Abstract
Recent years have witnessed a growing interest in a field of vaccinology that we have named vaccinomics. The overall idea behind vaccinomics is to identify genetic and other mechanisms and pathways that determine immune responses, and thereby provide new candidate vaccine approaches. Considerable data show that host genetic polymorphisms act as important determinants of innate and adaptive immunity to vaccines. This review highlights examples of the role of immunogenetics and immunogenomics in understanding immune responses to vaccination, which are highly variable across the population. The influence of HLA genes, non-HLA, and innate genes in inter-individual variations in immune responses to viral vaccines are examined using population-based gene/SNP association studies. The ability to understand relationships between immune response gene variants and vaccine-specific immunity may assist in designing new vaccines. At the same time, application of state-of-the-art next-generation sequencing technology (and bioinformatics) is desired to provide new genetic information and its relationship to the immune response.
Key words: genetic association, HLA, immunogenetics, polymorphisms, SNPs, vaccines, vaccinomics
Introduction
While readers of this journal are already familiar with the impact of pharmacogenetics and pharmacogenomics of drug therapy, such concepts have just recently begun to be applied to the development and delivery of vaccines. The application of the field of immunogenetics and immunogenomics to rational vaccine design has recently been labeled vaccinomics (1, 2). Accordingly, vaccinomics encompasses the field of predictive or individualized vaccinology as applied to a comprehensive understanding of the genetic and immunologic systems responsible for heterogeneity in vaccine-induced immune responses (3). Both environmental and host factors determine the adaptive immune responses to vaccination (4). These immune responses are highly variable across the population, suggesting a genetic basis. Further evidence comes from studies comparing vaccine-induced immune responses in monozygotic and dizygotic twins, which provides an estimate of the contribution of genetic factors by estimating the heritability of total variance that is due to genetics rather than environmental factors (5). Supporting the value of understanding the immunogenetic drivers of vaccine response, we demonstrated in a study of monozygotic and dizygotic twin pairs that the heritability of IgG levels (the proportion of genetic variance to overall variance) for measles, mumps, and rubella (MMR) vaccines was 88.5%, 38.8%, and 45.7%, respectively (6). In other twin studies, high heritability for antibody response was observed for hepatitis B (77%), oral polio (60%), tetanus toxoid (44%), and diphtheria (49%) vaccines (7). These data support the notion that genes play a defining function in the inter-individual variation of antibody responses following vaccination. By understanding these critical genetic determinants of immune response, we may develop the basis for an individualized approach to vaccination. In turn, understanding these genetic drivers could be used to enhance immune response, for example, in vaccine non-responders, and to inform new vaccine development (3,8,9).
The most frequently used method to investigate relationships between genetic polymorphisms and variations in immune responses to vaccination has been candidate gene and genome-wide association studies (GWAS). Such studies are important in providing the data necessary to support and pursue the novel and translational approach of directed vaccinology (1–3,9). Epidemiological and family vaccine studies have shown familial aggregation. Subsequently, many association studies have identified both HLA and non-HLA candidate gene markers, including genes in close linkage disequilibrium (LD) with the putative causative marker (3,9). These HLA and single-nucleotide polymorphism (SNP) findings emphasize the importance of identifying and replicating initial findings of genetic associations with vaccine-induced immune responses, as well as understanding the functional consequences of each gene/SNP association.
Importance of HLA Genes in Acquired Vaccine Immunity
Our immunogenetic studies involving well-characterized cohorts of healthy subjects from various populations have resulted in a better understanding of the role of host HLA genetic determinants in response to vaccines. Among the different genetic determinants that are recognized to affect adaptive immune response to viral vaccines, the HLA class I and class II genes represent one of the main focal points because of their biologic role of presenting pathogen-derived peptide epitopes to T cells and the extraordinary polymorphism in these genes. In our population-based studies, live attenuated measles, mumps, and rubella virus vaccines that stimulate both CD8+ and CD4+ T-cell responses were used. Peripheral blood mononuclear cells that contain dendritic cells, macrophages, and B cells were utilized as antigen-presenting cells, which can present exogenous viral antigens via cross-presentation by both class I and class II HLA molecules.
Clearly, immune responses after MMR, influenza, hepatitis B, and vaccinia vaccines are influenced by variation at the HLA loci and other immune regulatory genes (10–13). We have in part previously discussed some of these examples in other publications (1–3,9). Several alleles of the HLA loci have been linked with “responder” and “non-responder” phenotypes after MMR vaccination. For example, HLA class I, in particular the B*8 (p = 0.001), B*13 (p = 0.004), and B*44 (p = 0.03) alleles are associated with IgG seronegativity after a single dose of measles vaccine (14). We demonstrated that the A*29-C*16-B*44 haplotype is associated with low levels of IgG antibody to measles virus (p = 0.08) after two doses of measles vaccine (Table I) (15). Furthermore, the A*26-C*12-B*38 haplotype is associated with high lymphoproliferative responses to measles vaccine (p = 0.02).
Table I.
