the sessions ranged between 1.3 and 1.9 on a −3 to 3 scale for the CBT and the CR condition respectively. A repeated measurement analysis of variance (rmANOVA) with the 3 study centers and the 2 treatment conditions as between-subject factors and the therapeutic alliance scale of the BPSR as the dependent variable revealed no significant time × study center × treatment condition interaction (F8, 224 = 0.692, P = .698). Furthermore, there were no significant main effects for the factors time (P = .962), study center (P = .146), and treatment condition (P = .578). This rmANOVA was based on the 70 CBT and the 50 CR patients who completed the BPSR therapeutic alliance items at the end of session 3, 6, 9, 12, and 15.
Medication
At randomization, all patients were on antipsychotic medication. In the first 6 months after inclusion, 96 CBT patients and 91 CR patients still accepted any antipsychotic medication. Between the seventh and the 12th month, 90/99 (91%) accepted antipsychotic medication in the CBT group and 80/99 (81%) in the CR group. These differences are not significant (Fisher’s exact test, P = .213 and P = .065). The cumulated median chlorpromazine equivalence doses49 up to the 12-month assessment were 137 600 in the CBT and 135 400 in the CR (P = .785, Wilcoxon-Test). Sixty-six CBT and 72 CR patients received antidepressant medication at randomization. The proportion of patients with favorable or adequate medication compliance in the CBT was 94/99 between month 2 and 6 and 90/99 between month 7 and 12. In the CR, the proportions were 88/99 and 81/99 (Fisher’s exact tests, P = .238 and .117). The number of patients reporting at least moderate side effects of medication was 54/99 (6-month assessment) and 61/99 (12-month assessment) in the CR and 64/99 and 72/99 in the CBT (Fisher’s exact test, P = .353 and P = .509).
Adverse Events
Twenty-three adverse events (12 CBT, 11 CR) were observed in 15 patients (10 CBT, 5 CR, P = 0.31, Hazard ratio = 2.02, 95% CI: = 0.69–5.90). One CBT patient died after aspiration due to vomiting which was regarded as accident and not as adverse event of treatment. Details regarding adverse events will be published elsewhere.
Outcomes
Figure 2 and table 2 report the major result of this study. Ninety of 99 CBT patients and 79 of 99 CR patients (Fisher exact test, P = .05) were available at the 11- or 12-month assessment.
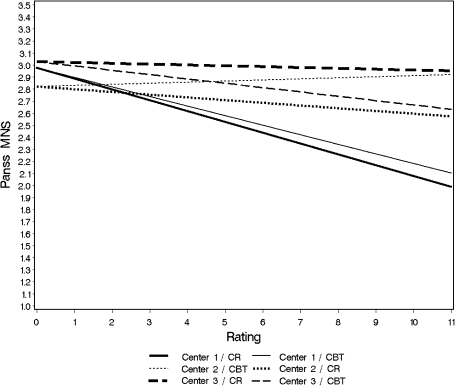
Fig. 2.
Multilevel linear mixed model for the primary endpoint: estimated course of negative symptoms (positive and negative syndrome scale-Modified negative syndrome [PANSS-MNS]) from baseline (assessment 0) over 10 monthly ratings to 1-year follow-up (assessment 11) for Cognitive behavioral therapy (CBT) and Cognitive remediation (CR) stratified for centers.
Table 2.
Multilevel Linear Mixed Model for the Primary Endpoint (PANSS-MNS), Analysis of Study Condition and Treatment Center
| Center | Therapy | n | Estimated at Baseline | Slopea | Estimated at 12 mo | Estimated Difference (CBT-CR) at 12 mo | P | Estimated Effect size of CBT vs CR at 12 mo | 95% CI for Effect Size |
| 1 | CR | 33 | 2.98 | −0.090 | 2.00 | 0.12 | .39 | 0.17 | −0.31–0.65 |
| CBT | 33 | 2.98 | −0.078 | 2.12 | |||||
| 2 | CR | 33 | 2.83 | −0.024 | 2.57 | 0.36 | .02 | −0.43 | −0.92–0.06 |
| CBT | 33 | 2.83 | 0.009 | 2.94 | |||||
| 3 | CR | 33 | 3.06 | −0.009 | 2.97 | −0.35 | .02 | 0.45 | −0.03–0.94 |
| CBT | 33 | 3.06 | −0.040 | 2.62 | |||||
| Overall | CR | 99 | 2.97 | −0.045 | 2.48 | 0.090 | .36 | 0.12 | −0.16–0.40 |
| CBT | 99 | 2.97 | −0.037 | 2.57 |
Note: CBT, Cognitive behavioral treatment; CR, Cognitive remediation; PANSS, positive and negative syndrome scale; MNS, Modified negative syndrome.
