Abstract
Cocaine interacts with monoamine transporters and sigma (σ) receptors, providing logical targets for medication development. In the present study, in vitro and in vivo pharmacological studies were conducted to characterize SN79, a novel compound which was evaluated for cocaine antagonist actions. Radioligand binding studies showed that SN79 had a nanomolar affinity for σ receptors and a notable affinity for 5-HT2 receptors, and monoamine transporters. It did not inhibit major cytochrome P450 enzymes, including CYP1A2, CYP2A6, CYP2C19, CYP2C9*1, CYP2D6, and CYP3A4, suggesting a low propensity for potential drug–drug interactions. Oral administration of SN79 reached peak in vivo concentrations after 1.5 h and exhibited a half-life of just over 7.5 h in male, Sprague–Dawley rats. Behavioral studies conducted in male, Swiss Webster mice, intraperitoneal or oral dosing with SN79 prior to a convulsive or locomotor stimulant dose of cocaine led to a significant attenuation of cocaine-induced convulsions and locomotor activity. However, SN79 produced sedation and motor incoordination on its own at higher doses, to which animals became tolerant with repeated administration. SN79 also significantly attenuated the development and expression of the sensitized response to repeated cocaine exposures. The ability of SN79 to significantly attenuate the acute and subchronic effects of cocaine provides a promising compound lead to the development of an effective pharmacotherapy against cocaine.
Electronic supplementary material
The online version of this article (doi:10.1208/s12248-011-9274-9) contains supplementary material, which is available to authorized users.
Key words: behavior, cocaine, monoamine transporter, pharmacokinetics, sigma receptor
INTRODUCTION
Monoamine transporters, particularly dopamine, have been classically considered the main site of action for cocaine. The binding of cocaine to dopamine transporters prevents the reuptake of dopamine into the presynaptic nerve terminal, thereby increasing its concentration in the synapse (1,2). Although constitutive knockout of dopamine transporters has not been particularly effective in mitigating the rewarding effects of cocaine (3,4), recent DAT knock in studies have shown the importance of DAT binding in cocaine’s reinforcing effects (5). However, medications targeting the specific cocaine binding site and sparing other elements of DAT function are yet to be developed. Alternatively, the dual knockout of dopamine and serotonin transporters can yield promising results with regard to reducing certain behavioral actions of cocaine in mice (6). In addition, select compounds that act at monoamine transporters, such as phenyltropane analogs (i.e., RTI-336 (3β-(4-chlorophenyl)-2β-[3-(4′-methylphenyl)isoxazol-5-yl]tropane hydrochloride, RTI-177 (3β-(4-chlorophenyl)-2β-[3-phenylisoxazol-5-yl]tropane hydrochloride) have been proposed for development as potential replacement therapy for cocaine abuse (7–9). These findings are consistent with the ability of cocaine to interact not only with dopamine but other monoamine transporters (10).
Interestingly, many compounds that act at monoamine transporters, including cocaine, also interact with sigma (σ) receptors (11–13). Cocaine appears to act as an agonist at σ receptors (14,15), and selective antagonists and antisense oligonucleotides at σ receptors can attenuate an array of cocaine-induced effects in animals (12,14,16,17). Together, these earlier data indicate that σ receptors may serve both as a direct target for cocaine and an indirect modulator of dopamine, and perhaps other monoamine transporters (9,14,18–23).
For this project, SN79 (6-acetyl-3-(4-(4-(4-fluorophenyl)piperazin-1-yl)butyl)benzo[d]oxazol-2(3H)-one) was synthesized and displayed high affinity for σ receptors, along with noticeable affinities for monoamine transporters (24). The ability of SN79 to interact with both σ receptors and monoamine transporters suggested its potential for mitigating the actions of cocaine. Therefore, SN79 was tested for its effectiveness in reducing several acute and subchronic effects of cocaine, including convulsions, hyperlocomotion, and the development and expression of sensitization. Since σ receptors have an important role in motor function, SN79 was also tested in motor incoordination and catalepsy studies under acute and subchronic conditions. To further evaluate the medication development potential of the compound, in vitro assays were used to determine potential interactions with cytochrome P450 enzymes. Preliminary in vivo studies were also performed to determine the half-life of the compound following oral dosing, and the ability of SN79 when dosed orally to attenuate select behavioral effects of cocaine.
MATERIALS AND METHODS
Synthesis of SN79
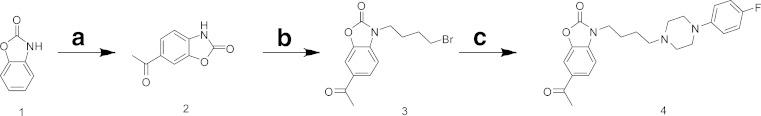
The synthetic scheme for SN79 is shown in Fig. 1. Acylation of commercially available 2(3H)-benzoxazolone (1) using acetic anhydride through a Friedel-Crafts type reaction gave a 6-acetylbenzo[d]oxazol-2(3H)-one (2) intermediate which underwent reaction with 1,4-dibromobutane to give a bromomethylene intermediate (3). This intermediate was alkylated with 1-(4-fluorophenyl)piperazine to give 6-acetyl-3-(4-(4-(4-florophenyl)piperazin-1-yl)butyl)benzo[d]oxazol-2(3H)-one (4) (SN79), which was transformed into the hydrochloride salt for biological testing. The CHN and spectral analysis of SN79 were satisfactory and consistent with its assigned structure.
Fig. 1.
Synthesis of SN79. a (CH3CO)2O, AlCl3, 75°C, 4 h, 28%; b 1,4-dibromobutane, K2CO3, DMF, 60°C, 2 h, 57%; c 1-(4-fluorophenyl)piperazine, K2CO3, DMF, 60°C, 4 h, 76%
Drugs
Compounds were obtained from the following sources: cocaine hydrochloride (Sigma, St. Louis, MO), (+)-pentazocine succinate (NIDA Chemical Synthesis Program, Rockville, MD). The radioligands were purchased from Perkin Elmer (Boston, MA). The other chemicals used for the radioligand binding assays were obtained from standard commercial sources (Sigma-Aldrich, St. Louis, MO).
SN79 has a high partition coefficient. It precipitates out of solution when it is frozen and defrosted, and is then difficult to resolubilize. Therefore, fresh stock solutions of SN79 were prepared each day by sonicating the compound for dissolution in distilled water (for in vitro assays and p.o. administration studies) or saline (for i.p. administration studies). When prepared in this manner, dissolved SN79 does not precipitate out of solution. All other compounds were prepared as previously described.
Animals
Male, Swiss Webster mice (18–28 g) and Sprague–Dawley rats (200–220 g) were obtained from Harlan (Indianapolis, IN; Frederick, MD). The mice were housed in groups of five with a 12:12-h light/dark cycle with food and water available ad libitum. The rats had a polyethylene cannula inserted in the right jugular vein and were housed individually. The animals were randomly assigned to different treatment groups. Each animal was used in only one experiment. All procedures involving animals were performed as approved by the Institutional Animal Care and Use Committee at the University of Mississippi and/or West Virginia University.
Radioligand Binding Assays
The affinity of SN79 for σ and non-σ binding sites was determined by competition binding assays in homogenates of rat brain tissues. The receptor binding assays were performed as described earlier (25,26). The total reaction volume in each tube was 500 μl. The assays were terminated by the addition of ice-cold buffer, followed by filtration through glass fiber filters presoaked in polyethyleneimine (1%) to minimize nonspecific binding. Counts were extracted from the filters using Ecoscint (National Diagnostics, Manville, NJ) for at least 8 h prior to counting. To further evaluate the selectivity of SN79, the compound was subject to NOVAScreen (Caliper Life Sciences, Hanover, MD) at additional targets. Further details of each assay condition can be accessed through their website at www.caliperls.com.
Cytochrome P450 Interactions
The effect of SN79 on a number of human cytochrome P450 enzymes was assessed by NovaScreen CYPScreen (Caliper Life Sciences, Hanover, MD). The specific cytochrome P450 enzymes selected were those most likely to cause problematic drug–drug interactions, with nicotinamide adenine dinucleotide phosphate (NADPH) being added to the substrate to catalyze the reaction. SN79 was evaluated at a single screening concentration of 10 μM, with 50% used as the criterion for significant inhibition at this concentration. Additional details of the assay conditions can be accessed through the Caliper Life Sciences website at www.caliperls.com.
