Abstract
The growing public health awareness of prematurity and its complications has prompted careful evaluation of the timing of deliveries by clinicians and hospitals. Preterm birth is associated with significant morbidity and mortality, and affects over half a million births in the United States each year. In some situations, however, a late-preterm birth or early-term birth is the optimal outcome for the mother, baby, or both, due to conditions that can result in worse outcomes if pregnancy is allowed to continue. These conditions may be categorized as placental, maternal, fetal, or all of these such as placenta previa, preeclampsia, and multiple gestations respectively. Some risks associated with early delivery are common to all conditions, including prematurity-related morbidities such as respiratory distress syndrome and intraventricular hemorrhage, as well as maternal delivery-related morbidities such as failed induction and cesarean delivery. However, when continuation of the pregnancy is associated with more risks such as hemorrhage, uterine rupture and stillbirth, preterm delivery maybe indicated. In February 2011, the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the Society for Maternal Fetal Medicine held a workshop on the “Timing of Indicated Late Preterm and Early Term Births”. The goal of the workshop was to synthesize the available information regarding conditions that may result in medically-indicated late preterm and early term births in order to determine the potential risks and benefits of delivery versus continued pregnancy, determine the optimal gestational age for delivery of affected pregnancies when possible, and inform future research regarding these issues. Based on available data and expert opinion, optimal timing for delivery for specific conditions was determined by consensus, along with associated risks and benefits.
Preterm birth, defined as delivery prior to 37 completed weeks’, is a public health priority, affecting over half a million pregnancies in the United States each year. Because preterm birth can result in significant morbidities and mortality, considerable effort and expense have been focused on understanding and preventing this devastating pregnancy outcome. In 2005, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) held a workshop focusing on those deliveries occurring between 34 and before 37 weeks gestation. The workshop highlighted the importance of this growing segment of preterm births, coined the designation “late preterm delivery”(34 0/7 – 36 6/7 weeks), and provided details of the morbidities associated with these deliveries (1). Although at less risk than infants born before 34 weeks gestation, infants born late preterm are more likely to have long-term neurodevelopmental problems and infant death than those born at term (2,3). In addition, neonates born between 34 and 37 weeks account for most admissions to the NICU and for large proportion of health care expenditures. In a large study (4), over one third of elective (not medically indicated) cesarean deliveries at term occurred before 39 weeks of gestation, with neonates born before 39 weeks being at increased risk for significant complications compared to those born after 39 weeks. The March of Dimes, NICHD, Society for Maternal Fetal Medicine, and American College of Obstetricians and Gynecologists have since championed the idea of preventing unnecessary preterm births and “early term births” (37 0/7 – 38 6/7 weeks). Several large health care groups have pushed to decrease the number of non-indicated deliveries before 39 weeks, with demonstrable success (5,6). Nationally, the percentage of infants born in the late preterm period declined 3 percent, from 9.1% in 2006 to 8.8% in 2008, after rising 25 percent from 7.3% to 9.1% between 1990 and 2006 (7).
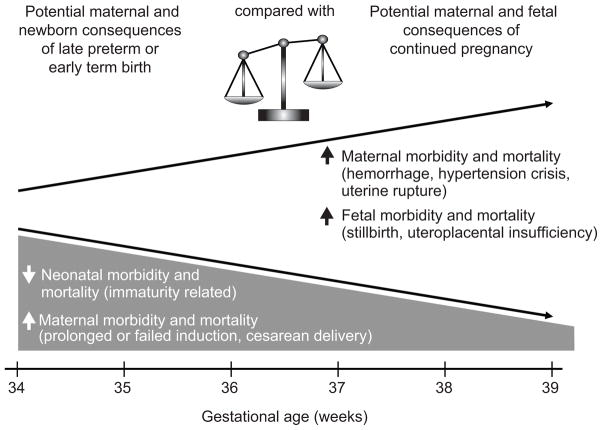
Addressing the issue of late preterm and early term birth requires balancing the risks of continuing the pregnancy versus the risk of delivery before term. (Fig. 1) While there is clear benefit to allowing uncomplicated pregnancies to reach full term, continuing pregnancy in the face of medical or obstetrical complications can potentially increase the risk to mother and/or fetus. In addition to potential acute complications from continuing the pregnancy in the presence of medical or obstetrical complications, fetal exposure to a hostile intrauterine environment has the potential to impact long term outcomes of the offspring through altered fetal programming (8). To better understand the obstetric, fetal and maternal conditions that could lead to late preterm or early term birth, the NICHD and Society for Maternal Fetal Medicine held a workshop in February 2011 on the “Timing of Indicated Late Preterm and Early Term Births”. The goals of the workshop were to synthesize the available information regarding conditions that can result in indicated late preterm and early term births in order to determine the potential risks and benefits of delivery versus continued pregnancy, determine the optimal gestational age for delivery of affected pregnancies when possible, and inform future research regarding these issues.
Figure 1.
Conceptual diagram representing the competing risks and benefits of indicated late preterm or early term birth vs. pregnancy continuation. The figure does not represent specific magnitudes or rates of changes in risks as these will vary according to the specific pregnancy complication and individual circumstances.
Neonatal morbidity and mortality decreases with advancing gestation, these are typically related to prematurity. For these deliveries in the late preterm or early term period, maternal morbidity and mortality generally are higher as a result of prolonged or failed induction and resultant cesarean deliveries.
