Abstract
In vitro dissolution testing is an important tool used for development and approval of generic dosage forms. The objective of this article is to summarize how dissolution testing is used for the approval of safe and effective generic drug products in the United States (US). Dissolution testing is routinely used for stability and quality control purposes for both oral and non-oral dosage forms. The dissolution method should be developed using an appropriate validated method depending on the dosage form. There are several ways in which dissolution testing plays a pivotal role in regulatory decision-making. It may be used to waive in vivo bioequivalence (BE) study requirements, as BE documentation for Scale Up and Post Approval Changes (SUPAC), and to predict the potential for a modified-release (MR) drug product to dose-dump if co-administered with alcoholic beverages. Thus, in vitro dissolution testing plays a major role in FDA’s efforts to reduce the regulatory burden and unnecessary human studies in generic drug development without sacrificing the quality of the drug products.
KEY WORDS: bioequivalence, biopharmaceutics, generic drugs, in vitro dissolution, quality by design
INTRODUCTION
Dissolution testing has emerged as a very important tool in the generic pharmaceutical industry. It is very widely used in formulation development, in monitoring the manufacturing process and as a quality control test. It can also be used to predict the in vivo performance of certain products. Dissolution testing has been successfully used for development and approval of generic solid oral dosage forms. Most recently, the use of dissolution testing has been extended to other solid generic dosage forms; in these cases it is generally called as in vitro release testing or simply drug release testing (1,2). Finally, dissolution testing plays significant role in identifying the need for the bioequivalence (BE) studies related to Scale-Up and Post-Approval Changes (SUPAC) (3,4).
The purpose of this article is to summarize how dissolution testing is used for the approval of safe and effective generic drug products in the USA. This article also reflects the current thinking of the US-Food and Drug Administration (US-FDA), on using dissolution and other in vitro drug release testing in consistently producing high-quality generic drug products and reducing the regulatory burden for the pharmaceutical industry.
ROLE OF DISSOLUTION TESTING IN APPROVAL OF GENERIC DRUG PRODUCTS
In vitro dissolution testing (dissolution) plays a critical role in the life cycle of a generic drug product. In developing a dissolution test for a generic product intended to be marketed in the USA, investigators should consider the official methods and standards published in the United States Pharmacopeia (USP). The USP describes seven different dissolution apparatuses which can be used to develop an appropriate dissolution method based on the drug product characteristics (5,6). The dissolution method should be sufficiently rugged and reproducible for daily operations, capable of being transferred between laboratories, and adequately discriminating to distinguish any changes that could affect the product’s in vivo performance (7). The Division of Bioequivalence (DBE), in the Office of Generic Drugs, Center for Drug Evaluation and Research, US-FDA asks investigators to conduct comparative dissolution testing using at least 12 dosage units each of test and reference products. Dissolution data should be generated by sampling the dissolution medium at time points appropriate to characterize the dissolution profile. It is suggested that three to four or more dissolution time points (other than zero), equally spaced, be utilized for rapidly dissolving drugs (8). For dissolution of extended-release (ER) formulations, more sampling time points are suggested, in order to adequately characterize the complete dissolution profile.
DISSOLUTION TESTING RECOMMENDATIONS FOR SOLID ORAL GENERIC DRUG PRODUCTS
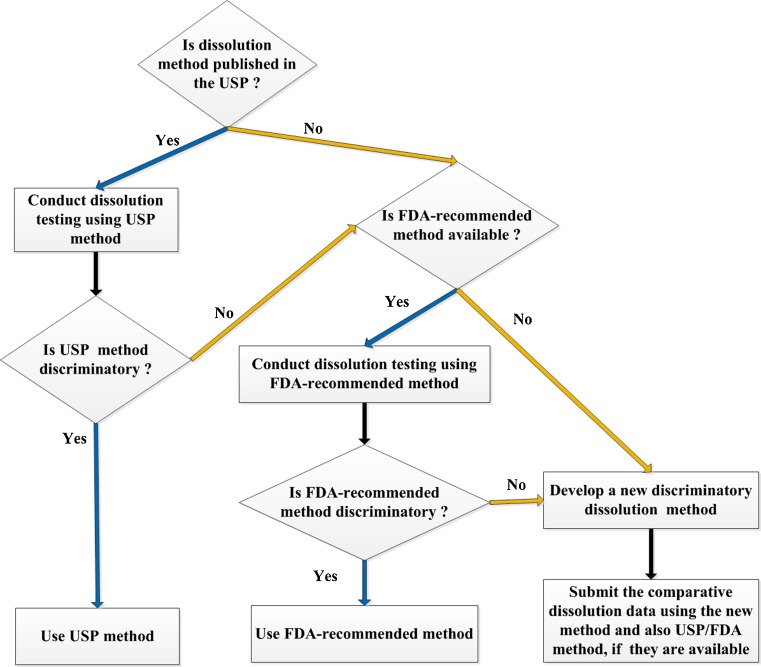
One of the first steps during the BE review of a potential new generic drug product is an assessment of whether the dissolution method proposed for the product is the appropriate one. As depicted in Fig. 1, the DBE recommends that for generic drug products, if a USP method is available for the product, then dissolution should be conducted using that method. If there is no USP method available then the dissolution testing should be conducted using a method recommended by the FDA (FDA-recommended method). The FDA posts a list of its recommended dissolution methods (http://www.accessdata.fda.gov/scripts/cder/dissolution/). An applicant may develop their own dissolution method if the FDA-recommended method is inadequate for their product. It is worth noting that even if the USP method is used, the DBE asks generic applicants to submit to the Abbreviated New Drug Application (ANDA) comprehensive dissolution testing data. Thus, an applicant should characterize comparative dissolution testing using at least 12 dosage units each of test and reference products, whether it proposes to use a USP method, FDA-recommended method, or its own method. In cases where the applicant develops their own method, both data using their method as well as data from the FDA-recommended method should be submitted for comparison.