In Vivo Metabolism Studies
Blood samples were taken from male, Sprague–Dawley rats (n = 5) outfitted with indwelling catheters in the jugular vein. An initial blood volume of 0.1 ml was taken to clear the line of heparinized saline. A fresh syringe was used to withdraw a 0.15-ml blood sample. This and all subsequent blood samples were placed in microfuge tubes and allowed to clot for 30 min at 0°C. The rats were administered 20 mg/kg SN79 using oral gavage. Timed blood samples were then collected for up to 36 h. For each blood sample, serum was separated by centrifugation at 3,000 rpm for 20 min at 4°C. SN79 was quantified using ultra performance liquid chromatography (details to be reported in another manuscript).
Convulsions
The mice were pretreated (i.p.) with saline (n = 10) or SN79 (0.1–10 mg/kg, n = 30), then challenged 15 min later with a convulsive dose of cocaine (70 mg/kg, i.p., n = 40). The mice were then individually placed in their own plastic chamber (59 × 43 × 13 cm) and observed for the next 30 min for convulsions. Convulsions were operationally defined as a loss of righting reflexes for at least 5 s combined with the presence of clonic limb movements or popcorn jumping.
Some of the mice were pretreated (p.o.) with distilled water (n = 48) or SN79 (10 mg/kg, n = 20), then challenged 60 min later with i.p. saline (n = 10) or cocaine (60–80 mg/kg, i.p., n = 58). The treated animals were observed for the next 30 min for the presence of convulsions as described above.
Locomotor Activity
The mice were acclimated to the treatment room for 30 min and then individually to a testing chamber of an automated activity monitoring system (San Diego Instruments, San Diego, CA) for 15 min. The mice were injected (i.p.) with saline (n = 24) or SN79 (0.1–10 mg/kg, n = 100), then challenged 15 min later with a locomotor stimulatory dose of cocaine (20 mg/kg, i.p., n = 62) or saline (n = 62). The total locomotor activity (ambulatory, fine, and rearing movements) of the mice was recorded for the next 30 min as the number of disruptions made by them in the 16 × 16 photobeam grids of the plexiglass testing chambers.
The mice were also pretreated (p.o.) with either distilled water (0.1 ml/10 g, n = 16) or SN79 (10–25 mg/kg, n = 40), and locomotor activity was recorded for 60 min prior to receiving an i.p. injection of either a stimulant dose of cocaine (20 mg/kg, n = 50) or saline (0.1 ml/10 g, n = 6). Total locomotor activity was recorded for the next 120 min.
Development of Sensitization
The mice were randomly divided into six treatment groups as shown in Table I. The mice were pretreated (i.p.) with saline (n = 16) or SN79 (0.1–10 mg/kg, n = 32), then challenged 15 min later with a locomotor stimulatory dose of cocaine (10 mg/kg, i.p., n = 24) or saline (n = 24). After the treatment, locomotor activity was quantified as described above. This procedure was conducted at the same time every day, for five consecutive days (days 1–5). A 10-day drug-free period followed to wash out the drugs and their metabolites from the mice. On day 15, all of the mice were pre-administered saline (n = 48) followed by cocaine (10 mg/kg, i.p., n = 48), and their locomotor activity was quantified.
Table I.
Treatment Schedule for Sensitization Experiments
| Group | Day 1–5 | Day 6–14 | Day 15 |
|---|---|---|---|
| Development of sensitization | |||
| 1 | Sal + Sal | NT | Sal + Coc |
| 2 | Sal + Coc | NT | Sal + Coc |
| 3 | SN (1 mg/kg) + Coc | NT | Sal + Coc |
| 4 | SN (10 mg/kg) + Coc | NT | Sal + Coc |
| 5 | SN (1 mg/kg) + Sal | NT | Sal + Coc |
| 6 | SN (10 mg/kg) + Sal | NT | Sal + Coc |
| Expression of sensitization | |||
| 1 | Sal + Sal | NT | Sal + Coc |
| 2 | Sal + Sal | NT | SN (1 mg/kg) + Coc |
| 3 | Sal + Sal | NT | SN (3 mg/kg) + Coc |
| 4 | Sal + Sal | NT | SN (10 mg/kg) + Coc |
| 5 | Sal + Coc | NT | Sal + Coc |
| 6 | Sal + Coc | NT | SN (1 mg/kg) + Coc |
| 7 | Sal + Coc | NT | SN (3 mg/kg) + Coc |
| 8 | Sal + Coc | NT | SN (10 mg/kg) + Coc |
All compounds were administered intraperitoneally. n = 6–8 per group
NT no treatment, Coc cocaine (10 mg/kg), Sal saline, SN SN79
Expression of Sensitization
The mice were divided randomly into eight treatment groups as shown in Table I. For five consecutive days at the same time (days 1–5), the mice were pretreated (i.p.) with saline (n = 48), then challenged 15 min later with cocaine (10 mg/kg, i.p., n = 24) or saline (n = 24), and their locomotor activity was recorded. A 10-day drug-free period followed to wash out the drugs and their metabolites from the mice. On day 15, the mice were administered (i.p.) saline (n = 12) or SN79 (1–10 mg/kg, n = 36), followed 15 min later with cocaine (10 mg/kg, i.p. n = 48). The locomotor activity was then recorded for the next 30 min.
Rotor Rod Test
The mice were trained to maintain their balance for 2 min on the rotor rod (San Diego Instruments, San Diego, CA) every morning and evening for three consecutive days. The rod accelerated to a speed of 6 rpm in 10 s and continued at the same speed until 2 min. The mice that failed to balance on the rotor rod at the end of the training sessions on the third day were excluded from the study. On the fourth day, the mice were treated (i.p.) with saline (n = 10) or SN79 (1, 10 mg/kg, n = 20). Their latency to fall was recorded at 10, 20, 30, 40, and 60 min after the treatment.
Catalepsy Test
The mice were administered (i.p.) either saline (n = 10) or SN79 (1, 10 mg/kg, n = 20). Their front paws were then placed on a horizontal metallic rod that was raised 5 cm above the bench top. The latency of the mice to bring their front paws down on the bench top was measured, with a cutoff time of 60 s. The cataleptic mice remain on the bar while the normal mice return to the bench top almost immediately. The measurements were made at 0, 10, 20, 30, 40, and 60 min after the treatment.
Chronic Rotor Rod Test
The mice were tested on the rotor rod as described above for five consecutive test days. On each of the test days, the mice were administered (i.p.) either saline (n = 7) or SN79 (10 mg/kg, n = 7).
Data Analysis
The data from the radioligand binding assays were analyzed using GraphPad Prism 4.0 (San Diego, CA) to calculate IC50 values. Apparent Ki values were then calculated using the Cheng–Prusoff equation, and Kd values were determined in separate saturation assays. The data from the cocaine-induced convulsion studies were analyzed using Fisher’s exact tests (GraphPad InStat, San Diego, CA). The data from the locomotor activity measurements, development and expression of sensitization studies, and catalepsy tests were analyzed using a one-way analysis of variance (ANOVA) followed by Dunnett’s or Tukey’s post hoc comparisons, wherever applicable. The acute and subchronic rotor rod test data were analyzed using a two-way ANOVA with post hoc Bonferroni’s tests (GraphPad Prism, San Diego, CA). P < 0.05 was considered statistically significant for all behavioral tests.
RESULTS
Affinity and Selectivity of SN79 for Sigma Receptors
The affinities of SN79 for σ1 and σ2 receptors, as well as 61 other receptors, transporters, ion channels, and binding sites are listed in Table II and in Supplementary Table I. Because SN79 precipitates out of concentrated solutions following freezing and defrosting, initial binding studies reported in abstract form that SN79 was a selective σ2 receptor ligand (24), an artifact which resulted from testing the σ2 receptor subtype first. In the current study, fresh stock solutions of SN79 were prepared from two different synthetic batches of the compound. The affinity of SN79 for σ1 receptors was 28.03 ± 3.39 nM (batch 1) and 25.22 ± 2.12 nM (batch 2). The affinity of SN79 for σ2 receptors was 6.89 ± 0.09 nM, which was consistent with earlier reported Ki values (24). The averages of all assays from the combined batches are summarized in Table II and show that SN79 binds to both σ1 and σ2 receptors in the nanomolar range. SN79 also exhibits moderate affinity for serotonin transporters and norepinephrine transporters, as well as some affinity for dopamine transporters. In addition, SN79 displayed a moderate affinity for 5-HT2 receptors and negligible affinity (>10,000 nM affinity or >1,000-fold selectivity compared to σ binding) for all other binding sites tested, which are listed in Supplementary Table I.
Table II.