Potential maternal consequences of continued pregnancy in the setting of complicated conditions, maternal morbidity and mortality are related to hemorrhage, hypertensive crises and uterine rupture. Continued pregnancy in these settings also can result in fetal morbidity and mortality due to stillbirth and uteroplacental insufficiency.
The workshop focused on three topic areas that reflect the most common reasons that may lead to consideration of early delivery: placental/uterine, fetal, and maternal conditions. For each condition, the epidemiology, risks, advantages of early delivery, and maternal/perinatal risks of continued pregnancy were discussed. In addition, the workshop developed recommendations regarding gestational age for delivery to optimize maternal, fetal and neonatal outcomes (Table 1), and identified high priority research gaps (Box 1) related to these conditions. For many conditions, delivery recommendations in Table 1 are based on the presence of the specific condition without additional complications (see footnote). In situations where co-morbidities are present, even earlier delivery may be indicated.
Table 1.
Guidance regarding timing of delivery when conditions complicate pregnancy at or after 34 weeks’ gestation
| Placental and uterine issues | Gestational age* for delivery | Grade of recommendation^^ |
|---|---|---|
| • Placenta previa** | 36–37 weeks | B |
|
| ||
| • Suspected placenta accreta/increta/percreta with placenta previa** | 34–35 weeks | B |
|
| ||
| • Prior classical cesarean (upper segment uterine incision)** | 36–37 weeks | B |
|
| ||
| • Prior myomectomy necessitating cesarean delivery** | 37–38 weeks (may require earlier delivery, similar to prior classical cesarean, in situations with more extensive or complicated myomectomy) | B |
|
| ||
| Fetal issues | ||
|
| ||
| • Fetal growth restriction-singleton | 38–39 weeks: | |
| • Otherwise uncomplicated, no concurrent findings | B | |
| 34–37 weeks: | ||
| • Concurrent conditions (oligohydramnios, abnormal Doppler studies, maternal risk factors, co-morbidity) | B | |
| Expeditious delivery regardless of gestational age | ||
| • Persistent abnormal fetal surveillance suggesting imminent fetal jeopardy | ||
|
| ||
| • Fetal growth restriction-twin gestation | 36–37 weeks: | |
| • Dichorionic-diamniotic twins with isolated fetal growth restriction | B | |
| 32–34 weeks: | ||
| • Monochorionic-diamniotic twins with isolated fetal growth restriction | B | |
| • Concurrent conditions (oligohydramnios, abnormal Doppler studies, maternal risk factors, co-morbidity) | B | |
| Expeditious delivery regardless of gestational age | ||
| • Persistent abnormal fetal surveillance suggesting imminent fetal jeopardy | ||
|
| ||
| • Fetal congenital malformations** | 34–39 weeks: | B |
| • Suspected worsening of fetal organ damage | ||
| • Potential for fetal intracranial hemorrhage (eg Vein of Galen aneurysm, Neonatal alloimmune thrombocytopenia) | ||
| • When delivery prior to labor is preferred (eg EXIT procedure) | ||
| • Previous fetal intervention | ||
| • Concurrent maternal disease (eg preeclampsia, chronic hypertension) | ||
| • Potential for adverse maternal effect from fetal condition | ||
| Expeditious delivery regardless of gestational age | B | |
| • When intervention is expected to be beneficial | ||
| • Fetal complications develop (abnormal fetal surveillance, new onset hydrops fetalis, progressive/new onset organ injury) | ||
| • Maternal complications develop (mirror syndrome) | ||
|
| ||
| • Multiple gestations: Dichorionic/Diamniotic** | 38 weeks | B |
|
| ||
| • Multiple gestations: Monochorionic/Diamniotic** | 34–37 weeks | B |
|
| ||
| • Multiple gestations: Di/Di or Mono/Di with single fetal death** | If occurs at or after 34 weeks, consider delivery (recommendation limited to pregnancies at or after 34 weeks. If occurs before 34 weeks, individualize based on concurrent maternal/fetal conditions) | B |
|
| ||
| • Multiple gestations: Monochorionic/Monoamniotic** | 32–34 weeks | B |
|
| ||
| • Multiple gestations: Monochorionic/Monoamniotic with single fetal death** | Consider delivery. Individualized according to gestational age and concurrent complications | B |
|
| ||
| • Oligohydramnios – isolated and persistent** | 36–37 weeks | B |
|
| ||
| Maternal and Obstetrical issues | ||
|
| ||
| Maternal issues | ||
|
| ||
| • Chronic hypertension – no medications** | 38–39 weeks | B |
|
| ||
| • Chronic hypertension – controlled on medication** | 37–39 weeks | B |
|
| ||
| • Chronic hypertension – difficult to control (requiring frequent medication adjustments)** | 36–37 weeks | B |
|
| ||
| • Gestational hypertension*** | 37–38 weeks | B |
|
| ||
| • Preeclampsia – severe** | At diagnosis (recommendation limited to pregnancies at or after 34 weeks) | C |
|
| ||
| • Preeclampsia – mild** | 37 weeks | B |
|
| ||
| • Diabetes – pregestational well controlled** | LPTB/ETB not recommended | B |
|
| ||
| • Diabetes – pregestational with vascular disease** | 37–39 weeks | B |
|
| ||
| • Diabetes – pregestational, poorly controlled** | 34–39 weeks (individualized to situation) | B |
|
| ||
| • Diabetes – gestational well controlled on diet** | LPTB/ETB not recommended | B |
|
| ||
| • Diabetes – gestational well controlled on medication** | LPTB/ETB not recommended | B |
|
| ||
| • Diabetes – gestational poorly controlled on medication** | 34–39 weeks (individualized to situation) | B |
|
| ||
| Obstetrical issues | ||
|
| ||
| • Prior stillbirth-unexplained** | LPTB/ETB not recommended Consider amniocentesis for fetal pulmonary maturity if delivery planned at <39 weeks | B C |
|
| ||
| • Spontaneous Preterm Birth: Preterm Premature Rupture Of Membranes (PROM)** | 34 weeks (recommendation limited to pregnancies at or after 34 weeks) | B |
|
| ||
| • Spontaneous Preterm Birth: Active preterm labor** | Delivery if progressive labor or additional maternal/fetal indication | B |
Gestational age is in completed weeks, thus “34 weeks” includes 34 weeks and 0 days through 34 weeks and 6 days.
