Abstract
Background
A small group of children have second and even more cerebrospinal fluid (CSF) shunt infections (SI). We sought to describe the treatment approaches used for, and the microbiology of, repeated shunt infections.
Methods
The study population included 31 children with second shunt infection (SI-2) among those undergoing initial CSF shunt placement and treatment for initial infection at Primary Children’s Medical Center. CSF shunt infection was defined as: 1) presence of bacteria in Gram stain and/or culture of CSF, wound, and/or pseudocyst; 2) visible hardware; or 3) abdominal pseudocyst; or (4) presence of bacteria in a blood culture in children with a ventriculoatrial shunt. Infection rates were generated using per-patient denominators, and the concordance of organisms across infections was summarized.
Results
Of the 31 children with SI-2, most were less than 6 months of age at initial shunt placement (81%), male (77%), and with ventriculoperitoneal shunts (71%). Eighteen developed SI-3 and 8 developed SI-4. Infection rates were 60% (95% confidence interval (CI): 42–75%, n=18/30) for SI-3 and 47% (95% CI: 26–69%, n=8/17) for SI-4. The median time to SI-3 was 477 days (range 5–828) and to SI-4 was 2137 days (range 9–2137). Gram-positive organisms predominated (93% of SI-2, 94% of SI-3). The majority of second shunt infections demonstrated Gram-stain concordance with both the initial (first) shunt infection (58%, 95% CI: 41–74%) and with the following (third) shunt infection (78%, 95% CI: 55–91%).
Conclusions
Children with second shunt infection experience high subsequent re-infection rates with a long time to re-infection.
Keywords: shunt, infection, children
Introduction
Infection is frequent in children with hydrocephalus treated by cerebrospinal fluid (CSF) shunt placement, with reported per-patient infections rates ranging from 6.5 to 23.5%[1–7] and occurring in approximately 11% of children within 24 months of uncomplicated initial CSF shunt placement. [8] Most shunt infections (SI) occur within one month of CSF shunt procedures. [9] A high percentage of pathogens originate from skin and the enteric system and include Staphylococcus epidermidis, Staphylococcus aureus, and Gram-negative bacilli. [10, 11] Thus, the prevailing hypothesis for CSF shunt infection is that the infecting organism is introduced during surgical manipulation of the site.
A subset of children goes on to experience multiple CSF shunt infections. The relationship between initial and subsequent CSF shunt infection is poorly understood. Some studies have distinguished between a second or subsequent infection if the isolated organism differs from that of the prior infection (re-infection), and an incompletely treated infection if the isolated organism was of the same genus and species and occurred within one month of completion of therapy from the prior infection (relapse). [10] However, this categorization has not been consistently adopted in the literature.
The two largest cohorts of children with multiple shunt infections published in the literature to date have described 18 and 10 children, respectively, with re-infection rates of 26% and 24%, respectively. [12, 13] Investigators have noted considerable variation in treatment duration and methods of medical and surgical management of shunt infections that might explain re-infections. [9, 10, 14, 15] Furthermore, while some studies have differentiated between first and second infections, [13] none has examined second and subsequent CSF shunt infections.
Using a dataset of children who underwent initial CSF shunt placement and initial CSF shunt infection at a large, tertiary-care, academic children’s hospital, we recognized an opportunity to assemble the largest population of children with multiple CSF shunt reinfections in the literature to date. Our intent in this study was to examine characteristics of second and subsequent CSF shunt infections in children. The aims of this study are to describe (1) treatment approaches used in, and (2) microbiology of, repeated shunt infections, including examining emerging antimicrobial resistance between first and second CSF shunt infections.
Materials and Methods
Conceptual framework
First CSF shunt infection is called SI-1, second CSF shunt infection is called SI-2, and all subsequent CSF shunt infections are numbered in order of occurrence.
Study Population
The study population consists of 31 children who developed second shunt infection (SI-2) after undergoing both initial CSF shunt placement and treatment for initial CSF shunt infection at Primary Children’s Medical Center (PCMC). (Figure 1) Entry into the larger study cohort required a child to undergo initial CSF shunt placement at PCMC between 1/1/1997 and 12/31/2006. Candidates for entry into the cohort were initially identified by a query of the Intermountain Healthcare administrative dataset for an admission with a primary International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) procedure code for placement of an extracranial ventricular shunt (02.3x); chart review was then conducted to ascertain whether children underwent initial CSF shunt placement during these admissions. After review of medical records for 909 candidates, we determined that 579 children had undergone initial CSF shunt placement at PCMC. For each child, chart review of subsequent CSF shunt-related admissions before 12/31/2006 was performed; we determined 122 children subsequently developed initial shunt infection. For each child with initial shunt infection, a query of the administrative dataset for subsequent admissions with an ICD-9-CM diagnosis code 996.63 was performed to ascertain candidates for SI-2 and subsequent infection admissions. Focused chart review of all subsequent admissions, with an emphasis on the candidate infection admissions, generated the study population of 31 children with SI-2.