Binding Affinities of SN79 for σ Receptors and Monoamine Transporters
| Radioligand | Nonspecific binding | Tissue | K i | |
|---|---|---|---|---|
| Sigma receptors: | ||||
| σ1 | 5 nM [3H](+)-pentazocine | 10 μM haloperidol | Rat brain | 27 ± 2 |
| σ2 | 3 nM [3H]di-o-tolylguanidine | 10 μM haloperidol | Rat brain | 7 ± 0.09 |
| Monoamine transporters: | ||||
| Dopamine | 0.5 nM [3H]WIN 35,428 | 50 μM cocaine | Rat striatum | 2,610 ± 57 |
| Serotonin | 0.2 nM [3H]paroxetine | 1.5 μM imipramine | Rat brainstem | 159 ± 15 |
| Norepinephrine | 0.5 nM [3H]nisoxetine | 4 μM desipramine | Rat cerebral cortex | 177 ± 14 |
Affinities (K i in nanomolars) were determined in the rat brain. The values in this table represent the mean ± S.E.M. from replicate assays
Lack of Inhibition of Major Cytochrome P450 Isozymes by SN79
Table III summarizes data obtained from NovaScreen CYPScreen analysis with SN79. SN79 (10 μM) does not significantly inhibit any of the following major cytochrome P450 isozymes: CYP1A2, CYP2A6, CYP2C19, CYP2C9*1, CYP2D6, and CYP3A4.
Table III.
Human Cytochrome P450 Inhibition Studies
| Cytochrome P450 | Substrate | % Inhibition by SN79 |
|---|---|---|
| 1A2 | 5 μM 7-ethoxy-3-cyanocoumarin | 17.8% |
| 2A6 | 3 μM coumarin | −10.4% |
| 2C19 | 2.5 μM 7-ethoxy-3-cyanocoumarin | 34.8% |
| 2C9*1 | 37.5 μM 7-methoxy-4-trifluoromethyl-coumarin | 15.3% |
| 2D6 | 0.5 μM AMMC | −1.7% |
| 3A4 | 0.5 μM dibenzyl fluorescein | 29.0% |
Duplicate assays were conducted for the inhibition of human recombinant cytochrome P450 enzymes expressed in SF9 cells by SN79. NADPH was added to the substrate to catalyze the reaction. SN79 was evaluated at a single screening concentration of 10 μM, with 50% used as the criterion for the significant inhibition at this concentration. The negative values represent slight induction. SN79 did not significantly inhibit or induce the major cytochrome P450 enzymes tested. AMMC = 3-[2-(N,N-diethyl-N-methylamino)ethyl]-7-methoxy-4-methylcoumarin
In Vivo Metabolism Studies
Plasma pharmacokinetic parameters associated with oral dosing of SN79 are: area under the curve, AUC (117.34 ± 1.53 μg-h/ml); half-life, T1/2 (7.51 ± 0.68 h); maximum concentration, Cmax (0.21 ± 0.08 μg/ml); mean residence time (9.90 ± 1.34 h); volume of distribution, Vd (115.10 ± 1.06 l/kg); apparent oral clearance at steady state, CLss/F (0.18 ± 0.02 l/h/kg); time to reach maximum concentration, Tmax (1.50 ± 0.05 h).
Effect of SN79 on Cocaine-Induced Convulsions
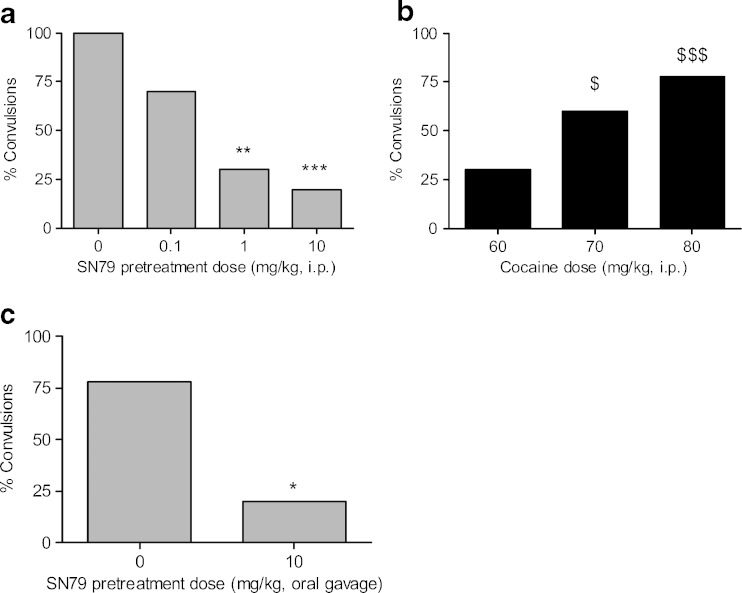
Intraperitoneal pretreatment with increasing doses of SN79 prior to administering a convulsive dose of cocaine led to a dose-dependent decline in the percentage of mice exhibiting convulsions as shown in Fig. 2a. This effect was significant at the SN79 pretreatment doses of 1 and 10 mg/kg, i.p. (p < 0.005 and p < 0.001, respectively), where the percentage of cocaine-induced convulsions was reduced to 30% and 20%, respectively.
Fig. 2.
a Effect of SN79 on cocaine-induced convulsions. Male, Swiss Webster mice were pretreated (i.p.) with SN79 (0.1–10 mg/kg) or saline, followed 15 min later with a convulsive dose of cocaine (70 mg/kg, i.p.). SN79 significantly attenuated cocaine-induced convulsions. **p < 0.005, ***p < 0.001, Fisher’s exact tests, n = 10 per group. b Dose response of cocaine-induced convulsions (60–80 mg/kg, i.p.), 1 h after oral administration of distilled water. Cocaine at 70 and 80 mg/kg produced convulsions in a significant percentage of mice when compared to a saline treatment group. $ p < 0.05, $$$ p < 0.001, Fisher’s exact tests, n = 9–10 per group. c Orally administered SN79 (10 mg/kg) attenuated cocaine-induced (80 mg/kg, i.p.) convulsions (*p < 0.05, Fisher’s exact tests), in a pattern similar to i.p. administration of SN79
Following oral administration of distilled water, cocaine produced convulsions in a dose-dependent manner (Fig. 2b). The Fisher’s exact tests confirmed that the changes were significant at the following i.p. doses of cocaine: 70 mg/kg (60% convulsions, p < 0.05) and 80 mg/kg (78%, p < 0.001) when compared to i.p. injections of saline which failed to produce convulsions in any animals tested. The convulsive effects of cocaine were reduced by 60 min pretreatment of orally administered SN79 (10 mg/kg). SN79 reduced the percentage of mice exhibiting convulsions due to the 70 mg/kg dose of cocaine from 60% to 10% (difference of 50%), but the change was not quite statistically significant (p = 0.057); orally administered SN79 significantly reduced the percentage of mice exhibiting convulsions following the 80-mg/kg dose of cocaine from 78% to 20% convulsions (difference of 58%, p < 0.05; Fig. 2c).
Effect of SN79 on Cocaine-Induced Locomotor Activity
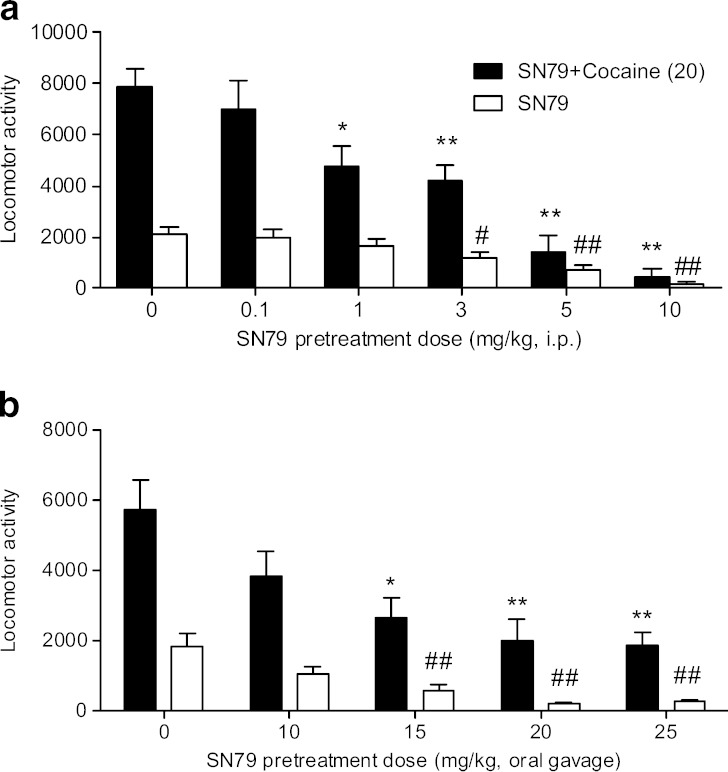
SN79 pretreatment prior to a locomotor stimulant dose of cocaine caused a significant attenuation of the locomotor hyperactivity (F(5,56) = 16.53, p < 0.0001), as shown in Fig. 3a. Post hoc Dunnett’s tests were conducted, and the attenuation of cocaine-induced hyperactivity was significant at four doses of SN79: 1 mg/kg (q = 3.07, p < 0.05), 3 mg/kg (q = 3.65, p < 0.01), 5 mg/kg (q = 6.42, p < 0.01), and 10 mg/kg (q = 7.35, p < 0.01).
