Abstract
Purpose
Cysteine-rich angiogenic inducer 61 (Cyr61) is an extracellular matrix protein involved in the transduction of growth factor and hormone signaling. Previous studies have suggested that Cyr61 may be a marker for a more aggressive phenotype. In this study, we evaluated the association between Cyr61 staining intensity and subsequent recurrence after surgical treatment of clinically localized prostate cancer.
Experimental Design
A study of 229 men with recurrence and 229 controls matched on age, race, pathologic stage, and Gleason sum nested in a cohort of men who underwent radical prostatectomy for clinically localized prostate cancer, utilizing immunohistochemistry analysis of tissue microarray (TMA) sections, was conducted. Odds ratios (OR) of recurrence and 95% confidence intervals (CIs) were estimated using conditional logistic regression.
Results
Recurrence was identified in 12.2% of cases, and in 24.0% of controls that had at least 1 TMA spot containing cancer with a staining intensity of 3 (P = 0.001). Taking into account age, pathologic stage and grade, presurgery prostate-specific antigen concentration, and calendar of surgery as a measure of tissue block storage time, men with a Cyr61 staining intensity of 3 were 56% less likely to recur than men with a lower staining intensity (OR = 0.44, 95% CI = 0.22-0.90).
Conclusions
High Cyr61 staining intensity in adenocarcinoma was associated with a lower risk of recurrence after surgical treatment of prostate cancer independent of pathologic tumor characteristics. If validated in other sample sets, Cyr61 may serve as a tissue biomarker for stratifying men for risk of recurrence and thus could inform treatment decision making.
A significant amount of attention has recently been focused on the clinical value of prostate cancer screening (1, 2). Much of the apparent controversy involves the use of the blood marker, prostate-specific antigen (PSA), as a stand-alone screening tool. It is apparent from these and other studies that although PSA has had a significant impact in decreasing the number of individuals who, on initial presentation, have advanced disease, we are over-treating a majority of men who have a form of the disease that will not result in cause-specific morbidity or mortality. With the relatively high rate of screening, the slow nature of disease progression, and the fact that most men do not seem to have a form of the disease that is considered to be clinically significant, the negative impact on quality of life resulting from prostate cancer treatment needs to be addressed.
Prostate cancer remains a major problem in the United States and throughout the world. In the United States alone, it is estimated that 27,630 patients died from prostate cancer in 2009 alone, making it the second leading cause of cancer deaths in men (3). Furthermore, 7 times that number will have a diagnosis of the disease this coming year. Currently, at the time of diagnosis, most cases present as localized disease and are treated by radical prostatectomy, radiation therapy, or active surveillance. Many aspects of diagnosis would be greatly enhanced by the identification and implementation of biomarkers for the prostate cancer. A marker able to distinguish prostate cancer with the potential to progress would be of particular utility in helping to determine which individuals should pursue a pathway such as active surveillance versus those who need more definitive or even adjuvant therapy.
Cysteine-rich angiogenic inducer 61 (Cyr61), also known as CCN1, is an extracellular matrix protein involved in the transduction of growth factor and hormone signaling (4). Members of the CNN family have highly regulated expression, are important in the regulation of growth and development, and have been linked to a variety of pathologic disorders, including cancer. Cyr61 commonly exhibits altered expression in several types of cancers, including breast, ovarian, hepatocellular, lung, and colorectal cancer (5). In a variety of tissues, Cyr61 has been shown to influence many signaling pathways involved in the regulation of normal physiologic functions. The biological mechanisms through which Cyr61 exerts its influence on the cellular environment remain elusive. This may be, in part, a consequence of context-dependent signaling by Cyr61 resulting in the activation of different downstream pathways in response to varying stimuli or in differing cells or tissues. Cyr61 signals through interaction with integrins but downstream effects vary greatly depending on the combination of integrins bound (6–9).
