Abstract
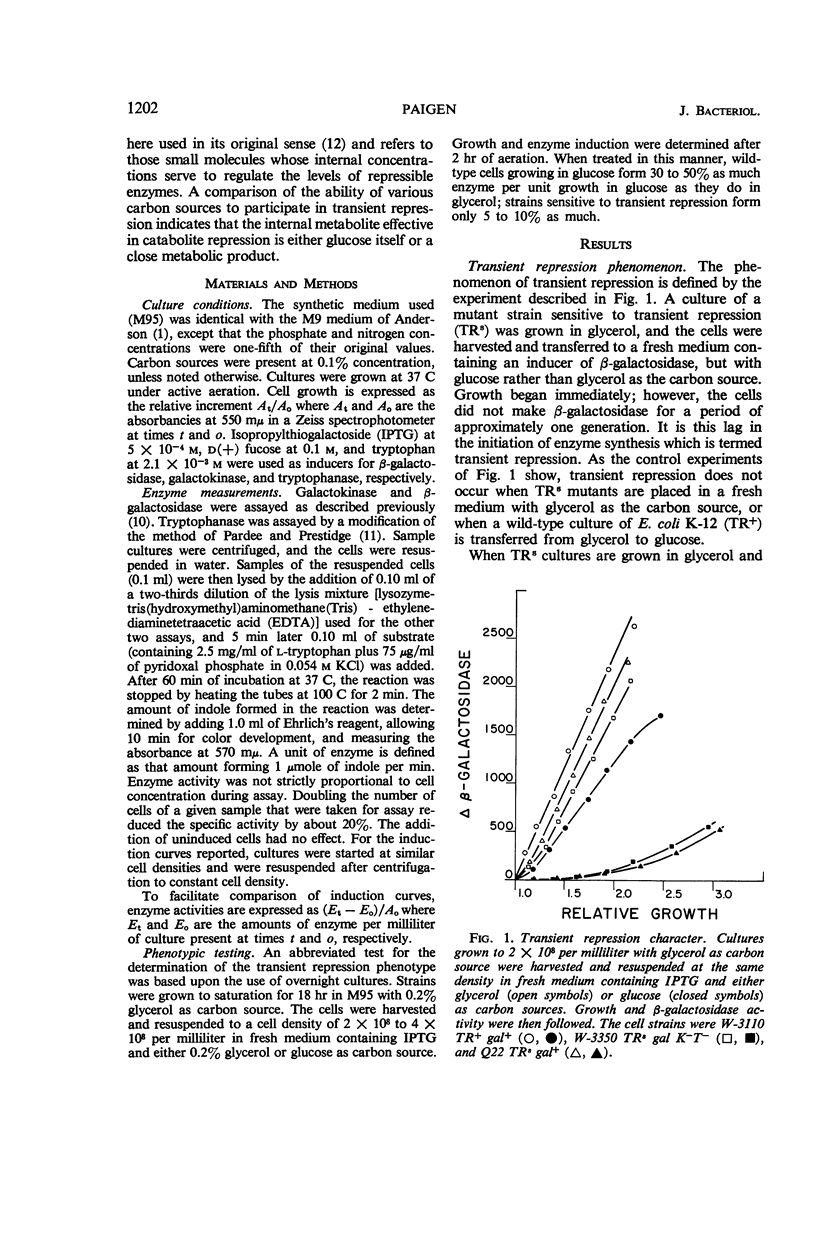
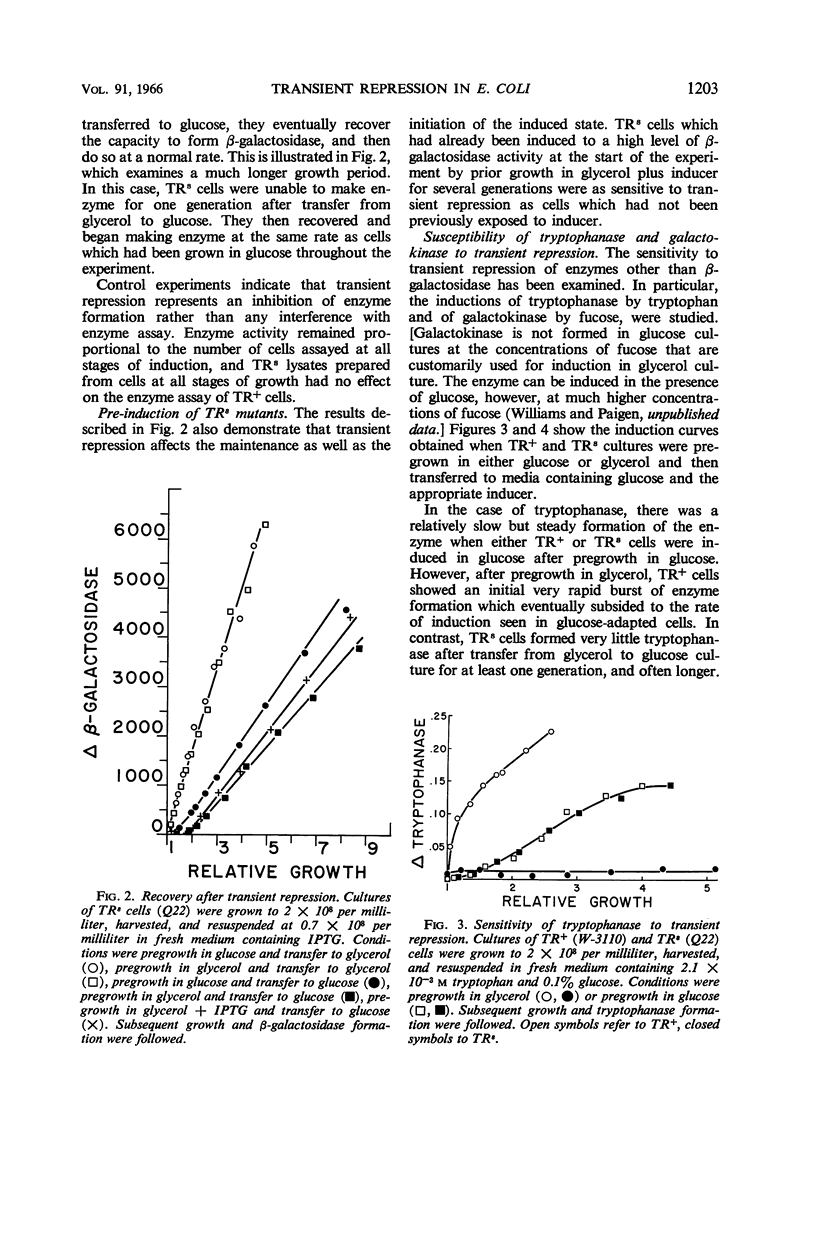
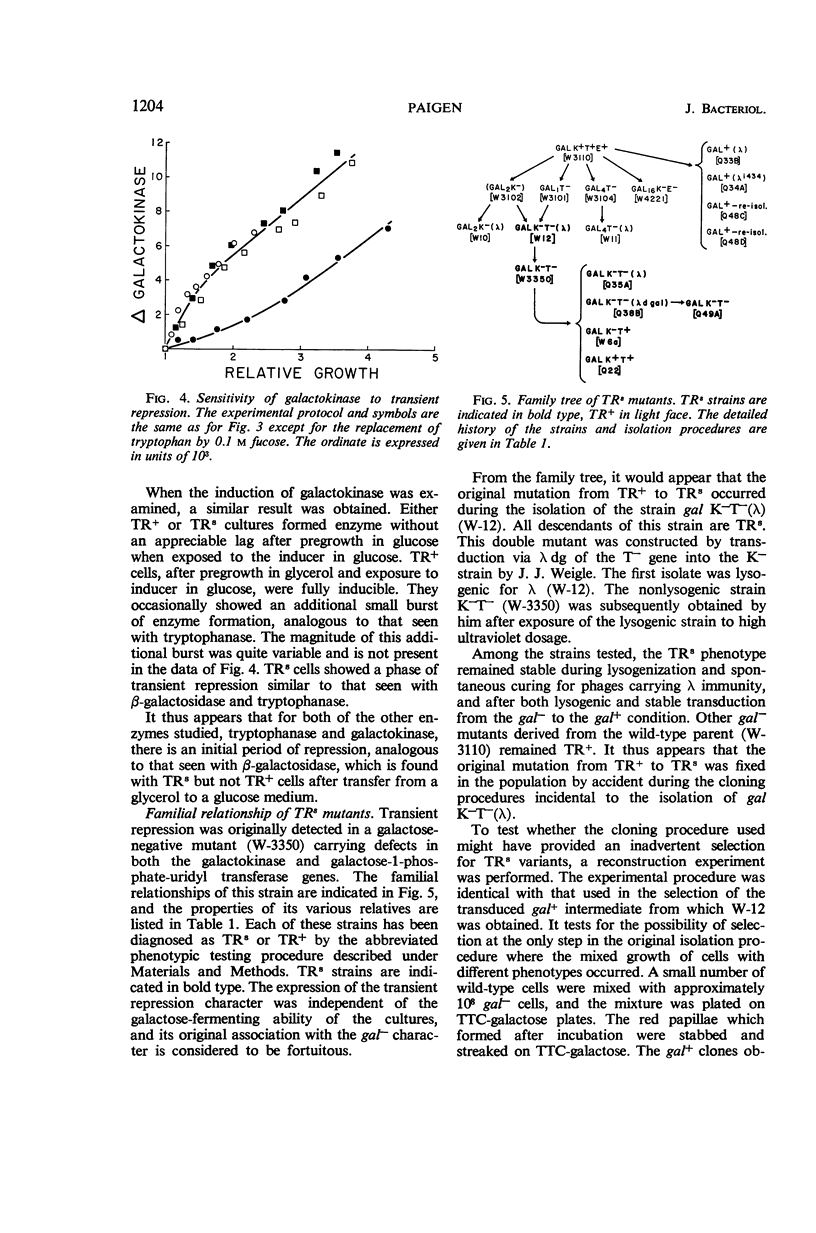
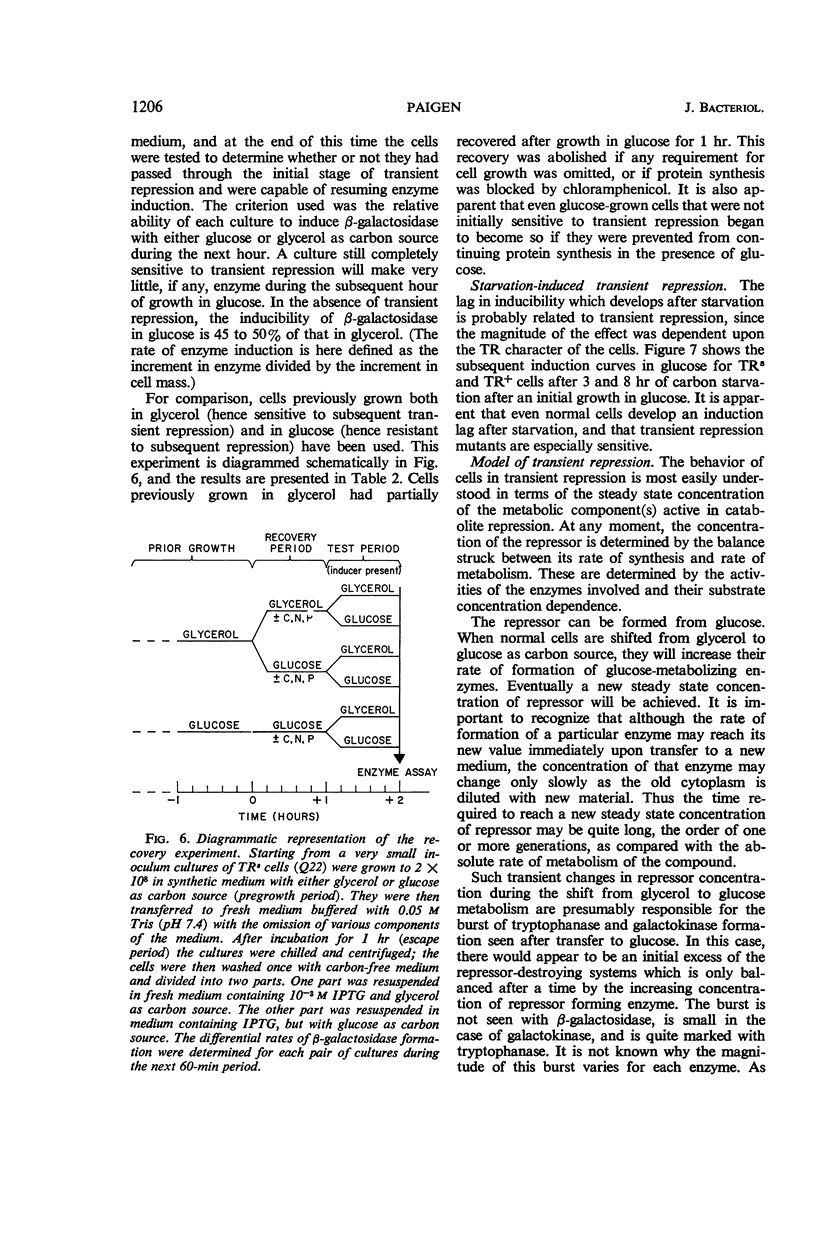
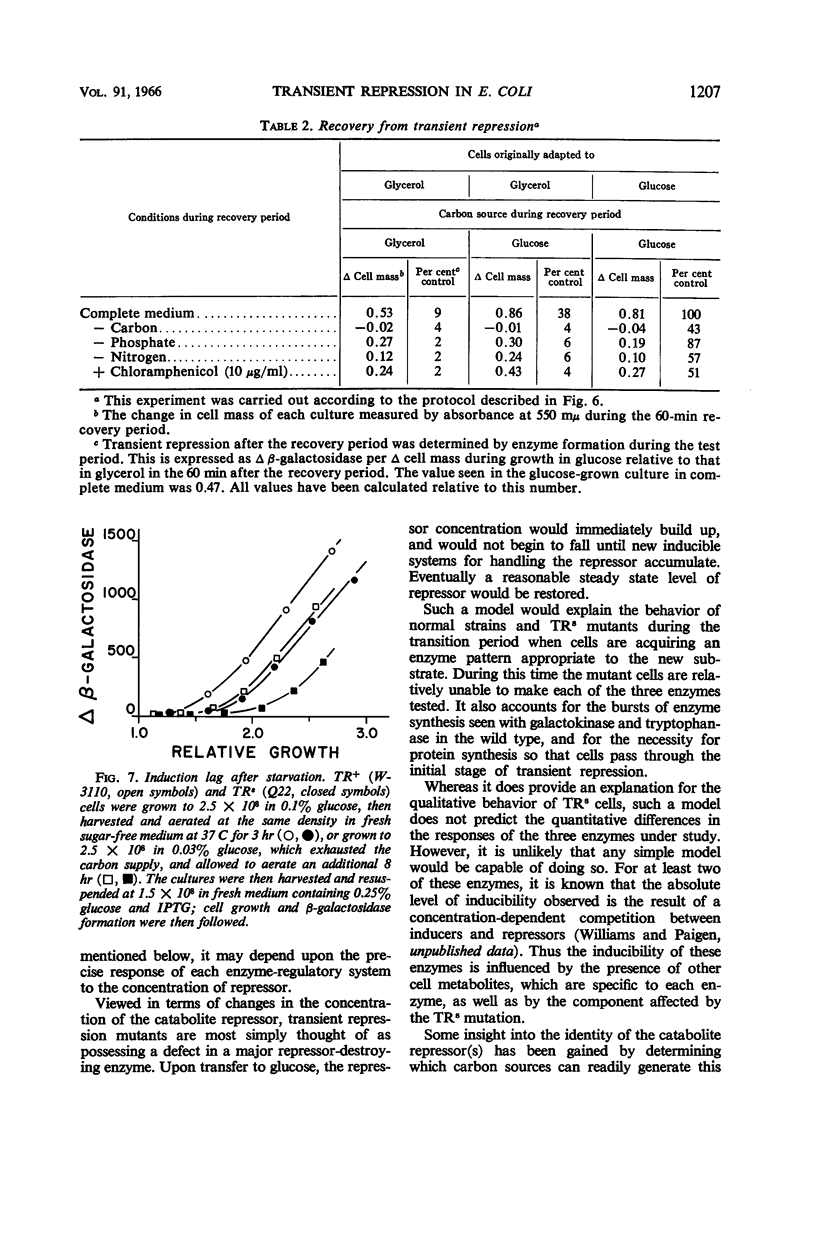
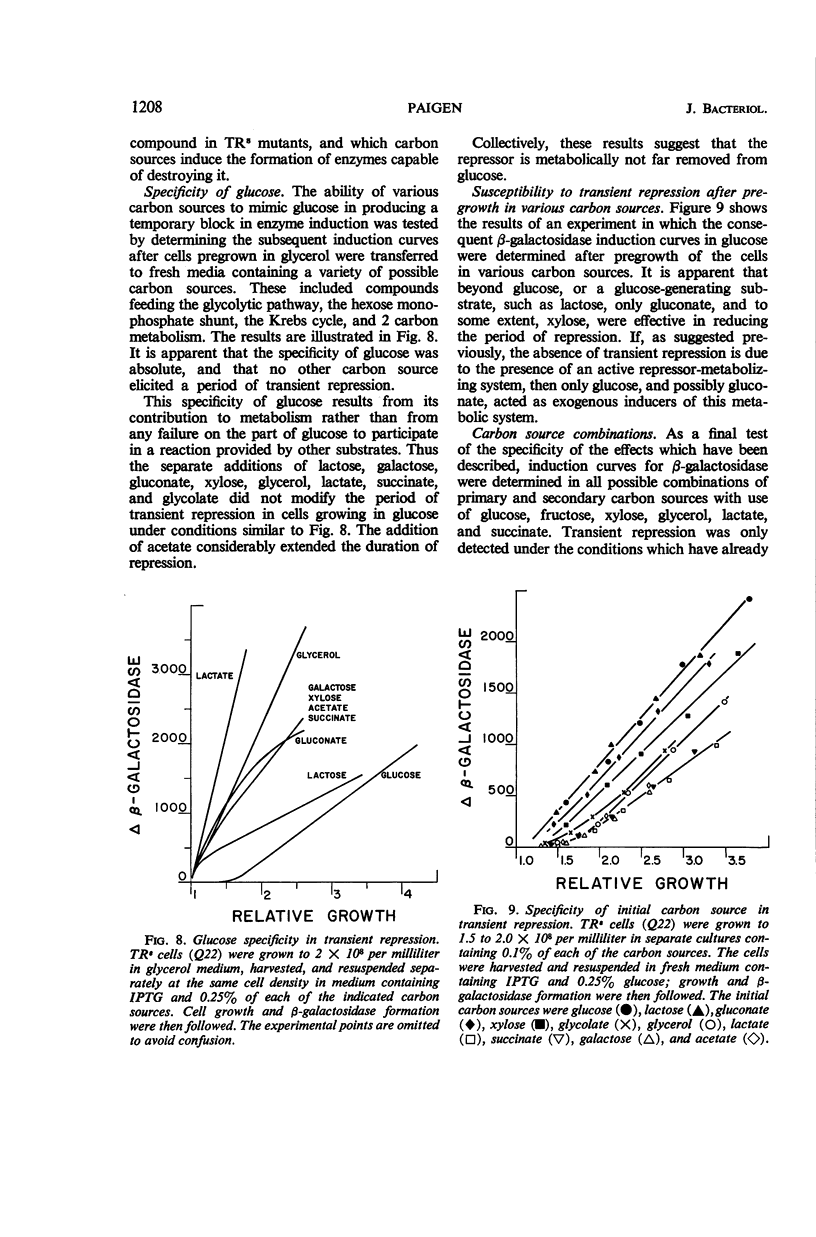
Paigen, Kenneth (Roswell Park Memorial Institute, Buffalo, N.Y.). Phenomenon of transient repression in Escherichia coli. J. Bacteriol. 91:1201–1209. 1966.—A family of mutants has been obtained in Escherichia coli K-12 in which β-galactosidase is not inducible for approximately one cell generation after the cells are transferred to glucose from other carbon sources. After that period; the enzyme can be induced at the level appropriate to