Estimated difference per month.
LMM for the primary endpoint (Intention To Treat analysis, ITT): the model assumes identical intercepts (estimated baseline values) for all patients of the same center based on random allocation. Patients of center 2 had the lowest values for the negative syndrome (mean item score 2.83) at the start of the study, followed by center 1 (2.98, difference to center 1: 0.15) and center 3 (3.06, difference to center 1: 0.23). The interaction between center and therapy was strong (figure 2). Only center 3 reached the expected superiority of the CBT with 0.35 points less at the end of the study compared with CR. In center 1, there was nearly no difference between the 2 treatments. In center 2, a superiority of CR of 0.36 points over CBT was observed. The pre-post effect sizes in the total sample were −0.42 (95% CI: −0.70 to −0.13) for the CBT and −0.53 (95% CI: −0.82 to −0.25) for the CR condition. In order to investigate factors that might be associated with the differences between the centers, several fixed (all baseline characteristics as listed in table 1) and time-varying effects (course of symptoms, therapeutic alliance, number of sessions conducted) were analyzed but no indication for moderation effects could be found.
Seventy-four of 99 CBT patients and 56 of 99 CR patients were available for the per-protocol analysis (Fisher exact test, P = .01) which confirms the overall results of the ITT-analysis. The negative symptom score decreased in both groups without significant difference posttreatment (estimated baseline value: 2.97 decreasing to 2.553 at 12 mo in CR and 2.57 at 12 mo in CBT, P = .905). The center effect was observed again.
Table 3 gives an overview over the analysis on the secondary endpoints. For each variable, ANCOVA has been computed. There was no significant difference between the study groups regarding the secondary endpoints.
Table 3.
Analysis of Secondary Endpoints, ANCOVAs Comparing Post Treatment Values Between Groups Controlling for Baseline Value and Study Center
| CBT | CR | Effect sizea | Pb | n | |||||
| Pre | Post | dc (95% CI) | Pre | Post | dd (95% CI) | (95% CI) | |||
| PANSS positive | 1.5 (0.4) | 1.5 (0.5) | 0.01 (−0.27 to 0.29) | 1.5 (0.4) | 1.6 (0.7) | 0.09 (−0.19 to 0.37) | −0.09 (−0.37 to 0.19) | .500 | 198 |
| PANSS negative | 2.7 (0.7) | 2.3 (0.8) | −0.47 (−0.76 to −0.19) | 2.6 (0.7) | 2.3 (0.7) | −0.59 (−0.84 to −0.27) | 0.07 (−0.21 to 0.34) | .642 | 198 |
| PANSS general | 1.9 (0.4) | 1.8 (0.4) | −0.31 (−0.59 to –0.03) | 1.9 (0.5) | 1.8 (0.5) | −0.28 (−0.56 to 0.00) | −0.02 (−0.31 to 0.25) | .950 | 198 |
| SANS affective blunting | 2.3 (0.9) | 2.1 (1.1) | −0.19 (−0.47 to 0.09) | 2.4 (0.9) | 2.0 (1.0) | −0.37 (−0.66 to −0.09) | 0.04 (−0.24 to 0.32) | 0.263 | 198 |
| SANS alogia | 1.3 (1.0) | 0.9 (0.9) | −0.44 (−0.73 to −0.16) | 1.4 (1.0) | 0.9 (1.0) | −0.50 (−0.79 to −0.22) | −0.03 (−0.31 to 0.25) | 0.862 | 198 |
| SANS apathy | 2.2 (1.0) | 2.0 (1.1) | −0.24 (−0.52 to 0.04) | 2.2 (0.8) | 1.9 (0.9) | −0.31 (−0.59 to –0.03) | 0.06 (−0.22 to 0.34) | 0.812 | 198 |
| SANS anhedonia | 2.8 (1.0) | 2.6 (1.1) | −0.17 (−0.45 to 0.11) | 2.6 (1.0) | 2.6 (1.0) | −0.07 (−0.34 to 0.21) | 0.02 (−0.26 to 0.30) | 0.608 | 198 |