Uncomplicated, thus no fetal growth restriction, superimposed preeclampsia, etc. If these are present, then the complicating conditions take precedence and earlier delivery may be indicated
Maintenance antihypertensive therapy should not be used to treat gestational hypertension LPTB: Late preterm birth at 34 weeks 0 days through 36 weeks 6 days ETB: Early term birth at 37 weeks 0 days through 38 weeks 6 days
Grade of Recommendations are based on the following: Recommendations and/or conclusions are based on (A) good and consistent scientific evidence; (B) limited or inconsistent scientific evidence; (C) primarily on consensus and expert opinion. The recommendations regarding expeditious delivery for imminent fetal jeopardy were not given a grade. The recommendation regarding severe preeclampsia is largely based on expert opinion, however higher level evidence is not likely to be forthcoming as this condition is believed to carry significant maternal risk with limited potential fetal benefit from expectant management after 34 weeks.
While the workshop focused on conditions that might benefit from late preterm or early term delivery, this list is not exhaustive. Other conditions, such as cholestasis of pregnancy, history of uterine rupture/dehiscence, and vasa previa, were identified as being appropriate to consider for late preterm or early term delivery but were not reviewed at the meeting.
Caveats
A number of caveats need to be taken into account when deciding on the optimal timing of delivery, including the potential for conflict between maternal and neonatal benefit, the role of amniocentesis for determining fetal lung maturity, the need to individualize care, and the need for accurate pregnancy dating. Determination of the optimal timing of delivery is complex as multiple factors may contribute to the risk of mortality and morbidity. In some circumstances the major reason for delivery may be to limit maternal risk, while in other cases delivery is pursued primarily for fetal benefit. In certain conditions, it is necessary to weigh opposing maternal and fetal risks of continued pregnancy versus early delivery, while in others there is benefit to both from early delivery, but the optimal timing to limit risk to the mother versus fetus needs to be considered. This document is not intended to serve as a standard of care, the individualization of clinical management is warranted. Decision-making regarding the optimal timing of delivery should incorporate the totality and relative magnitude of maternal/fetal risks, the presence of maternal co-morbidities or underlying risk factors, practice setting, and patient preferences. This conference focused on those indicated deliveries occurring in the late preterm and early term periods, from 34 weeks to 36 weeks 6 days and from 37 to 38 weeks 6 days gestational age respectively. Because of this, accurate gestational age determination is needed to optimally use these recommendations in clinical practice. The terminology used to report gestational age varies between publications. For clarity, in this manuscript we refer to gestational age in completed weeks, thus “34 weeks” includes 34 weeks and 0 days through 34 weeks and 6 days. Finally, this manuscript focuses on timing of delivery after 34 weeks. Some of the conditions addressed may occur before 34 weeks and their management may be different than what is proposed in this document. For example, management of severe preeclampsia before 34 weeks may depend on various findings relating to end-organ damage, but delivery is recommended after 34 weeks regardless of presence or absence of end-organ damage.
The utility of amniocentesis prior to delivery was discussed across the conditions. In general, the recommendation was that if an indication for delivery was present, the use of amniocentesis to assess fetal lung maturity would not assist in guiding management. This is in part due to the rationale that if significant maternal and/or fetal risk exists, delivery should occur regardless of biochemical maturity and if delivery could be deferred due to the absence of pulmonary maturity, then there is not a stringent indication for prompt delivery. Additionally, it is recognized that a mature fetal lung profile denoting the presence of pulmonary surfactant does not necessarily translate to maturity of other organ systems (9). Thus, unless amniocentesis is recommended for a specific indication, it is not otherwise addressed.
Another issue that was relevant for most conditions was the administration of antenatal corticosteroids (ACS) prior to the indicated preterm birth, especially for patients naive to ACS or who have an immature lung profile if amniocentesis was performed. A course of ACS is recommended for women at risk for preterm birth before 34 weeks. The benefit of ACS after 34 weeks is unclear, and there are ongoing studies evaluating this question (10). Current recommendations do not include ACS administration prior to delivery at or after 34 0/7 weeks gestation (11,12). Although there is one trial that found improved respiratory outcomes in infants delivered to women who received ACS prior to elective cesarean delivery at term, the data are limited (13).
