Abstract
During fetal life, vagal sensory fibers establish a reproducible distribution in the gut that includes an association with myenteric ganglia. Previous work has shown that netrin is expressed in the bowel wall and, by acting on its receptor, deleted in colorectal cancer (DCC), mediates the guidance of vagal sensory axons to the developing gut. Because the highest concentration of netrins in fetal bowel is in the endoderm, we tested the hypothesis that the ingrowth of vagal afferents to the gut would be independent of the presence of enteric neurons, although enteric neurons might influence the internal distribution of these fibers. Surprisingly, experiments indicated that the vagal sensory innervation is intrinsic neuron-dependent. To examine the vagal innervation in the absence of enteric ganglia, fetal Ret -/- mice were labeled by applying DiI bilaterally to nodose ganglia. In Ret -/- mice, DiI-labeled vagal sensory axons descended in paraesophageal trunks as far as the proximal stomach, which contains neurons, but did not enter the aganglionic bowel. To determine whether neurons produce netrins, enteric neural-crest-derived cells (ENCDCs) were immunoselected from E15 rat gut. Transcripts encoding netrin-1 and -3 were not detected in the ENCDCs, but appeared after they had given rise to neurons. When these neurons were co-cultured with cells expressing c-Myc-tagged netrin-1, the neurons displayed netrin-1, but not c-Myc, immunoreactivity. Enteric neurons thus synthesize netrins. The extent to which neuronal netrin accounts for the dependence of the vagal sensory innervation on intrinsic neurons, remains to be determined.
Keywords: vagus nerve, nodose ganglia, enteric nervous system, axon guidance, gastrointestinal tract
INTRODUCTION
The innervation of the gastrointestinal tract has two components: intrinsic and extrinsic. The intrinsic element is the enteric nervous system (ENS), which is able to control enteric behaviors in the absence of input from the brain or spinal cord (Langley, 1921). Although the ENS is capable of independent function, the ENS normally operates in coordination with a critical extrinsic innervation through which the bowel and central nervous system (CNS) interact. The extrinsic innervation is composed of vagal and spinal sensory nerves, vagal and sacral pre-ganglionic axons, and sympathetic input from pre- and paravertebral ganglia. Of the extrinsic components, the largest numbers of axons are sensory; moreover, the majority of vagal axons carry signals from the bowel to the brain (Powley and Phillips, 2002; Blackshaw et al., 2007).
Netrins have been established to be important guidance molecules for CNS axons and can exert effects that are attractive (Serafini et al., 1996; Astic et al., 2002) or repellent (Colamarino and Tessier-Lavigne, 1995). Previous work has shown that netrins also function as guidance molecules in the developing bowel. Both migrating crest-derived cells and vagal sensory axons are attracted to sources of netrins; this effect, in both cases, is mediated by the netrin receptor, deleted in colorectal cancer (DCC) (Jiang et al., 2003; Ratcliffe et al., 2006). The specific roles played by netrins/DCC in the establishment of the vagal sensory innervation are not yet clear. Netrin/DCC may help to attract vagal sensory axons to the bowel and/or they may function within the gut to direct vagal sensory axons to their correct destinations. Vagal sensory axons are found in long terminals between the cells of the longitudinal and circular smooth muscle layers (intramuscular arrays; IMAs), in intraganglionic laminar endings (IGLEs) and in the lamina propria of the mucosa (Powley and Phillips, 2002; Blackshaw et al., 2007). The locations of these terminals is physiologically important because the function of the various endings differ; IMAs appear to be stretch receptors (Phillips and Powley, 2000), IGLEs respond to tension in the gut wall and vagal terminals in the lamina propria are functional mucosal receptors (Blackshaw et al., 2007).
The current study examined the development of the association between vagal sensory axons and enteric neurons. Netrin expression in the bowel wall is maximal in the endodermal epithelium, although netrin expression is also detectable in the outer gut mesenchyme, where the primordial myenteric plexus develops. We tested the hypothesis that vagal sensory axons would be attracted to the bowel by endoderm and/or mesenchyme-derived netrin and thus the vagal innervation would be independent of the presence of intrinsic neurons. Surprisingly, however, observations showed that the development of the vagal sensory innervation is dependent upon enteric neurons and that enteric neurons express netrins. Vagal sensory axons are unable to innervate developing Ret -/- bowel, which lacks enteric neurons. These neurons, moreover, contain netrins, which they acquire by biosynthesis rather than uptake.
METHODS
Animals
Timed pregnant mice (CD-1) and rats (Sprague Dawley) were obtained from Charles River (Waltham, MA). Mice with a targeted mutation in Ret were bred at Columbia University on a mixed background (129S1/SvmJ:C57BL/6; gift from Dr. F. Costantini, Columbia University) (Schuchardt et al., 1994; Rothman et al., 1996). Gestation was dated from the day a vaginal plug was discovered, which was considered E0. PCR genotyping of fetuses was done as described previously (Schuchardt et al., 1994). When used for experiments, animals were sacrificed by exposure to CO2 gas. The Animal Care and Use Committee of Columbia University and the Animal Research Ethics Board at McMaster University have approved this procedure.
Histochemistry and immunocytochemistry
Vagal sensory fibers were identified in the developing gut by applying the lipophilic dye, 1,1’-dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine perchlorate (DiI; Molecular Probes, Eugene, OR) to the nodose ganglia of fetal mice with the Ret wild type allele (Ret+/+) and of fetal mice heterozygous (Ret +/-) and homozygous (Ret -/-) for the Ret null mutation, as previously described (Ratcliffe et al., 2006). Fetuses were fixed for 24 hours at 4°C in a solution containing 4% phosphate-buffered formaldehyde (freshly prepared from paraformaldehyde; pH 7.4) and subsequently incubated in phosphate buffered saline (PBS; pH 7.4) containing 0.1% sodium azide for 4 (E12) or 6 (E16) weeks at 37°C to allow DiI to reach the most distal nerve terminals. Fetuses were cryoprotected with 30% sucrose, embedded in Neg-50 Frozen Section Medium (Richard-Allan Scientific, Kalamazoo, MI), frozen in liquid nitrogen, and sectioned at 10 μm. Sections were thaw-mounted onto Colorfrost/Plus microscope slides (Fisher Scientific Company L.L.C, Pittsburg, PA) and mounted in glycerol containing 2.7 mg/ml 1,4 diazabicyclo (2,2,2) octane (Sigma-Aldrich Co., St. Louis, MO) to prevent photobleaching of fluorophores.