Fig. 3.
a Effect of SN79 on cocaine-induced and basal locomotor activity. Male, Swiss Webster mice were pretreated (i.p.) with either SN79 (0.1–10 mg/kg) or saline followed 15 min later with a locomotor stimulant dose of cocaine (20 mg/kg, i.p., black bars) or saline (white bars). SN79 significantly attenuated the locomotor stimulant effects of cocaine, as well as basal locomotor activity. Data are expressed as mean ± S.E.M., *p < 0.05, **p < 0.01 (for comparison with cocaine), # p < 0.05, ## p < 0.01 (for comparison with saline), n = 10–12 per group. b Effect of orally administered SN79 on cocaine-induced and basal locomotor activity. Locomotor activity was recorded for 1 h following orally administered SN79 (white bars), which caused significant sedative effects at 15–25 mg/kg, p.o. Animals pretreated with oral administration of distilled water (0 mg/kg SN79 pretreatment) were then challenged with either a stimulant dose of cocaine (20 mg/kg, i.p., black bar) or saline (i.p., white bar); all other animals pretreated with oral dosing with SN79 (10–25 mg/kg) were challenged with cocaine (20 mg/kg, i.p., black bars). Cocaine-induced (20 mg/kg, i.p.) increases in locomotor activity were significantly attenuated by pretreatment with 15, 20, and 25 mg/kg, p.o. SN79. Data are expressed as mean ± S.E.M., *p < 0.05, **p < 0.01 (SN79 + cocaine compared to dH20 + cocaine), ## p < 0.01 (SN79 compared to distilled water + saline), n = 10 per group
Apart from counteracting the effects due to cocaine, SN79 by itself also caused a significant decline in locomotor activity (F(5,56) = 9.59, p < 0.0001). Post hoc Dunnett’s tests showed that the locomotor activity due to administration of SN79 was significantly different from that due to saline treatment at the following doses of SN79: 3 mg/kg (q = 2.75, p < 0.05), 5 mg/kg (q = 4.13, p < 0.01), and 10 mg/kg (q = 5.70, p < 0.01).
Orally dosed SN79 alone (10–25 mg/kg) also displayed significant sedative effects (F(4,45) = 10.09, p < 0.0001). Post hoc Dunnett’s tests confirmed that SN79 at the following doses decreased locomotor activity compared to saline: 15 mg/kg (q = 3.87, p < 0.01), 20 mg/kg (q = 5.10, p < 0.01), and 25 mg/kg (q = 4.88, p < 0.01), as shown in Fig. 3b. Following the 60-min pretreatment period, an i.p. injection of a stimulant dose of cocaine (20 mg/kg) was administered, and there was a significant reduction in locomotor activity in SN79-treated animals (F(5,50) = 5.06, p < 0.001). Post hoc Tukey’s multiple comparison tests revealed that pretreatment with SN79 15, 20 or 25 mg/kg significantly attenuated the cocaine-induced increases in locomotor activity when compared to distilled water pretreatment (q = 4.71, p < 0.05; q = 5.65, p < 0.01; and q = 5.89, p < 0.01, respectively). The initial 30 min of the challenge period was also analyzed in the same manner and was found to exhibit similar trends.
Effect of SN79 on the Development of Cocaine-Induced Sensitization
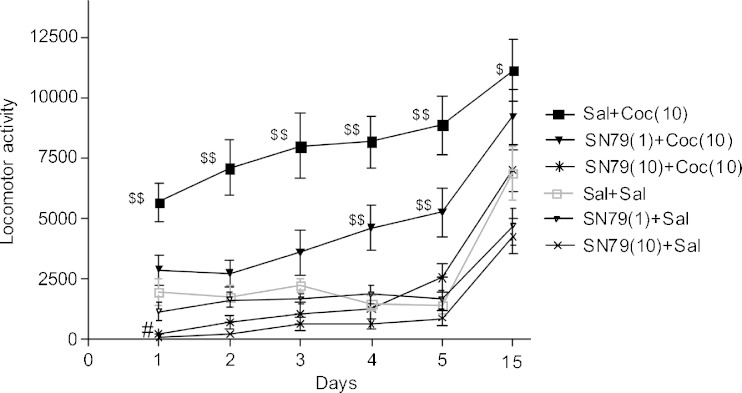
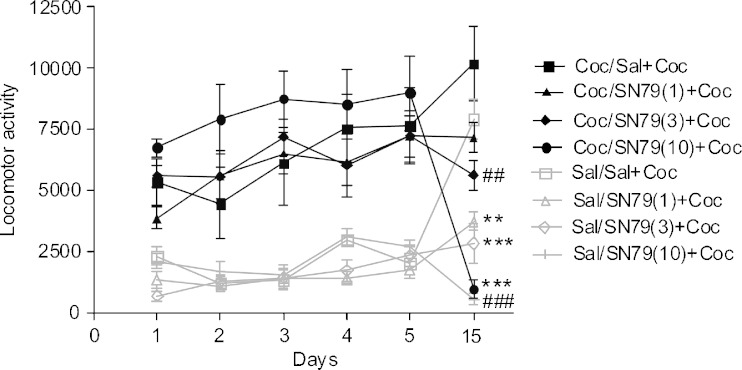
There was a significant difference between the experimental groups on all of the treatment days: day 1 (F(5,42) = 17.05, p < 0.0001); day 2 (F(5,42) = 18.04, p < 0.0001); day 3 (F(5,42) = 14.42, p < 0.0001); day 4 (F(5,42) = 19.95, p < 0.0001); day 5 (F(5,42) = 17.59, p < 0.0001); day 15 (F(5,42) = 6.77, p < 0.0001). Post hoc Dunnett’s test confirmed that on day 1, only the cocaine-treated mice displayed significantly higher locomotor activity in comparison to the saline-treated mice (q = 5.19, p < 0.01). In contrast, the group of mice treated with 10 mg/kg of SN79 alone exhibited significantly lower locomotor activity when compared with the saline-treated mice on day 1 (q = 2.63, p < 0.05). The locomotor activity displayed by the rest of the groups on day 1 was not significantly different from the saline treatment group. With the exception of the cocaine treatment group that showed an elevated locomotor response on days 2 and 3, none of the other groups had significantly different locomotor activity as compared to the saline control group (q = 6.47, p < 0.01 and q = 5.71, p < 0.01, respectively). On days 4 and 5, both the saline + cocaine (10 mg/kg) treatment group and the SN79 (1 mg/kg) + cocaine (10 mg/kg) treatment group displayed significantly higher locomotor activity than the saline treatment group (q = 7.31, p < 0.01; q = 7.19, p < 0.01 and q = 3.40, p < 0.01; q = 3.70, p < 0.01, respectively). On the challenge day (day 15), when all of the groups were administered cocaine, the mice that received cocaine on days 1–5 exhibited significantly higher locomotor activity than the mice that received saline on days 1–5 (q = 2.97, p < 0.05), a pattern characteristic of sensitization. On the challenge day, locomotor activity of the rest of the groups was not significantly different from the group that received saline on days 1–5. The results are depicted in Fig. 4.
Fig. 4.
Effect of SN79 on the development of sensitization to cocaine. Male, Swiss Webster mice were injected (i.p.) with either saline (Sal) or SN79 (1 or 10 mg/kg) followed 15 min later with either saline or cocaine (Coc, 10 mg/kg) once a day for 5 days. A 10-day drug-free period followed. On day 15, all of the mice were injected (i.p.) with saline, followed 15 min later with cocaine (10 mg/kg). Mice that received SN79 (1 and 10 mg/kg) on days 1–5 did not exhibit a sensitized response to cocaine on day 15. Data are represented as mean ± S.E.M., # p < 0.05, $ p < 0.05, $$ p < 0.01 vs. saline group (gray line), post hoc Dunnett’s tests, n = 8 per group
Effect of SN79 on the Expression of Cocaine-Induced Sensitization
On days 1–5, the cocaine-treated mice displayed significantly higher locomotor activity than the saline-treated animals, and the analysis of variance confirmed a significant difference between the treatment groups. The data were as follows: day 1 F(7,40) = 22.35, p < 0.0001; day 2 F(7,40) = 8.67, p < 0.0001; day 3 F(7,40) = 12.51, p < 0.0001; day 4 F(7,40) = 8.46, p < 0.0001; day 5 F(7,40) = 10.58, p < 0.0001. On day 15, there was also a significant difference between the treatment groups (F(7,40) = 20.26, p < 0.0001), with SN79 attenuating locomotor activity both in mice that were sensitized (cocaine treatments on days 1–5) and not sensitized (saline treatments on days 1–5; Fig. 5). Post hoc Tukey’s tests confirmed that pretreatment with the following doses of SN79 prior to cocaine on day 15 caused a significant decline both in the group treated with saline on days 1–5 (1 mg/kg q = 5.48, p < 0.01; 3 mg/kg q = 6.65, p < 0.001; 10 mg/kg q = 9.64, p < 0.001) and the group treated with cocaine on days 1–5 (3 mg/kg q = 5.98, p < 0.01; 10 mg/kg q = 12.08, p < 0.001).