| SANS attention | 1.6 (1.0) | 1.2 (1.1) | −0.35 (−0.63 to −0.07) | 1.5 (1.1) | 1.3 (1.2) | −0.18 (−0.47 to 0.09) | −0.08 (−0.36 to 0.19) | 0.350 | 198 |
| SANS total | 2.0 (0.6) | 1.7 (0.8) | −0.40 (−0.68 to −0.12) | 2.0 (0.6) | 1.8 (0.7) | −0.39 (−0.67 to −0.11) | −0.03 (−0.31 to 0.25) | 0.758 | 198 |
| CDSS total | 0.4 (0.4) | 0.2 (0.4) | −0.37 (−0.65 to −0.09) | 0.4 (0.4) | 0.2 (0.4) | −0.46 (−0.75 to −0.19) | 0.05 (−0.23 to 0.33) | 0.499 | 198 |
| CGI—severity | 4.2 (0.8) | 3.9 (0.9) | −0.37 (−0.65 to −0.09) | 4.1 (0.8) | 3.9 (1.0) | −0.13 (−0.41 to 0.15) | −0.06 (−0.34 to 0.21) | 0.166 | 197 |
| GAF | 59.3 (9.6) | 63.1 (12.1) | 0.35 (0.07 to 0.63) | 59.2 (7.9) | 61.2 (11.3) | 0.20 (−0.08 to 0.48) | 0.17 (−0.11 to 0.45) | 0.144 | 198 |
| SCL-90-R—GSI | 1.0 (0.7) | 0.9 (0.7) | −0.12 (−0.40 to 0.16) | 0.9 (0.5) | 0.7 (0.6) | −0.26 (−0.55 to 0.02) | 0.26 (−0.03 to 0.54) | 0.053 | 187 |
Note: CBT, Cognitive behavioural therapy; CR, Cognitive remediation; PANSS, Positive and negative syndrome scale; SANS, Scale for the assessment of negative symptoms; CDSS, Calgary Depression Rating Scale for Schizophrenia; CGI, Clinical global impression; GAF, Global assessment of functioning; SCL, Symptom Checklist.
Effect size of the CBT compared with CR at posttreatment assessment.
Result of ANCOVA’s.
Pre-post effect size of CBT.
Pre-post effect size of CR.
Rehospitalization: 28 CBT patients (29.5%, SE 4.7) and 31 CR patients (35.1%, SE 5.1) have been readmitted to hospital within the 1-year treatment phase. This difference is not significant (Logrank-test, P = .390)
Discussion
The efficacy of CBT for the reduction of negative symptoms is analyzed in this RCT using rigorous methodology: randomized multicenter design, systematic recruitment, adequate statistical power, blind assessments which are controlled for rater shift, audio recording of treatment sessions, manualized treatment, data monitoring by an external institution, external statistician, and statistical analysis with adequate handling of missing data. The study has been conducted according to GCP standards of the International Conference on Harmonisation whenever applicable in a psychotherapy trial.
Since patients with severe positive and depressive symptoms and with moderate extrapyramidal symptoms were excluded from the study, we can conclude that the majority of patients have primary negative symptoms.3 With mean PANSS scores of 3.0 for the MNS (2.6 for the standard negative factor) and 1.5 for the positive syndrome, this sample represents an outpatient population with moderate negative symptoms and rather mild positive symptoms. In addition, more than 80% of the patients present with these symptoms for more than 6 months. Two studies even on inpatients report lower scores for negative symptoms (negative symptoms mean score: 1.96, Bechdolf et al50; 2.5: Valmaggia et al51). In CR studies, the mean score on the PANSS Negative Syndrome Scale ranged from 2.0 to 2.5 for the CR and the unspecific or TAU condition (Lindenmayer et al52, McGurk et al53, Penades et al54). Thus, the sample characteristics are adequate for a clinical trial on negative symptoms and clearly different from those of studies on positive symptoms.