Numerous gene expression analyses have shown altered Cyr61 expression in various cancers, including breast, ovarian, hepatocellular, lung, and colorectal cancers, and depending on cancer type Cyr61 can either enhance or inhibit tumor growth (5, 9, 10–13). A previous study showed that the mRNA encoding Cyr61 was downregulated in prostate cancer in comparison with normal adjacent to cancer tissue (14). Furthermore, in the nonprostate cancer tissue obtained from men with prostate cancer, Cyr61 expression was significantly higher than benign tissue from men with benign prostatic hyperplasia or donor prostates (15). Sakamoto et al. showed by in situ hybridization that Cyr61 localizes to basal cells in normal prostate tissues (16). Additional evidence substantiating the importance of Cyr61 in prostate cancer indicated that transfection of Cyr61 enhances prostate cancer cell migration, invasion, and proliferation and that Cyr61 regulates Rac1 signaling, a mechanism by which Cyr61 may exert its influence on growth and motility (17). This work also showed the association between Cyr61 and metastatic capability in prostate cancer cell lines. Most recently, we observed that Cyr61 is elevated in cancers with Gleason scores of 8 and greater as compared with those with Gleason score of less than 8 (18). This finding spurred our interest in its potential utility in the identification of prostate cancers with the potential to eventually be lethal.
On the basis of the findings that Cyr61 seems to be a potentially interesting molecule both in the pathobiology of prostate cancer and as a tissue-based biomarker, we pursued the question whether Cyr61 staining levels might be able to predict which men with prostate cancer are more likely to recur after surgical treatment in a study in which the currently used clinical prognostic indicators of pathologic stage and Gleason sum and presurgery PSA are taken into account.
Materials and Methods
This study was approved by the Johns Hopkins University School of Medicine Institutional Review Board.
Study population and design
We previously developed a case–control study nested in the cohort of 4,860 men who underwent radical retropubic prostatectomy (RRP) for clinically localized prostate cancer at the Johns Hopkins Hospital between 1993 and 2005 and who had not had hormonal or radiation therapy prior to RRP. This study was designed to facilitate the evaluation of prognostic and risk factors for recurrence following RRP. Cases were the 524 men who experienced biochemical recurrence, metastasis, or prostate cancer death after surgery. For each case, we used incidence density sampling to select a control who had not experienced recurrence by the date of recurrence and who was matched on pathologic stage, Gleason sum, age, and race.
Tissues and tissue microarrays
A set of 16 tissue microarrays (TMAs) was constructed at the Department of Pathology TMA core facility at Johns Hopkins University School of Medicine for the 524 matched cases and controls. Matched pairs were placed on the same TMA so that a subset of the TMAs could be used depending on the combination of the feasibility of the assay and sample size calculations. RRP specimens were dissected immediately after surgical removal and immersed in 10% neutral buffered formalin prior to routine paraffin embedding and processing. As previously described by Kononen et al. (19), paired prostate cancer and noncancer tissues were spotted in triplicate from each RRP specimen. For this analysis, we used 10 of the TMAs. After immunohistochemical (IHC) staining, complete data in TMA spots with cancer were available for a total of 229 cases and 229 matched controls. Of these, 196 cases and 196 matched controls had TMA spots with noncancer tissue. In addition, 6 matched pairs without complete data in TMA spots with cancer had at least 1 TMA spot with noncancer tissue.
Immunohistochemistry and scoring
Cyr61 rabbit polyclonal antibody, epitope 163-240, obtained from Santa Cruz (sc-13100) was used for staining slides and TMAs. Immunostaining for Cyr61, including deparaffinization and antigen retrieval, was carried out using a Ventana Benchmark XT autostainer (Ventana Medical Systems, Inc.) and the I-view diaminobenzidine tetrahydrochloride (DAB) detection kit (Ventana Medical Systems, Inc.). Four micron thick TMA sections were incubated for 90 minutes with EDTA buffer and then with primary antibodies (1:200) for 32 minutes at room temperature. The reaction was visualized by peroxide/DAB and slides were counterstained with hematoxylin. Given the consistently diffuse and uniform intensity of Cyr61 expression by the tumor (or benign) glandular epithelial cells in a given TMA spot (Fig. 1), the assessment of area of staining (percentage of cells stained) in each TMA spot was not required. A visual intensity score was assigned for each spot by a urologic pathologist, using a 3-tier intensity system. Strong intensity staining was scored as 3, moderate as 2, weak as 1, and no staining as 0. This visual scoring method has been previously validated by comparison of semiquantitative visual intensity scoring results to quantitative analysis of Cyr61 staining intensity obtained by image analysis, described previously (18).