The types of risks and benefits of early delivery generally are similar regardless of obstetrical or medical condition. Newborn risks include gestational age dependent morbidities (e.g. respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis, hyperbilirubinemia, feeding difficulties, temperature instability, among others). Maternal risks of early delivery include complications associated with labor induction (including increased risk of cesarean delivery, hemorrhage, infection, prolonged hospital stay). The benefits of early delivery may include avoidance of stillbirth or fetal compromise due to uteroplacental insufficiency, removal of the fetus from a hostile uterine environment, as well as resolution of the underlying condition (e.g. preeclampsia) before it worsens or secondary conditions develop.
Informed decision-making and family counseling should consider the risks of the underlying condition and concurrent complications, if any, as well as the potential for reduction of prematurity-related neonatal morbidities if pregnancy is continued, versus the likelihood of stillbirth, neonatal death and long-term sequelae. When considering fetal conditions, the results of fetal testing, such as NST and/or BPP, should be taken into consideration. A reassuring fetal testing may allow delay of delivery by few days, either to allow further maturation or cervical ripening. However, antenatal testing is imperfect. Stillbirth is possible and has been reported for several conditions (e.g. diabetes, cholestasis) even shortly after a “reassuring” antenatal test (14–16). Antenatal testing only assesses “chronic” utero-placental insufficiency and not more acute phenomena such as abruption, change in maternal state, and infection; all of which can potentially impact the risk/benefit ratio. On the other hand, timing of delivery in conditions affecting the mother may be dictated by the maternal status independent of the fetal testing. It is critical that the totality of the clinical picture be taken into account when deciding on the optimal timing of delivery. The risks and benefits of continued pregnancy versus late preterm or early term delivery specific to each condition are discussed individually. Overall the available data are limited, and thus the provided recommendations are largely based on consensus expert opinion and extrapolations from relevant articles.
Placental and uterine issues
The goals of late preterm and early term birth for pregnancies complicated by placental and uterine conditions are to avoid acute catastrophic maternal complications and to limit the potential for fetal death or compromise. Additionally, early delivery can avert an emergent unscheduled delivery performed under suboptimal circumstances. Relevant conditions include placenta previa, placenta accreta/increta/percreta (hereafter referred to as “placenta accreta”), chronic abruptio placentae and conditions that carry a significant risk of uterine rupture such as prior classical cesarean delivery and prior myomectomy.
Placenta previa occurs in 0.3–0.5% of pregnancies (17). Placenta accreta, the term being used overall for abnormal trophoblastic invasion into the myometrium and/or adjacent maternal tissues, occurs in 1 in 333 deliveries (18), in 0.2% of all pregnancies in women with a prior cesarean delivery, and in 3.3% of those with both prior cesarean delivery and placenta previa. The frequency of placenta accreta is increasing. Both placenta previa and accreta can result in severe obstetrical hemorrhage with subsequent maternal shock, need for transfusion, disseminated intravascular coagulation, hysterectomy, damage to adjacent organs, intensive care unit (ICU) admission, and death. Placenta previa is likely to result in hemorrhage before delivery of the fetus. For the asymptomatic patient near term, the risk of continuing pregnancy is of an unscheduled delivery due to hemorrhage or labor. Suboptimal timing can result in decreased availability of needed resources (e.g. blood products, dedicated operating room staff and surgical specialists), and in fetal/neonatal hypoxemia/acidemia resulting from maternal hypovolemic shock. Of 230 cases with placenta previa, risk of an emergent bleed for hemorrhage was 4.7% at 35 weeks, 15% at 36 weeks, 30% at 37 weeks and 59% at 38 weeks (19,20). A decision analysis and expert opinion recommended delivery at 36–37 weeks’ gestation in women with uncomplicated placenta previa (17, 20). Forty-four percent of women with placenta accreta will require emergency surgery if delivery is planned after 36 weeks (21). Case controlled studies of placenta accreta have demonstrated that catastrophic bleeding is common after 36 weeks and planned delivery at 34–35 weeks after antenatal steroids resulted in decreased blood loss and blood transfusions (21,22). A decision analysis came to similar conclusions that in the setting of placenta accreta, delivery after 34 weeks without confirmation of fetal lung maturity results in the highest quality-adjusted life years (23).
Chronic abruptio placentae is not well-defined in the literature, but has been described as intermittent or persistent uterine bleeding in the third trimester without other evident cause. Typically, those with abruptio placentae and maternal hemodynamic compromise or non-reassuring fetal testing are delivered rather than managed conservatively. Between 0.65–1.0% of pregnancies are complicated by abruptio placentae, but the incidence of chronic abruption is unknown. Risk factors include trauma, preeclampsia, maternal vascular disease and substance use. Complications of the condition include uteroplacental insufficiency which in turn can result in fetal growth restriction and stillbirth, as well as complications similar to those noted for placenta previa. While early delivery could potentially avoid further placental separation with subsequent acute hemorrhage requiring emergent delivery, the lack of a standard definition and limited data regarding the clinical course of this condition preclude recommendations regarding the optimal timing of delivery.