For immunocytochemisty, tissues were permeabilized and blocked by incubation in PBS containing 0.4% Triton X-100 and 4% normal goat serum. Primary antibodies were applied overnight in a humidified chamber. These included rabbit antibodies to PGP9.5 (ubiquitin carboxyl-terminal hydrolase isozyme L1; dilution 1:1000; AbD Serotec, Oxford, UK), chicken antibodies to mouse/human netrin-1 (1 μg/ml; Aves Labs, Inc., Tigard, OR), and monoclonal mouse antibodies to c-Myc (9E10; 1 μg/ml; Santa Cruz Biotechnology, Santa Cruz, CA). Double-labeling was used to locate DiI and the immunoreactivity of PGP9.5 simultaneously. For this purpose, tissues were permeabilized with 0.3% Tween 20, which does not extract DiI (Lukas et al., 1998), and sites of anti-PGP9.5 binding were detected by incubation for 3 hours at room temperature with goat anti-rabbit antibodies labeled with Alexa 488 (diluted 1:200; Molecular Probes). For double label immunocytochemistry of fetal stomach with antibodies to PGP9.5 and netrin-1, sites of antibody binding were detected with goat anti-rabbit antibodies labeled with Alexa 488 (diluted 1:200; Molecular Probes) and goat anti-chicken antibodies labeled with Alexa 594 (diluted 1:200; Molecular Probes). In these experiments, nuclei were identified by staining DNA with bisbenzimide (1μg/ml in PBS; Sigma); this staining facilitated identification of fetal structures in the otherwise unstained slides. For triple label immunocytochemistry of tissue culture material, sites of antibody binding were detected with goat anti-rabbit antibodies labeled with Alexa 350 (diluted 1:200; Molecular Probes), goat anti-chicken antibodies labeled with Alexa 488 (diluted 1:200; Molecular Probes) and goat anti-mouse antibodies labeled with Alexa 594 (diluted 1:200; Molecular Probes). Photomicrographs were obtained with a Q Imaging digital camera attached to a Leica DMRXA2 microscope and an Apple computer with Openlab software (Improvision Inc., Lexington, MA). An image editing software program, Adobe Photoshop 10.0 (Adobe Systems Inc., San Jose, CA) was used to merge images and to adjust brightness and contrast.
Isolation of enteric neural-crest-derived cells
To obtain enteric neural-crest-derived cells (ENCDCs), gut was removed from E15 fetal rats and dissociated with collagenase. The resulting cellular suspension was purified by immunoselection with rabbit polyclonal antibodies to the common neurotrophin receptor p75NTR (gift from Dr. M. V. Chao, New York University, NY), as described previously (Chalazonitis et al., 1994). Both the positively (crest-derived) and negatively (non-crest-derived) immunoselected cells were re-suspended in RNAlater (Applied Biosystems/Ambion, Austin, TX) for subsequent RNA extraction. The crest-derived cells were also used in tissue culture experiments as described below.
Tissue Culture
ENCDCs
For subsequent RNA analysis, ENCDCs were plated in monolayers in 35 mm tissue culture dishes (BD Falcon, BD Biosciences, San Jose, CA; 8 × 105 cells/dish) and maintained in medium consisting of Dulbecco’s Minimal Essential Media with F12 (DMEM/F12; GIBCO, Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (GIBCO), 0.6% D-glucose, and 1% penicillin-streptomycin (GIBCO). The cultures were incubated at 37°C in an atmosphere containing 5% CO2 for 6 days. To promote neuronal differentiation, the Ret ligand, glial cell line-derived neurotrophic factor (GDNF; 10 ng/ml; rat recombinant, R&D Systems, Minneapolis, MN) was added to the culture media after 24 and 72 hours of incubation. Following culture, the cells were re-suspended in RNAlater (Ambion, Applied Biosystems, Austin TX) for storage until RNA was extracted.
ENCDCs co-cultured with 293-EBNA cells
To distinguish neuronal uptake of netrin from its biosynthesis, ENCDCs were induced with GDNF to differentiate into neurons, which were exposed to a labeled extrinsic source of netrin-1. Labeled extrinsic netrin-1 was obtained from stably transfected 293-EBNA cells expressing c-Myc-tagged netrin-1 (gift from Dr. M. Tessier-Lavigne, Genentech, Inc., South San Francisco CA)(Keino-Masu et al., 1996). Control cultures included ENCDCs exposed to GDNF cultured alone or with parental 293-EBNA cells (Invitrogen). Both netrin-1-secreting and control 293-EBNA cells were maintained as cell aggregates prior to culture (Ratcliffe et al., 2006). All cells were arranged on a base of polymerized collagen (20 μl). The ENCDCs were concentrated by centrifugation and 2 μl (containing approximately 1 × 103 cells) was plated evenly on the collagen base and sandwiched under a second 2 μl layer of collagen. The second collagen layer spread the cells centrifugally, leaving room in the middle of the collagen base to place the 293-EBNA cell aggregates. After the 293-EBNA cells were added, a third collagen layer (4 μl) was applied. Collagen was polymerized for 10 minutes at room temperature; growth medium, as described above, was added and the cultures were incubated at 37°C in an atmosphere containing 5% CO2. GDNF (10 ng/ml) was added to the culture media after 24 and 72 hours of incubation. All cultures were maintained for 6 days, fixed overnight in 4% formaldehyde and processed for immunocytochemistry.
RT-PCR
Total RNA was isolated according to manufacturer’s instructions from ENCDCs and non-ENCDCs from E15 rat intestine, as well as from ENCDCs that had differentiated into neurons in culture (Trizol; Invitrogren). The isolated RNA (1-2 μg) was then converted to cDNA in a 30 μl reaction volume containing random hexamer primers (0.3 μg; Invitrogen), dNTPs (0.5 mM; Promega Corporation, Madison, WI), RNasin (40 U; Promega), and Moloney Murine Leukemia Virus (M-MLV) Reverse Transcriptase (400 U; Invitrogen). PCR was used to amplify the resulting cDNA to detect transcripts encoding netrin-1 and netrin-3. cDNA encoding netrin-1 was amplified with the primers designed from the GenBank sequence NM_008744 (antisense: 5’-TGACTGTAGGCACAACACGG-3’; sense : 5’-CTCCATGTTGAATCTGCAGC-3’; amplified product: 153 bp) for 35 cycles (94°C for 60 sec, 58 °C for 45 sec, and 72°C for 60 sec). cDNA encoding netrin-3 was amplified with the primers designed from the GenBank sequence NM_019047 (antisense : 5 ’- GCCTCGCTCTTGTATTCCTG-3’; sense: 5’-GGCACTGTGAGGGTTACGTT-3’; amplified product: 222 bp) for 35 cycles (94°C for 60 sec, 58°C for 45 sec, and 72°C for 60 sec). The identities of the netrin-1 (Jiang et al., 2003) and netrin-3 PCR products were confirmed by sequencing (MOBIX Lab, McMaster University, Hamilton, ON).
Gel electrophoresis and immunoblotting
Brain (n=3) and gut (small and large intestine) were harvested from Ret +/+ (n = 1), Ret +/- (n = 5) and Ret -/- (n = 2) littermates at E14. The tissues were washed with PBS, and homogenized in 300 μl of 50 mM Tris buffer (pH 7.4) containing EDTA (1.0 mM), EGTA (2.0 mM), phenylmethanesulfonyl fluoride (1.0 mM), aprotinin (100 μg/ml), and leupeptin (100 μg/ml) (Li et al., 2004). Protein from netrin-1-secreting and parental 293-EBNA cells was also extracted for use as positive and negative controls; protein was extracted according to the manufacturer’s instructions using RIPA Lysis Buffer (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Relative quantities of netrin protein were analyzed by immunoblotting. For this purpose, 20 μg of brain, gut or 293-EBNA cell proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE; 10% resolving gel). The separated proteins were then electroblotted onto Immun-Blot PVDF membranes (Bio-Rad Laboratories, Canada, Ltd., Mississauga, ON) and immersed in blocking buffer containing 5% non-fat dry milk in Tris-base sodium chloride buffer with 0.5% Tween 20 (TBST; pH 7.4) for 1 hour at room temperature. The blot was incubated overnight at 4°C with rat monoclonal antibodies to mouse netrin-1 (2μl/ml; R&D Systems) or with antibodies to β-actin (1:1000; Cell Signaling Technology, Inc., Danvers, MA). After washing in TBST, the blot was incubated with horseradish peroxidase-labeled goat anti-rat (1:2000; Santa Cruz Biotechnology) or goat anti-rabbit (1:4000, Santa Cruz Biotechnology) secondary antibodies to identify, respectively, netrin-1 and β-actin immunoreactivity. The blot was finally washed with TBST and developed with a chemiluminescent substrate (ECL; GE Healthcare, Piscataway, NJ).