Associations Between HLA Gene Polymorphisms and Humoral Immune Responses After Two Doses of MMR Vaccine
| HLA Gene | Genes and alleles (p value) | Effect on antibody level | Number of subjects | References |
|---|---|---|---|---|
| Measles vaccine | ||||
| HLA class I | B*3503 (p = 0.01) | Increased | 346 | Not published |
| HLA class II | DRB1*0701 (p = 0.03), DQA1*0201 (p = 0.03) | Decreased | 346 | Not published |
| HLA class I haplotype | A*29-C*16-B*44 (p = 0.08) | Decreased | 346 | (15) |
| HLA class II haplotype | DRB1*15/16-DQB1*06-DPB1*04 (p = 0.02) | Increased | 346 | (15) |
| HLA class II haplotype | DRB1*15/16-DQB1*06-DPB1*03 (p = 0.09) | Increased | 346 | (15) |
| Rubella vaccine | ||||
| HLA class I | A*2705 (p = 0.001), A*5701 (p = 0.03) | Decreased | 738 | (16) |
| HLA class I | A*4501 (p = 0.02) | Increased | 738 | (16) |
| HLA class II | DPA1*0201 (p = 0.005), DPB1*0301 (p = 0.01), DPB1*1301 (p = 0.04) | Decreased | 738 | (16) |
| HLA class II | DPB1*0401 (p = 0.001) | Increased | 738 | (16) |
| HLA class I haplotype | A*03-C*07-B*07 (p = 0.04) | Decreased | 738 | (16) |
| HLA class II haplotype | DRB1*04-DQB1*03-DPB1*03 (p = 0.01) | Decreased | 738 | (16) |
| HLA class II haplotype | DRB1*15/16-DQB1*06-DPB1*03 (p = 0.005) | Decreased | 738 | (16) |
| Mumps vaccine | ||||
| HLA class II | DQB1*0303 (p = 0.04) | Decreased | 346 | (18) |
| HLA class I haplotype | A*26-C*12-B*38 (p = 0.007) | Increased | 346 | (15) |
| HLA class I haplotype | A*29-C*16-B*44 (p = 0.03) | Decreased | 346 | (15) |
In regard to rubella vaccine response, low-rubella IgG antibody levels are associated with B*2705 (p = 0.028), whereas B*4501 alleles are associated with high antibody levels after two doses of rubella vaccine (16). Importantly, we demonstrated that B*2705 alleles are associated with low rubella-induced antibody levels in two independent cohorts of subjects (16). In regard to cellular immunity, B*3503 (p = 0.031) and C*1502 (p = 0.035) alleles are associated with high levels of lymphocyte proliferation to rubella virus, and B*1302 (p = 0.02), B*3701 (p = 0.01), and B*3801 (p = 0.007) alleles are associated with high levels of lymphoproliferation to mumps virus following two doses of MMR vaccine (17,18). Important associations of class I alleles with cytokine responses to rubella vaccine were also discovered. Differences in rubella-specific immune response among common HLA alleles were assessed simultaneously via a global test to collectively determine whether at least one HLA allele in each gene locus was associated with a modified immune response to rubella vaccine. The relationships between alleles of the HLA-B (*3901, *4001, *4102, *4403) (global p value = 0.03) and HLA-C (*0303, *1601, *1703) (global p value = 0.02) loci, and rubella-specific TNF-α production point to the potential significance of HLA class I molecules in the inflammatory immune response (19).
The role of HLA class II genes in immunity to vaccines is also crucial. In a cohort of 346 healthy children, DQB1*0201 (p = 0.04), DQB1*0402 (p = 0.02), DQA1*0401 (p = 0.003), DRB1*0301 (p = 0.02), DRB1*0801 (p = 0.02), DRB1*1201 (p = 0.04), and DRB1*1302 (p = 0.01) alleles were significantly associated with low lymphoproliferative responses to mumps vaccine (18). Interestingly, subjects who carried the DRB1*03-DQB1*02-DPB1*04 haplotype had high lymphoproliferative responses to both measles (p = 0.01) and mumps viruses (p = 0.006) (15). A strong association was found between many alleles of the HLA-DQA1 (*0103, *0301, *0303) (global p value = 0.02) and HLA-DQB1 (*0202, *0302, *0603) (global p value = 0.007) loci and inter-individual variations in rubella virus-induced IL-2 secretion (19). Consistent associations were found with rubella-induced antibody levels that confirmed our earlier reported HLA associations in replication studies we performed (16). Specifically, DPA1*0201 alleles are consistently associated with low levels of rubella-induced antibodies, whereas DPB1*0401 alleles are associated with high-antibody levels in two separate study cohorts (Table I) (16). Furthermore, the relationship of DRB1*04-DQB1*03-DPB1*03 and DRB1*15/16-DQB1*06-DPB1*03 haplotypes with low levels of rubella virus-specific antibodies were found in these two separate studies (16). Future studies designed to perform definitive mechanistic experiments that might allow attribution of direct “cause and effect” of HLA genotype on phenotype are currently in progress.
Immune responses after rubella vaccine are also modulated by genes in the class III region in extended haplotypes. Using a method that accounts for linkage-phase ambiguity by an expectation maximization algorithm (20), we found an association involving haplotypes expanding across the HLA class I region, ten SNP haplotypes (LTA-TNF-LST1), and the HLA class II region and rubella-specific antibodies (global p value = 0.03). An example of a specific haplotype associated with high levels of humoral immune response to rubella is the A*02-C*03-B*15-AAAACGGGGC-DRB1*04-DQA1*03-DQB1*03-DPA1*01-DPB1*04 haplotype (p = 0.002) (21). Such population-based gene association studies, and the insights they provide, are critical to identifying and solving the problem of low and/or waning immunity and in advancing the science by pursuing the immunogenetic basis for variation among vaccinated individuals (22,23).