Fig. 1.
Decision tree for ANDA sponsors to select a dissolution method for their generic product
In cases where neither a USP and nor FDA-recommended method is available, an appropriate new dissolution method should be developed. The new dissolution method development report should be submitted to the ANDA, so that the DBE can evaluate the feasibility of the new method. The new dissolution method development report should include a pH solubility profile of the drug substance, dissolution profiles at different rotational speeds and dissolution media. Dissolution profiles should be generated using at least three dissolution media for example, pH 1.2, 4.5, and 6.8 buffers. Water may also be tested as an additional dissolution medium for method optimization. It may be difficult to achieve sink conditions for poorly water-soluble drugs; therefore a suitable surfactant in an appropriate concentration can be used in the dissolution medium for these drug products (9).
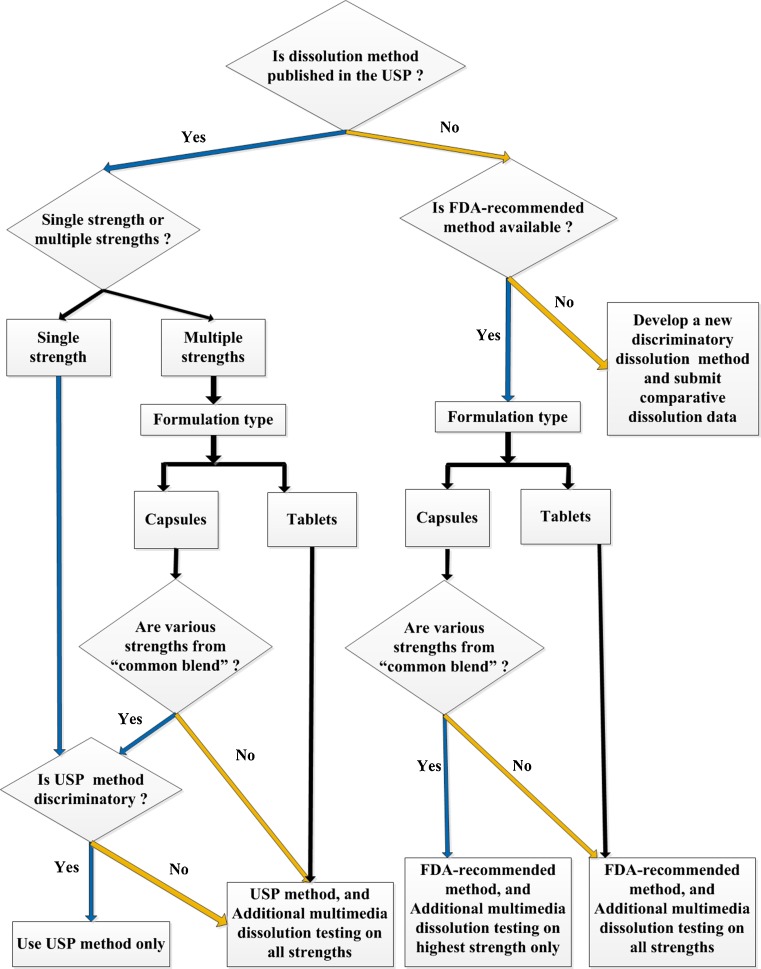
For immediate-release (IR) generic products, dissolution testing using a single method may be sufficient. For ER products, if a USP method is available then dissolution testing recommendations are based on the product formulation and number of strengths to be marketed. As presented in Fig. 2, if the USP method is adequately discriminating and the drug product is to be marketed in only one strength then dissolution data generated using only the USP method may be sufficient (9). In the case of multiple-strength ER capsule drug products, the dissolution testing recommendations are based on the formulation design. If multiple strengths of an ER capsule product are produced from a “common blend” then dissolution data generated using only the USP method may be sufficient, provided that the USP method is adequately discriminating. For multiple strengths of an ER tablet drug product, and for multiple strengths of an ER capsule product which are not produced from a “common blend”, dissolution testing in addition to the USP method is recommended, in order to provide the FDA with sufficient information to determine the optimal and most discriminating dissolution method for the product. The additional dissolution testing should be conducted using at least three dissolution media, for example, pH 1.2, 4.5, and 6.8 buffers (8,9). Water may also be tested as a possible dissolution medium during the method optimization process (8,9). If the applicant proposes to use a dissolution method other than the USP method, then it should submit dissolution data generated on 12 units per strength for all strengths, for both the test (generic) and reference products using both the USP method and the applicants newly developed method.