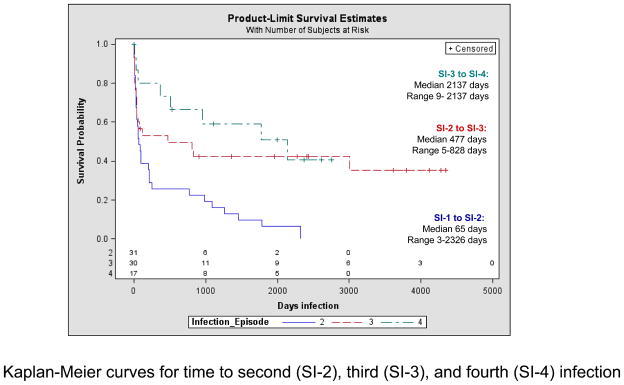
Figure 1.
Kaplan-Meier curves for time to second (SI-2), third (SI-3), and fourth (SI-4) infection
Data Collection
For every child who developed SI-2, we extracted information from the medical record about patient factors, initial shunt placement, and all subsequent infection admissions. Patient factors collected included gender, race/ethnicity, and etiology of hydrocephalus. Initial shunt placement data included age at initial shunt placement, type of shunt placed (including multiple shunts), and numbers of revisions between initial CSF shunt and initial infection.
CSF shunt infections were ascertained by medical record review. While SI-1 episodes occurred before 12/31/06, SI-2 and all subsequent infections were ascertained before 6/28/10. An infection was defined as one or more of the following criteria: 1) presence of bacteria in a Gram stain and/or culture of CSF, wound swab, and/or pseudocyst fluid; or 2) documentation of visible hardware; or 3) abdominal pseudocyst. In children with a ventriculoatrial shunt in place, presence of bacteria in a blood culture was also considered a CSF shunt infection. This definition of infection was arrived at by consensus among pediatric neurosurgeons representing 4 clinical centers in the Hydrocephalus Clinical Research network. [16] It did not include cultures that demonstrated growth in broth only. Each infection episode met above criteria, but also included some duration of antibiotic treatment and two surgical interventions.
Data collected about SI-1 and SI-2 included treatment and culture details. Treatment data included date and time of initial infection surgery, initial surgical approach to infection (i.e., full removal and EVD placement followed by new shunt placement versus shunt externalization), location of initial infection surgery (i.e. bedside versus operating room), date and time of final infection surgery, type of shunt. Culture data included microorganism recovered, Gram-stain (e.g. Gram-positive or Gram-negative), antibiotic susceptibilities, presence and timing of new external ventricular drain (EVD)-associated ventriculitis, presence and timing of bacteremia, date and time of first positive culture, negative CSF cultures 3 days and 7 days prior to final infection surgery, and antibiotic treatment during SI-1 to assess for emergence of antimicrobial resistance at SI-2. Cultures refer to the type of culture that determined the presence of infection, most typically CSF.
For SI-3 and all subsequent infections, we ascertained date of first infection surgery, date and time of first positive culture, microorganism recovered, and Gram-stain. For each child, we also collected the date of last care received within Intermountain Healthcare before 6/28/10 and, where applicable, date of death or shunt removal.
Statistical Analysis
Univariate analysis was performed to describe the study population. All infection rates were calculated using patient, rather than procedure, denominators. For infection rates, we used all children with the infection in the numerator and all susceptible children in the denominator. Given the variable follow-up for the children who remained in the population, we also generated infection-free survival curves for all children with SI-2, SI-3, and SI-4. To generate time to shunt infection, we used the dates of first positive culture for the infection episode and date of final infection surgery for the preceding infection episode. Median time to re-infection for subsequent infections, as well as range, were calculated from Kaplan-Meier curves. We generated 95% confidence intervals (CIs) for these estimates of infection rates using the Wilson score method without continuity correction. [17] Finally, we used univariate analysis to describe the microbiology of repeated shunt infections. SAS 9.2 was used for all analyses and construction of Figures 2, 3, and 4.