Non-HLA Genetic Polymorphisms and Vaccine Response
A significant observation that comes from many of the HLA association studies linked to vaccines suggests that non-HLA genes also contribute to the heterogeneity of vaccine-induced immunity. Hohler et al. found that approximately 40% of the genetic contribution to the hepatitis B (HBsAg) vaccine antibody response is related to HLA genes (24). Interestingly, the heritability of the HBsAg vaccine response attributed to the HLA-DRB1 locus was projected to be 25%, leaving the remaining heritability of 36% to other genetic loci. Our recent study has shown that inter-individual variation in the HLA genes accounts for ~20% of the overall genetic variation in rubella vaccine-induced antibodies (16). Guided by this understanding, we conducted a genotyping study in 738 children to determine associations between candidate SNPs and haplotypes and immune measures following two doses of rubella vaccine (25,29,32,36). Our data suggest that the variability of both rubella vaccine-induced humoral and cytokine responses is significantly modulated by cytokine and cytokine receptor genetic variants. For example, an increased representation of minor alleles for two promoter SNPs (rs2844482, p = 0.0002 and rs2857708, p = 0.001) of the TNFA gene were associated with two-fold increases in rubella-specific IgG levels (25). Furthermore, IL-6 production was associated (p ≤ 0.01) with intronic SNPs (rs5745993, rs17882988, rs472093, rs5746059, and rs590977) in the TNFRSF1B gene, while several promoter and intronic SNPs in the IL12B gene were significantly associated (p < 0.001) with higher IL-6 production after rubella vaccination. Individual SNPs and haplotypes in the IL12B and TNFA/TNFRSF1B genes were associated with immunity to rubella vaccine. Specifically, the TNFA haplotype AAACGGGGC and IL12B promoter haplotype TAG were associated with high rubella-specific IgG (p < 0.001) and IL-6 secretion (p = 0.008), respectively. A common haplotype in the TNFRSF1B gene was associated with low (p < 0.001) IL-6 secretion (25). Thus, cytokines play an essential role in the modulation of immune responses and cytokine production is influenced by the rate of transcription of their cytokine and cytokine receptor genes. As an example, SNPs in these cytokine genes can affect mRNA splicing, stability, and structure of RNA molecules, or protein folding (26). Understanding of such polymorphisms may assist in designing new vaccines that include cytokines to “replace” those not produced natively and reinstate an optimal Th1/Th2/T17 cell equilibrium that would provide protective immunity after vaccination (27,28).
Innate Gene Polymorphisms and Vaccine Immunity
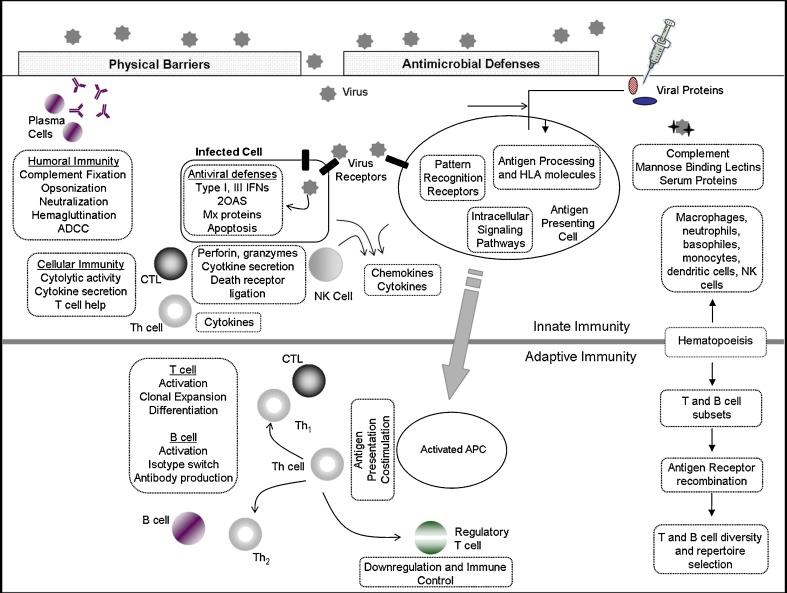
The immune system comprises a complex set of signaling pathways that function not as discrete parts, but rather as a network of interrelated pathways that have dynamic additive, subtractive, synergistic, and regulatory network effects on one another and combine to form an integrated response that we have termed “the immune response network theory”(2) (Fig. 1). As an example, the role of polymorphisms in the Toll-like receptor (TLR), vitamin A and D receptors, antiviral effector, and other innate immune response (IR) genes in vaccine-induced immunity is of growing importance and recognition. We studied the association of polymorphisms in these candidate innate IR genes to rubella vaccine-induced humoral and cellular immune responses. Overall, eight significant SNP associations (p < 0.05) were found between the vitamin A (RARB), RIG-I, and TRIM (TRIM 5 and TRIM 22) genes and humoral immunity after rubella vaccine (29). Our association study determined that the non-synonymous SNP rs3740996 in the TRIM5 gene was associated with rubella-specific antibody response (p = 0.01). This specific SNP was found to have an important functional role (29–31). This same study also found 22 important associations (range of p values 0.002–0.05) between genetic variants in the vitamin A receptor family (RARA, RARB, and RARG), vitamin D receptor, RXRA genes, and rubella-specific (IFN-γ, IL-2, IL-10, TNF-α, and GM-CSF) cytokine responses (32). Additional studies are necessary to replicate and validate these findings.
Fig. 1.