glucose-grown cultures of the parent cells. Among a wide variety of carbon sources, the only one capable of eliciting a state of transient repression is glucose. Conversely, transient repression occurs when cells are transferred to glucose from any of a variety of other carbon sources. The only exceptions to this so far discovered are lactose, gluconate, and xylose. Susceptibility to transient repression in mutants can also be induced in glucose-grown cells by a period of starvation. Mutant cells which have become susceptible to transient repression lose susceptibility in the presence of glucose only when they are under conditions which permit active protein synthesis. The presence of an inducer of β-galactosidase is not required during this time, nor does pre-induction for β-galactosidase diminish the susceptibility of mutants. At least two other catabolite repression-sensitive enzymes (galactokinase and tryptophanase) are also sensitive to transient repression, and the two phenomena are probably related. The absolute specificity of glucose and the pattern of response seen after growth in different carbon sources suggest that the endogenous metabolite which produces these repressions is far more readily derived from glucose in metabolism than it is from any other exogenous carbon source.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. H. Growth Requirements of Virus-Resistant Mutants of Escherichia Coli Strain "B". Proc Natl Acad Sci U S A. 1946 May;32(5):120–128. doi: 10.1073/pnas.32.5.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epps H. M., Gale E. F. The influence of the presence of glucose during growth on the enzymic activities of Escherichia coli: comparison of the effect with that produced by fermentation acids. Biochem J. 1942 Sep;36(7-9):619–623. doi: 10.1042/bj0360619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F. FACTORS INFLUENCING THE ENZYMIC ACTIVITIES OF BACTERIA. Bacteriol Rev. 1943 Sep;7(3):139–173. doi: 10.1128/br.7.3.139-173.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- MAGASANIK B., NEIDHARDT F. C. Inhibitory effect of glucose on enzyme formation. Nature. 