The intensities of netrin-1 and β-actin immunostaining were digitized using an Epson Perfection 4870 scanner (Epson Canada Ltd., Toronto, ON) and optical density was determined using ImageJ software (National Institutes of Health, Bethesda, MD). The relative mean netrin-1/β-actin intensity was calculated and compared in Ret sufficient (Ret +/+ and Ret +/-) and Ret deficient (Ret -/-) mice. Student’s t-test (two-tailed; unpaired) was used for this purpose.
RESULTS
Vagal sensory axons fail to innervate Ret -/- bowel
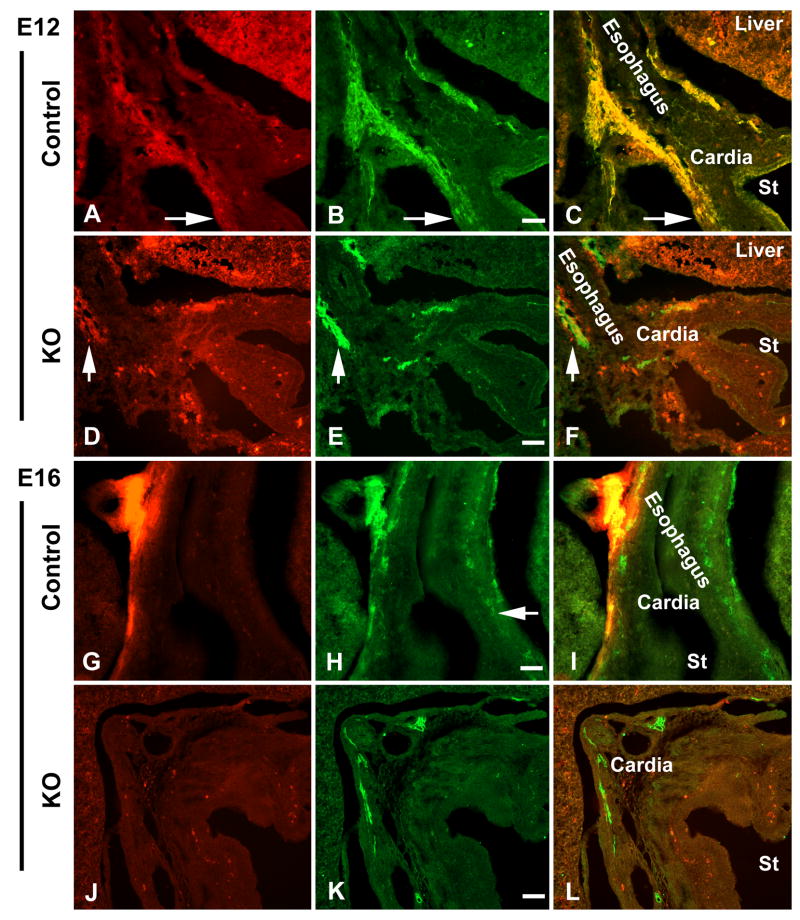
To identify descending vagal sensory axons in the E12 and E16 fetal bowel, DiI was applied bilaterally to the developing nodose ganglia. DiI has been demonstrated to move distally in the plasma membrane to reach the terminals of vagal sensory axons, which can then be positively identified by the fluorescence of DiI (Ratcliffe et al., 2006). In Ret +/+ and in Ret +/- fetal mice at E12, DiI-labeled vagal sensory axons were found in the outer wall of the esophagus and adjacent stomach (Fig. 1A, C). In contrast, no DiI-labeled axons were seen distal to the esophagogastric junction in E12 Ret -/- gut, although paraesophageal DiI-labeled vagal axons were identified (Fig. 1D, F). In Ret +/+ and Ret +/- preparations at E16, DiI-labeled paraesophageal axons were seen to descend into the stomach (Fig. 1G, I) and proximal small intestine (not illustrated). Again, as at E12, no DiI-labeled fibers were found below the cardiac stomach or small bowel in Ret -/- animals at E16 (Fig. 1J, L), despite the presence of DiI-labeled vagal axons in the outer wall of the esophagus (Supplementary Material, Fig. 1) and in the esophagogastric junction. Interestingly, DiI-labeled vagal sensory axons began to innervate the wall of the Ret -/- mouse esophagus at E16 (Supplementary Material, Fig. 1), just as they have been found to do in wild-type mice (Ratcliffe et al., 2006).
Figure 1.
Vagal sensory axons fail to innervate Ret -/- gut. Vagal sensory axons are visualized by applying DiI bilaterally to the nodose ganglia of Ret-sufficient (control) and -deficient (knockout; KO) fetal mice at E12 and E16 (A, D, G, J). Nerves are identified with antibodies to PGP9.5 (B, E, H, K). All images are from coronal-sectioned material; the esophagus, cardia and lumen of the stomach (St) are labeled in the merged images (C, F, I, L). (A) Both paraesophageal bundles and gastric vagal sensory axons are found in the E12 control Ret +/- gut (arrow). (B and C) DiI-labeled vagal axons are double-labeled with antibodies to PGP9.5 (arrow). (D and F) While DiI-labeled vagal axons can be seen in the paraesophageal bundles (arrow), no DiI-labeled gastric vagal sensory axons are found in the E12 Ret -/- stomach. Autofluorescent cells are visualized in the stomach wall. (E) Enteric neurons are absent in the body of the Ret -/- stomach at E12. (G and I) At E16, DiI-labeled vagal fibers can be identified in the wall of the esophagus and adjacent cardiac stomach. (H) Enteric ganglia, visualized with antibodies to PGP9.5, are present in the presumptive myenteric plexus of the Ret +/+ bowel (arrow). (J and L) No DiI-labeled vagal sensory axons are identified distal to the cardiac stomach in the E16 stomach of Ret -/- mice. Autofluorescent cells are visualized in the stomach wall. (K) Enteric nerves, labeled with antibodies to PGP9.5 in the cardiac stomach, are absent in the body of the Ret -/- stomach at E16. Bars = 50 μm (B, E, H, K).
PGP9.5 immunoreactivity was located simultaneously in the DiI-labeled preparations to confirm both the neuronal identity of the DiI-labeled fibers and the phenotype of the transgenic mice. DiI-fluorescence was coincident with PGP9.5-immunoreactivity in vagal fibers in the Ret +/+ and Ret +/- fetuses, verifying the neuronal origin of the DiI-labeled fibers (Fig. 1B, C, H, I). As expected, there were no enteric ganglia distal to the cardiac stomach in E12 and E16 Ret -/-mice (Fig. 1E, K).