Infection with a viral pathogen or immunization with a viral vaccine stimulates a cascading network of integrated immune pathways. Non-specific innate responses activated by pattern recognition receptors serve to elicit IFN responses and to activate antigen presenting cells in order to properly initiate adaptive immunity. Type I and III IFNs initiate various antiviral proteins and responses such as: 2OAS, MX proteins and PKR, which inhibit viral replication in infected cells and render uninfected cells resistant to viral infection. Complement and mannose-binding lectins opsonize and neutralize viral particles, which are cleared by phagocytic cells such as macrophages. Pro-inflammatory cytokines and chemokine secretion attracts effector leukocytes into infected tissues. Neutrophils and eosinophils infiltrate inflamed tissues and destroy infected cells through the release of toxic granule contents including: DNases, RNases, lytic enzymes, antimicrobial peptides, and reactive oxygen/nitrogen species. Innate immunity responds to infection immediately and serves to halt or delay viral replication and to initiate robust, antigen-specific adaptive responses. Activated antigen presenting cells acquire antigen, upregulate co-stimulatory molecules and cytokine secretion and traffic to the neighboring lymph nodes, where viral antigens are presented to T cells. T-helper cells differentiate into Th1 cells supplying IL-2, IFN-γ, and other cytokines to support CTL activation, clonal expansion, and lytic activity, Th2 cells which supply necessary cytokines (IL-4, IL-5) and co-stimulatory signals (CD40L) for B-cell maturation, replication, and isotype switching, and regulatory T cells, which downregulate immune responses and allow a return to homeostasis. B cells differentiate into plasma cells and secrete antibodies, which agglutinate, opsonize, and neutralize viral particles, fix complement, and allow for antibody dependent cell cytotoxicity (ADCC). Activated CD8+ T cells lyse infected cells through perforin, granzymes and through death receptors such as FasL. Cytokine secretion (IFN-γ, TNF-α) by T lymphocytes can also have direct antiviral activity. Together, these humoral and adaptive responses halt viral replication, lyse-infected cells and remove viral particles from the host. Virus-specific lymphocyte numbers then contract to a small, long-lived memory population capable of rapidly responding to subsequent infection with a viral pathogen
TLRs are pattern recognition receptors that can contribute to viral detection by sensing RNA and viral proteins, leading to induction of cytokines and interferon response (33). In our study, a TLR3 gene SNP (rs5743305, −8441A > T) was found to be associated with rubella-specific GM-CSF production (32). SNPs (rs3740996 and rs10838525) in the innate TRIM5 gene coding regions were associated with rubella-specific TNF-α and IL-2/GM-CSF, respectively. These two SNPs have been previously demonstrated to have functional roles in regard to antiviral activity (30,31,34,35). Furthermore, we found 23 associations (p < 0.05) between genetic variants within the 2′-5′-oligoadenylate synthetase (OAS) gene, and rubella-induced IL-2, IL-10, IL-6 secretion and antibody levels (36). Three OAS1 gene SNPs (rs3741981/Ser162Gly, rs1051042/Thr361Arg, rs2660), located in an LD or haplotype block of functional significance were associated with an increase in rubella-specific IL-2 production (p < 0.02) (36). Thus, the immune response to rubella vaccination is restricted by more than just HLA-specific polymorphisms, and additional genes that influence vaccine response should be identified and studied. These non-HLA genes (TLRs, RIG-I, TRIM, and others) are often targets of novel and effective adjuvants for subunit vaccines. For example, the MPL adjuvant signals through TLR4 and has been tested in multiple vaccines (37). Synthetic lipopeptides, polyinosinic–polycytidylic acid [poly(I/C)] and CpG oligodeoxynucleotides, that signal through TLR2, TLR3, and TLR9, respectively, have also been evaluated as adjuvants to stimulate innate immunity (38–40). In this regard, specific human polymorphisms in innate immune sensor genes may potentially limit the efficacy of such vaccine adjuvants.
Genome-Wide Association Studies and Next-Generation Sequencing
The enhancement in SNP genotyping methodologies and progress in the human genome sequence project (41) have facilitated genome-wide SNP association and linkage studies for identification of novel genetic associations between SNPs and variation in markers of immunity to pathogens (42). Remarkably, such studies are rare and population-based studies identifying the genetic and functional basis for many viral vaccine-induced immune response variations have not yet been performed. This includes validating and replicating genotype–phenotype association data in independent cohorts, whether obtained from candidate, genome-wide association, or genome-wide expression microarray studies (43,44). For example, using a system biology approach, Querec et al. analyzed human peripheral blood mononuclear cells following primary vaccination with YF-17D yellow fever vaccine (45), to predict the resulting adaptive immune response. Similar measles, mumps, rubella, influenza, and smallpox vaccine GWA, microarray and next-generation sequencing (NGS/mRNA-Seq) studies are underway in our laboratory. On the other hand, GWAS platforms do not interrogate all common genetic variants and will not allow detection of rare variants. Utilization of state-of-the-art technology, such as NGS, is needed to provide new information, such as rare polymorphisms, alternative splice variants, copy number variants, and their relationship to immune response (2,46). NGS can also be useful to whole genome, fine-mapping genetic regions of importance or deep profiling and targeted sequencing of genes of interest (2,46). Combining immunogenetic and NGS data will lead to a new understanding of the fundamental mechanisms that determine the immunogenetic drivers of the immune response to vaccination. Though generating large amounts of sequence data will create a bioinformatics challenge for the scientific community, this new technology opens a new opportunity for future vaccine studies and provides a foundation for NGS-based vaccinology.