1956 Oct 13;178(4537):801–802. doi: 10.1038/178801b0. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J. The repression of constitutive beta-galactosidase in Escherichia coli by glucose and other carbon sources. Biochem J. 1962 Mar;82:489–493. doi: 10.1042/bj0820489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCFALL E., MANDELSTAM J. SPECIFIC METABOLIC REPRESSION OF THREE INDUCED ENZYMES IN ESCHERICHIA COLI. Biochem J. 1963 Nov;89:391–398. doi: 10.1042/bj0890391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEIDHARDT F. C., MAGASANIK B. Reversal of the glucose inhibition of histidase biosynthesis in Aerobacter aerogenes. J Bacteriol. 1957 Feb;73(2):253–259. doi: 10.1128/jb.73.2.253-259.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEIDHARDT F. C. Mutant of Aerobacter aerogenes lacking glucose repression. J Bacteriol. 1960 Oct;80:536–543. doi: 10.1128/jb.80.4.536-543.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAIGEN K. CHANGES IN THE INDUCIBILITY OF GALACTOKINASE AND BETA-GALACTOSIDASE DURING INHIBITION OF GROWTH IN ESCHERICHIA COLI. Biochim Biophys Acta. 1963 Oct 1;77:318–328. doi: 10.1016/0006-3002(63)90502-x. [DOI] [PubMed] [Google Scholar]
- PARDEE A. B., PRESTIDGE L. S. The initial kinetics of enzyme induction. Biochim Biophys Acta. 1961 Apr 29;49:77–88. doi: 10.1016/0006-3002(61)90871-x. [DOI] [PubMed] [Google Scholar]