Enteric neurons express netrin-1 and netrin-3
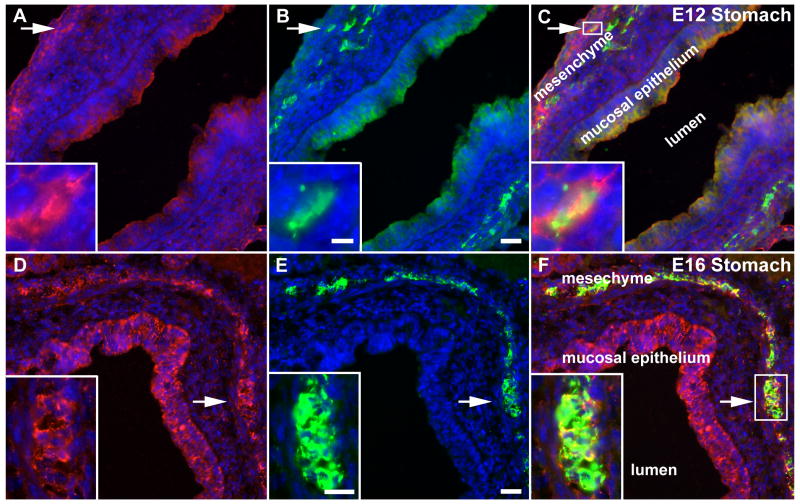
Netrin-1 immunoreactivity was located in developing mouse stomach at E12 (Fig. 2A, C) and E16 (Fig. 2D, F). Antibodies to PGP9.5 were used to identify neurons and neurites in the same sections (Fig. 2B, E). As has been reported previously (Jiang et al., 2003; Ratcliffe et al., 2006), netrin-1 immunoreactivity was most abundant at both E12 and E16 in the mucosal epithelium and outer mesenchyme. Neurons were located close to the netrin-1-immunoreactive mesenchyme at E12 (Fig. 2A-C) and were found within it at E16 (Fig. 2D-F). At both ages, coincident netrin-1 and PGP9.5 immunoreactivities were observed in primordial myenteric neurons (Fig. 2 insets). These observations are consistent with the idea that developing myenteric neurons contain netrin-1 and do so as early as E12.
Figure 2.
Enteric neurons in the wild-type stomach are netrin-immunoreactive at E12-E16. Stomach from E12 and 16 fetal mice was immunostained to locate netrin-1 (red; A, D) and the neuronal marker, PGP9.5 (green; B, E) in the bowel wall. Nuclei are identified by staining DNA with bisbenzimide (blue). The lumen of the stomach is labeled (lumen) and the images are merged in C and F. (A-C; E12) Netrin-1 immunoreactivity (A, C) is found in the mucosal epithelium and outer mesenchyme (labeled in C). PGP9.5 immunoreactivity (B) overlaps the netrin-immunoreactive band in the outer mesenchyme. At high magnification, a subset of neurons (B; arrow and inset) are also netrin-1-immunoreactive (A, C; arrow, box and inset). (D-F; E16) The gastric mucosal epithelium and outer mesenchyme (labeled in F) are still netrin-1-immunoreactive (D, F). PGP.5 immunoreactivity corresponds in location to the band of netrin-1 immunoreactivity in the outer mesenchyme (E, F). At high magnification, many neurons (E; arrow and inset) are also netrin-1-immunoreactive (E, F; arrow, box and inset). Bars = 25 μm (B, E); 5 μm (B; inset); 15 μm (E; inset).
RNA was extracted from ENCDCs and non-ENCDCs isolated from E15 rat gut by positive and negative immunoselection with antibodies to p75NTR. Transcripts encoding netrin-1 and netrin-3 were detected in the non-ENCDCs but were not detected in the ENCDCs (Fig. 3). ENCDCs were then cultured under conditions that promote neuronal differentiation. RNA was extracted from the enteric neurons that developed after 6 days in culture. In contrast to freshly immunoselected ENCDCs, ENCDCs were found to express transcripts encoding both netrin-1 and netrin-3 after time had been allowed for neuronal differentiation (Fig. 3).
Figure 3.
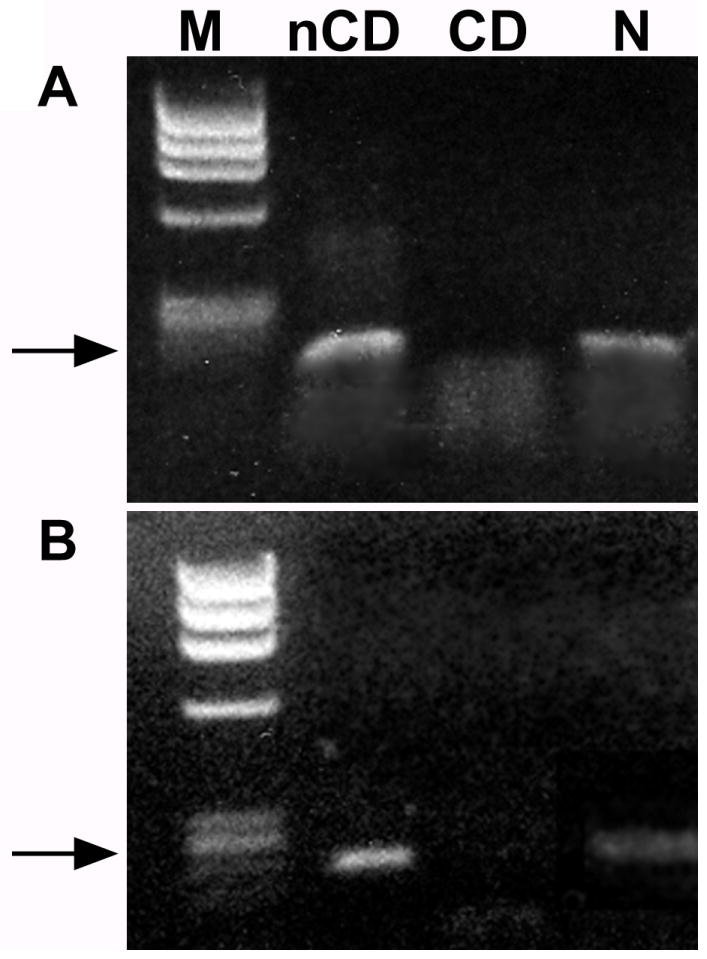
Enteric neurons, but not their crest-derived precursors, express mRNA encoding netrin-1 and netrin-3. (A) mRNA was isolated from non-crest derived (nCD), crest-derived and from crest-derived cells that had been cultured and differentiated into enteric neurons (N). Transcripts encoding netrin-1 are present in the nCD and N, but not in the CD cells. Arrow = 153 bp. (B) Transcripts encoding netrin-3 are present in the nCD and N, but not in the CD cells. Arrow = 222 bp.