Fig. 1.
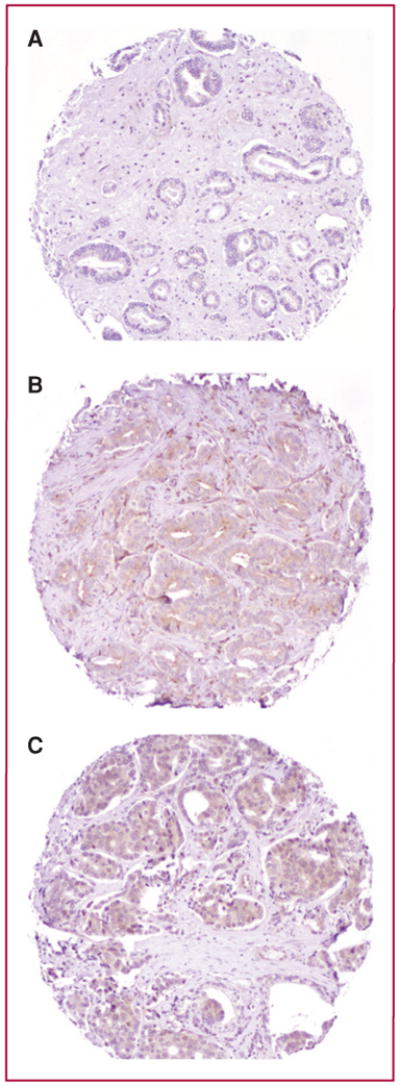
Photomicrographs of TMA spots illustrating the spectrum of immunoexpression of Cyr61 in prostate carcinoma. A, weak Cyr61 staining in Gleason score 3 + 3 = 6 prostate carcinoma. B, moderate intensity Cyr61 staining in Gleason score 3 + 4 = 7 prostate carcinoma. C, strong intensity Cyr61 staining in Gleason score 4 + 4 = 8 prostate carcinoma. Magnification, 200×.
Statistical analysis
We estimated odds ratios (ORs) of recurrence and 95% confidence intervals (CIs) by Cyr61 staining intensity, using conditional logistic regression, to take into account the matching factors: age, race, pathologic stage, and Gleason sum. In a second analysis, we further adjusted for calendar year of surgery because tissue block storage time may influence antigenicity and for presurgery PSA concentration. We estimated the association between Cyr61 staining intensity and recurrence separately for TMA spots with cancer (229 pairs) and TMA spots with normal tissue (202 pairs). We modeled staining intensity by using indicator variables corresponding to 1, 2, and 3 versus 0. For each man, we used the spot with the highest staining intensity, based on the hypothesis that greater staining intensity would be associated with a higher risk of recurrence. We also modeled staining intensity by using a binary variable with cutpoints at ≥1 (vs. 0), ≥2 (vs. <2), and 3 (vs. <3). All analyses were carried out using SAS, Version 9.1. Statistical tests were 2-sided, and P < 0.05 were considered to be statistically significant.
Results
The characteristics of the 229 men with recurrence and the 229 matched men with no recurrence are shown in Table 1. In the controls, Cyr61 staining intensity in TMA spots with cancer (using the TMA spot with the highest staining intensity) was not associated with Gleason sum or presurgery PSA concentration, but the proportion of T3b or worse disease was more common in TMA spots with higher staining intensity than in those with low staining intensity (Table 2). Mean age and the proportion of men who were not white also seemed to differ across Cyr61 staining intensity. The median Cyr61 staining intensity in the TMA spots containing cancer did not differ by Gleason sum in the controls (<8: 0.5, ≥8: 0.5).