Fig. 2.
Decision-tree for ANDA sponsors for submitting dissolution testing data for an extended-release solid oral generic drug product. {“Common blend”: A batch of final blend that can be packed in different amounts providing various strengths of the capsule product. Multimedia: lower pH, e.g. 1.2; medium pH, e.g. 4.5; higher pH, e.g. 6.8 and water}
If a USP method is not available for the ER dosage form, but an FDA-recommended method is available, then it is recommended that dissolution be conducted using the FDA-recommended method. Whether additional dissolution testing is recommended depends upon the product formulation. For multiple strengths of ER tablets and ER capsules where the various strengths are not produced from a “common blend”, additional dissolution testing is recommended on all the strengths. If the multiple strengths of the ER capsule product line are produced from a “common blend”, then dissolution testing in addition to the FDA-recommended method is recommended only on the highest strength. The additional dissolution testing should be conducted using at least three dissolution media, for example pH 1.2, 4.5, and 6.8 buffers; water may also be used for method optimization (9). If the applicant proposes to use a dissolution method other than the FDA-recommended method, then it should submit dissolution data generated on 12 units per strength for all strengths, for both the test (generic) and reference products using both the FDA-recommended method and the applicants newly developed method.
For delayed-release (DR) solid oral dosage forms, the dissolution testing should demonstrate that (a) the product is stable under the acidic conditions of the stomach (for example pH 1.2); and (b) release in the pH present in the intestine. If a USP method is available then dissolution should be conducted using that method. If there is no USP method, then for all the strengths of a DR product the DBE recommends that dissolution testing should be conducted under acidic conditions (pH 1.2) for 2 h followed by neutral medium (e.g., pH 6.8). In general, DR products should display acid resistance under the dissolution testing conditions (8,9). Currently, the DBE does not request additional multimedia dissolution testing for DR products demonstrating biphasic release (10).
As stated above, for some ER and DR drug products, a USP and/or FDA-recommended method is available, but not necessarily suitable for the generic test product. In this case, the DBE encourages the applicant to develop the most suitable and adequately discriminating dissolution to distinguish any changes that could affect the test product’s in vivo performance. In such a case, the DBE requests the submission of a dissolution method development report. In addition, it is recommended that the submission contain results of comparative test and reference dissolution profiles generated using the USP and/or the FDA-recommended method and the applicant’s proposed method. In this way, the appropriateness of the new proposed method can be carefully evaluated.
DISSOLUTION TESTING AND BCS-BASED BIOWAIVERS FOR GENERIC DRUG PRODUCTS
In recent times, the regulatory perspective of dissolution has shifted due to improved knowledge and understanding of dissolution science and mechanisms. FDA’s Guidance for Industry on the Biopharmaceutics Classification System (BCS Guidance) emphasizes how the dissolution test can be used to grant biowaivers for highly soluble and highly permeable drugs formulated in rapidly dissolving IR solid oral dosage forms (11).
The BCS is a framework for classifying drug substances based on their solubility and permeability, and for providing a scientific rationale to justify the granting of biowaivers for certain products (11,12). The BCS system when integrated with the dissolution of the drug product takes into account three major factors; namely, dissolution, solubility, and intestinal permeability. It is well-established that these three factors govern the rate and extent of drug absorption from IR solid oral dosage forms (11,12).