We found an attrition rate regarding follow-up participation of 9% in CBT and 20% in CR. CBT showed a significantly lower attrition. Attrition rates in methodologically well-designed studies according to Wykes et al16 lay between 3.9% over 5 weeks (Lewis et al55) and 27.2% for 3 months (Tarrier et al56) for the CBT. In CR studies (Wykes et al57; Reeder et al58), attrition rates at posttreatment for the CR condition were in a range from 9.3% over 3 months (Wykes et al57) and 20.0% over 4 months.54 Interpretation of attrition should account for the fact that in Germany all patients have unrestricted access to psychiatric care (including psychotherapy) outside of studies.
Similar to attrition, the extent of treatment participation was different between the groups. 42% of CR patients as opposed to only 25% of CBT patients attended less than 14 sessions. For CBT, these rates are comparable to Garety et al59 who reported a rate of 65%–70% of patients receiving at least 12 of 20 sessions.
The major objective was to analyze whether CBT has specific effects on negative symptoms when compared with CR which is similar regarding therapeutic attention but which is different regarding the postulated mechanisms of change. The major result is that there was no significant difference regarding negative symptoms between the groups. This is against the hypothesis of specific effects of CBT. This result is unbiased regarding the course of the therapeutic alliance, adherence to medication, dose of medication, change of rater’s standards over time, and the baseline parameters. A possible explanation for the major finding is based on the assumption that patients with negative symptoms are characterized by low expectations of pleasure, success, and social acceptance. It could be the case that both interventions helped patients to experience pleasure and success, which could lead to decreased negative symptoms. Questionnaire data about self-concepts before and after the treatment are available in this study and will be reported elsewhere in order to empirically investigate this speculation.
Patients in both groups showed improvements of negative symptoms over time. The within-group effect size of −0.49 in the CBT group meets the expectation as described in the power calculation. These effect sizes are similar to those of CBT for persistent positive symptoms (ES = 0.37, Wykes et al16), SST (ES = 0.40, Kurtz et al35) for negative symptoms, and second-generation antipsychotic medication for the reduction of negative symptoms (ES = 0.39, Leucht et al9). Thus, in general, the effect of CBT meets the expectation. Even if this effect size should be considered moderate it seems to be relevant for the reduction of negative symptoms and indicates that even chronic symptoms can be improved over time. CR showed an even higher effect of −0.53 which was against our expectation based on previous research. Based on the Cochrane meta-analysis of McGrath and Hayes60 and own negative results on CR for the reduction of negative symptoms,33 we hypothesized in the planning phase of this trial that CR should have no effect on negative symptoms. According to recent literature, it has to be expected that CR should result in small effects. Pfammatter et al61 summarized a number of meta-analyses on CR and found an effect size of 0.24 for the reduction of negative symptoms. McGurk et al41 found an ES of 0.28 for the reduction of the overall symptomatology. Twamley et al62 emphasized that there is a huge heterogeneity regarding patient characteristics, treatment modalities, assessments, and further methodological aspects which limits the possibility of aggregate analyses. Thus, the CR pre-post effect size of 0.53 in this study exceeds the expectations even based on current evidence. This finding may be due to the intensive care patients received during study participation. The participation in monthly assessments may well improve the effects of study treatments. This consideration applies also on our CBT, which would result in a somewhat lower “real” effect size than 0.42. The study design does not allow differentiating between the courses under TAU and the effect of psychotherapeutic attention in both interventions. For this purpose, a third study condition had been required which was not included for methodological reasons, in particular statistical power and the feasibility of recruitment.
The present study has several limitations. First, the attrition rate and the rate of patients not treated according to manual were significantly higher in the CR control condition. Thus, if there were a dose-response relationship in CR, the effect of CR would have been underestimated. Second, the present RCT does not address the underlying mechanisms of change. Therefore, the question whether the same mechanisms of change were active in both conditions remains open. Furthermore, the generalizability of the results is restricted since patients showed only moderate negative symptoms, had at least a minimum of social contacts, were compliant with their medication regimen, and were in general cooperative. Accordingly, the results might be generalized primarily to outpatients who ask for treatment by themselves.