New Vaccine Development
Novel vaccine development, including the concept of personalized vaccines (3), is naturally a multi-step process, and the field of vaccinomics is at the beginning of this process. We and others have identified significant associations between inter-individual variations in vaccine-induced responses and genetic variants in immune response and innate genes. Our research demonstrates that humoral and cellular immunity to vaccines is the result of multigenic influences, signifying that host polymorphisms play a critical role in determining responses to vaccines (15,21,47,48). Information on these associations may aid in designing vaccines that avoid immunogenetic restrictions. Peptide vaccine studies in animal models offer evidence of this theory (49–52). As stated by Spielberg (53), “Just as pharmacogenetics has suggested ways of designing drugs to minimize population variability, understanding mechanisms of immunogenetic variation may lead to new vaccines designed specifically to minimize immunogenetically based vaccine failure.” This concept encapsulates the thrust of our immunogenetics work and its relationship to the field of vaccinomics and vaccine development. In a recent review, Vandenbroek and Goris (54) point out that “cytokine gene polymorphisms may be gateways to novel targets for immunotherapy.” Jin and Wang (26) state, “the lesson learned from HLA is that polymorphism can occur preferentially in the functional domains of a given molecule with dramatic effects on epitope selection and presentation.” As previously mentioned, we synthesized these insights into the concept of the “immune response network theory” (9,22) stating that immunity to a vaccine is driven by the complex interactions of individual and groups of genes and epigenetic interactions, and therefore can be determined and, in the future, predicted (3,55).
As a specific example of a directed vaccine development pathway informed by vaccinomics, we developed a mass spectrometry approach to identifying naturally processed vaccinia and measles-derived peptides isolated from specific HLA class I (A*0201, B*1501, and C*03) and class II (DRB1*0301) molecules associated with smallpox vaccine- and measles vaccine-induced immune responses, respectively (56,57). Using mass spectrometry, we identified 13 immunogenic measles virus-derived DRB1*0301-bound peptides that may be potentially used in generating a new measles vaccine formulation, and these peptide epitopes were chosen to avoid our previously identified HLA polymorphic restrictions (57–59). By identifying such genetically restricted peptides, and using information regarding peptide/HLA promiscuity, including HLA supertypes, new multi-peptide vaccines administered with specific adjuvants could be developed and tested. As another example of these ideas, HLA and cytokine gene polymorphisms were demonstrated to be associated with responses to recombinant hepatitis B vaccine (HBV), including antibody non-response (60). Designing a novel HBV vaccine that consists of peptides with adjuvants (for example, GM-CSF) could avoid these immunogenetic restrictions (61,62). Likewise, our studies have found a non-synonymous SNP (rs3796504) in the SLAM receptor gene associated with a 70% decline in measles-specific IgG levels (63). Possibly, this SNP impacts the capacity of measles virus to attach to its cellular receptor, and hence prevents generation of a protective immune response. Vaccine immunogenicity could be improved by a vaccine virus genetically engineered with binding specificities that allow attachment in the presence and absence of such a cellular receptor genetic variant. Thus, vaccinomics serves to identify mechanisms and pathways that direct immune responses, and provide new vaccine approaches. While additional studies are required to further explore and better understand genetic determinants of immune response to vaccines, it is clear that vaccinomics will bring new scientific insights and approaches to the field of vaccinology. While just at the beginning of this scientific revolution, we believe this approach will drive a “second golden era” of vaccine development (1).
Acknowledgments
We thank our subjects and their parents who participated in our studies and the Mayo Clinic Vaccine Research Group staff. We thank Richard B. Kennedy for help in preparing the figure. This work was supported by NIH grants AI 48793, AI 33144 and 1 UL1 RR024150-01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health, and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Disclosure
Dr. Poland is the chair of a Safety Evaluation Committee for an investigational vaccine trials being conducted by Merck Research Laboratories. Dr. Poland offers consultative advice on new vaccine development to Merck & Co., Inc., Avianax, Theraclone Sciences (formally Spaltudaq Corporation), MedImmune LLC, Liquidia Technologies, Inc., Emergent BioSolutions, Novavax, Dynavax, EMD Serono, Inc., Novartis Vaccines and Therapeutics and PAXVAX, Inc.
References
- 1.Poland GA. Pharmacology, vaccinomics, and the second golden age of vaccinology. Clin Pharmacol Ther. 2007;82(6):623–626. doi: 10.1038/sj.clpt.6100379. [DOI] [PubMed] [Google Scholar]
- 2.Poland GA, Ovsyannikova IG, Jacobson RM, Smith DI. Heterogeneity in vaccine immune response: the role of immunogenetics and the emerging field of vaccinomics. Clin Pharmacol Ther. 2007;82(6):653–664. doi: 10.1038/sj.clpt.6100415. [DOI] [PubMed] [Google Scholar]