According to the BCS Guidance, biowaivers may be granted for BCS class 1 (high-solubility and high-permeability drug substance) products if the drug product is rapidly dissolving. An IR drug product is considered rapidly dissolving when no less than 85% of the labeled amount of the drug substance dissolves within 30 min, using USP Apparatus 1 at 100 rpm (or Apparatus 2 at 50 rpm) in a volume of 900 ml or less in each of the following media: (a) 0.1N HCl or Simulated Gastric Fluid USP without enzymes; (b) a pH 4.5 buffer; and (c) a pH 6.8 buffer or Simulated Intestinal Fluid USP without enzymes (11).
For a rapidly dissolving IR generic product containing highly soluble and highly permeable drug substances, applicants may request that the agency grant a BCS-based biowaiver, if the following conditions are met:
The reference listed drug (RLD) product is rapidly dissolving; and
The test product exhibits dissolution profiles similar to the RLD product under all test conditions as defined in the guidance (11).
ANDA applicants may consult FDA’s Guidance for Industry (13): Individual Products Bioequivalence Recommendations Guidances for information about some potential BCS Class 1 drugs for which a BCS biowaiver option may be feasible.
DISSOLUTION TESTING AND OTHER BIOWAIVERS FOR GENERIC DRUG PRODUCTS—PREAPPROVAL
Comparative dissolution testing can be used to grant biowaivers for different strengths for IR dosage forms as long as certain conditions are met (8,9).
If BE to the reference listed drug has been established for the one strength (generally the highest) of a generic drug product line then, as per FDA’s General Guidance on Bioavailability and Bioequivalence for Orally Administered Drug Products (BA/BE Guidance) (9), an in vivo BE study requirements can be waived for one or more additional strengths based on dissolution tests if the drug product is in the same dosage form, but in a different strength and proportionally similar in its active and inactive ingredients to the strength on which acceptable in vivo BE testing was conducted (9).
For IR generic drug products, dissolution profiles in one medium are usually sufficient to support waivers of in vivo testing if an appropriate regulatory dissolution method is established, and if the dissolution results indicate that the dissolution characteristics of the product are not dependent on the product strength, i.e., various strengths have similar dissolution. If an appropriate dissolution method is not established then dissolution data in three media (pH 1.2, 4.5, and 6.8) are recommended (8,9).
Dissolution can also be used to support applicant requests for biowaivers for various strengths of a modified-release (MR) drug product line. In this case, the DBE may decide that it is unnecessary to conduct in vivo studies on one or more strengths based on acceptable dissolution performance, proportional similarity among strengths, and an acceptable in vivo study on one (generally the highest) strength. The scope of dissolution testing differs based on whether the MR formulation is an ER capsule, ER tablet, DR capsule, or DR tablet (9).
For ER-beaded capsule formulations, in vivo BE studies are recommended for one strength, usually the highest. Other strengths can be deemed bioequivalent to the corresponding strengths of the RLD if BE is established in vivo for one strength, the various strengths differs only in the number of beads containing the active moiety, and dissolution profiles are similar for each strength using the recommended dissolution method (9). For ER tablet formulations, the in vivo BE studies are recommended for one strength, usually the highest. The other strengths of the product line may be deemed bioequivalent to the corresponding strengths of the RLD. The criteria for this determination are that (1) the lower strengths are in same dosage form; (2) active and inactive ingredients are proportionally similar; (3) the drug release mechanism from all strengths of the formulations is the same; and (4) the dissolution profiles of the strength that underwent acceptable in vivo BE testing and the other strength(s) is (are) similar in at least three dissolution media (e.g., pH 1.2, 4.5 and 6.8) (9). To support biowaiver requests for various strengths of a DR product line demonstrating biphasic release, the DBE considers dissolution data in a manner similar to the process for IR products in that dissolution testing using standard dissolution method for the product is recommended and multimedia dissolution testing is generally not requested. For MR products containing both DR and ER components, the DBE may request additional dissolution testing of strengths other than the one used for in vivo testing; the scope of the request will depend on whether the product is a capsule or tablet, as described above.
ROLE OF DISSOLUTION TESTING IN POST-PPROVAL CHANGES OF GENERIC DRUG PRODUCTS
FDA’s SUPAC Guidance for Industry provides a more simplified path to the industry to test and report the manufacturing changes of various types of pharmaceutical products formulations. Comparative dissolution testing between the pre-manufacturing change (Bio-batch) and post-manufacturing change plays very significant role in approval of SUPAC changes. The SUPAC Guidances define the various categories of these post-approval changes in form of “Levels” (3,4).