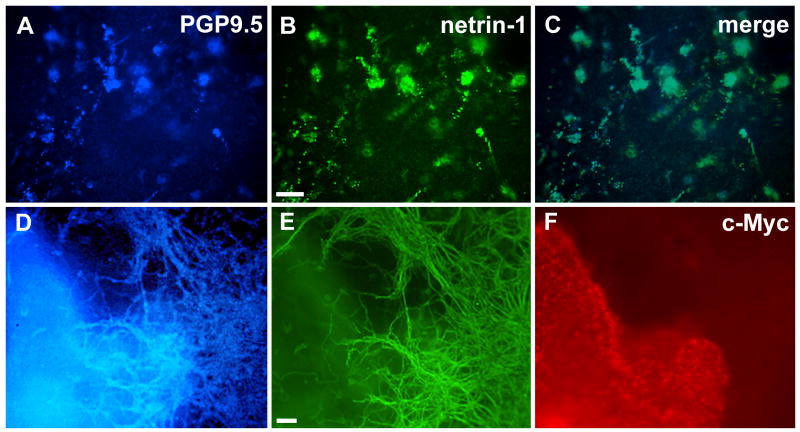
To determine whether enteric neurons take up as well as synthesize netrins, ENCDCs were co-cultured with stably transfected 293-EBNA cells expressing c-Myc-tagged netrin-1 for 6 days in 3-dimensional collagen gels. Controls included ENCDCs cultured alone or with parental non-transfected 293-EBNA cells. PGP9.5 immunoreactivity was used to identify enteric neurons in the cultures. PGP9.5-immunoreactive enteric neurons were also netrin-1-immunoreactive (Fig. 4A-C). To distinguish the c-Myc-tagged netrin that transfected 293-EBNA cells secreted from the non-tagged netrin that enteric neurons synthesize, the cultures were immunostained with antibodies to c-Myc. Enteric neurons and the neurites to which they gave rise showed coincident PGP9.5 and netrin-1 immunoreactivities but were never c-Myc immunoreactive (Fig. 4D-F). In control experiments, in which crest-derived cells were cultured with parental 293-EBNA cells, the 293-EBNA cells, as expected, did not express c-Myc (data not shown).
Figure 4.
Crest-derived cells, immunoselected from E15 rat gut, were cultured for 6 days in 3-dimensional collagen gels ± c-Myc-tagged netrin-1-secreting 293-EBNA cells. (A) PGP9.5 immunoreactivity (blue). (B) Netrin-1 immunoreactivity (green). (C) Merged image. After 6 days in single culture, the crest-derived cells have differentiated into enteric neurons and are PGP9.5-immunoreactive. (A) The same PGP 9.5-immunoreactive cells are netrin-1-immunoreactive (B, C). (D) PGP9.5 immunoreactivity (blue). (E) Netrin-1 immunoreactivity (green). (F) c-Myc- immunoreactivity (red). After 6 days in culture, PGP9.5-immunoreactive neurons have differentiated and given rise to an exuberant outgrowth of neurites (D). Netrin-1-immunoreactity is coincident with that of PGP9.5 within the neurites (E). Note that the neurites are all free of c-Myc immunoreactivity (F). The plane of focus in panels D and E is through the neurite outgrowth and thus neuronal cell bodies, which lie deep within the collagen gel, are not resolved. Bars = 100 μm (B, E).
Netrin-1 protein is not decreased in Ret -/- gut
Because both non-crest derived components of the bowel wall and enteric neurons can synthesize netrins, it is necessary to distinguish the roles played by the netrins that each secrete. Netrin protein was therefore quantified in immunoblots of protein extracted from the bowel of E14 Ret +/+, Ret +/- and Ret -/- fetal mice. This age was selected because it is a time when most of the bowel will have received colonists from the neural crest (Anderson et al., 2006). It is also a time likely to be relevant to the navigation of vagal sensory axons in the bowel (Ratcliffe et al., 2006). Controls included protein extracted from the brains of the same animals and from both the netrin-1-secreting and non-secreting parental 293-EBNA cells. Densitometry was used to quantify netrin immunoreactivity, which was normalized to the immunoreactivity of β-actin in the same samples (ImageJ software; NIH).
Netrin-1 immunoreactivity was detected in preparations of gut from each type of mouse although netrin-1 immunoreactivity was more abundant in netrin-1-secreting 293-EBNA cells and E14 brain (Fig. 5A). No netrin-1 immunoreactivity was seen in immunoblots from control non-netrin-secreting cells (parental 293-EBNA cells; Fig. 5A). The ratio of netrin-1/β-actin intensity was found to be 0.14 (n = 1) in Ret +/+ gut, and the mean ratio to be 0.16 (n = 5; ± 0.02 SEM) in Ret +/- gut and 0.14 (n = 2; ± 0.04 SEM) in Ret -/- gut (Fig. 5B). The mean ratio of netrin-1/β-actin intensity in the brain was 0.47 (n = 3; ± 0.02 SEM; Fig. 5B). The netrin-1/β-actin intensity in Ret-sufficient (Ret +/+ and Ret +/-) was thus not significantly different from that in Ret-deficient (Ret -/-) bowel although netrin-1 is significantly less concentrated in the fetal bowel than in the brain (p < 0.0001; Fig. 5B). The same results were obtained in a replicate immunoblot.
Figure 5.
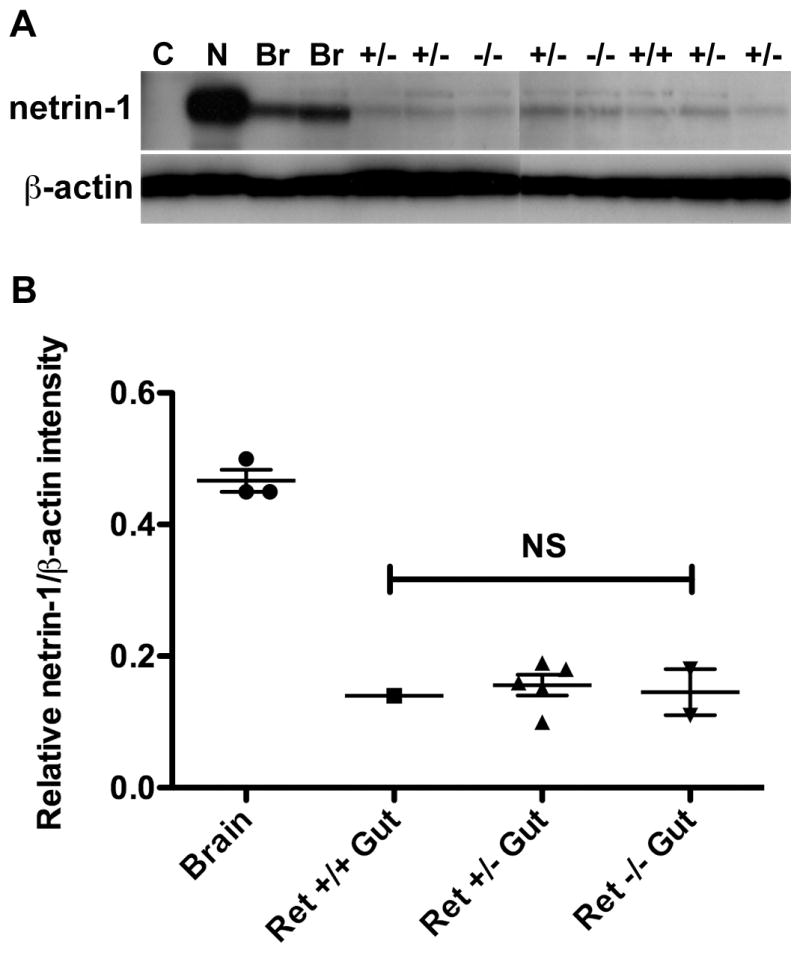
Total netrin-1 protein is not decreased in Ret -/- gut. (A) Protein was isolated from 293-EBNA control (C) and netrin-1-secreting cells (N), from E14 mouse brain (Br; n = 3) and from one litter of E14 Ret +/+ (n = 1), Ret +/- (n = 5) and Ret -/- gut (n = 2). The n values refer to numbers of individual mice. Immunoblotting reveals netrin-1 protein in all examples, except for the control cell line negative control. Levels of β-actin protein are similar in all samples. (B) The relative intensity of netrin-1 immunoreactivity was compared to that of β-actin in protein extracted from E14 brain and Ret +/+, Ret +/- and Ret -/- gut. There is no statistical difference in mean relative netrin-1/β-actin intensity between Ret-sufficient (Ret +/+ and Ret +/-) and Ret-deficient (Ret -/-) gut. Dot plot: mean ± SEM.