Table 1. Characteristics of 229 prostate cancer recurrence cases and 229 matched controls nested in the Johns Hopkins Hospital recurrence cohort.
| Cases | Controls | P | |
|---|---|---|---|
| Mean age, y | 59.4 | 59.6 | Matched |
| Race, n (%) | |||
| White | 187 (81.6) | 196 (85.6) | |
| African American | 21 (9.2) | 15 (6.5) | Matched |
| Other | 21 (9.2) | 18 (7.9) | |
| Mean presurgery PSA concentration, ng/mL | 11.9 | 9.9 | 0.01 |
| Mean pathologic Gleason sum | 7.0 | 7.0 | Matched |
| Pathologic stage | |||
| T2 | 39 (17.0) | 39 (17.0) | |
| T3a | 123 (53.7) | 124 (54.2) | Matched |
| T3b or worse | 67 (29.3) | 66 (28.8) | |
| Mean time from prostatectomy to recurrence or last follow-up, y | 2.6 | 6.0 | <0.0001 |
Table 2. Characteristics of 229 controls by Cyr61 staining intensity in TMA spots containing cancer, Johns Hopkins Hospital recurrence cohort.
| Cyr61 staining intensitya | P | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||
| Mean age, y | 60.2 | 60.2 | 57.2 | 60.0 | 0.04 |
| Race, n (%) | |||||
| White | 59 (76.6) | 51 (86.4) | 35 (92.1) | 51 (92.7) | 0.06 |
| African American | 10 (13.0) | 4 (6.8) | 1 (2.6) | 0 (0) | |
| Other | 8 (10.4) | 4 (6.8) | 2 (5.3) | 4 (7.3) | |
| Mean presurgery PSA concentration, ng/mL | 11.0 | 9.2 | 8.4 | 10.1 | 0.15 |
| Mean pathologic Gleason sum | 7.0 | 7.1 | 6.8 | 7.0 | 0.31 |
| Pathologic stage | |||||
| T2 | 16 (20.8) | 7 (11.9) | 8 (21.1) | 8 (14.6) | 0.005 |
| T3a | 50 (64.9) | 29 (49.2) | 22 (57.9) | 23 (41.8) | |
| T3b or worse | 11 (14.3) | 23 (39.0) | 8 (21.1) | 24 (43.6) | |
| Mean time from prostatectomy to last follow-up, y | 5.9 | 6.1 | 6.1 | 6.1 | 0.97 |
Used the TMA spot with the highest staining intensity.
Cyr61 staining intensity in TMA spots containing cancer was not linearly associated with risk of recurrence, which was lowest in men with at least 1 TMA spot containing cancer with a staining intensity of 3 when compared with 0 (Table 3). Recurrence was identified in 12.2% of cases and 24.0% of controls had at least 1 TMA spot containing cancer with a staining intensity of 3, a difference that was statistically significant (P = 0.001). Taking into account the matching factors age, race, and pathologic stage and grade, men who had at least 1 TMA spot containing cancer with a staining intensity of 3 were less likely to recur than men with a staining intensity of less than 3. After further adjusting for calendar year of surgery and presurgery PSA concentration, the association remained inverse and statistically significant (Table 3); men who had a staining intensity of 3 had a 56% lower risk of recurrence than men with a staining intensity of less than 3. Reflecting the nonlinear association, Cyr61-positive staining when defined as ≥2 (vs. <2) or ≥1 (vs. 0) staining intensity in at least 1 TMA spot containing cancer was not associated with the risk of recurrence.