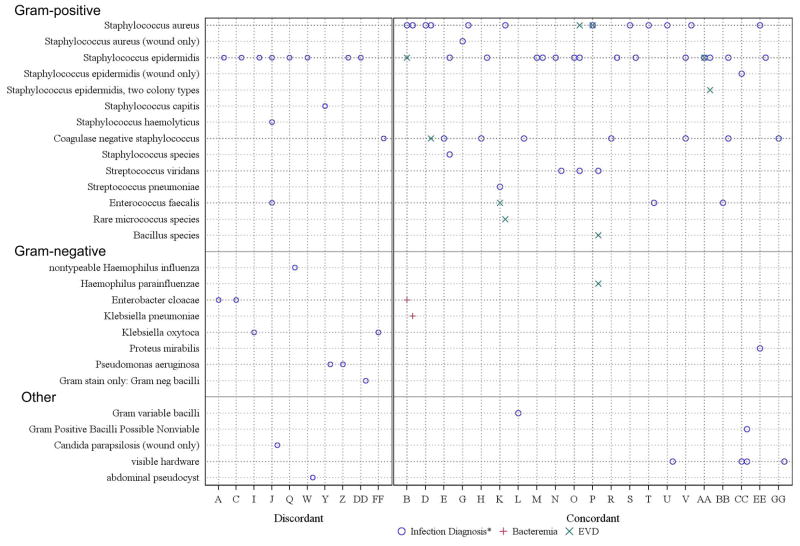
Figure 2.
* diagnosed by CSF culture or blood culture in a ventriculoatrial shunt, and where indicated, visible hardware and abdominal pseudocyst
CSF shunt infection microbiology for each patient at SI-1 and SI-2 (n=31). The x-axis represents each patient. The y-axis represents the organism obtained at the time of infection diagnosis. SI-1 organism(s) are shown on the vertical line for each patient. SI-2 organism(s) are shown slightly to the right of the vertical line for each patient. Organisms associated with infection diagnosis are represented by a blue circle, organisms associated with ventriculitis are represented by a green X, and organisms that caused bacteremia are represented by a red cross. The 10 patients on the left side of the figure demonstrated Gram stain discordance between SI-1 and SI-2, whereas the 21 patients on the right side of the figure demonstrated Gram stain concordance between SI-1 and SI-2.
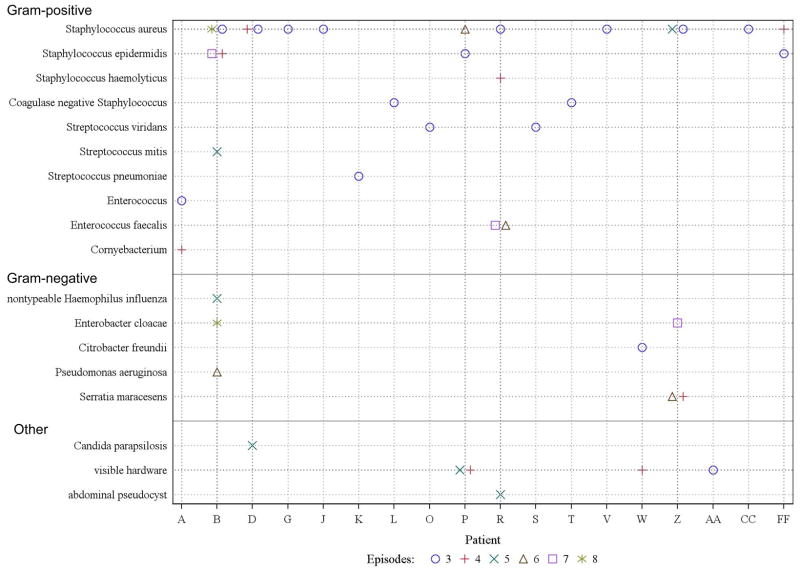
Figure 3.
CSF shunt infection microbiology for patient with SI-3 and subsequent infections (n=18). The x-axis represents each patient. The y-axis represents the organism obtained at the time of infection diagnosis (CSF culture or blood culture in a ventriculoatrial shunt, and where indicated, visible hardware and abdominal pseudocyst). Organisms for each infection episode are represented either on, or (when multiple infection episodes involved the same organism) next to, the vertical line for each patient. SI-3 is represented by a blue circle, SI-4 by a red cross, SI-5 by a green X, SI-6 by a brown triangle, SI-7 by a purple square, and SI-8 by a green star.