In conclusion, an important question for further studies is whether a combination of CBT and CR strategies might be even more effective for the treatment of patients with negative symptoms.
Funding
This study was funded publicly by the German Research Foundation (Deutsche Forschungsgemeinschaft, grants Kl 1179/2-1 and Kl 1179/3-1).
Acknowledgments
We thank Johannes Harbort, Michael Ruch, Hanna Smoltczyk, Maria Weickert for their contribution as study raters, Klaus Hesse, Sabine Kossow, Maria Memmou for their contribution as study therapists, Birgit Conradt for her contribution as supervisor, Nicole Frommann for her contribution to the treatment manuals, and Ute E. Jakobi-Malterre for writing assistance. The authors declare that there is no conflict of interest regarding this article.
References
- 1.Häfner H, an der Heiden W. The course of schizophrenia in the light of modern follow-up studies: the ABC and WHO studies. Eur Arch Psychiatry Clin Neurosci. 1999;249(suppl 4):14–26. doi: 10.1007/pl00014180. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter WT, Heinrichs DW, Wagman AM. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry. 1988;145:578–583. doi: 10.1176/ajp.145.5.578. [DOI] [PubMed] [Google Scholar]
- 3.Peralta V, Cuesta MJ. Clinical models of schizophrenia: a critical approach to competing conceptions. Psychopathology. 2000;33:252–258. doi: 10.1159/000029154. [DOI] [PubMed] [Google Scholar]
- 4.Kirkpatrick B, Fenton WS, Carpenter WT, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32:214–219. doi: 10.1093/schbul/sbj053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lencz T, Smith CW, Auther A, Correll CU, Cornblatt B. Nonspecific and attenuated negative symptoms in patients at clinical high-risk for schizophrenia. Schizophr Res. 2004;68:37–48. doi: 10.1016/S0920-9964(03)00214-7. [DOI] [PubMed] [Google Scholar]
- 6.Mason O, Startup M, Halpin S, Schall U, Conrad A, Carr V. Risk factors for transition to first episode psychosis among individuals with ‘at-risk mental states'. Schizophr Res. 2004;71:227–237. doi: 10.1016/j.schres.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Thorup A, Petersen L, Jeppesen P, et al. Integrated treatment ameliorates negative symptoms in first episode psychosis–results from the Danish OPUS trial. Schizophr Res. 2005;79:95–105. doi: 10.1016/j.schres.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 8.Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373:31–41. doi: 10.1016/S0140-6736(08)61764-X. [DOI] [PubMed] [Google Scholar]
- 9.Leucht S, Arbter D, Engel RR, Kissling W, Davis JM. How effective are second-generation antipsychotic drugs? A meta-analysis of placebo-controlled trials. Mol Psychiatry. 2009;14:429–447. doi: 10.1038/sj.mp.4002136. [DOI] [PubMed] [Google Scholar]
- 10.Green MF, Nuechterlein KH. The MATRICS initiative: developing a consensus cognitive battery for clinical trials. Schizophr Res. 2004;72:1–3. doi: 10.1016/j.schres.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Mäkinen J, Miettunen J, Isohanni M, Koponen H. Negative symptoms in schizophrenia—a review. Nord J Psychiatry. 2008;62:334–341. doi: 10.1080/08039480801959307. [DOI] [PubMed] [Google Scholar]
- 12.Gould RA, Mueser KT, Bolton E, Mays V, Goff D. Cognitive therapy for psychosis in schizophrenia: an effect size analysis. Schizophr Res. 2001;48:335–342. doi: 10.1016/s0920-9964(00)00145-6. [DOI] [PubMed] [Google Scholar]
- 13.Pilling S, Bebbington P, Kuipers E, et al. Psychological treatments in schizophrenia: I. Meta-analysis of family intervention and cognitive behaviour therapy. Psychol Med. 2002;32:763–782. doi: 10.1017/s0033291702005895. [DOI] [PubMed] [Google Scholar]
