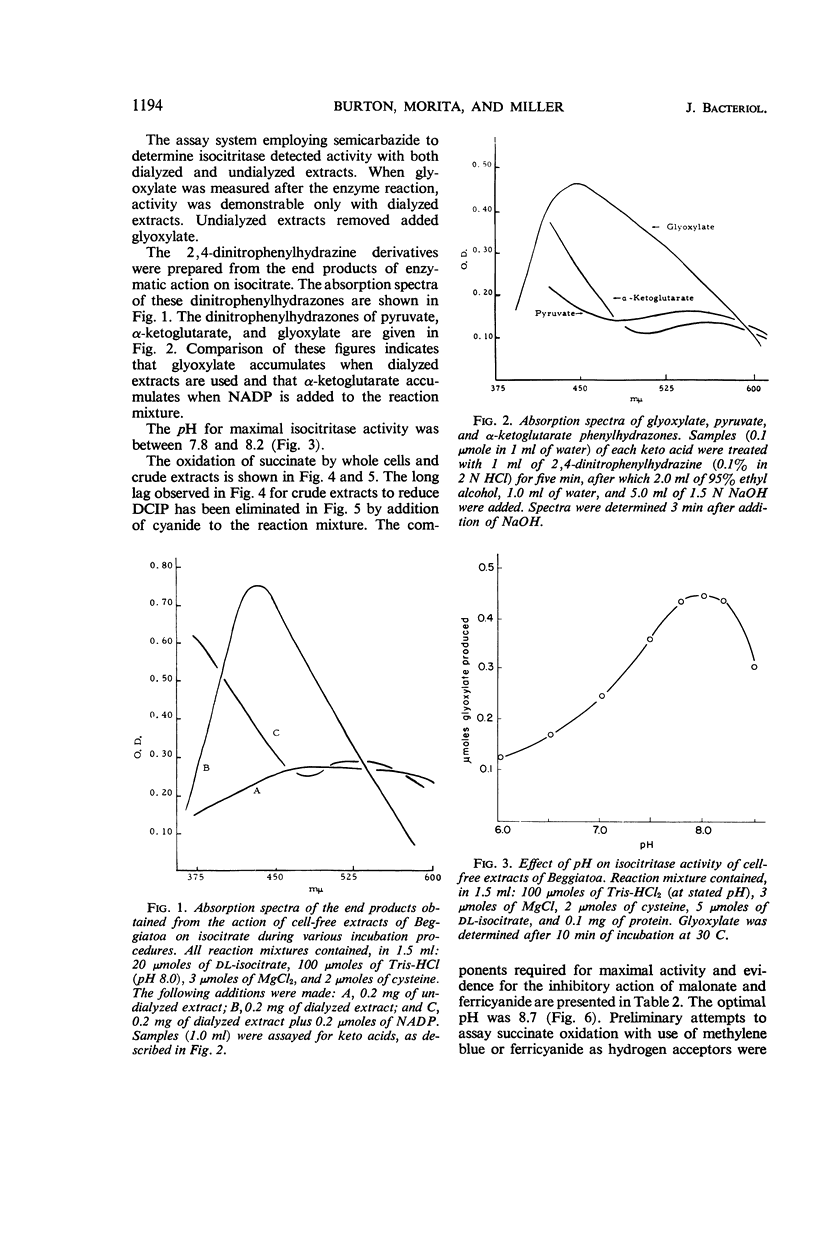
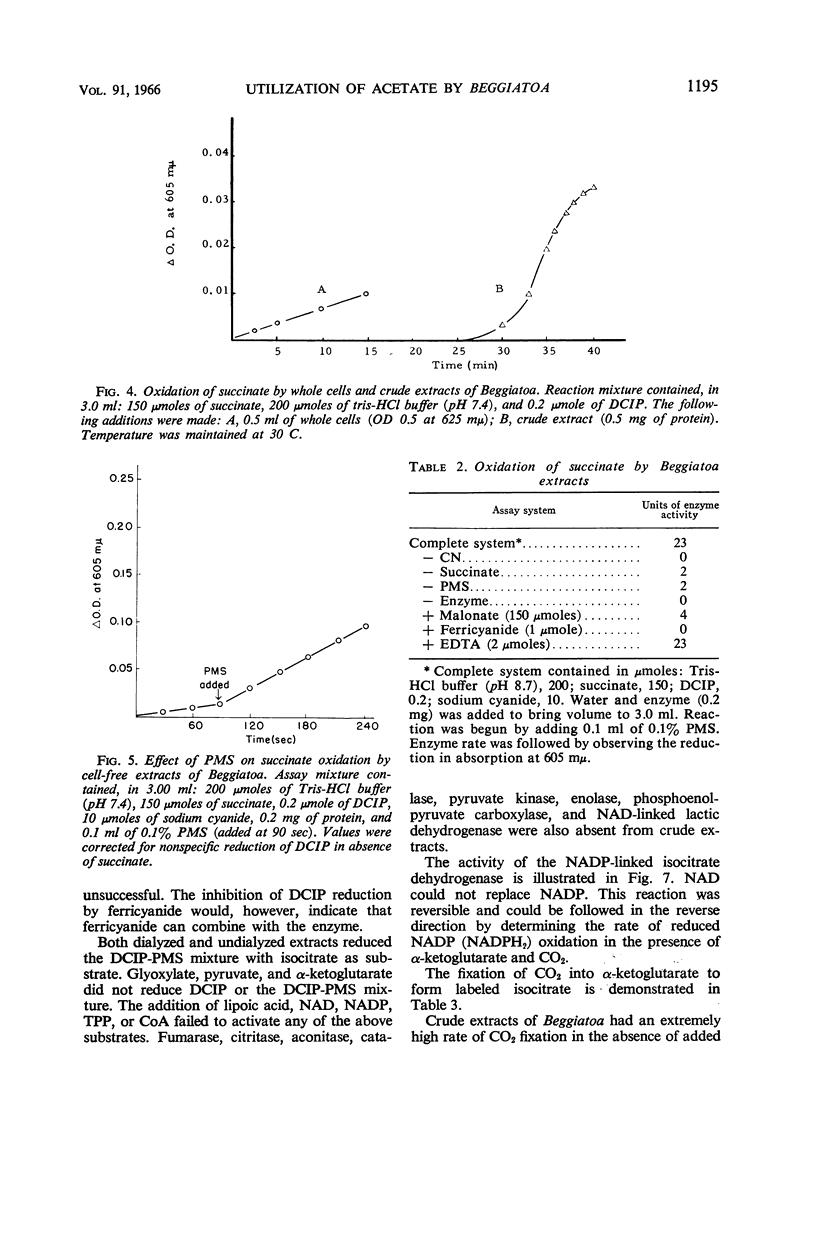
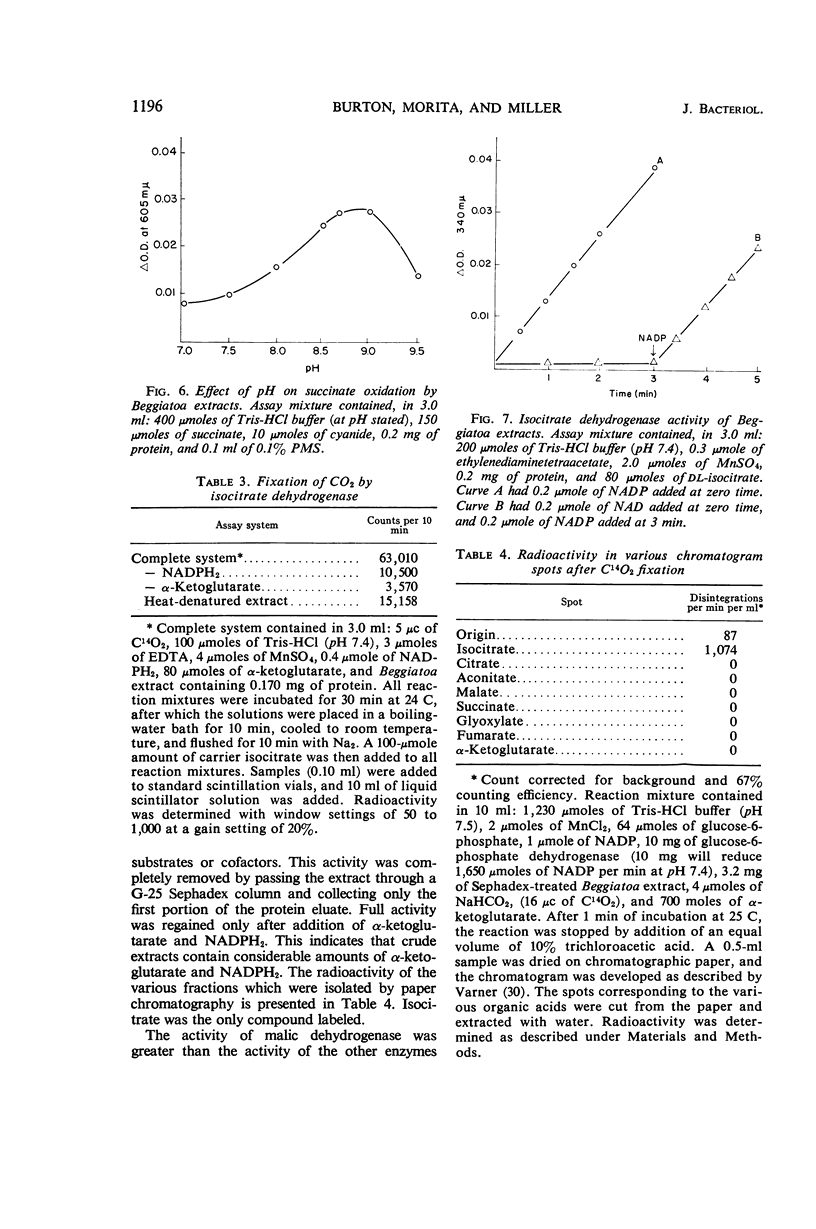
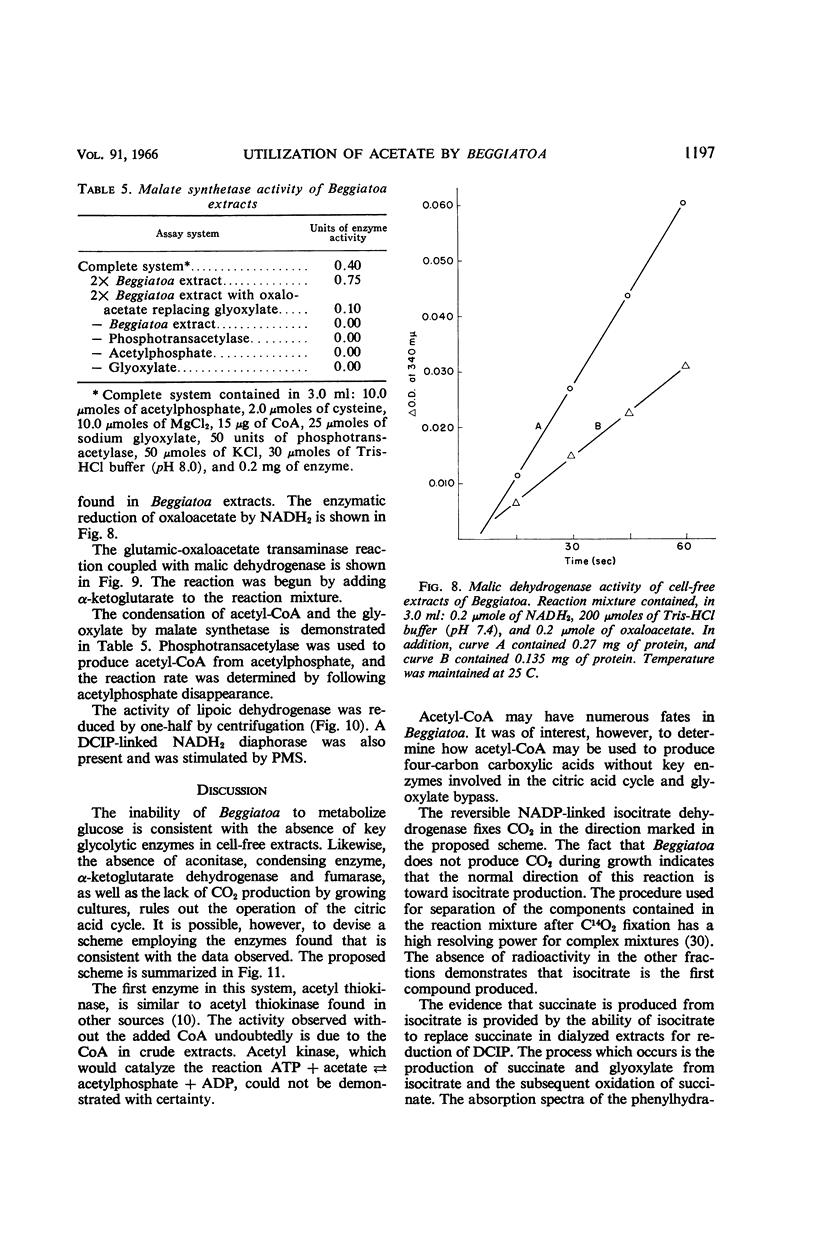
Abstract
Burton, Sheril D. (Institute of Marine Science, University of Alaska, College), Richard Y. Morita, and Wayne Miller. Utilization of acetate by Beggiatoa. J. Bacteriol. 91:1192–1200. 1966.—A proposed system which would permit acetate incorporation into four-carbon compounds without the presence of key enzymes of the citric acid cycle or glyoxylate cycle is described. In this system, acetyl-coenzyme A (CoA) is condensed with glyoxylate to form malate, which, in turn, is converted to oxaloacetate. Oxaloacetate then reacts with glutamate to produce α-ketoglutarate, which is subsequently converted to isocitrate. Cleavage of isocitrate produces glyoxylate and succinate. Thus, the proposed system is similar to the glyoxylate bypass in that malate is produced