Table 3. Association between Cyr61 staining intensity in TMA spots containing cancer and risk of recurrence after prostatectomy for clinically localized prostate cancer, 229 cases and 229 matched controls nested in the Johns Hopkins Hospital recurrence cohort 1993–2005.
| Cyr61 staining intensitya | Number of cases/controls | OR (95% CI)b | OR (95% CI)c |
|---|---|---|---|
| 0 | 83/77 | 1.0 (ref) | 1.0 (ref) |
| 1 | 69/59 | 1.03 (0.63–1.68) | 1.11 (0.57–2.17) |
| 2 | 49/38 | 1.11 (0.65–1.87) | 1.57 (0.77–3.22) |
| 3 | 28/55 | 0.40 (0.21–0.76) | 0.54 (0.24–1.22) |
| <3 | 201/174 | 1.0 (ref) | 1.0 (ref) |
| 3 | 28/55 | 0.39 (0.22–0.68) | 0.44 (0.22–0.90) |
| <2 | 152/136 | 1.0 (ref) | 1.0 (ref) |
| ≥2 | 77/93 | 0.73 (0.50–1.08) | 0.96 (0.57–1.64) |
| <1 | 83/77 | 1.0 (ref) | 1.0 (ref) |
| ≥1 | 146/152 | 0.88 (0.58–1.32) | 1.08 (0.62–1.88) |
Used the TMA spot with the highest staining intensity.
Matched analysis.
Matched analysis further adjusted for calendar year of surgery and presurgery PSA concentration.
Among the 202 matched pairs that had noncancer containing TMA spots, Cyr61 staining intensity was not associated with risk of recurrence. In 11.3% of cases and 10.9% of controls, at least 1 spot with a staining intensity of 3 was present, a difference that was not statistically significant (P = 0.87). Cyr61 staining intensity, whether defined as 3 (vs. <3), ≥2 (vs. <2) or ≥ 1 (vs. 0), in noncancer containing TMA spots was not associated with risk of recurrence (Table 4). To determine whether the noncancer containing spots correlated with the cancer containing spots from the same individuals, we compared the agreement in Cyr61 staining intensity of 3 versus not 3 (staining intensity of 0, 1, and 2) between cancer and normal tissue in the same man. A staining intensity of 3 is the cutpoint that was associated with recurrence in our study. The agreement is as follows (cancer/normal tissue): 3/3, 6.9%; 3/not 3, 19.4%; not 3/3, 3.2%; and not 3/not 3, 70.4%. Taking into account chance agreement, the κ statistic is 0.27, which indicates that the agreement in Cyr61 staining intensity of 3 between tumor and associated normal tissue is not high.
Table 4. Association between Cyr61 staining intensity in TMA spots containing noncancer tissue and risk of recurrence after prostatectomy for clinically localized prostate cancer, 202 cases and 202 matched controlsa nested in the Johns Hopkins Hospital recurrence cohort 1993–2005.
| Cyr61 staining intensityb | Number of cases/controls | OR (95% CI)c | OR (95% CI)d |
|---|---|---|---|
| 0 | 111/95 | 1.0 (ref) | 1.0 (ref) |
| 1 | 42/48 | 0.74 (0.44–1.25) | 0.77 (0.40–1.48) |
| 2 | 26/37 | 0.56 (0.30–1.05) | 0.67 (0.29–1.57) |
| 3 | 23/22 | 0.84 (0.43–1.65) | 1.23 (0.51–2.95) |
| <3 | 179/180 | 1.0 (ref) | 1.0 (ref) |
| 3 | 23/22 | 1.05 (0.56–1.97) | 1.44 (0.64–3.28) |
| <2 | 153/143 | 1.0 (ref) | 1.0 (ref) |
| ≥2 | 49/59 | 0.72 (0.44–1.20) | 0.96 (0.49–1.88) |
| <1 | 111/95 | 1.0 (ref) | 1.0 (ref) |
| ≥1 | 91/107 | 0.71 (0.47–1.07) | 0.83 (0.48–1.42) |
The control analysis consisted of 202 cases and 202 controls (196 pairs + 6 pairs that were designated to have prostate cancer, but for whom the TMA spot did not contain the disease).
Used the TMA spot with the highest staining intensity.
Matched analysis.
Matched analysis further adjusted for calendar year of surgery and presurgery PSA concentration.