Fig. 5.
Effect of SN79 on the expression of sensitization due to subchronic treatment with cocaine. Male, Swiss Webster mice were injected (i.p.) with saline (Sal, gray lines) or cocaine (Coc, 10 mg/kg, black lines), once a day for 5 days. Following a 10-day drug-free period (day 15), mice were injected (i.p.) with saline (Sal) or SN79 (1, 3, 10 mg/kg), followed 15 min later with cocaine (Coc, 10 mg/kg). SN79 (3 and 10 mg/kg) treatment blocked the expression of sensitization to cocaine. Data are represented as mean ± S.E.M., **p < 0.01, ***p < 0.001 and ## p < 0.01, ### p < 0.001 post hoc Tukey’s tests (comparing saline + cocaine treatment with SN79 + cocaine treatment in groups treated during days 1–5 with either saline or cocaine, respectively), n = 6 per group
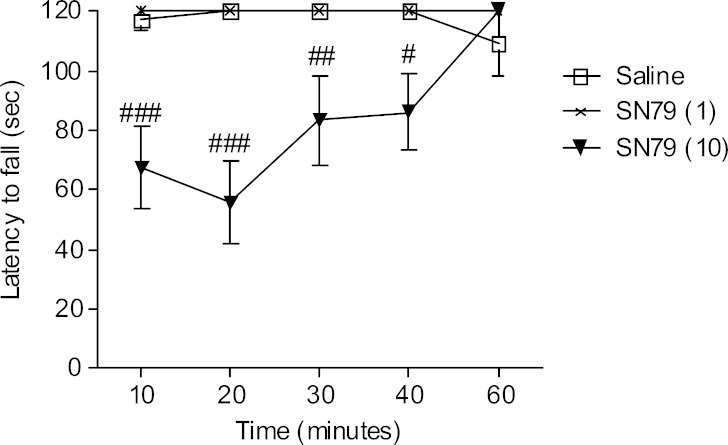
Effect of SN79 in the Rotor Rod Test
The administration of a high dose of SN79 (10 mg/kg, i.p.) led to significant motor incoordination in the mice. A two-way ANOVA showed a significant effect of treatment (F(2,135) = 36.39, p < 0.0001) and treatment × time interaction (F(8,135) = 3.92, p < 0.005). Post hoc Bonferroni’s tests showed that the motor incoordination effect of SN79 was more pronounced at earlier time points: 10, 20, 30, and 40 min (Fig. 6; t = 4.52, p < 0.001; t = 5.87, p < 0.001; t = 3.35, p < 0.01; t = 3.09, p < 0.05, respectively). After 60 min of treatment, the latency to fall from the rotor rod was not significantly different from the saline-treated mice (t = 0.99, ns). A low dose of SN79 (1 mg/kg, i.p.) did not significantly affect the latency to fall from the rotor rod. The data are represented in Fig. 6.
Fig. 6.
Effect of SN79 on motor coordination in mice. Male, Swiss Webster mice that were trained to balance on a rotor rod were administered (i.p.) either saline or SN79 (1, 10 mg/kg). SN79 (10 mg/kg) caused motor incoordination in mice. The data are represented as mean ± S.E.M. A two-way ANOVA was conducted followed by Bonferroni’s post hoc tests. # p < 0.05, ## p < 0.01, ### p < 0.001, n = 10 per group
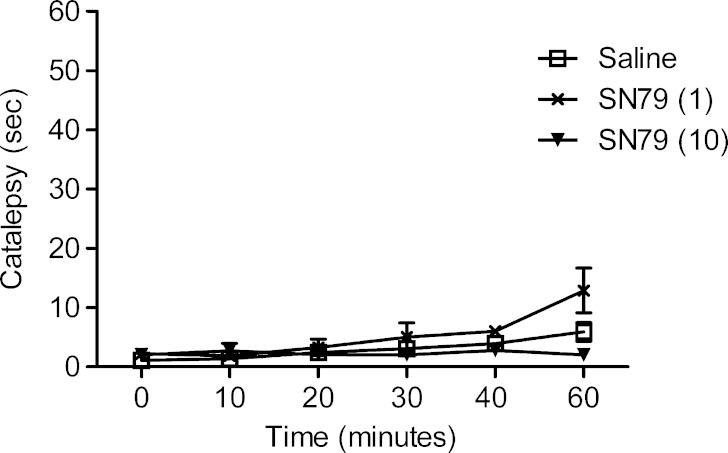
Effect of SN79 in the Catalepsy Test
Treatment of the mice with either the low or high dose of SN79 (1 or 10 mg/kg, i.p.) did not significantly alter the latency to bring the paws down to the ground from the bar at the 10-, 20-, 30-, and 40-min time points. However, at the 60-min time point, the analysis of variance showed a significant difference between the experimental groups (F(2,27) = 5.28, p < 0.05). Post hoc Dunnett’s test revealed no significant differences between each of the SN79 doses (1 and 10 mg/kg, i.p.) when compared with saline (q = 2.06, ns and q = 1.15, ns). The results are depicted in Fig. 7.
Fig. 7.
Effect of SN79 treatment on catalepsy. Male, Swiss Webster mice were administered (i.p.) either saline or SN79 (1, 10 mg/kg) and at 0, 10, 20, 30, 40, 60 min after treatment, their front paws were placed on a horizontal rod 5 cm above the bench top, and the latency to return their paws back to the bench top was recorded as an index of catalepsy. SN79 treatment did not significantly differ from the saline treatment. The data are represented as mean ± S.E.M., n = 10 per group
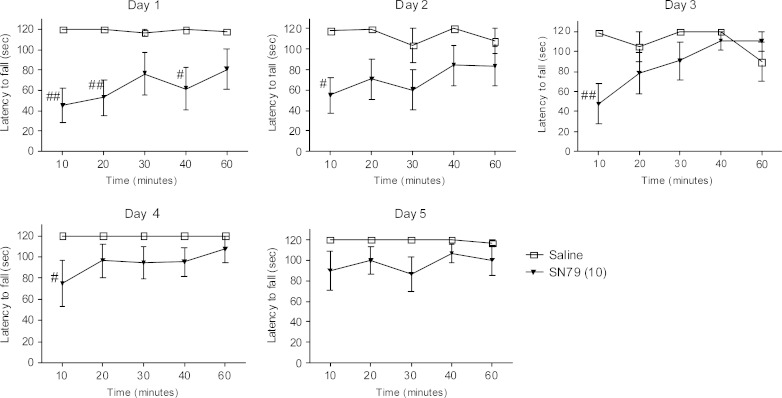
Effect of Subchronic Treatment with SN79 on Motor Incoordination
The mice treated with a high dose of SN79 (10 mg/kg, i.p.) for five consecutive days developed tolerance to the acute effects of the drug on motor incoordination. A two-way ANOVA on day 1 showed a significant effect of SN79 treatment (F(1,60) = 40.57, p < 0.0001). Post hoc Bonferroni’s tests showed that SN79 at 10, 20, and 40 min showed significant motor incoordination (t = 3.86, p < 0.01; t = 3.44, p < 0.01; t = 2.97, p < 0.05, respectively). A two-way ANOVA on day 2 showed a significant effect of SN79 treatment (F(1,60) = 20.55, p < 0.0001). The post hoc Bonferroni’s test showed that SN79 at 10 min showed significant motor incoordination (t = 2.96, p < 0.05). On day 3, a two-way ANOVA showed a significant effect of treatment (F(1,60) = 6.77, p < 0.05) and treatment × time interaction (F(4,60) = 2.80, p < 0.05). The post hoc Bonferroni’s test showed significant motor incoordination by SN79 treatment at 10 min (t = 3.57, p < 0.01). On day 4, a two-way ANOVA showed a significant effect of treatment (F(1,60) = 13.51, p < 0.005). Post hoc Bonferroni’s test showed a significant motor incoordination effect of SN79 treatment at 10 min (t = 2.82, p < 0.05). By day 5 of the treatment, a two-way ANOVA showed a significant effect of treatment (F(1,60) = 11.58, p < 0.01). However, post hoc Bonferroni’s tests did not show any significant effect of SN79 on motor incoordination at any time point. The results of each of the 5 days are represented in Fig. 8.