Several conditions carry a significant risk of uterine rupture. Prior cesarean delivery with a vertical incision involving the upper muscular portion of the uterus (e.g. classical cesarean) accounted for 9% of indicated repeat cesarean deliveries in one large prospective observational study (24). The prevalence of prior classical cesarean affecting pregnancy is 0.3–0.4%, with a risk of uterine rupture in subsequent pregnancies ranging between 1–12%. Similarly, myomectomy can involve the muscular portion of the myometrium, but the frequency of this condition complicating pregnancy is unknown. When myomectomy involves the muscular portion of the myometrium, delivery by cesarean is typically recommended (in contrast to myomectomy of a pedunculated myoma where vaginal delivery remains an option). One study found uterine rupture risks of 0.49–0.7% after laparoscopic myomectomy and 1.7% after resection at laparotomy, however it is unknown if any differences in risk relate to the surgical technique or the characteristics of patients selected for one approach over the other. Little is known regarding the impact of the location of the leiomyoma (e.g. transmural versus intramural versus serosal and upper versus lower uterine segment), extent of resection (number excised) on uterine rupture risk. Risks of uterine rupture include maternal hemorrhage and hypovolemic shock, as well as need for transfusion and emergency laparotomy. Importantly, uterine rupture can occur prior to the onset of labor. After uterine rupture, the fetus is at risk for stillbirth, as well as hypoxia/acidosis and its sequelae. Importantly, uterine rupture in women with prior uterine surgery involving the muscular portion can occur prior to the onset of labor (25,26). Early delivery can avert the risk of uterine rupture and its sequelae. Based on cohort studies and a decision analysis, delivery at 36–37 weeks is recommended in the setting of prior classical cesarean, with an estimated trade-off of 22 cases of RDS to prevent one case of HIE with uterine rupture (24,27,28). While the potential risk for uterine rupture after myomectomy is low, the consequences can be catastrophic. Thus when cesarean is planned for women with prior myomectomy, the strategy of delivery at 37–38 weeks may be considered, with individualization based on the type and extent of the myomectomy surgery.
Fetal issues
A number of fetal conditions place the fetus at risk for stillbirth, hypoxia/acidosis, and/or cardiac failure. Examples include fetal growth restriction, congenital malformations, multiple gestations and isolated oligohydramnios. Late preterm birth or early term birth may be beneficial under such circumstances to avoid further fetal risk secondary to a progressively, or even stable, hostile intrauterine environment.
Fetal growth restriction in singleton gestations has been variably defined using birth weight cutoffs of less than the third, fifth or 10th percentiles. Since many of these fetuses are constitutionally small, and because fetuses can experience growth failure despite being larger than an arbitrary cutoff, some investigators have advocated incorporation of maternal and paternal characteristics to derive “individualized fetal growth assessment” as a more predictive marker of fetal risk from poor intrauterine growth (29). In twin gestations, fetal growth restriction has been defined using standard cutoffs similar to singletons or growth curves specific to twins, with either method identifying about 20–25% of twin fetuses to be below the 10th percentile for all fetuses. Additionally, discordance in fetal size between twins of 20% or more is used to delineate a fetus that is not reaching its potential for intrauterine growth. The major aim of early delivery in the setting of fetal growth restriction is to minimize fetal and newborn risks, particularly fetal death and long term neurologic sequelae. The presence of concurrent medical or obstetrical conditions may shift the optimal timing of delivery towards an earlier gestation (Table 1).
Over 2% of pregnancies are complicated by major congenital anomalies, and 1% have anomalies that require delivery planning. The risks of complications dependent on the particular anomaly. Examples of maternal complications include anxiety, pregnancy-related hypertension, mirror syndrome, polyhydramnios (with its risks of respiratory compromise, abruption placentae or preterm labor), PROM or maternal respiratory compromise. The optimal gestational age of delivery to minimize fetal and newborn complications cannot be generalized as some fetal abnormalities require expeditious delivery while others can be managed expectantly. Many fetal anomalies do not necessitate either late preterm or early term birth, and these fetuses are better served by allowing time for growth and maturation in-utero. Some overarching recommendations are provided in Table 1.
Multiple gestations, including dichorionic/diamniotic and monochorionic/diamniotic twins, whether uncomplicated or affected by a single fetal death, carry increased maternal and fetal risks. Three percent of deliveries are twins and 50% of these deliver preterm with 30% delivering in the late preterm period. In addition to preterm birth, twin pregnancies are at increased risk for fetal growth restriction, congenital anomalies (monochorionic twins) as well as issues specific to multiples (twin-twin transfusion syndrome [3–4%], fetal death [1–2% per week in monochorionic gestations after 32 weeks] and discordant fetal growth). In addition, maternal conditions such as gestational diabetes, preeclampsia, abruptio placentae, placenta previa, and postpartum hemorrhage are more common in twin pregnancies. Early delivery may reduce the risk of stillbirth, and allow timing of delivery to optimize staffing and resources for newborn care in complex cases. Uncomplicated dichorionic twins have optimal outcomes when delivered at 38 weeks. Uncomplicated diamniotic-monochorionic twins have higher risks of stillbirth, thus a late preterm delivery (34–37 weeks) is recommended. Because of the high risk of cord entanglement resulting in fetal death in monoamniotic twins, delivery at 32 to 34 weeks is recommended. In the setting of a multiple gestation with one fetal demise, early delivery may prevent a second fetal death. In dichorionic pregnancy with a single fetal demise, expectant management to 37 weeks with weekly surveillance is generally recommended; however due to limited data, delivery between 34 and 36 weeks may also be reasonable. In monochorionic (mono- and di-amniotic) gestations with a single fetal demise, neurological injury may not be preventable due to the acute cardiovascular effects in the surviving co-twin, however early delivery might avert stillbirth in the live fetus. Regardless of chorionicity, maternal anxiety in these situations cannot be underestimated and may be a factor in the clinical judgment regarding the timing of delivery. Considerations regarding delivery timing for multiple gestations are presented in Table 1.