Discussion
In this study, we observed that men whose prostate cancers had higher Cyr61 staining intensity were less likely to recur after surgical treatment than those with lower staining intensities, a finding that was independent of currently used prognostic indicators including pathologic stage, Gleason sum, and presurgery PSA concentration. On the basis of our previous IHC results showing elevated Cyr61 expression in those with high Gleason scores (18), we hypothesized that Cyr61 would also be elevated in men whose prostate cancer recurred after treatment. Contrary to our hypothesis, higher Cyr61 expression in prostate cancer tissue was statistically significantly associated with a lower risk of recurrence. Explanations for why higher expression of Cyr61 was associated with a lower risk of prostate cancer recurrence after surgical treatment are unknown. This is clearly an area that requires further investigation but may represent a biologically important distinction in the disease. Nevertheless, ours is one of the first reports on a single tissue-based biomarker that may have utility in predicting future risk of recurrence based upon this TMA designed to evaluate the question.
In contrast to our prior investigation (18), in the current study, we found no difference in tumor Cyr61 expression between the controls with low and high Gleason scores. We did perform a reanalysis of both sets of slides and noted a lower staining intensity in the current study than in our previous work. We ruled out differences in the Cyr61 IHC protocol as the explanation for the difference in staining intensity. Finally, the number of recurrences in the initial TMA is too small to conclude anything about the association with recurrence. We have now reevaluated these data and found that although our numbers of recurrences have increased to 17 (from 11 previously), the hazard ratio (HR; adjusted for Gleason sum) is 1.29 (95% CI = 0.59–2.80) and when not adjusted for Gleason score the HR = 2.00 (95% CI = 0.89–4.30). These data indicate that there is neither a negative or positive relationship between Cyr61 staining in the first TMA, but it was not powered to evaluate this.
The results obtained in this study support that the tissue levels of Cyr61 might predict risk of prostate cancer recurrence after surgical treatment beyond the currently used pathologic and clinical indicators. Thus, Cyr61 may be a clinically useful biomarker and should be examined in expanded sample sets obtained from other institutions.
Translational Relevance.
One of the most important questions in the field of prostate cancer is differentiating the individuals with disease that has the potential to progress from those that will not. In this article, we have need a tissue microarray that has been specifically designed as a case-controlled study to determine whether Cyr61 staining is additive to the currently used tools including prostate-specific antigen and Gleason score. Our findings show that Cyr61 staining does indeed add to the ability of these criteria to determine risk for progression of prostate cancer. To our knowledge, this is one of the few biomarkers that have been studied in this fashion and much less has been determined to have this clinical potential.
Acknowledgments
The authors thank Dr. Angelo M. De Marzo and Helen L. Fedor, The Brady Urological Research Institute Prostate Specimen Repository, Johns Hopkins University School of Medicine, for the generation of the TMAs for this nested case–control study, funded in part by the Prostate SPORE Pathology Core (P50 CA58236) and a DOD grant (DAMD 17-03-0273).
Grant Support: Supported by the NCI Prostate Cancer SPORE grant (P50 CA58236), the Patana Fund of the Brady Urological Institute, and a grant from the Patrick C. Walsh Fund of the Brady Urological Institute. A.M. Mondul was supported by the National Institutes of Health, National Research Service Award T32 CA009314.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–8. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 2.Andriole GL, Crawford ED, Grubb RL, III, Buys SS, Chia D, Church TR, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–9. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jemal A, Center MM, Ward E, Thun MJ. Cancer occurrence. Methods Mol Biol. 2009;471:3–29. doi: 10.1007/978-1-59745-416-2_1. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Du X. Functional properties and intracellular signaling of CCN1/Cyr61. J Cell Biochem. 2007;100:1337–45. doi: 10.1002/jcb.21194. [DOI] [PubMed] [Google Scholar]