- 3.Poland GA, Ovsyannikova IG, Jacobson RM. Personalized vaccines: the emerging field of vaccinomics. Expert Opin Biol Ther. 2008;8(11):1659–1667. doi: 10.1517/14712598.8.11.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marchant A, Pihlgren M, Goetghebuer T, Weiss HA, Ota MO, Schlegel-Hauter SE, et al. Predominant influence of environmental determinants on the persistence and avidity maturation of antibody responses to vaccines in infants. J Infect Dis. 2006;193(11):1598–1605. doi: 10.1086/503775. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson RM, Ovsyannikova IG, Targonski PV, Poland GA. Studies of twins in vaccinology. Vaccine. 2007;25(16):3160–3164. doi: 10.1016/j.vaccine.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 6.Tan PL, Jacobson RM, Poland GA, Jacobsen SJ, Pankratz SV. Twin studies of immunogenicity—determining the genetic contribution to vaccine failure. Vaccine. 2001;19:2434–2439. doi: 10.1016/S0264-410X(00)00468-0. [DOI] [PubMed] [Google Scholar]
- 7.Newport MJ, Goetghebuer T, Weiss HA. The MRC Gambia Twin Study Group, Whittle H, Siegrist C-A, et al. Genetic regulation of immune responses to vaccines in early life. Genes Immun. 2004;5(2):122–129. doi: 10.1038/sj.gene.6364051. [DOI] [PubMed] [Google Scholar]
- 8.Poland GA, Jacobson RM, Ovsyannikova IG. Trends affecting the future of vaccine development and delivery: the role of demographics, regulatory science, the anti-vaccine and consumer culture and vaccinomics. Vaccine. 2009;27(25–26):3240–3244. doi: 10.1016/j.vaccine.2009.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poland GA, Ovsyannikova IG, Jacobson RM. Application of pharmacogenomics to vaccines. Pharmacogenomics. 2009;10(5):837–852. doi: 10.2217/pgs.09.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hohler T, Gerken G, Notghi A, Lubjuhn R, Taheri H, Protzer U, et al. HLA-DRB1*1301 and *1302 protect against chronic hepatitis B. J Hepatol. 1997;26(3):503–507. doi: 10.1016/S0168-8278(97)80414-X. [DOI] [PubMed] [Google Scholar]
- 11.Gelder CM, Lambkin R, Hart KW, Fleming D, Williams OM, Bunce M, et al. Associations between human leukocyte antigens and nonresponsiveness to influenza vaccine. J Infect Dis. 2002;185:114–117. doi: 10.1086/338014. [DOI] [PubMed] [Google Scholar]
- 12.Ovsyannikova IG, Jacobson RM, Vierkant RA, Pankratz SV, Jacobsen SJ, Poland GA. Associations between human leukocyte antigen (HLA) alleles and very high levels of measles antibody following vaccination. Vaccine. 2004;22:1914–1920. doi: 10.1016/j.vaccine.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 13.Poland GA, Ovsyannikova IG, Jacobson RM. Immunogenetics of seasonal influenza vaccine response. Vaccine. 2008;26S:D35–D40. doi: 10.1016/j.vaccine.2008.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobson RM, Poland GA, Vierkant RA, Pankratz VS, Schaid DJ, Jacobsen SJ, et al. The association of class I HLA alleles and antibody levels following a single dose of measles vaccine. Hum Immunol. 2003;64(1):103–109. doi: 10.1016/S0198-8859(02)00741-3. [DOI] [PubMed] [Google Scholar]
- 15.Ovsyannikova IG, Pankratz VS, Vierkant RA, Jacobson RM, Poland GA. Human leukocyte antigen haplotypes in the genetic control of immune response to measles-mumps-rubella vaccine. J Infect Dis. 2006;193(5):655–663. doi: 10.1086/500144. [DOI] [PubMed] [Google Scholar]
- 16.Ovsyannikova IG, Jacobson RM, Vierkant RA, O'Byrne MM, Poland GA. Replication of rubella vaccine population genetic studies: validation of HLA genotype and humoral response associations. Vaccine. 2009;27(49):6926–6931. doi: 10.1016/j.vaccine.2009.08.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ovsyannikova IG, Jacobson RM, Vierkant RA, Jacobsen SJ, Pankratz VS, Poland GA. The contribution of HLA class I antigens in immune status following two doses of rubella vaccination. Hum Immunol. 2004;65:1506–1515. doi: 10.1016/j.humimm.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Ovsyannikova IG, Jacobson RM, Dhiman N, Vierkant RA, Pankratz VS, Poland GA. Human leukocyte antigen and cytokine receptor gene polymorphisms associated with heterogeneous immune responses to mumps viral vaccine. Pediatrics. 2008;121:e1091–e1099. doi: 10.1542/peds.2007-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ovsyannikova IG, Ryan JE, Vierkant RA, O'Byrne MM, Jacobson RM, Poland GA. Influence of host genetic variation on rubella-specific T cell cytokine responses following rubella vaccination. Vaccine. 2009;27:3359–3366. doi: 10.1016/j.vaccine.2009.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaid DJ, Rowland CM, Tines DE, Jacobson RM, Poland GA. Score tests for association between traits and haplotypes when linkage phase is ambiguous. Am J Hum Genet. 2002;70:425–434. doi: 10.1086/338688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ovsyannikova IG, Vierkant RA, Pankratz VS, Jacobson RM, Poland GA. Extended LTA, TNF, LST1 and HLA gene haplotypes and their association with rubella vaccine-induced immunity. PLoS ONE. 2010;5(7):e11806. doi: 10.1371/journal.pone.0011806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poland GA, Ovsyannikova IG, Jacobson RM. Vaccine immunogenetics: bedside to bench to population. Vaccine. 2008;26:6183–6188. doi: 10.1016/j.vaccine.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poland GA, Ovsyannikova IG, Jacobson RM. Genetics and immune response to vaccines. In: Kaslow RA, McNicholl JM, Hill AVS, editors. Genetic susceptibility to infectious diseases. New York, New York: Oxford University Press; 2008. pp. 414–429. [Google Scholar]