Isolated oligohydramnios has been defined by some as a single vertical pocket of 2.0 cm or less and by others as an amniotic fluid index of 5.0 cm or less. Using the single vertical pocket, oligohydramnios has been reported in 2.3% of pregnancies at 34–36 weeks and in 3% at 37 weeks or more. Using the amniotic fluid index, oligohydramnios is present in 4.8% of pregnancies at 34–36 weeks and 10% at 37 weeks or more. Oligohydramnios is associated with non-reactive non-stress tests (1.5-fold increase), fetal heart rate decelerations (1.8-fold), fetal intolerance in labor, and stillbirth (4.5-fold), as well as Apgar score ≤3 at 5 minutes (11-fold) and meconium aspiration (12-fold). Oligohydramnios in the presence of normal fetal growth may be less ominous than when it is associated with abnormal fetal growth. However, ultrasound is insensitive in the diagnosis of fetal growth restriction when population-based nomograms are used. The advantage of early delivery is the avoidance of stillbirth, but the maternal risks of early delivery are those related to labor induction and cesarean delivery. The optimal definition of oligohydramnios has not been determined. There is no consensus on which method to use in defining oligohydramnios in the context of timing of delivery. There is no evidence that one method is a better predictor of adverse neonatal outcome than the other. While either has been used to diagnose oligohydramnios, the single deepest pocket has higher specificity in the preterm period and would lead to lower rates of delivery (30). In the setting of otherwise uncomplicated isolated and persistent oligohydramnios, delivery at 36–37 weeks is recommended (Table 1). Whether a different threshold would be needed if the single deepest pocket versus the AFI is used remains unknown. Given that amniotic fluid evaluation is a component of fetal surveillance tests, it would be prudent not to make the decision regarding timing of delivery for oligohydramnios in isolation and to include the results of the complete fetal testing (BPP or modified BPP) along with other clinical parameters such as maternal condition and fetal growth.
Maternal and Obstetrical issues
Maternal and obstetrical issues that may require consideration of late preterm or early term delivery can be divided into those that are acute or those that have ongoing risk. Acute issues include preterm labor and preterm rupture of the membranes (preterm PROM), while those with ongoing risk include hypertensive diseases and diabetes, and obstetrical complications such as preeclampsia, or prior stillbirth,
Pregnancy-related hypertension (gestational hypertension and preeclampsia) occurs in about 30% of nulliparous pregnancies (31). The majority of preeclampsia (80%) occurs after 38 weeks of gestation. The risks of continued pregnancy in the setting of gestational hypertension or preeclampsia include development of severe preeclampsia and its complications [hypertensive crisis, HELLP syndrome (hemolysis, elevated liver enzymes and low platelets), abruptio placentae, renal failure, disseminated intravascular coagulation (DIC), eclampsia, death] and fetal morbidity (fetal growth restriction, asphyxia after hypertensive crisis and placental abruption, and death). Gestational diabetes occurs in up to 15% of pregnancies, however this rate may change if the criteria for its diagnosis are changed (32). Three to 4% of pregnant women have pregestational diabetes. Early delivery in the setting of diabetes may reduce the risk of macrosomia and associated birth trauma, and avoidance of stillbirth. Women with diabetes are at increased risk for uncontrolled hyperglycemia and cesarean delivery. The optimal timing of delivery in gestational hypertension, preeclampsia and diabetes depends on the severity of the condition, presence of co-morbidities, whether treatment with medication was needed, and the occurrence of superimposed obstetrical conditions (Table 1).
Recurrence of stillbirth is estimated to occur in up to 8%, and is more likely with prior earlier loss, recurrent losses, accompanied fetal growth restriction, and black race (33). Women with a history of stillbirth are also at increased risk for other complications including fetal growth restriction, preterm birth, and preeclampsia. Continued pregnancy in the setting of a prior stillbirth may also result in maternal anxiety. Fetal risks include stillbirth. In the absence of fetal growth restriction and co-morbidities, a history of an unexplained stillbirth is not an indication for late preterm or early term birth. Delivery prior to 39 weeks gestation has not been proved to reduce the risk of recurrent stillbirth or adverse pregnancy outcomes in women with prior stillbirths (Table 1). Given the lack of demonstrable benefit for early delivery in this situation, amniocentesis for lung maturity may be prudent if scheduled delivery is intended before 39 weeks gestation due to maternal anxiety and/or preferences. Importantly, however, a mature pulmonary profile in the late preterm or early term period does not assure the absence of neonatal complications.
Women presenting with preterm labor or PROM may proceed to deliver spontaneously, but this does not occur in all cases. Expectant management of late preterm or early term PROM is associated with increased risks of chorioamnionitis and fetal death from umbilical cord compression, but the latency until delivery is usually brief in most cases. Because of this short latency, newborn outcomes are unlikely to be improved with expectant management after late preterm or early term PROM, and delivery is recommended with preterm PROM occurring at or after 34 weeks gestation or in patients with PROM who have reached 34 weeks’.
Another frequent reason for late preterm or early term delivery is augmentation of labor or repeat cesarean delivery because the patient presents with contractions. The decision to augment contractions or to proceed with a previously planned cesarean delivery for women in labor in the late preterm or early term period should be reserved for those with progressive labor or a concurrent medical or obstetric complication.