In addition to chemistry and manufacturing documentation, comparative dissolution data are required to support the approval of intended change. According to the SUPAC Guidance, if a product is an IR solid oral dosage form, for any level 1 or level 2 change, dissolution testing may be adequate (no BE study) to support agency approval of the post-approval change. For “site change” level 3 changes, dissolution testing may be adequate (no BE study) to support approval of the post-approval change. For all other level 3 changes, both dissolution testing and an in vivo BE study are necessary to support the approval of the post-approval change. However, if an acceptable and validated in vivo/in vitro correlation (IVIVC) is available then the requirement for BE study may be waived (3).
For all level 1 changes to approved MR generic formulations, it is necessary to conduct comparative dissolution testing on the pre-change and post-change products using a standard dissolution method (4). To support most level 2 changes, it is necessary to compare pre-change and post-change products in the compendial dissolution medium plus generate comparative multi-point dissolution profiles in at least three other media (e.g., water, 0.1N HCl, and USP buffered media at pH values of 4.5 and 6.8). It is recommended that pre-change and post-change product dissolution profiles be compared statistically, generally by using the f2 similarity factor test to confirm mathematically that the pre-change and post-change product dissolution profiles are not significantly different (8). Note that if the agency determines that the above comparative dissolution testing is acceptable, then it is not necessary to conduct an in vivo BE study of the post-approval formulation.
For all level 3 changes to MR products, and for some level 2 changes, it is necessary to conduct an in vivo study (new product verses reference listed drug) to demonstrate that the post-approval product is bioequivalent to the corresponding RLD. However, an established IVIVC for the product can significantly reduce the regulatory and economical burden for obtaining agency approval of level 2 and level 3 changes to MR products. If an established IVIVC is available, then comparative pre-change and post-change product dissolution profiles using only the standard regulatory dissolution method may be adequate to support approval of the change(s) (3,4). Therefore establishing an IVIVC at the development stage of the product can greatly reduce the number of BE studies required for any SUPAC type of changes and save resources and reduce the regulatory burden for applicants.
DISSOLUTION TESTING AND ALCOHOL-INDUCED DOSE-DUMPING OF GENERIC MR ORAL DRUG PRODUCTS
MR drug products are intended to release the drug slowly into the systemic circulation and offer the advantage to patients that fluctuations in plasma concentrations are minimized. The in vivo release mechanism can be altered if an MR formulation is administered with alcohol, leading to dose dumping. The consequences of dose dumping of certain drugs could lead to serious or even fatal adverse events in some patients (14).
FDA recognizes that alcohol-induced dose dumping can pose a serious safety concern for some MR drug products. In response to the agency’s concerns about this safety issue, the DBE adopted a policy of requesting information on in vitro dose dumping in the presence of alcohol in its review of ANDAs for certain classes of MR drug products, for example all MR opioid products (14). The DBE’s current policy is to request that ANDA applicants perform an in vitro dose dumping in alcohol test on a particular generic product if the approval package for the corresponding RLD shows that in vitro dose dumping in alcohol test results were requested by the agency for the New Drug Application (NDA) for the product. The MR drug products for which the DBE requests the in vitro dose dumping in alcohol test can be located in FDA’s Guidance for Industry, Individual Products Bioequivalence Recommendations Guidances (13).
The in vitro dose dumping in alcohol test is a dissolution test designed to assure that any potential for in vivo dose dumping in the presence of alcohol for a generic MR drug product is either not present or, if present, not significantly different from that of its corresponding RLD. Since patients may be switched from the RLD to the corresponding approved generic version, it is important to provide assurance that ethanol will have the same effects on drug release from a generic MR formulation and the RLD.
Briefly, the in vitro dose dumping in alcohol test compares the dissolution performance of the generic (test) product and the RLD at various ethanol concentrations (13–17). Differing amounts of ethanol are added to 0.1 N HCl media on a volume/volume (v/v) basis to give the following percentages:
0% ethanol (no ethanol added)
5% ethanol
20% ethanol
40% ethanol
The baseline medium of 0.1N HCl is selected to approximate conditions in the stomach, since most of a dose of ethanol is absorbed through the gastric mucosa. The various percentages of ethanol are considered to be representative of consumption of beer (5% ethanol), mixed drinks (20% ethanol), and neat liquor (40% ethanol). The in vitro dose dumping in alcohol test should be performed for each strength of the test product and reference product, regardless of whether the MR dosage form is a tablet or capsule. Twelve units of the test product and 12 units of the RLD are tested separately in 900 mL volumes of each medium. Samples of the media are taken once every 15 min until 2 h is reached. The percent of labeled amount of the active drug dissolved in the medium (percentage dissolved) is calculated for each sample. Dissolution data are expressed as percentage dissolved.
The DBE evaluates the results of the in vitro data submitted by the ANDA applicants, to address the performance of the drug release mechanism in the presence of alcohol, and consider the results in deciding whether two products are bioequivalent (16,17).