- 14.Rector NA, Beck AT. Cognitive behavioral therapy for schizophrenia: an empirical review. J Nerv Ment Dis. 2001;189:278–287. doi: 10.1097/00005053-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Tarrier N, Wykes T. Is there evidence that cognitive behaviour therapy is an effective treatment for schizophrenia? A cautious or cautionary tale? Behav Res Ther. 2004;42:1377–1401. doi: 10.1016/j.brat.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 16.Wykes T, Steel C, Everitt B, Tarrier N. Cognitive behavior therapy for schizophrenia: effect sizes, clinical models, and methodological rigor. Schizophr Bull. 2008;34:523–537. doi: 10.1093/schbul/sbm114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmermann G, Favrod J, Trieu VH, Pomini V. The effect of cognitive behavioral treatment on the positive symptoms of schizophrenia spectrum disorders: a meta-analysis. Schizophr Res. 2005;77:1–9. doi: 10.1016/j.schres.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 18.Pinto A, La Pia S, Mennella R, Giorgio D, DeSimone L. Cognitive-behavioral therapy and clozapine for clients with treatment-refractory schizophrenia. Psychiatr Serv. 1999;50:901–904. doi: 10.1176/ps.50.7.901. [DOI] [PubMed] [Google Scholar]
- 19.Sensky T, Turkington D, Kingdon D, et al. A randomized controlled trial of cognitive-behavioral therapy for persistent symptoms in schizophrenia resistant to medication. Arch Gen Psychiatry. 2000;57:165–172. doi: 10.1001/archpsyc.57.2.165. [DOI] [PubMed] [Google Scholar]
- 20.Tarrier N, Yusupoff L, Kinney C, et al. Randomised controlled trial of intensive cognitive behaviour therapy for patients with chronic schizophrenia. BMJ. 1998;317:303–307. doi: 10.1136/bmj.317.7154.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniels LA. group cognitive-behavioral and process-oriented approach to treating the social impairment and negative symptoms associated with chronic mental illness. J Psychother Pract Res. 1998;7:167–176. [PMC free article] [PubMed] [Google Scholar]
- 22.Bailer J, Takats I, Westermeier C. Die Wirksamkeit individualisierter Kognitiver Verhaltens therapie bei schizophrener Negativsymptomatik und sozialer Behinderung: Eine kontrollierte Studie. Z Klin Psychol Psychother. 2001;30:268–278. [Google Scholar]
- 23.Cather C. Functional cognitive-behavioural therapy: a brief, individual treatment for functional impairments resulting from psychotic symptoms in schizophrenia. Can J Psychiatry. 2005;50:258–263. doi: 10.1177/070674370505000504. [DOI] [PubMed] [Google Scholar]
- 24.NICE. The Guidelines Manual. London, UK: NICE; 2010. Available at: www.nice.org.uk. [Google Scholar]
- 25.Kingdon D, Turkington D. Cognitive Therapy of Schizophrenia. New York, NY: The Guilford Press; 2005. [Google Scholar]
- 26.Bleuler E. Dementia praecox oder Gruppe der Schizophrenien. In: Aschaffenburg G, editor. Handbuch der Psychiatrie. Leipzig, Germany: Franz Deuticke; 1911. [Google Scholar]
- 27.Wittorf A, Wiedemann G, Buchkremer G, Klingberg S. Quality and correlates of specific self-esteem at the beginning stabilisation phase of schizophrenia. Psychiatry Res. 2010;179:130–138. doi: 10.1016/j.psychres.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 28.Beck AT, Rector NA, Stolar N, Grant PM. Schizophrenia. Cognitive Theory, Research, and Therapy. New York, NY: The Guildford Press; 2009. [Google Scholar]
- 29.Rector NA, Beck AT, Stolar N. The negative symptoms of schizophrenia: a cognitive perspective. Can J Psychiatry. 2005;50:247–257. doi: 10.1177/070674370505000503. [DOI] [PubMed] [Google Scholar]