Summary
With the push to reduce preterm birth and the recent public health awareness of the complications of prematurity, it is important to realize that some preterm births benefit the mother, baby or both. In specific situations, a preterm birth is the optimal outcome to pregnancy. Given the unique situations individual patients present with, the evidence to help determine the best timing for delivery may be difficult to find and generalize. We have reviewed many of the common conditions that could benefit from early delivery to help, and provide guidance to the practitioner using available evidence and expert opinion.
This document reflects the opinions of the workshop participants, and its contents do not necessarily represent the official views of either the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institutes of Health, or the Society for Maternal-Fetal Medicine.
Footnotes
Dr. Spong, Associate Editor of Obstetrics & Gynecology, was not involved in the review or decision to publish this article.
Financial Disclosure: The authors did not report any potential conflicts of interest.
References
- 1.Raju TN, Higgins RD, Stark AR, Leveno KJ. Optimizing care and outcome for late-preterm (near-term) infants: a summary of the workshop sponsored by the National Institute of Child Health and Human Development. Pediatrics. 2006 Sep;118(3):1207–14. doi: 10.1542/peds.2006-0018. [DOI] [PubMed] [Google Scholar]
- 2.Behrman RE, Butler AS, editors. Committee on understanding premature birth and assuring healthy outcomes, Board on Health Sciences Policy. Institute of Medicine. National Academies Press; 2005. Preterm birth: causes, consequences and prevention. [PubMed] [Google Scholar]
- 3.Mathews TH, MacDorman MF. National vital statistics report. 17. Vol. 58. Hyattsville, MD: National Center for Health Statistics; 2010. Infant mortality statistics from the 2006 period linked birth/infant death dataset. [PubMed] [Google Scholar]
- 4.Tita AT, Landon MB, Spong CY, Lai Y, Leveno KJ, Varner MW, Moawad AH, Caritis SN, Meis PJ, Wapner RJ, Sorokin Y, Miodovnik M, Carpenter M, Peaceman AM, O’Sullivan MJ, Sibai BM, Langer O, Thorp JM, Ramin SM, Mercer BM Eunice Kennedy Shriver NICHD Maternal-Fetal Medicine Units Network. Timing of elective repeat cesarean delivery at term and neonatal outcomes. N Engl J Med. 2009 Jan 8;360(2):111–20. doi: 10.1056/NEJMoa0803267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donovan EF, Lannon C, Bailit J, Rose B, Iams JD, Byczkowski T Ohio Perinatal Quality Collaborative Writing Committee. A statewide initiative to reduce inappropriate scheduled births at 36(0/7)-38(6/7) weeks’ gestation. Am J Obstet Gynecol. 2010 Mar;202(3):243.e1–8. doi: 10.1016/j.ajog.2010.01.044. Erratum in: Am J Obstet Gynecol. 2010 Jun;202(6):603. [DOI] [PubMed] [Google Scholar]
- 6.Oshiro B, Henry E, Wilson J, et al. Decreasing elective deliveries before 39 weeks of gestation in an integrated health care system. Obstet Gynecol. 2009;113:804–11. doi: 10.1097/AOG.0b013e31819b5c8c. [DOI] [PubMed] [Google Scholar]
- 7.Martin JA, Osterman MJK, Sutton PD. NCHS Data Brief No. 39. May, 2010. Are Preterm Births on the Decline in the United States? Recent Data From the National Vital Statistics System. [PubMed] [Google Scholar]
- 8.Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007 May;261(5):412–7. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 9.Bates E, Rouse DJ, Mann ML, Chapman V, Carlo WA, Tita AT. Neonatal outcomes after demonstrated fetal lung maturity before 39 weeks of gestation. Obstet Gynecol. 2010 Dec;116(6):1288–95. doi: 10.1097/AOG.0b013e3181fb7ece. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. [accessed 4/5/2011]; http://www.bsc.gwu.edu/mfmu/Projects/brieftrl.cgi#ALPS.