DISCUSSION
Experiments were carried out to determine whether the innervation of the gastrointestinal tract by vagal afferents requires that enteric neurons be present. Abnormalities of the ENS have been well characterized in mice lacking Ret activity (Schuchardt et al., 1994); however, no attempt has previously been made to discern how the presence or absence of enteric ganglia affect the ability of primary afferent neurons to project axons to correct locations in the fetal bowel. Because enteric ganglia fail to develop distal to the cardiac stomach in Ret -/- animals, these mice enable the role of enteric ganglia in establishing the afferent innervation of the gut to be examined. Although the survival of enteric neurons is Ret-dependent and Ret and/or GDNF play a role in the development of sympathetic and other parasympathetic neurons (Enomoto et al., 2000; Enomoto et al., 2001; Young et al., 2004), our data suggest that vagal sensory neurons are Ret-independent. The nodose ganglia developed and projected axons toward the bowel in Ret -/- mice. In fact, paraesophageal vagal trunks were seen in Ret-deficient as well as in the Ret-sufficient animals. These trunks moreover, in both Ret-sufficient and -deficient mice, contained fibers that were labeled with DiI that had been applied to the nodose ganglia. The Ret deficiency, therefore, did not prevent vagal sensory axons from projecting to the gut. The defect in the sensory projection in Ret -/- mice was thus manifested in the terminal projections of DiI-labeled fibers within the gut, rather than in the proximal extension of axons to the organ.
The dependence of the vagal sensory innervation on the presence of enteric neurons implies that these neurons express and secrete molecule(s), which either attract vagal sensory axons or enable them to survive. Vagal terminals, such as IGLEs, are selectively located in or on myenteric ganglia (Powley and Phillips, 2002); therefore, a means of guiding or attracting these axons to the developing neurons must exist. Because netrins and their receptor, DCC, mediate the guidance of vagal sensory axons to the fetal mouse gut (Ratcliffe et al., 2006) and vagal sensory axons express DCC and are netrin-responsive, they probably would be attracted to enteric neurons if these neurons were to secrete netrins. We thus asked whether enteric neurons are a source of netrins in the bowel. Although ENCDC did not express netrins immediately after their immunoselection from fetal rat gut, their successors did so when the ENCDC were allowed to develop into enteric neurons in culture. These neurons expressed transcripts encoding netrins-1 and -3. When co-cultured with 293-EBNA cells stably transfected to secrete c-Myc-tagged netrin-1, the enteric neurons were found to be netrin-1-, but not c-Myc-immunoreactive, demonstrating that enteric neurons synthesize, rather than take up, netrins. There are thus at least three sources of netrins in the fetal gut: endoderm, outer gut mesenchyme (Jiang et al., 2003) (Ratcliffe et al., 2006) and enteric neurons.
It might be argued that the vagal sensory innervation of the bowel could fail in Ret -/- mice if vagal sensory axons were to be attracted to the gut by the Ret ligand, GDNF, or if the nodose neurons were to depend on GDNF for survival. Addition of GDNF to culture medium, however, is not necessary for the in vitro survival of nodose ganglion neurons or for their extension of neurites toward explants of bowel (Ratcliffe et al., 2006). If vagal axons were to be GDNF-dependent, moreover, then these axons would be unable to respond to enteric GDNF and navigate in Ret -/- mice; that, however, does not appear to be the case. As noted above, the nodose ganglia develop in Ret -/- mice and project axons in the normal way as far as the cardiac stomach. Vagal fibres stop progressing when they reach the aganglionic bowel.
It is interesting that in contrast to the vagal sensory innervation of the foregut, spinal sensory nerves do innervate the aganglionic hindgut in mice that lack either endothelin 3 (Edn3ls/ls) or the endothelin B receptor (Ednrbs-l). Slowly adapting afferent responses to distension can be recorded from the rectum of the aganglionic Ednrbs-l mouse as well as from its wild-type littermates (Spencer et al., 2008). Spinal sensory axons, moreover, are present in the longitudinal muscle of the aganglionic Ednrbs-l tissue but they do not grow into the circular muscle. In wild-type mice, rectal intraganlionic laminar endings (rIGLEs) are present and likely to be the receptors that respond to distension. The sensory endings in the longitudinal muscle thus are mislocated in the Ednrbs-l gut and are abnormal in form; nevertheless, a functional sensory innervation is established in the aganglionic rectum. The Edn3ls/ls hindgut also contains coarse nerve bundles that are comprised of pelvic splanchnic nerves and spinal afferents (Payette et al., 1987). The extrinsic innervation in both Ednrbs-l mice and Edn3ls/ls mice is thus different from the vagal innervation that is the focus in the current study of Ret -/- foregut. While there is an extrinsic innervation of the two forms of aganglionic hindgut, in neither case is the pattern of this innervation totally normal. Conceivably, therefore, the mislocation and aberrant location of the extrinsic sensory innervation of the aganglionic hindgut reflects the absence of intrinsic neurons and/or netrins. We propose that the vagal innervation is particularly sensitive to the presence of enteric ganglia. We have demonstrated that the axons of nodose neurons are attracted to netrins (Ratcliffe et al., 2006). Whether spinal afferents are similarly attracted is a good question that merits future investigation.
Netrin expression in the fetal gut has previously been found in the endoderm and in the outer gut mesenchyme, which includes the location of primordial myenteric ganglia (Jiang et al., 2003). We now find that ENCDCs lack the ability to produce netrins at the moment of their immuno-isolation although non-ENCDCs contain transcripts encoding netrins- 1 and -3. The neurons that developed from ENCDC, however, acquired the ability to express transcripts encoding netrins-1 and -3. These observations suggest that netrin expression is a developmentally regulated function in enteric derivatives of the neural crest. The early crest-derived émigrés express DCC and can follow netrin gradients, which are important in their perpendicular change of direction from a predominantly proximo-distal migratory trajectory to a radial migration (Jiang et al., 2003). One set of radially migrating crest-derived cells follows the mucosally produced netrin gradient and ultimately gives rise to the submucosal plexus, while another set migrates into the pancreatic buds toward the pancreatic source of netrin and ultimately forms intrapancreatic ganglia. It seems reasonable that cells that follow netrin gradients would themselves have to refrain from secreting their own netrin, which would disrupt the gradients the cells have to follow. On the other hand, once the crest-derived cells have reached their destination in the bowel and are no longer migrating, but differentiating as neurons, the tasks they face change. Settled neurons become targets that have to attract an innervation. The sessile terminally differentiated progeny of migrating precursors can now use the very signal that they once followed to attract axons that have to find them; therefore, netrin expression is a function of differentiated enteric neurons and not a function of their precursors. PGP9.5-immunoreactive neurons were found to co-express netrin-1 immunoreactivity in the mouse stomach as early as E12 and netrin-1-containing neurons became more numerous at E16. ENCDC enter the murine foregut at E9.5-10 (Rothman and Gershon, 1982; Anderson et al., 2006); therefore, colonization of the bowel by ENCDC precedes the appearance of netrin-1-immunoreactive enteric neurons, which in turn precede the descent of vagal sensory axons to the stomach (Ratcliffe et al., 2006).