Fig. 8.
Effect of subchronic treatment of SN79 on motor incoordination. Male, Swiss Webster mice that were trained to balance on the rotor rod were injected (i.p.) once a day with saline or SN79 (10 mg/kg), and rotor rod performance was evaluated for five consecutive days. Subchronic SN79 treatment caused tolerance to the motor incoordination observed on day 1. The data are represented as mean ± S.E.M. A two-way ANOVA was conducted followed by Bonferroni’s post hoc tests. # p < 0.05, ## p < 0.01 as compared to the saline control, n = 7 per group
DISCUSSION
In the present study, radioligand binding assays revealed that SN79 possesses nanomolar affinity for σ receptors, a notable affinity for 5-HT2 receptors, and significant affinities for monoamine transporters. In contrast, it has negligible (>10,000 nM) affinity for 57 other binding sites. Although in initial testing, SN79 was reported to be a selective σ2 receptor ligand (24); this turned out to be an artifact resulting from the compound precipitating out of solution following freezing and defrosting, and happenstance of using fresh solutions when running σ2 receptor assays in the initial testing. When fresh solutions were used to generate the data reported herein for all σ receptor binding assays, SN79 was found to exhibit a significant affinity for both σ1 and σ2 receptors, a finding which was confirmed using two synthetic batches of SN79.
Under in vitro conditions, SN79 did not inhibit any of the major cytochrome P450 drug metabolizing enzymes tested herein suggesting a low propensity for drug–drug interactions should it undergo further development as a pharmacotherapy. In addition, SN79 had a 7.5-h in vivo half-life in rats, reaching peak plasma concentrations after oral dosing in about 1.5 h, suggesting favorable pharmacokinetic parameters for the development of an orally administered medication.
In the first part of the study, SN79 attenuated the convulsive effects of cocaine. This was consistent with earlier studies where agonists and antagonists at σ receptors exacerbated and attenuated the convulsive effects of cocaine respectively (14). The interaction of SN79 with 5-HT2 receptors could also contribute to anticonvulsant effects since earlier studies showed that pharmacological antagonists at 5-HT2 receptors prevented the convulsive effects of cocaine (2). However, direct interactions with monoamine transporters are unlikely to convey anticonvulsive effects since earlier studies showed that the seizurogenic effects of cocaine were actually mediated by serotonin transporters and may be inhibited through σ receptors, whereas the role of norepinephrine and dopamine transporters in mediating these effects seemed negligible (27). It is noteworthy that the anticonvulsive actions of SN79 against cocaine were observed following both intraperitoneal and oral dosing. These findings are significant because cocaine-induced convulsions are resistant to treatment with currently available anticonvulsive drugs (28,29). Since cocaine is responsible for more serious intoxications and emergency department mentions than any other illicit substance (30), compounds like SN79 provide a potentially new option for mitigating this adverse event.
In the second part of the study, SN79 dose dependently decreased cocaine-induced locomotor activity, further confirming putative antagonist actions at σ receptors. The 1 mg/kg, i.p. dose of SN79 attenuated the stimulant effects of cocaine without any effects on its own, a pattern that is consistent with the actions of an antagonist. Orally administered SN79 at 15, 20, and 25 mg/kg was able to attenuate the stimulant effects of cocaine; however, each of these doses produced sedative effects in the absence of cocaine.
When administered at higher doses, SN79 (i.p. and p.o.) itself caused sedative effects. The decrease in basal locomotor activity observed at high doses of SN79 indicates several possibilities. First, SN79 may act as a σ receptor antagonist that at higher doses, disrupts inherent motor tone. Second, SN79 may act as an inverse agonist at σ receptors, causing a decrease in motor activity at high doses. This is possible because σ receptors are densely located in the motor areas of the brain and have been implicated in motor functions (31,32). Third, SN79 at higher doses may change the conformation of σ receptors, inhibiting signal transduction in σ-enriched motor areas (33,34). Fourth, psychostimulants increase locomotor activity by increasing dopamine levels in the striatum (35). Sigma receptor agonists modulate dopamine transporter function and the release of dopamine (36); SN79 could block these functions of endogenous σ ligands and cause sedation.
It is unclear that other, non-σ interactions of SN79 can reduce basal locomotor activity and cocaine-induced locomotor stimulant effects. Cocaine, for example, has been reported to increase locomotor activity in SERT and NET knockout mice relative to wild-type mice, indicating heightened responses to the drug (37). 5-HT2 agonists and antagonists also have variable effects on cocaine-induced locomotor activity, depending on the specific subtype involved (38). When considered together, the profile of SN79 to antagonize cocaine-induced hyperlocomotion most likely involves σ receptors (39–46).
Apart from these acute effects, repeated treatment with cocaine leads to neuroadaptations in the brain reward system (47). One of the long lasting measurable behavioral manifestations of these neuroadaptations is sensitization (47). SN79 prevented the development of sensitization to cocaine. A number of earlier, less selective σ receptor antagonists, such as BMY14802, NPC16377, rimcazole, and SR31742A also prevented the development of cocaine-induced sensitization (48,49). Recently, there has been some evidence that the 5-HT2 receptor antagonist ritanserin may be involved in the attenuation of cocaine-induced development of sensitization (50). However, σ receptor antagonists with negligible affinity for 5-HT2 receptors also prevented the development of sensitization to cocaine, indicating that an interaction with 5-HT2 sites is not necessary for mitigating the sensitizing effects of cocaine (51). Along with the many other mechanisms already known to contribute to cocaine-induced sensitization (52), collectively, the data suggest an important role of σ receptors in the development of sensitization.
SN79 also blocked the expression of cocaine-induced sensitization, but this occurred at a dose that caused sedation on its own. The sedative effect of SN79, however, did not appear to be the cause of the reduction in the expression of sensitization, since the animals became tolerant to the sedative effects of SN79 over time. Also, other σ receptor antagonists like CM156, which has a high affinity for σ receptors but does not cause significant sedation, can attenuate the expression of sensitization (53).
From a mechanistic aspect, σ receptors appear to play an important role in cocaine-induced neuroadaptations for several reasons. First, during cocaine-induced sensitization, there are changes in the dopamine transporter and dopamine levels in the brain (54). Sigma receptors have been known to modulate dopamine transporter function and dopamine release via protein kinase C, the calcium/calmodulin-dependent kinase II system, ceramide, and changes in calcium levels (36,55,56). Second, activation of σ receptors can indirectly increase phosphorylation of CREB via ceramide, which is an important mediator of neuroadaptations in the brain (57–59). Therefore, administering SN79 to block σ receptors may be effective in preventing the occurrence of sensitization. In addition to σ receptors, SN79 by its interaction with 5-HT2 receptors can also contribute to the attenuation of expression of cocaine-induced sensitization (50). However, other σ receptor antagonists with negligible affinity for 5-HT2 receptors can also attenuate the expression of sensitization emphasizing the contributive and not dominant role of 5-HT2 receptors (53).
Collectively, the results suggest that targeting σ receptors with SN79 is effective in blocking the acute and subchronic effects of cocaine. However, the sedative effects observed at high doses and the narrow therapeutic range (1–10 mg/kg, i.p.) may limit its clinical usefulness. Therefore, the rotor rod and catalepsy tests were performed to evaluate its implications on motor side effects. Following a single administration, SN79 did not cause catalepsy, but at a high dose of 10 mg/kg, i.p., SN79 caused motor incoordination, in addition to the acute sedative effect observed in the locomotor studies.
From a clinical viewpoint, medications for psychiatric conditions are taken on a repeated basis. Therefore, the effects of repeated dosing of SN79 on these side effects were evaluated. Tolerance to sedation and the motor incoordination were observed, and this could result from several mechanisms. First, pharmacokinetic tolerance could be developing, whereby SN79 may activate metabolizing enzymes that lead to its faster removal from the body. Second, SN79 may lead to the development of pharmacodynamic tolerance whereby changes may occur in the downstream signaling of σ receptors or monoaminergic pathways, in their efficiency of coupling to signaling pathways, or even the downregulation of target proteins.
In summary, SN79 reduced the toxic and stimulant effects of cocaine and also caused motor side effects such as sedation and incoordination with acute treatment. Although SN79 has a somewhat narrow therapeutic range, continued characterization and development of SN79 and related compounds will be important because they may have the therapeutic potential for treating not only the acute effects of cocaine, but also subchronic effects even when introduced after subjects have been exposed for some time to cocaine.