DISSOLUTION TESTING AND GENERIC DESI DRUG APPROVAL
From 1938 to 1962, FDA approved drugs based on safety only. In 1962, The Kefauver and Harris Amendment to the Food and Drug Cosmetic Act required that the drug products approved in USA should not only be safe but also effective. This requirement for drug effectiveness was applied retrospectively to all the products approved between 1938 and 1962. The National Academy of Science/National Research Council (NAS/NRC) was charged by the FDA with the responsibility of initiating the Drug Efficacy Study to review the effectiveness of these drugs approved between 1938 and 1962. NAS/NRC reports, which classified these previously approved formulations as “effective” or “less than effective”, were reviewed by the FDA which then published its conclusions as Drug Efficacy Study Implementation (DESI) notices in the Federal Register (18–20). Dissolution data plays a very important role in approval of new generic drug products for selected drugs on the DESI-effective list. DESI-effective generic drug products without known BE problems have a therapeutic equivalence code of AA in the Orange Book (21), and are eligible for biowaivers of in vivo BE study requirements. To support the DESI biowaiver requests, the DBE asks ANDA applicants to submit formulation data and dissolution testing data. If there is a USP dissolution method available, then dissolution testing data using USP method may be adequate for the submission. When there is no USP dissolution method for the product but there is a FDA-recommended method, dissolution testing using the FDA-recommended method may be adequate. DESI-based biowaivers can be granted for acceptable formulations if test product dissolution profiles are similar to corresponding RLD dissolution profiles. The continued widespread application of DESI-based biowaivers within the DBE is one additional situation which emphasizes the importance of the dissolution testing in approval of safe and effective generic drugs for marketing.
DISSOLUTION TESTING FOR OTHER ORAL DOSAGE FORMS
Suspensions can be considered to be similar to disintegrated forms of solid formulations. Bioavailability of various poorly soluble drugs administrated as suspension formulations has been reported to be dissolution rate-limited (22). For dissolution testing of oral suspensions, the DBE generally recommends the use of USP apparatus 2 (paddle) at 25 or 50 rpm. It is worth noting that a dosage unit for a suspension is the labeled strength and the in vitro testing of suspensions should be conducted using a total of 12 units of both test product and RLD product from 12 different containers.
Liquid-filled capsules can be composed of hydrophilic or lipophilic drug substances (1,23). For liquid-filled capsules containing lipophilic drugs, the DBE asks applicants to develop a “quantitative rupture” in vitro drug release test; where, after the rupture of the capsule shell the drug is released and measured in the medium. The DBE believes that this method provides an accurate assessment of the rate at which a capsule shell ruptures by quantifying the amount of active pharmaceutical ingredient (API) dispersed in the surrounding medium. DBE recognizes that developing “quantitative rupture” drug release test for capsules containing lipophilic drugs formulated in oils may be a challenge. Therefore, the DBE encourages the firms to develop an appropriate method using a USP apparatus. Generally, a suitable surfactant in an appropriate concentration can be used in the aqueous medium for these formulations. As with other dissolution tests, the use of organic solvents in the dissolution media is not encouraged. However, as with other types of formulations, DBE is open to any other appropriate new validated in vitro method which the applicant provides strong scientific evidence of suitability for their drug product.
Chewing gums are sometimes used as drug delivery dosage forms. Drug release from the chewing gum formulation is complex since it is based not only on the solubilization of the drug but also on the shear forces encountered by the formulation (23). The USP currently does not describe a drug release test for the chewing gum formulation. However, the European Pharmacopeia has published a method for drug release testing of these formulations (24). For a proposed generic chewing gum formulation, FDA requests dissolution testing. Biowaivers may be granted for some strengths provided that the dissolution testing is acceptable and other requirements are met. For chewing gums, the DBE currently accepts the use of an appropriate in vitro dissolution method using the apparatus described in the European Pharmacopeia or any other apparatus which the DBE deems suitable for this purpose.
DISSOLUTION TESTING OF NON-ORAL DOSAGE FORMS
As with oral formulations, release of the API from a non-oral formulation is the key to the performance of a drug product. Therefore, the DBE includes evaluation of drug release testing in its review of BE submissions of potential generic non-oral dosage forms. For non-oral dosage forms, the test is referred as “drug release” rather than “dissolution” testing (1,2). It is believed that, in addition to other quality checks, the use of a proper drug release test as a quality control tool will greatly assist manufacturers to consistently reproduce high quality non-oral generic drug products.