- 24.Hohler T, Reuss E, Evers N, Dietrich E, Rittner C, Freitag CM, et al. Differential genetic determination of immune responsiveness to hepatitis B surface antigen and to hepatitis A virus: a vaccination study in twins. Lancet. 2002;360(9338):991–995. doi: 10.1016/S0140-6736(02)11083-X. [DOI] [PubMed] [Google Scholar]
- 25.Dhiman N, Haralambieva IH, Kennedy RB, Vierkant RA, O'Byrne MM, Ovsyannikova IG, et al. Assesment of SNP/haplotype associations in cytokine and cytokine receptor genes and immunity to rubella vaccination. Immunogenetics. 2010;62(4):197–210. doi: 10.1007/s00251-010-0423-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin P, Wang E. Polymorphism in clinical immunology—from HLA typing to immunogenetic profiling. J Transl Med. 2003;1(1):8. doi: 10.1186/1479-5876-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perera LP, Waldmann TA, Mosca JD, Baldwin N, Berzofsky JA, Oh SK. Development of smallpox vaccine candidates with integrated IL-15 that demonstrate superior immunogenicity, efficacy and safety in mice. J Virol. 2007;81(16):8774–8783. doi: 10.1128/JVI.00538-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keen LJ. The extent and analysis of cytokine and cytokine receptor gene polymorphism. Transpl Immunol. 2002;10(2–3):143–146. doi: 10.1016/S0966-3274(02)00061-8. [DOI] [PubMed] [Google Scholar]
- 29.Ovsyannikova IG, Haralambieva IH, Dhiman N, O'Byrne MM, Pankratz VS, Jacobson RM, et al. Polymorphisms in the vitamin A receptor and innate immunity genes influence the antibody response to rubella vaccination. J Infect Dis. 2010;201(2):207–213. doi: 10.1086/649588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sawyer SL, Wu LI, Akey JM, Emerman M, Malik HS. High-frequency persistence of an impaired allele of the retroviral defense gene TRIM5alpha in humans. Curr Biol. 2006;16(1):95–100. doi: 10.1016/j.cub.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 31.van Manen D, Rits MA, Beugeling C, van Dort K, Schuitemaker H, Kootstra NA. The effect of Trim5 polymorphisms on the clinical course of HIV-1 infection. PLoS Pathog. 2008;4(2):e18. doi: 10.1371/journal.ppat.0040018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ovsyannikova IG, Dhiman N, Haralambieva IH, Vierkant RA, O'Byrne MM, Jacobson RM, et al. Rubella vaccine-induced cellular immunity: evidence of associations with polymorphisms in the Toll-like, vitamin A and D receptors, and innate immune response genes. Hum Genet. 2010;127:207–221. doi: 10.1007/s00439-009-0763-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowie AG, Haga IR. The role of Toll-like receptors in the host response to viruses. Mol Immunol. 2005;42(8):859–867. doi: 10.1016/j.molimm.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Javanbakht H, An P, Gold B, Petersen DC, O'Huigin C, Nelson GW, et al. Effects of human TRIM5alpha polymorphisms on antiretroviral function and susceptibility to human immunodeficiency virus infection. Virology. 2006;354(1):15–27. doi: 10.1016/j.virol.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 35.Goldschmidt V, Bleiber G, May M, Martinez R, Ortiz M, Telenti A. Role of common human TRIM5alpha variants in HIV-1 disease progression. Retrovirology. 2006;3:54. doi: 10.1186/1742-4690-3-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haralambieva IH, Dhiman N, Ovsyannikova IG, Vierkant RA, Pankratz VS, Jacobson RM, et al. 2′-5′-Oligoadenylate synthetase single-nucleotide polymorphisms and haplotypes are associated with variations in immune responses to rubella vaccine. Hum Immunol. 2010;71(4):383–391. doi: 10.1016/j.humimm.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316(5831):1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 38.Lau YF, Tang LH, Ooi EE. A TLR3 ligand that exhibits potent inhibition of influenza virus replication and has strong adjuvant activity has the potential for dual applications in an influenza pandemic. Vaccine. 2009;27(9):1354–1364. doi: 10.1016/j.vaccine.2008.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cooper CL, Davis HL, Morris ML, Efler SM, Adhami MA, Krieg AM, et al. CPG 7909, an immunostimulatory TLR9 agonist oligodeoxynucleotide, as adjuvant to Engerix-B HBV vaccine in healthy adults: a double-blind phase I/II study. J Clin Immunol. 2004;24(6):693–701. doi: 10.1007/s10875-004-6244-3. [DOI] [PubMed] [Google Scholar]
- 40.Belyakov IM, Isakov D, Zhu Q, Dzutsev A, Klinman D, Berzofsky JA. Enhancement of CD8+ T cell immunity in the lung by CpG oligodeoxynucleotides increases protective efficacy of a modified vaccinia Ankara vaccine against lethal poxvirus infection even in a CD4-deficient host. J Immunol. 2006;177(9):6336–6343. doi: 10.4049/jimmunol.177.9.6336. [DOI] [PubMed] [Google Scholar]
- 41.Manolio TA, Brooks LD, Collins FS. A HapMap harvest of insights into the genetics of common disease. J Clin Invest. 2008;118(5):1590–1605. doi: 10.1172/JCI34772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirschhorn JN, Daly MJ. Genome-wide association studies for common diseases and complex traits. Genetics. 2005;6:95–108. doi: 10.1038/nrg1521. [DOI] [PubMed] [Google Scholar]
- 43.Igl BW, Konig IR, Ziegler A. What do we mean by ‘replication’ and ‘validation’ in genome-wide association studies? Hum Hered. 2009;67(1):66–68. doi: 10.1159/000164400. [DOI] [PubMed] [Google Scholar]