Although it is clear that netrins are able to attract vagal sensory axons, it is unlikely that netrins are the only attractive molecules to which these axons respond. The knockout of Ret removes not only the enteric neuronal source of netrins, but whatever additional chemoattractants these neurons might secrete. In fact, because non-neuronal sources of netrins in the enteric epithelium and mesenchyme remain intact in the Ret -/- bowel, it is curious that the effect of removing the neuronal source of netrin is so complete. Vagal fibers might have been expected to become lost in the mesenchyme without the short-range netrin-mediated attraction to their neuronal targets; however, the fibers do not even enter or survive in the aganglionic bowel. IMAs and mucosal projections (Powley and Phillips, 2002) as well as IGLEs associated with ganglia, are absent from the Ret -/- gut. Conceivably, the survival of vagal sensory axons within the bowel is dependent on the presence of intrinsic neurons. Intrinsic neurons and/or their axons might release factors that are required for the growth/maintenance of vagal sensory fibers. If so, then vagal terminals might simply degenerate when deprived of intrinsic neuron-supplied factors.
Vagal sensory axons may have to grow, temporarily at least, to primordial myenteric ganglia before branching to subsidiary targets in order to survive within the gut. The vagal innervation descends in the outer gut mesenchyme and thus encounters the primordial myenteric plexus before the endoderm. All vagal sensory axons might thus have to interact with myenteric neurons in order to form terminals in the ganglionic proximity (IGLEs) as well as in the lamina propria and musculature (IMAs). It follows that if the postulated myenteric “relay stations” were to be absent, the whole innervation would fail and vagal sensory axons would form no endings in aganglionic bowel. This process is not dissimilar to the “handshake hypothesis”, which has been used to explain the thalamic innervation of targets in the cerebral cortex (Molnar and Blakemore, 1995). In that case, cells of the preplate form an axonal scaffold that guides thalamic fibers and act as temporary targets for them (Molnar et al., 1998). Specific ablation of preplate neurons impairs connections from the neocortex to the rest of the brain (Xie et al., 2009). Myenteric neurons may play a similar role for the vagal sensory innervation of the gut. The fetal bowel contains significantly more IGLEs than IMAs (personal observations), which is consistent with the proposed role of myenteric neurons as “relay stations”. DCC, furthermore, is a dependence receptor (Mehlen and Mazelin, 2003); thus, the lack of netrin-secreting enteric neurons that potentially attract DCC-expressing axons, could lead to the death of that population and the subsequent overall failure of vagal sensory axons to innervate aganglionic segments of bowel. Because nodose axons survive well in vitro in the absence of co-cultured enteric neurons (Ratcliffe et al., 2006), their putative intrinsic neuronal dependence would have to apply to their ability to survive within the microenvironment of the developing bowel. With regard to the netrin-mediated chemoattractive effects of intrinsic neurons, it seems likely that netrins would be released, not only from sources within ganglia, but also by intrinsic axons that innervate effectors, such as smooth muscle cells, outside of ganglia. These intrinsic fibers might attract extrinsic sensory axons to travel with them to give rise to IMAs and mucosal projections. The absence of the intrinsic innervation of effectors in Ret -/- mice thus could contribute to the global absence of vagal sensory axons in the aganglionic Ret -/- gut. It also seems likely that the complexity of the vagal sensory innervation is sufficiently great that more than a single class of attractive molecule or attraction alone is adequate to sculpt the extrinsic sensory innervation of the bowel. Repulsive effects, such as those mediated by laminin (Ratcliffe et al., 2008), are probably necessary to refine the actions of netrins.
Vagal sensory axons may initially be attracted by the long-range effect of netrin secreted by all sources in the developing gut wall. Subsequently, the vagal fibers within the bowel wall respond to short-range guidance cues, including netrins, secreted by enteric neurons. Simultaneously, subsets of vagal sensory axons are repelled from regions of the bowel that do not normally receive a vagal innervation. These ideas are supported by prior demonstration that laminin converts netrin-mediated attraction into repulsion (Höpker et al., 1999; Ratcliffe et al., 2008). According to this model, certain vagal sensory axons, upon entering the gut wall, would be attracted toward aggregating intrinsic neurons because of the netrins they secrete. Depending on the timing of the growth of vagal sensory fibers, some might be attracted to myenteric neurons as they aggregate and become incorporated into the developing ganglia, consistent with the ultrastructural identification of IGLE varicosities around enteric neurons and within myenteric ganglia (Powley et al., 2008). Late-arriving vagal axons, however, would encounter ganglia that are becoming ensheathed with a basal lamina that is rich in laminin-111 (Payette et al., 1988). The balance between initial attraction and sudden laminin-induced repulsion would account for the presence of IGLEs in periganglionic sheaths, as shown by both light (Zagorodnyuk et al., 2001) and electron (Powley et al., 2008) microscopy. Differential long- and short-range actions of netrins have been described and depend on differences in the sensitivity of responding neurons (Kennedy et al., 2006). Whatever the nature of netrin or other guidance cues is, the ultimate formation of an extrinsic sensory innervation remains dependent on intrinsic ganglia.
Supplementary Material
Figure 1. Vagal sensory axons are visualized by applying DiI bilaterally to the nodose ganglia of a Ret-deficient fetal mouse at E16 (A, C, D, F). Nerves are labeled with antibodies to PGP9.5 (B, C, E, F). A different coronal section of the same fetus is illustrated in Figure 1 (J-L). DiI-labeled vagal axons (arrow, C) are seen in the outer wall of the esophagus. Fine DiI-labeled fibers are found innervating the esophageal musculature (arrowheads, F). Bars = 50 μm (B); 100 μm (E).
Acknowledgments
Mice with a targeted mutation in Ret were a gift from Dr. F. Costantini (Columbia University). 293-EBNA cells expressing c-Myc-tagged netrin-1 were a gift from Dr. M. Tessier-Lavigne (Genentech, Inc.). We thank Zaiqi Wu and Odysse Michos (laboratory of Dr. F. Costantini) for their assistance in breeding and genotyping the Ret transgenic mice, Zhishan Li for his advice and Rajka Borojevic for her excellent technical assistance. Sources of financial support: CIHR Operating Grant and the McMaster Children’s Hospital (EMR); NIH NS 15547 and 12969 (MDG).