Electronic Supplementary Materials
Below is the link to the electronic supplementary material.
Binding affinities of SN79 (DOC 124 kb)
Acknowledgments
We appreciate the technical expertise of Jamaluddin Shaikh, Michael Seminerio, and Yantong Xu for conducting some of the radioligand binding assays. We also appreciate the technical assistance of Caroline Croom, Bahbak Shariat-Madar, and Brittany Spitznogle during some of the convulsion and locomotor studies.
This study was supported by grants from the National Institute on Drug Abuse (DA011979, DA013978, DA023205). Nidhi Kaushal received a Natural Products Neuroscience Fellowship through a COBRE grant from the National Center for Research Resources (P20 RR021929).
Footnotes
Reprint requests should be made to: Rae R. Matsumoto, Basic Pharmaceutical Sciences, West Virginia University, Morgantown, WV 26506; email: rmatsumoto@hsc.wvu.edu.
References
- 1.Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-G. [DOI] [PubMed] [Google Scholar]
- 2.Ritz MC, George FR. Cocaine-induced convulsions: pharmacological antagonism at serotonergic, muscarinic and sigma receptors. Psychopharmacol. 1997;129:299–310. doi: 10.1007/s002130050197. [DOI] [PubMed] [Google Scholar]
- 3.Rocha BA, Fumagalli F, Gainetdinov RR, Jones SR, Ator R, Giros B, et al. Cocaine self-administration in dopamine-transporter knockout mice. Nat Neurosci. 1998;1:132–137. doi: 10.1038/1152. [DOI] [PubMed] [Google Scholar]
- 4.Sora I, Wichems C, Takahashi N, Li XF, Zeng Z, Revay R, et al. Cocaine reward models: conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc Natl Acad Sci USA. 1998;95:7699–7704. doi: 10.1073/pnas.95.13.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomsen M, Han DD, Gu HH, Caine SB. Lack of cocaine self-administration in mice expressing a cocaine-insensitive dopamine transporter. J Pharmacol Exp Ther. 2010;331:204–211. doi: 10.1124/jpet.109.156265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, et al. Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci USA. 2001;89:5300–5305. doi: 10.1073/pnas.091039298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czoty PW, Martelle JL, Carroll FI, Nader MA. Lower reinforcing strength of the phenyltropane cocaine analogs RTI-336 and RTI-177 compared to cocaine in nonhuman primates. Pharmacol Biochem Behav. 2010;96:274–278. doi: 10.1016/j.pbb.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhar MJ, McGirr KM, Hunter RG, Lambert PD, Garrett BE, Carroll FI. Studies of selected phenyltropanes at monoamine transporters. Drug Alcohol Depend. 1999;56:9–15. doi: 10.1016/S0376-8716(99)00005-8. [DOI] [PubMed] [Google Scholar]
- 9.Cao J, Kulkarni SS, Husbands SM, Bowen WD, Williams W, Kopajtic T, et al. Dual probes for the dopamine transporter and sigma1 receptors: novel piperazinyl alkyl-bis(4′-fluorophenyl)amine analogues as potential cocaine-abuse therapeutic agents. J Med Chem. 2003;46:2589–2598. doi: 10.1021/jm030008u. [DOI] [PubMed] [Google Scholar]
- 10.Han DD, Gu HH. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol. 2006;6:6. doi: 10.1186/1471-2210-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharkey J, Glen KA, Wolfe S, Kuhar MJ. Cocaine binding at sigma receptors. Eur J Pharmacol. 1988;149:171–174. doi: 10.1016/0014-2999(88)90058-1. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto RR, Hewett KL, Pouw B, Bowen WD, Husbands SM, Cao JJ, et al. Rimcazole analogs attenuate the convulsive effects of cocaine: correlation with binding to sigma receptors rather than dopamine transporters. Neuropharmacology. 2001;41:878–886. doi: 10.1016/S0028-3908(01)00116-2. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto K. Sigma-1 receptors and selective serotonin reuptake inhibitors: clinical implications of their relationship. CNS Agents Med Chem. 2009;9:197–204. doi: 10.2174/1871524910909030197. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto RR, Liu Y, Lerner M, Howard EW, Brackett DJ. Sigma receptors: potential medications development target for anti-cocaine agents. Eur J Pharmacol. 2003;469:1–12. doi: 10.1016/S0014-2999(03)01723-0. [DOI] [PubMed] [Google Scholar]
- 15.Nuwayhid SJ, Werling LL. Sigma2 (σ2) receptors as a target for cocaine action in the rat striatum. Eur J Pharmacol. 2006;535:98–103. doi: 10.1016/j.ejphar.2005.12.077. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto RR, McCracken KA, Pouw B, Miller J, Bowen WD, Williams W, et al. N-alkyl substituted analogs of the sigma receptor ligand BD1008 and traditional sigma receptor ligands affect cocaine-induced convulsions and lethality in mice. Eur J Pharmacol. 2001;411:261–273. doi: 10.1016/S0014-2999(00)00917-1. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto RR, McCracken KA, Pouw B, Zhang Y, Bowen WD. Involvement of sigma receptors in the behavioral effects of cocaine: evidence from novel ligands and antisense oligodeoxynucleotides. Neuropharmacology. 2002;42:1043–1055. doi: 10.1016/S0028-3908(02)00056-4. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto RR. Targeting sigma receptors: novel medication development for drug abuse and addiction. Expert Rev Clin Pharmacol. 2009;2:351–358. doi: 10.1586/ecp.09.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bermack JE, Debonnel G. Modulation of serotonergic neurotransmission by short- and long-term treatments with sigma ligands. Br J Pharmacol. 2001;134:691–699. doi: 10.1038/sj.bjp.0704294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bermack JE, Debonnel G. The role of sigma receptors in depression. J Pharmacol Sci. 2005;97:317–336. doi: 10.1254/jphs.CRJ04005X. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Alvear GM, Werling LL. Regulation of [3H]dopamine release from rat striatal slices by sigma receptor ligands. J Pharmacol Exp Ther. 1994;271:212–219. [PubMed] [Google Scholar]
- 22.Gonzalez-Alvear GM, Werling LL. σ1 Receptors in rat striatum regulate NMDA-stimulated [3H]dopamine release via a presynaptic mechanism. Eur J Pharmacol. 1995;294:713–719. doi: 10.1016/0014-2999(95)00617-6. [DOI] [PubMed] [Google Scholar]
- 23.Reith ME. Cocaine receptors on monoamine transporters and sodium channels. NIDA Res Monogr. 1988;88:23–43. [PubMed] [Google Scholar]
- 24.Narayanan S, Mésangeau C, Shaikh J, Kaushal N, Matsumoto RR, Poupaert JH, McCurdy CR. Design, synthesis and evaluation of the first highly selective sigma-2 receptor ligand. 234th ACS National Meeting; 2007 August 19–23, Boston, MA.