- 5.Planque N, Perbal B. A structural approach to the role of CCN (CYR61/CTGF/NOV) proteins in tumourigenesis. Cancer Cell Int. 2003;3:15. doi: 10.1186/1475-2867-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen CC, Young JL, Monzon RI, Chen N, Todorovic V, Lau LF. Cytotoxicity of TNFalpha is regulated by integrin-mediated matrix signaling. EMBO J. 2007;26:1257–67. doi: 10.1038/sj.emboj.7601596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen NS, Leu J, Todorovic V, Lam SC, Lau LF. Identification of a novel integrin alphavbeta3 binding site in CCN1 (CYR61) critical for pro-angiogenic activities in vascular endothelial cells. J Biol Chem. 2004;279:44166–76. doi: 10.1074/jbc.M406813200. [DOI] [PubMed] [Google Scholar]
- 8.Menendez JA, Vellon L, Mehmi I, Teng PK, Griggs DW, Lupu R. A novel CYR61-triggered ‘CYR61-alphavbeta3 integrin loop’ regulates breast cancer cell survival and chemosensitivity through activation of ERK1/ERK2 MAPK signaling pathway. Oncogene. 2005;24:761–79. doi: 10.1038/sj.onc.1208238. [DOI] [PubMed] [Google Scholar]
- 9.Lin MT, Chang CC, Lin BR, Yang HY, Chu CY, Wu MH, et al. Elevated expression of Cyr61 enhances peritoneal dissemination of gastric cancer cells through integrin alpha2beta1. J Biol Chem. 2007;282:34594–604. doi: 10.1074/jbc.M706600200. [DOI] [PubMed] [Google Scholar]
- 10.Bleau AM, Planque N, Perbal B. CCN proteins and cancer: two to tango. Front Biosci. 2005;10:998–1009. doi: 10.2741/1594. [DOI] [PubMed] [Google Scholar]
- 11.Chen PP, Li WJ, Wang Y, Zhao S, Zhao S, Li DY, Feng LY, et al. Expression of Cyr61, CTGF, and WISP-1 correlates with clinical features of lung cancer. PLoS One. 2007;2:e534. doi: 10.1371/journal.pone.0000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng P, Wang B, Ren EC. Cyr61/CCN1 is a tumor suppressor in human hepatocellular carcinoma and involved in DNA damage response. Int J Biochem Cell Biol. 2008;40:98–109. doi: 10.1016/j.biocel.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 13.Holloway SE, Beck AW, Girard L, Jaber MR, Barnett CC, Jr, Brekken RA, et al. Increased expression of Cyr61 (CCN1) identified in peritoneal metastases from human pancreatic cancer. J Am Coll Surg. 2005;200:371–7. doi: 10.1016/j.jamcollsurg.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Pilarsky CP, Schmidt U, Eissrich C, Stade J, Froschermaier SE, Haase M, et al. Expression of the extracellular matrix signaling molecule Cyr61 is downregulated in prostate cancer. Prostate. 1998;36:85–91. doi: 10.1002/(sici)1097-0045(19980701)36:2<85::aid-pros3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 15.Prakash K, Pirozzi G, Elashoff M, Munger W, Waga I, Dhir R, et al. Symptomatic and asymptomatic benign prostatic hyperplasia: molecular differentiation by using microarrays. Proc Natl Acad Sci U S A. 2002;99:7598–603. doi: 10.1073/pnas.112191399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakamoto S, Yokoyama M, Aoki M, Suzuki K, Kakehi Y, Saito Y. Induction and function of CYR61 (CCN1) in prostatic stromal and epithelial cells: CYR61 is required for prostatic cell proliferation. Prostate. 2004;61:305–17. doi: 10.1002/pros.20098. [DOI] [PubMed] [Google Scholar]
- 17.Sun ZJ, Wang Y, Cai Z, Chen PP, Tong XJ, Xie D. Involvement of Cyr61 in growth, migration, and metastasis of prostate cancer cells. Br J Cancer. 2008;99:1656–67. doi: 10.1038/sj.bjc.6604712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Antonio KB, Toubaji A, Albadine R, Mondul AM, Platz EA, Netto GJ, et al. Expression of the extracellular matrix associated protein Cyr61 is linked with the development of prostate cancer. J Urol. 2010;183:1604–10. doi: 10.1016/j.juro.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kononen J, Bubendorf L, Kallioniemi A, Bärlund M, Schraml P, Leighton S, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–947. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]