from glyoxylate and acetyl-CoA, but differs from both the citric acid cycle and the glyoxylate bypass, since citrate and fumarate are not involved. Fumarase, aconitase, catalase, citritase, pyruvate kinase, enolase, phosphoenolpyruvate carboxylase, lactic dehydrogenase, α-ketoglutarate dehydrogenase, and condensing enzyme were not detectable in crude extracts of Beggiatoa. Succinate was oxidized by a soluble enzyme not associated with an electron-transport particle. Isocitrate was identified as the sole compound labeled when C14O2 was added to a reduced nicotinamide adenine dinucleotide, CO2 generating system (crystalline glucose-6-phosphate dehydrogenase and glucose-6-phosphate) in the presence of α-ketoglutarate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANDURSKI R. S. Further studies on the enzymatic synthesis of oxalacetate from phosphorylenolpyruvate and carbon dioxide. J Biol Chem. 1955 Nov;217(1):137–150. [PubMed] [Google Scholar]
- BURTON S. D., MORITA R. Y. EFFECT OF CATALASE AND CULTURAL CONDITIONS ON GROWTH OF BEGGIATOA. J Bacteriol. 1964 Dec;88:1755–1761. doi: 10.1128/jb.88.6.1755-1761.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAUST L., WOLFE R. S. Enrichment and cultivation of Beggiatoa alba. J Bacteriol. 1961 Jan;81:99–106. doi: 10.1128/jb.81.1.99-106.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLAN N. O., COLOWICK S. P., NEUFELD E. F. Pyridine nucleotide transhydrogenase. II. Direct evidence for and mechanism of the transhydrogenase reaction. J Biol Chem. 1952 Mar;195(1):107–119. [PubMed] [Google Scholar]
- KAPLAN N. O., COLOWICK S. P., NEUFELD E. F. Pyridine nucleotide transhydrogenase. III. Animal tissue transhydrogenases. J Biol Chem. 1953 Nov;205(1):1–15. [PubMed] [Google Scholar]
- NOVELLI G. D. Methods for determination of coenzyme A. Methods Biochem Anal. 1955;2:189–213. doi: 10.1002/9780470110188.ch7. [DOI] [PubMed] [Google Scholar]
- OLSON J. A. The purification and properties of yeast isocitric lyase. J Biol Chem. 1959 Jan;234(1):5–10. [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- SANADI D. R., LITTLEFIELD J. W. Studies on alpha-ketoglutaric oxidase. I. Formation of "active" succinate. J Biol Chem. 1951 Dec;193(2):683–689. [PubMed] [Google Scholar]
- SANADI D. R., SEARLS R. L. Reversible reduction of thioctamide catalyzed by the alpha-ketoglutaric dehydrogenase complex. Biochim Biophys Acta. 1957 Apr;24(1):220–221. doi: 10.1016/0006-3002(57)90179-8. [DOI] [PubMed] [Google Scholar]
- SCOTEN H. L., STOKES J. L. Isolation and properties of Beggiatoa. Arch Mikrobiol. 1962;42:353–368. doi: 10.1007/BF00409071. [DOI] [PubMed] [Google Scholar]
- SMITH R. A., GUNSALUS I. C. Isocitritase; enzyme properties and reaction equilibrium. J Biol Chem. 1957 Nov;229(1):305–319. [PubMed] [Google Scholar]