- 30.Grant PM, Beck AT. Defeatist beliefs as a mediator of cognitive impairment, negative symptoms, and functioning in schizophrenia. Schizophr Bull. 2009;35:798–806. doi: 10.1093/schbul/sbn008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grant PM, Beck AT. Asocial beliefs as predictors of asocial behavior in schizophrenia. Psychiatry Res. 2010;177:65–70. doi: 10.1016/j.psychres.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Green MF, Nuechterlein KH. Should Schizophrenia be treated as a neurocognitive disorder? Schizophr Bull. 1999;25:309–318. doi: 10.1093/oxfordjournals.schbul.a033380. [DOI] [PubMed] [Google Scholar]
- 33.Wolwer W, Frommann N, Halfmann S, Piaszek A, Streit M, Gaebel W. Remediation of impairments in facial affect recognition in schizophrenia: efficacy and specificity of a new training program. Schizophr Res. 2005;80:295–303. doi: 10.1016/j.schres.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 34.Penn DL, Mueser KT, Tarrier N, et al. Supportive therapy for schizophrenia: possible mechanisms and implications for adjunctive psychosocial treatments. Schizophr Bull. 2004;30:101–112. doi: 10.1093/oxfordjournals.schbul.a007055. [DOI] [PubMed] [Google Scholar]
- 35.Kurtz MM, Mueser KT. A meta-analysis of controlled research on social skills training for schizophrenia. J Consult Clin Psychol. 2008;76:491–504. doi: 10.1037/0022-006X.76.3.491. [DOI] [PubMed] [Google Scholar]
- 36.Hayes RL, McGrath J. Cognitive Rehabilitation for People With Schizophrenia and Related Conditions (Cochrane Review) Oxford, UK: The Cochrane Library,; Issue 1, Update Software; 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bellucci DM, Glaberman K, Haslam N. Computer-assisted cognitive rehabilitation reduces negative symptoms in the severely mentally ill. Schizophr Res. 2003;59:225–232. doi: 10.1016/s0920-9964(01)00402-9. [DOI] [PubMed] [Google Scholar]
- 38.Velligan DI, Bow-Thomas CC, Huntzinger C, et al. Randomized controlled trial of the use of compensatory strategies to enhance adaptive functioning in outpatients with schizophrenia. Am J Psychiatry. 2000;157:1317–1323. doi: 10.1176/appi.ajp.157.8.1317. [DOI] [PubMed] [Google Scholar]
- 39.Medalia A, Revheim N, Casey M. Remediation of memory disorders in schizophrenia. Psychol Med. 2000;30:1451–1459. doi: 10.1017/s0033291799002913. [DOI] [PubMed] [Google Scholar]
- 40.Spaulding WD, Reed D, Sullivan M, Richardson C, Weiler M. Effects of cognitive treatment in psychiatric rehabilitation. Schizophr Bull. 1999;25:657–676. doi: 10.1093/oxfordjournals.schbul.a033409. [DOI] [PubMed] [Google Scholar]
- 41.McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164:1791–1802. doi: 10.1176/appi.ajp.2007.07060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klingberg S, Wittorf A, Wiedemann G. Disorganization and cognitive impairment in schizophrenia: independent symptom dimensions? Eur Arch Psychiatry Clin Neurosci. 2006;256:532–540. doi: 10.1007/s00406-006-0704-0. [DOI] [PubMed] [Google Scholar]
- 43.Klingberg S, Hesse K, Herrlich J, et al. Cognitive behavioral therapy on negative symptoms of schizophrenia disorders—background and therapy of the TONES-study. Nervenheilkunde. 2008;27:997–1006. [Google Scholar]
- 44.Klingberg S, Wittorf A, Herrlich J, et al. Cognitive behavioural treatment of negative symptoms in schizophrenia patients: study design of the TONES study, feasibility and safety of treatment. Eur Arch Psychiatry Clin Neurosci. 2009;259:149–154. doi: 10.1007/s00406-009-0047-8. [DOI] [PubMed] [Google Scholar]
- 45.Fluckiger C, Regli D, Zwahlen D, Hostettler S, Caspar F. The bern post session report 2000, patient and therapist versions: measuring psychotherapeutic processes. Z Klin Psychol Psychother. 2010;39:71–79. [Google Scholar]
- 46.Kemp R, David A. Psychological predictors of insight and compliance in psychotic patients. Br J Psychiatry. 1996;169:444–450. doi: 10.1192/bjp.169.4.444. [DOI] [PubMed] [Google Scholar]