References
- Anderson RB, Stewart AL, Young HM. Phenotypes of neural-crest-derived cells in vagal and sacral pathways. Cell Tissue Res. 2006;323:11–25. doi: 10.1007/s00441-005-0047-6. [DOI] [PubMed] [Google Scholar]
- Astic L, Pellier-Monnin V, Saucier D, Charrier C, Mehlen P. Expression of netrin-1 and netrin-1 receptor, DCC, in the rat olfactory nerve pathway during development and axonal regeneration. Neuroscience. 2002;109:643–656. doi: 10.1016/s0306-4522(01)00535-8. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Brookes SJ, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil. 2007;19:1–19. doi: 10.1111/j.1365-2982.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- Chalazonitis A, Rothman TP, Chen J, Lamballe F, Barbacid M, Gershon MD. Neurotrophin-3 induces neural crest-derived cells from fetal rat gut to develop in vitro as neurons or glia. J Neurosci. 1994;14:6571–6584. doi: 10.1523/JNEUROSCI.14-11-06571.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colamarino SA, Tessier-Lavigne M. The axonal chemoattractant netrin-1 is also a chemorepellent for trochlear motor axons. Cell. 1995;81:621–629. doi: 10.1016/0092-8674(95)90083-7. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Crawford PA, Gorodinsky A, Heuckeroth RO, Johnson EM, Jr, Milbrandt J. RET signaling is essential for migration, axonal growth and axon guidance of developing sympathetic neurons. Development. 2001;128:3963–3974. doi: 10.1242/dev.128.20.3963. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Heuckeroth RO, Golden JP, Johnson EM, Milbrandt J. Development of cranial parasympathetic ganglia requires sequential actions of GDNF and neurturin. Development. 2000;127:4877–4889. doi: 10.1242/dev.127.22.4877. [DOI] [PubMed] [Google Scholar]
- Höpker V, Shewan D, Tessier-Lavigne M, Poo M, Holt C. Growth-cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature. 1999;401:69–73. doi: 10.1038/43441. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Liu M, Gershon MD. Netrins and DCC in the guidance of migrating neural crest-derived cells in the developing bowel and pancreas. Dev Biol. 2003;258:364–384. doi: 10.1016/s0012-1606(03)00136-2. [DOI] [PubMed] [Google Scholar]
- Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SS-Y, Culotti JG, Tessier-Lavigne M. Deleted in colorectal cancer (DCC) encodes a netrin receptor. Cell. 1996;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- Kennedy TE, Wang H, Marshall W, Tessier-Lavigne M. Axon guidance by diffusible chemoattractants: a gradient of netrin protein in the developing spinal cord. J Neurosci. 2006;26:8866–8874. doi: 10.1523/JNEUROSCI.5191-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley JN. The Autonomic Nervous System, Part 1. Cambridge: W Heffer; 1921. [Google Scholar]
- Li ZS, Pham TD, Tamir H, Chen JJ, Gershon MD. Enteric dopaminergic neurons: definition, developmental lineage, and effects of extrinsic denervation. J Neurosci. 2004;24:1330–1339. doi: 10.1523/JNEUROSCI.3982-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas JR, Aigner M, Denk M, Heinzl H, Burian M, Mayr R. Carbocyanine postmortem neuronal tracing. Influence of different parameters on tracing distance and combination with immunocytochemistry. J Histochem Cytochem. 1998;46:901–910. doi: 10.1177/002215549804600805. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Mazelin L. The dependence receptors DCC and UNC5H as a link between neuronal guidance and survival. Biol Cell. 2003;95:425–436. doi: 10.1016/s0248-4900(03)00072-8. [DOI] [PubMed] [Google Scholar]
- Molnar Z, Adams R, Goffinet AM, Blakemore C. The role of the first postmitotic cortical cells in the development of thalamocortical innervation in the reeler mouse. J Neurosci. 1998;18:5746–5765. doi: 10.1523/JNEUROSCI.18-15-05746.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar Z, Blakemore C. How do thalamic axons find their way to the cortex? Trends Neurosci. 1995;18:389–397. doi: 10.1016/0166-2236(95)93935-q. [DOI] [PubMed] [Google Scholar]
- Payette RF, Tennyson VM, Pham TD, Mawe GM, Pomeranz HD, Rothman TP, Gershon MD. Origin and morphology of nerve fibers in the aganglionic colon of the lethal spotted (ls/ls) mutant mouse. J Comp Neurol. 1987;257:237–252. doi: 10.1002/cne.902570209. [DOI] [PubMed] [Google Scholar]
- Payette RF, Tennyson VM, Pomeranz HD, Pham TD, Rothman TP, Gershon MD. Accumulation of components of basal laminae: Association with the failure of neural crest cells to colonize the presumptive aganglionic bowel of ls/ls mutant mice. Dev Biol. 1988;125:341–360. doi: 10.1016/0012-1606(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Brain Res Rev. 2000;34:1–26. doi: 10.1016/s0165-0173(00)00036-9. [DOI] [PubMed] [Google Scholar]
- Powley TL, Phillips RJ. Musings on the wanderer: what’s new in our understanding of vago-vagal refexes? I. Morphology and topography of vagal afferents innervating the GI tract. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1217–1225. doi: 10.1152/ajpgi.00249.2002. [DOI] [PubMed] [Google Scholar]
- Powley TL, Wang XY, Fox EA, Phillips RJ, Liu LW, Huizinga JD. Ultrastructural evidence for communication between intramuscular vagal mechanoreceptors and interstitial cells of Cajal in the rat fundus. Neurogastroenterol Motil. 2008;20:69–79. doi: 10.1111/j.1365-2982.2007.00990.x. [DOI] [PubMed] [Google Scholar]
- Ratcliffe EM, D’Autreaux F, Gershon MD. Laminin terminates the Netrin/DCC mediated attraction of vagal sensory axons. Dev Neurobiol. 2008;68:960–971. doi: 10.1002/dneu.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe EM, Setru SU, Chen JJ, Li ZS, D’Autreaux F, Gershon MD. Netrin/DCC-mediated attraction of vagal sensory axons to the fetal mouse gut. J Comp Neurol. 2006;498:567–580. doi: 10.1002/cne.21027. [DOI] [PubMed] [Google Scholar]
- Rothman TP, Chen J, Howard MJ, Costantini FD, Pachnis V, Gershon MD. Increased expression of laminin-1 and collagen (IV) subunits in the aganglionic bowel of ls/ls, but not c-ret-/- mice. Dev Biol. 1996;178:498–513. doi: 10.1006/dbio.1996.0234. [DOI] [PubMed] [Google Scholar]
- Rothman TP, Gershon MD. Phenotypic expression in the developing murine enteric nervous system. J Neurosci. 1982;2:381–393. doi: 10.1523/JNEUROSCI.02-03-00381.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchardt A, D’Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Kerrin A, Zagorodnyuk VP, Hennig GW, Muto M, Brookes SJ, McDonnell O. Identification of functional intramuscular rectal mechanoreceptors in aganglionic rectal smooth muscle from piebald lethal mice. Am J Physiol Gastrointest Liver Physiol. 2008;294:G855–867. doi: 10.1152/ajpgi.00502.2007. [DOI] [PubMed] [Google Scholar]
- Xie YY, Jacobs E, Fisher R. Targeted ablation and reorganization of the principal preplate neurons and their neuroblasts identified by golli promoter transgene expression in the neocortex of mice. ASN Neuro. 2009;1:227–250. doi: 10.1042/AN20090038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young HM, Anderson RB, Anderson CR. Guidance cues involved in the development of the peripheral autonomic nervous system. Auton Neurosci. 2004;112:1–14. doi: 10.1016/j.autneu.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Chen BN, Brookes SJ. Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J Physiol. 2001;534:255–268. doi: 10.1111/j.1469-7793.2001.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1. Vagal sensory axons are visualized by applying DiI bilaterally to the nodose ganglia of a Ret-deficient fetal mouse at E16 (A, C, D, F). Nerves are labeled with antibodies to PGP9.5 (B, C, E, F). A different coronal section of the same fetus is illustrated in Figure 1 (J-L). DiI-labeled vagal axons (arrow, C) are seen in the outer wall of the esophagus. Fine DiI-labeled fibers are found innervating the esophageal musculature (arrowheads, F). Bars = 50 μm (B); 100 μm (E).