For ophthalmic suspensions, generic liposome formulations, and rectal and vaginal suppositories, if there is no USP or FDA-recommended method then the DBE encourages the firms to develop a method to characterize the in vitro release. For parenteral, implants, microparticles and suspensions, if there is no USP or FDA-recommended method then the DBE asks the firms to develop a drug release test using USP 4 (Flow-Through Cell), or, if applicable, apparatus 2 (paddle) or any other appropriate method, for comparative evaluation by the DBE.
Transdermal delivery systems are complex dosage forms for which the rate and extent of the drug release may be influenced by various parameters. Therefore, besides other quality control tests, quality and reproducibility of the transdermal products should be tested using in vitro drug release testing (1). Currently, USP describes three in vitro release testing methods (USP apparatus 5, 6 and 7) for these systems (6,22). The DBE encourages applicants to use these USP apparatuses to develop suitable in vitro release tests which can be used as appropriate quality control tests.
Semisolid dosage forms, including creams, gels, lotions and ointments, are generally intended for topical route of application (25). Currently DBE does not request in vitro release testing data in support of BE submissions to ANDAs for the generic drug products in this category. The DBE does not request an in vitro release test for approval of generic semisolid preparations; the test is also not needed as a routine batch-to-batch quality control test. Likewise, the DBE does not consider in vitro release testing as a surrogate for in vivo BE studies of generic semisolid drug products; thus, in vitro release testing cannot be used to support biowaivers of such products. However, in vitro release testing for semisolid drug products can be used in support of certain types of post-approval changes to generic semisolid formulations. Such in vitro release testing methods, to compare performance of certain types of pre-change and post-change formulations, can be developed using an open chamber diffusion cell system such as a Franz cell system. Thus, a validated in vitro release test method developed using an open-chamber diffusion cell system such as a Franz cell system finds its utility in support of some scale-up and post-approval changes of generic semisolid drug products (25).
IN VITRO DISSOLUTION TESTING OF GENERIC DRUG PRODUCTS: FUTURE ROLE
FDA strives to reduce the regulatory burden and unnecessary human studies without sacrificing the quality of the drug products. Dissolution testing of generic drug products plays a major role in this effort. For many years, the generic industry has used dissolution testing as a quality control tool to ensure consistent performance and high quality of the generic drug products.
In some cases, dissolution testing is useful as in vitro surrogate for product performance to guide formulation development and ascertain the need for BE studies (26). Since in vitro dissolution testing of generic solid dosage forms is a regulatory tool for biowaivers; efforts should be made to ensure that, as much as possible, in vitro dissolution methods reflect the in vivo performance of the generic drug products. In future it may be useful to develop dissolution methods using biorelevant media for formulations with dissolution rate-limited absorption; likewise, it can be helpful to develop an IVIVC or even in vivo/in vitro relationship for a new generic MR formulation. Predictive dissolution testing developed on the basis of human physiology can make significant contributions to rational generic drug development.
For approvals of generic drugs for solid oral dosage forms, it is recommended that sponsors develop meaningful in vitro dissolution testing procedures. Appropriate use of dissolution testing as a quality control tool has been successful in assuring the high quality and reproducible performance of solid oral generic drug products (26). Since the release of the API from the formulation is the key to the performance of a drug product the agency has extended the principle of drug release testing to other generic dosage forms. It is believed that, in addition to other quality checks, the use of a proper drug release test as a quality control tool will enable consistent manufacture of high-quality non-oral generic drug products. Dissolution and drug release testing will play an even wider role in regulating quality generic drug products in the future.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Xiaojian Jiang for her valuable suggestions.
REFERENCES
- 1.Siewert M, Dressman J, Brown CK, Shah VP. FIP/AAPS guidelines to dissolution/in vitro release testing of novel/special dosage forms. AAPS PharmSciTech. 2003;4(1):1. doi: 10.1208/pt040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah VP, Tymes NW, Skelly JP. In vitro release profile of clonidine transdermal therapeutic systems and scopolamine patches. Pharm Res. 1989;6:346–351. doi: 10.1023/A:1015962911344. [DOI] [PubMed] [Google Scholar]
- 3.Food and Drug Administration. Guidance for industry: immediate release solid oral dosage forms: scale-up and post-approval changes. Rockville, MD: US Department of Health and Human Services, FDA, Center for Drug Evaluation and Research; November 1995.
- 4.Food and Drug Administration. Guidance for industry: modified release solid oral dosage forms: scale-up and post-approval changes. Rockville, MD: US Department of Health and Human Services, FDA, Center for Drug Evaluation and Research; October 1997.
- 5.Chapter 711: Dissolution. In: United States Pharmacopeia 31 (USP 31): National Formulary 26 (NF 26). 2008. p. 267–274. 2010.