- 11.ACOG Committee Opinion No. 475. Antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2011 Feb;117(2 Pt 1):422–4. doi: 10.1097/AOG.0b013e31820eee00. [DOI] [PubMed] [Google Scholar]
- 12.Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA. 1995 Feb 1;273(5):413–8. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- 13.Stutchfield P, Whitaker R, Russell I Antenatal Steroids for Term Elective Caesarean Section (ASTECS) Research Team. Antenatal betamethasone and incidence of neonatal respiratory distress after elective caesarean section: pragmatic randomised trial. BMJ. 2005;331(7518):662. doi: 10.1136/bmj.38547.416493.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith CV, Nguyen HN, Kovacs B, McCart D, Phelan JP, Paul RH. Fetal death following antepartum fetal heart rate testing: a review of 65 cases. Obstet Gynecol. 1987 Jul;70(1):18–20. [PubMed] [Google Scholar]
- 15.Dayal AK, Manning FA, Berck DJ, Mussalli GM, Avila C, Harman CR, Menticoglou S. Fetal death after normal biophysical profile score: An eighteen-year experience. Am J Obstet Gynecol. 1999 Nov;181(5 Pt 1):1231–6. doi: 10.1016/s0002-9378(99)70114-6. [DOI] [PubMed] [Google Scholar]
- 16.Girz BA, Divon MY, Merkatz IR. Sudden fetal death in women with well-controlled, intensively monitored gestational diabetes. J Perinatol. 1992 Sep;12(3):229–33. [PubMed] [Google Scholar]
- 17.Oyelese Y, Smulian JC. Placenta previa, placenta accrete, and vasa previa. Obstet Gynecol. 2006 April;107(4):927–941. doi: 10.1097/01.AOG.0000207559.15715.98. [DOI] [PubMed] [Google Scholar]
- 18.Publications Committee, Society for Maternal-Fetal Medicine. Belfort M. Placenta accreta. Am J Obstet Gynecol. 2010;203(5):430–9. doi: 10.1016/j.ajog.2010.09.013. (Level III) [DOI] [PubMed] [Google Scholar]
- 19.Zlatnik MG, Cheng YW, Norton ME, Thiet MP, Caughey AB. Placenta previa and the risk of preterm delivery. J Matern Fetal Neonatal Med. 2007 Oct;20(10):719–723. doi: 10.1080/14767050701530163. [DOI] [PubMed] [Google Scholar]
- 20.Zlatnik MG, Little SE, Kohli P, Kaimal AJ, Stotland NE, Caughey AB. When should women with placenta previa be delivered? A decision analysis. J Reprod Med. 2010 Sep–Oct;55(9–10):373–381. [PMC free article] [PubMed] [Google Scholar]
- 21.Warshak C, Ramos G, Eskander R, Benirschke K, Saenz C, Kelly T, et al. Effect of predelivery diagnosis in 99 consecutive cases of placenta accreta. Obstet Gynecol. 2010;115:65–9. doi: 10.1097/AOG.0b013e3181c4f12a. [DOI] [PubMed] [Google Scholar]
- 22.O’Brien J, Barton J, Donaldson E. The management of placenta percreta: conservative and operative strategies. Am J Obstet Gynecol. 1996;175(6):1632–8. doi: 10.1016/s0002-9378(96)70117-5. [DOI] [PubMed] [Google Scholar]
- 23.Robinson B, Grobman W. Effectiveness of timing strategies for delivery of individuals with placenta previa and accreta. Obstet Gynecol. 2010;116(4):835–42. doi: 10.1097/AOG.0b013e3181f3588d. [DOI] [PubMed] [Google Scholar]
- 24.Landon MB, Hauth JC, Leveno KJ, et al. Maternal and perinatal outcomes associated with a trial of labor after prior cesarean section. N Engl J Med. 2004;351:2581. doi: 10.1056/NEJMoa040405. [DOI] [PubMed] [Google Scholar]
- 25.Spong CY, Landon MB, Gilbert S, Rouse DJ, Leveno KJ, Varner MW, Moawad AH, Simhan HN, Harper M, Wapner RJ, Sorokin Y, Miodovnik M, Carpenter M, Peaceman AM, O’Sullivan MJ, Sibai BM, Langer O, Thorp JM, Ramin SM, Mercer BM National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Risk of uterine rupture and adverse perinatal outcome at term after cesarean delivery. Obstet Gynecol. 2007 Oct;110(4):801–7. doi: 10.1097/01.AOG.0000284622.71222.b2. [DOI] [PubMed] [Google Scholar]
- 26.Eden RD, Parker RT, Gall SA. Rupture of the pregnant uterus: a 53-year review. Obstet Gynecol. 1986 Nov;68(5):671–4. [PubMed] [Google Scholar]
- 27.Chauhan SP, Magann EF, Wiggs CD, et al. Pregnancy after classic cesarean delivery. Obstet Gynecol. 2002;100( 5 Pt 1):946. doi: 10.1016/s0029-7844(02)02239-1. [DOI] [PubMed] [Google Scholar]
- 28.Stotland NF, Lipschitz LS, Caughey AB. Delivery strategies for women with a previous classic cesarean delivery: A decision analysis. Am J of Obstet Gynecol. 2002;187:1203. doi: 10.1067/mob.2002.127123. [DOI] [PubMed] [Google Scholar]
- 29.Gardosi J, Figueras F, Clausson B, Francis A. The customised growth potential: an international research tool to study the epidemiology of fetal growth. Paediatr Perinat Epidemiol. 2011 Jan;25(1):2–10. doi: 10.1111/j.1365-3016.2010.01166.x. [DOI] [PubMed] [Google Scholar]
- 30.Nabhan AF, Abdelmoula YA. Amniotic fluid index versus single deepest vertical pocket as a screening test for preventing adverse pregnancy outcome. Cochrane Database of Systematic Reviews. 2008;(3):Art. No.: CD006593. doi: 10.1002/14651858.CD006593.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts JM, Myatt L, Spong CY, Thom EA, Hauth JC, Leveno KJ, Pearson GD, Wapner RJ, Varner MW, Thorp JM, Jr, Mercer BM, Peaceman AM, Ramin SM, Carpenter MW, Samuels P, Sciscione A, Harper M, Smith WJ, Saade G, Sorokin Y, Anderson GB. Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units NetworkVitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010 Apr 8;362(14):1282–91. doi: 10.1056/NEJMoa0908056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryan EA. Diagnosing gestational diabetes. Diabetologia. 2011 Mar;54(3):480–6. doi: 10.1007/s00125-010-2005-4. Epub 2011 Jan 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reddy UM, Laughon SK, Sun L, Troendle J, Willinger M, Zhang J. Prepregnancy risk factors for antepartum stillbirth in the United States. Obstet Gynecol. 2010 Nov;116(5):1119–26. doi: 10.1097/AOG.0b013e3181f903f8. [DOI] [PMC free article] [PubMed] [Google Scholar]