- 47.Klingberg S, Wittorf A, Fischer A, Jakob-Deters K, Buchkremer G, Wiedemann G. Evaluation of a cognitive behaviourally oriented service for relapse prevention in schizophrenia. Acta Psychiatr Scand. 2010;121:340–350. doi: 10.1111/j.1600-0447.2009.01479.x. [DOI] [PubMed] [Google Scholar]
- 48.Feingold A. Effect sizes for growth-modeling analysis for controlled clinical trials in the same metric as for classical analysis. Psychol Methods. 2009;14:43–53. doi: 10.1037/a0014699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 50.Bechdolf A, Knost B, Kuntermann C, et al. A randomized comparison of group cognitive-behavioural therapy and group psychoeducation in patients with schizophrenia. Acta Psychiatr Scand. 2004;110:21–28. doi: 10.1111/j.1600-0447.2004.00300.x. [DOI] [PubMed] [Google Scholar]
- 51.Valmaggia LR, Van der Gaag M, Tarrier N, Pijnenborg M, Slooff CJ. Cognitive-behavioural therapy for refractory psychotic symptoms of schizophrenia resistant to atypical antipsychotic medication—randomised controlled trial. Br J Psychiatry. 2005;186:324–330. doi: 10.1192/bjp.186.4.324. [DOI] [PubMed] [Google Scholar]
- 52.Lindenmayer JP, McGurk SR, Mueser KT, et al. A randomized controlled trial of cognitive remediation among inpatients with persistent mental illness. Psychiatr Serv. 2008;59:241–247. doi: 10.1176/ps.2008.59.3.241. [DOI] [PubMed] [Google Scholar]
- 53.McGurk SR, Mueser KT, Derosa TJ, Wolfe R. Work, recovery, and comorbidity in schizophrenia: a randomized controlled trial of cognitive remediation. Schizophr Bull. 2009;35:319–335. doi: 10.1093/schbul/sbn182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Penades R, Catalan R, Salamero M, et al. Cognitive remediation therapy for outpatients with chronic schizophrenia: a controlled and randomized study. Schizophr Res. 2006;87:323–331. doi: 10.1016/j.schres.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 55.Lewis S, Tarrier N, Haddock G, et al. Randomised controlled trial of cognitive-behavioural therapy in early schizophrenia: acute-phase outcomes. Br J Psychiatry Suppl. 2002;43:s91–s97. doi: 10.1192/bjp.181.43.s91. [DOI] [PubMed] [Google Scholar]
- 56.Tarrier N, Wittkowski A, Kinney C, McCarthy E, Morris J, Humphreys L. Durability of the effects of cognitive-behavioural therapy in the treatment of chronic schizophrenia: 12-month follow-up. Br J Psychiatry. 1999;174:500–504. doi: 10.1192/bjp.174.6.500. [DOI] [PubMed] [Google Scholar]
- 57.Wykes T, Reeder C, Landau S, et al. Cognitive remediation therapy in schizophrenia: randomised controlled trial. Br J Psychiatry. 2007;190:421–427. doi: 10.1192/bjp.bp.106.026575. [DOI] [PubMed] [Google Scholar]
- 58.Reeder CH, Wykes T. Within-group differences in cognitive change and response to cognitive remediation therapy (CRT) Schizophr Bull. 2007;33:574. [Google Scholar]
- 59.Garety PA, Fowler DG, Freeman D, et al. Cognitive–behavioural therapy and family intervention for relapse prevention and symptom reduction in psychosis: randomised controlled trial. Br J Psychiatry. 2008;192:412–423. doi: 10.1192/bjp.bp.107.043570. [DOI] [PubMed] [Google Scholar]
- 60.McGrath J, Hayes RL. Cognitive rehabilitation for people with schizophrenia and related conditions. Cochrane Database Syst Rev. 2000 doi: 10.1002/14651858.CD000968. CD000968. DOI: 10.1002/14651858.CD000968. (3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pfammatter M, Junghan UM, Brenner HD. Efficacy of psychological therapy in schizophrenia: conclusions from meta-analyses. Schizophr Bull. 2006;32:S64–S80. doi: 10.1093/schbul/sbl030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Twamley EW, Jeste DV, Bellack AS. A review of cognitive training in schizophrenia. Schizophr Bull. 2003;29:359–382. doi: 10.1093/oxfordjournals.schbul.a007011. [DOI] [PubMed] [Google Scholar]