- 25.Matsumoto RR, Bowen WD, Tom MA, Vo VN, Truong DD, De Costa BR. Characterization of two novel sigma receptor ligands: antidystonic effects in rats suggest sigma receptor antagonism. Eur J Pharmacol. 1995;280:301–310. doi: 10.1016/0014-2999(95)00208-3. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto RR, Shaikh J, Wilson LL, Vedam S, Coop A. Attenuation of methamphetamine-induced effects through the antagonism of sigma receptors: evidence from in vivo and in vitro studies. Eur Neuropsycopharmacol. 2008;18:871–881. doi: 10.1016/j.euroneuro.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ritz MC, George FR. Cocaine-induced seizures and lethality appear to be associated with distinct central nervous system binding sites. J Pharmacol Exp Ther. 1993;264:1333–1343. [PubMed] [Google Scholar]
- 28.Dhuna A, Pascual-Leone A, Langendorf F, Anderson DC. Epileptogenic properties of cocaine in humans. Neurotoxicology. 1991;12:621–626. [PubMed] [Google Scholar]
- 29.Gasior M, Ungard JT, Witkin JM. Preclinical evaluation of newly approved and potential antiepileptic drugs against cocaine-induced seizures. J Pharmacol Exp Ther. 1999;290:1148–1156. [PubMed] [Google Scholar]
- 30.Vitale S, van de Mheen D. Illicit drug use and injuries: a review of emergency room studies. Drug Alcohol Depend. 2006;82:1–9. doi: 10.1016/j.drugalcdep.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 31.Bouchard P, Quirion R. [3H]1,3-di(2-tolyl)guanidine and [3H](+)pentazocine binding sites in the rat brain: autoradiographic visualization of the putative sigma1 and sigma2 receptor subtypes. Neuroscience. 1997;76:467–477. doi: 10.1016/S0306-4522(96)00221-7. [DOI] [PubMed] [Google Scholar]
- 32.Walker JM, Bowen WD, Patrick SL, Williams WE, Mascarella SW, Bai X, et al. A comparison of (−)-deoxybenzomorphans devoid of opiate activity with their dextrorotatory phenolic counterparts suggests role of σ2 receptors in motor function. Eur J Pharmacol. 1993;231:61–68. doi: 10.1016/0014-2999(93)90684-A. [DOI] [PubMed] [Google Scholar]
- 33.Jacobowitz DM, Kallarakal AT. Flotillin-1 in the substantia nigra of the Parkinson brain and a predominant localization in catecholaminergic nerves in the rat brain. Neurotox Res. 2004;6:245–257. doi: 10.1007/BF03033435. [DOI] [PubMed] [Google Scholar]
- 34.Trushina E, Du CJ, Parisi J, McMurray CT. Neurological abnormalities in caveolin-1 knock out mice. Behav Brain Res. 2006;172:24–32. doi: 10.1016/j.bbr.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 35.Delfs JM, Schreiber L, Kelley AE. Microinjection of cocaine into the nucleus accumbens elicits locomotor activation in the rat. J Neurosci. 1990;10:303–310. doi: 10.1523/JNEUROSCI.10-01-00303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Derbez AE, Mody RM, Werling LL. Sigma2-receptor regulation of dopamine transporter via activation of protein kinase C. J Pharmacol Exp Ther. 2002;301:306–314. doi: 10.1124/jpet.301.1.306. [DOI] [PubMed] [Google Scholar]
- 37.Hall FS, Li X-F, Randall-Thompson J, Sora I, Murphy DL, Lesch K-P, et al. Cocaine-conditioned locomotion in dopamine transporter, norepinephrine transporter and serotonin transporter knockout mice. Neurosci. 2009;162:870–880. doi: 10.1016/j.neuroscience.2009.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Filip M, Bubar MJ, Cunningham KA. Contribution of serotonin (5-hydroxytryptamine; 5-HT) 5-HT2 receptor subtypes to the hyperlocomotor effects of cocaine: acute and chronic pharmacological analyses. J Pharmacol Exp Ther. 2004;310:1246–1254. doi: 10.1124/jpet.104.068841. [DOI] [PubMed] [Google Scholar]
- 39.Carroll FI, Howell LL, Kuhar MJ. Pharmacotherapies for treatment of cocaine abuse: preclinical aspects. J Med Chem. 1999;42:2721–2736. doi: 10.1021/jm9706729. [DOI] [PubMed] [Google Scholar]
- 40.Xu F, Gainetdinov RR, Wetsel WC, Jones SR, Bohn LM, Miller GW, et al. Mice lacking the norepinephrine transporter are supersensitive to psychostimulants. Nat Neurosci. 2000;3:465–471. doi: 10.1038/74839. [DOI] [PubMed] [Google Scholar]
- 41.Reith MEA, Wiener HL, Fischette CT. Sertraline and cocaine-induced locomotion in mice I. Acute studies. Psychopharmacol. 1991;103:297–305. doi: 10.1007/BF02244282. [DOI] [PubMed] [Google Scholar]
- 42.Fletcher PJ, Phil D, Grottick AJ, Higgins GA. Differential effects of the 5-HT2A receptor antagonist M100,907 and the 5-HT2C receptor antagonist SB242,084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine induced reinstatement of responding. Neuropsychopharmacol. 2002;27:576–586. doi: 10.1016/S0893-133X(02)00342-1. [DOI] [PubMed] [Google Scholar]
- 43.McMahon LR, Cunningham KA. Antagonism of 5-hydroxytryptamine2A receptors attenuates the behavioral effects of cocaine in rats. J Pharmacol Exp Ther. 2001;297:357–363. [PubMed] [Google Scholar]
- 44.Lapa GB, Byrd GD, Lapa AA, Budygin EA, Childers SR, Jones SR, et al. The synthesis and biological evaluation of dopamine transporter inhibiting activity of substituted diphenylmethoxypiperidines. Bioorg Med Chem Lett. 2005;15:4915–4918. doi: 10.1016/j.bmcl.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 45.Licata SC, Schmidt HD, Pierce RC. Suppressing calcium/calmodulin-dependent protein kinase II activity in the ventral tegmental area enhances the acute behavioural response to cocaine but attenuates the initiation of cocaine-induced behavioural sensitization in rats. Eur J Neurosci. 2004;19:405–414. doi: 10.1111/j.0953-816X.2003.03110.x. [DOI] [PubMed] [Google Scholar]
- 46.Rubinstein M, Phillips TJ, Bunzow JR, Falzone TL, Dziewczapolski G, Zhang G, et al. Mice lacking dopamine D4 receptors are supersensitive to ethanol, cocaine, and methamphetamine. Cell. 1997;90:991–1001. doi: 10.1016/S0092-8674(00)80365-7. [DOI] [PubMed] [Google Scholar]
- 47.White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/S0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- 48.Ujike H, Kuroda S, Otsuki S. Sigma receptor antagonists block the development of sensitization to cocaine. Eur J Pharmacol. 1996;296:123–128. doi: 10.1016/0014-2999(95)00693-1. [DOI] [PubMed] [Google Scholar]
- 49.Witkin JM, Terry P, Menkel M, Hickey P, Pontecorvo M, Ferkany J, et al. Effects of the selective sigma receptor ligand, 6-[6-(4-hydroxypiperidinyl)hexyloxy]-3-methylflavone (NPC 16377), on behavioral and toxic effects of cocaine. J Pharmacol Exp Ther. 1993;266:473–482. [PubMed] [Google Scholar]
- 50.Ago Y, Nakamura S, Baba A, Matsuda T. Neuropsychotoxicity of abused drugs: effects of serotonin receptor ligands on methamphetamine- and cocaine-induced behavioral sensitization in mice. J Pharmacol Sci. 2008;106:15–21. doi: 10.1254/jphs.FM0070121. [DOI] [PubMed] [Google Scholar]
- 51.Liu Y, Matsumoto RR. Alterations in fos-related antigen-2 and σ1 receptor gene and protein expression are associated with the development of cocaine-induced behavioral sensitization: time course and regional distribution studies. J Pharmacol Exp Ther. 2008;327:187–195. doi: 10.1124/jpet.108.141051. [DOI] [PubMed] [Google Scholar]
- 52.Ron D, Jurd R. The “ups and downs” of signaling cascades in addiction. Sci STKE. 2005;309:re14. doi: 10.1126/stke.3092005re14. [DOI] [PubMed] [Google Scholar]
- 53.Xu YT, Kaushal N, Shaikh J, Wilson LL, Mesangeau C, McCurdy CR, et al. A novel substituted piperazine, 3-(4-(4-cyclohexylpiperazin-1-yl)butyl)benzo[d]thiazole-2(3H)-thione (CM156), attenuates the stimulant and toxic effects of cocaine in mice. J Pharmacol Exp Ther. 2010;333:491–500. doi: 10.1124/jpet.109.161398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-U. [DOI] [PubMed] [Google Scholar]
- 55.Crawford KW, Coop A, Bowen WD. σ2 Receptors regulate changes in sphingolipid levels in breast tumor cells. Eur J Pharmacol. 2002;443:207–209. doi: 10.1016/S0014-2999(02)01581-9. [DOI] [PubMed] [Google Scholar]
- 56.Riddle EL, Rau KS, Topham MK, Hanson GR, Fleckenstein AE. Ceramide-induced alterations in dopamine transporter function. Eur J Pharmacol. 2003;458:31–36. doi: 10.1016/S0014-2999(02)02727-9. [DOI] [PubMed] [Google Scholar]
- 57.Crawford KW, Bowen WD. Sigma-2 receptor agonists activate a novel apoptotic pathway and potentiate antineoplastic drugs in breast tumor cell lines. Cancer Res. 2002;62:313–322. [PubMed] [Google Scholar]
- 58.Won JS, Choi MR, Suh HW. Stimulation of astrocyte-enriched culture with C2 ceramide increases proenkephalin mRNA: involvement of cAMP-response element binding protein and mitogen activated protein kinases. Brain Res. 2001;903:207–215. doi: 10.1016/S0006-8993(01)02452-0. [DOI] [PubMed] [Google Scholar]
- 59.Hyman SE. Addiction to cocaine and amphetamine. Neuron. 1996;16:901–904. doi: 10.1016/S0896-6273(00)80111-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Binding affinities of SN79 (DOC 124 kb)