- 6.Chapter 724: Drug release. In: United States Pharmacopeia 31 (USP 31): National Formulary 26 (NF 26). 2008. p. 275–278. 2010.
- 7.Chapter 1092: The dissolution procedure. Development and validation. In: United States Pharmacopeia 31 (USP 31): National Formulary 26 (NF 26). 2008. p. 573–678.
- 8.Food and Drug Administration. Guidance for industry: dissolution testing of Immediate Release Solid Oral Dosage Forms. Rockville, MD: US Department of Health and Human Services, FDA, Center for Drug Evaluation and Research; August 1997.
- 9.Food and Drug Administration. Guidance for industry: bioavailability and bioequivalence studies for orally administered drug products—general considerations. Rockville, MD: US Department of Health and Human Services, FDA, Center for Drug Evaluation and Research; March 2003.
- 10.Anand O. Dissolution testing: an FDA perspective; AAPS workshop, physical pharmacy and biopharmaceutics. May 5–13, 2009. Baltimore, MD. In: http://mediaserver.aapspharmaceutica.com/meetings/09_PPB/Wed/Track_I/Om_Anand.pdf. Accessed 26 September 2010.
- 11.Food and Drug Administration. Guidance for industry: waiver of in vivo bioavailability and bioequivalence studies for immediate release solid oral dosage forms based on a biopharmaceutics classification system. Rockville, MD: US Department of Health and Human Services, FDA, Center for Drug Evaluation and Research; August 2000.
- 12.Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413–420. doi: 10.1023/A:1016212804288. [DOI] [PubMed] [Google Scholar]
- 13.Individual Product Bioequivalence Recommendations: In: US FDA. http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm075207.htm. Accessed 06 October 2010.
- 14.FDA Alert for Healthcare Professionals (July 2005): Hydromorphone Hydrochloride Extended-Release Capsules (marketed as Palladone™). 2005 July: http://www.fda.gov/cder/drug/InfoSheets/HCP/hydromorphoneHCP.pdf. Accessed 04 October 2010.
- 15.Chandaroy P, Jiang X, Lee C, Thompson C, Conner DP, Davit BM. In vitro alcohol-induced dose-dumping dissolution studies of generic modified-release oral drug products: American Association of Pharmaceutical Scientists 2008 annual meeting, Atlanta, November 2008: http://www.aapsj.org/abstracts/AM_2008/AAPS2008-001832.PDF. Accessed 20 February 2011.
- 16.FDA/CDER to Osmotica Pharmaceutical Corp. - petition partial approval and denial. In: http://www.regulations.gov/search/Regs/home.html#docketDetail?R=FDA-2009-P-0403. Accessed 6 October 2010.
- 17.FDA/Associate Commissioner for Policy and Planning - petition approval. Docket ID:FDA-2005-P-0366. In: http://www.regulations.gov/search/Regs/home.html#documentDetail?R=090000648044af45. Accessed 07 October 2010.
- 18.Kefauver-Harris Amendments. Public Law (PL) 87–781, 87th Congress. 10-10-1962.
- 19.Drug Efficacy Study: a report to the commissioner of Food and Drugs, National Academy of Sciences, National Research Council, Washington, DC, 1969.
- 20.Conner DP, Davit BM. Bioequivalence and drug product assessment, in vivo. In: Shargel L, Kanfer I, editors. Generic drug product development, solid oral dosage forms. Marcel Dekker, Inc: 2005. p 227–55.
- 21.Orange book: In:http://www.fda.gov/Drugs/DevelopmentApprovalProcess/ucm079068.htm#Therapeutic%20Equivalence-Related%20Terms. Accessed 06 October 2010.
- 22.Abdou HM. Dissolution. Remington: the science and practice of pharmacy. Nineteenth ed. Mack Publishing Company; 1995. p. 593–604.
- 23.Azarmi S, Roa W, Lobenberg R. Current perspectives in dissolution testing of conventional and novel dosage forms. Int J Pharm. 2007;328:12–21. doi: 10.1016/j.ijpharm.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Chewing gum, medicated, drug release. European Pharmacopoeia, Edition 4. Strasbourg, France: Directorate for the quality of medicines of the Council of Europe; 2002. p. 227–228.
- 25.Food and Drug Administration . Guidance for industry: nonsterile semisolid dosage forms: scale-up and post-approval changes. Rockville: US Department of Health and Human Services, FDA, Center for Drug Evaluation and Research; 1997. [Google Scholar]
- 26.Zhang H, Yu LX. Dissolution testing for solid oral drug products: theoretical considerations. Am Pharm Rev. 2004;7(5):26–31. [Google Scholar]