- 44.Chanock SJ, Manolio T, Boehnke M, Boerwinkle E, Hunter DJ, Thomas G, et al. Replicating genotype-phenotype associations. Nature. 2007;447(7145):655–660. doi: 10.1038/447655a. [DOI] [PubMed] [Google Scholar]
- 45.Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D, et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10(1):116–125. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dhiman N, Smith DI, Poland GA. Next-generation sequencing: a transformative tool for vaccinology. Expert Rev Vaccine. 2009;8(8):963–967. doi: 10.1586/erv.09.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ovsyannikova IG, Jacobson RM, Vierkant RA, Pankratz VS, Poland GA. HLA supertypes and immune responses to measles–mumps–rubella viral vaccine: findings and implications for vaccine design. Vaccine. 2007;25(16):3090–3100. doi: 10.1016/j.vaccine.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 48.Ovsyannikova IG, Vierkant RA, Pankratz VS, O'Byrne MM, Jacobson RM, Poland GA. HLA haplotype and supertype associations with cellular immune responses and cytokine production in healthy children after rubella vaccine. Vaccine. 2009;27:3349–3358. doi: 10.1016/j.vaccine.2009.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sinha P, Snyder JA, Kim EY, Moudgil KD. The major histocompatibility complex haplotypes dictate and the background genes fine-tune the dominant versus the cryptic response profile of a T-cell determinant within a native antigen: relevance to disease susceptibility and vaccination. Scand J Immunol. 2007;65(2):158–165. doi: 10.1111/j.1365-3083.2006.01891.x. [DOI] [PubMed] [Google Scholar]
- 50.Halassy B, Mateljak S, Bouche FB, Putz MM, Muller CP, Frkanec R, et al. Immunogenicity of peptides of measles virus origin and influence of adjuvants. Vaccine. 2006;24(2):185–194. doi: 10.1016/j.vaccine.2005.07.057. [DOI] [PubMed] [Google Scholar]
- 51.Putz MM, Ammerlaan W, Schneider F, Jung G, Muller CP. Humoral immune responses to a protective peptide-conjugate against measles after different prime-boost regimens. Vaccine. 2004;22(31–32):4173–4182. doi: 10.1016/j.vaccine.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 52.Pasquetto V, Bui HH, Giannino R, Mirza F, Sidney J, Oseroff C, et al. HLA-A*0201, HLA-A*1101, and HLA-B*0702 Transgenic mice recognize numerous poxvirus determinants from a wide variety of viral gene products. J Immunol. 2005;175(8):5504–5515. doi: 10.4049/jimmunol.175.8.5504. [DOI] [PubMed] [Google Scholar]
- 53.Spielberg SP. Therapeutics and toxicology. Curr Opin Pediatr. 1998;10:201–202. doi: 10.1097/00008480-199804000-00015. [DOI] [PubMed] [Google Scholar]
- 54.Vandenbroeck K, Goris A. Cytokine gene polymorphisms in multifactorial diseases: gateways to novel targets for immunotherapy? Trends Pharmacol Sci. 2003;24(6):284–289. doi: 10.1016/S0165-6147(03)00131-7. [DOI] [PubMed] [Google Scholar]
- 55.Kaslow RA, McNicholl JM, Hill AVS. Genetic susceptibility to infectious diseases. New York: Oxford University Press; 2008. [Google Scholar]
- 56.Johnson KL, Ovsyannikova IG, Mason CJ, Bergen HR, III, Poland GA. Discovery of naturally processed and HLA-presented class I peptides from vaccinia virus infection using mass spectrometry for vaccine development. Vaccine. 2009;28(1):38–47. doi: 10.1016/j.vaccine.2009.09.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson KL, Ovsyannikova IG, Poland G, Muddiman DC. Identification of class II HLA-DRB1*03-bound measles virus peptides by 2D-liquid chromatography tandem mass spectrometry. J Proteome Res. 2005;4:2243–2249. doi: 10.1021/pr0501416. [DOI] [PubMed] [Google Scholar]
- 58.Ovsyannikova IG, Johnson KL, Naylor S, Muddiman DC, Poland GA. Naturally processed measles virus peptide eluted from class II HLA-DRB1*03 recognized by T lymphocytes from human blood. Virology. 2003;312(2):495–506. doi: 10.1016/S0042-6822(03)00281-2. [DOI] [PubMed] [Google Scholar]
- 59.Ovsyannikova IG, Johnson KL, Muddiman DC, Vierkant RA, Poland GA. Identification and characterization of novel, naturally processed measles virus class II HLA-DRB1 peptides. J Virol. 2004;78(1):42–51. doi: 10.1128/JVI.78.1.42-51.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang C, Tang J, Song W, Lobashevsky E, Wilson CM, Kaslow RA. HLA and cytokine gene polymorphisms are independently associated with responses to hepatitis B vaccination. Hepatology. 2004;39(4):978–988. doi: 10.1002/hep.20142. [DOI] [PubMed] [Google Scholar]
- 61.Kim M-J, Nafziger AN, Harro CD, Keyserling HL, Ramsey KM, Drusano GL, et al. Revaccination of healthy nonresponders with hepatitis B vaccine and prediction of seroprotection response. Vaccine. 2003;21(11–12):1174–1179. doi: 10.1016/S0264-410X(02)00626-6. [DOI] [PubMed] [Google Scholar]
- 62.Lee HG, Lim J-S, Lee K-Y, Choi Y-K, Choe I-S, Chung T-W, et al. Peptide-specific CTL induction in HBV-seropositive PBMC by stimulation with peptides in vitro: novel epitopes identified from chronic carriers. Virus Res. 1997;50:185–194. doi: 10.1016/S0168-1702(97)00068-3. [DOI] [PubMed] [Google Scholar]
- 63.Dhiman N, Cunningham JM, Jacobson RM, Vierkant RA, Wu Y, Ovsyannikova IG, et al. Variations in measles vaccine-specific humoral immunity by polymorphisms in SLAM and CD46 measles virus receptors. J Allergy Clin Immunol. 2007;120(3):666–672. doi: 10.1016/j.jaci.2007.04.036. [DOI] [PubMed] [Google Scholar]