Results
Study Population
Characteristics of the 31 children with SI-2 are shown. (Table 1) The majority were under 6 months of age at initial shunt placement (n=25, 81%), male (n=24, 77%), and Caucasian (n=27, 87%) with ventriculoperitoneal shunts (n=22, 71%).
Table 1.
Characteristics of children who developed second CSF shunt infection (SI-2), n=31
| Characteristic | n (%) | Characteristic | n (%) |
|---|---|---|---|
| Age at initial shunt placement | Etiology of hydrocephalus | ||
| 0–30 days | 12 (39%) | IVH secondary to prematurity | 8 (26%) |
| 1–6 months | 13 (42%) | Other intracranial cyst | 7 (23%) |
| 6–48 months | 5 (16%) | Aqueductal stenosis | 5 (16%) |
| ≥ 48 months | 1 (3%) | Myelomeningocele | 3 (10%) |
|
|
|||
| Gender | Posterior fossa cyst | 2 (6%) | |
| Male | 24 (77%) | Post-infectious | 1 (3%) |
| Female | 7 (23%) | Posterior fossa tumor | 1 (3%) |
|
|
|||
| Race/ethnicity | Head injury | 1 (3%) | |
| Caucasian | 27 (87%) | Encephalocele | 1 (3%) |
| Latino | 3 (10%) | Communicating congenital | 1 (3%) |
| Other | 1 (3%) | Other congenital (eg schizencephaly) | 1 (3%) |
|
| |||
| Initial shunt location | Number of revisions before SI-1 | ||
| Ventriculoperitoneal | 22 (71%) | Zero | 18 (58%) |
| Ventriculoatrial | 5 (16%) | One | 3 (10%) |
| Multiple ventriculoperitoneal | 3 (10%) | Two | 8 (26%) |
| Epiduroperitoneal | 1 (3%) | Three | 1 (3%) |
| Four | 1 (3%) | ||
Hydrocephalus etiology includes intraventricular hemorrhage secondary to prematurity and intracranial cyst followed by aqueductal stenosis and myelomeningocele. Many children (n=18, 58%) had no CSF shunt revision procedures before development of initial CSF shunt infection.
Treatment approaches used in repeated CSF shunt infections
CSF shunt infections were diagnosed primarily by bacterial growth in CSF culture (SI-1 n=28, 91% and SI-2 n=25, 81%). (Table 2) The surgical approach to treatment of infection was usually removal of CSF shunt (SI-1 n=30, 97% and SI-2 n=27, 87%). During the study period, this was done frequently at the bedside (SI-1 n=21, 68% and SI-2 n=15, 48%) using conscious sedation and sterile technique. (Data not shown) The majority of children had negative CSF cultures 3 days (SI-1 n=22, 71% and SI-2 n=25, 81%) and 7 days (SI-1 n=21, 68% and SI-2 n=24, 77%) prior to final infection surgery. (Data not shown) The median duration of externalization/EVD placement was longer than two weeks (SI-1 16 days, SI-2 15 days). Of the four children with multiple shunts in place at the time of SI-2, two underwent full removal/EVD placement followed by new CSF shunt placement, and two underwent externalization.
Table 2.
Characteristics of infection episodes in children who developed second CSF shunt infection (SI-2), n=31
| Characteristic at Time of First CSF Shunt Infection (SI-1) | Characteristic at Time of Second CSF Shunt Infection (SI-2) | ||
|---|---|---|---|
| n (%) | n (%) | ||
| Age at infection | |||
| 0–30 days | 3 (10%) | 0–30 days | 0 (0%) |
| 1–6 months | 20 (64%) | 1–6 months | 11 (35%) |
| 6–48 months | 7 (23%) | 6–48 months | 13 (42%) |
| ≥ 48 months | 1 (3%) | ≥ 48 months | 7 (23%) |
|
| |||
| Diagnosis of infection | |||
| CSF culture | 25 (81%) | CSF culture | 22 (71%) |
| CSF & wound culture | 3 (10%) | CSF & wound culture | 3 (10%) |
| CSF & visible hardware | 0 (0%) | CSF & visible hardware | 1 (3%) |
| CSF Gram stain | 0 (0%) | CSF Gram stain | 1 (3%) |
| Wound culture | 1 (3%) | Wound culture | 1 (3%) |
| Visible hardware & wound culture | 1 (3%) | Visible hardware & wound culture | 0 (0%) |
| Visible hardware alone | 0 (0%) | Visible hardware alone | 2 (6%) |
| Abdominal pseudocyst | 0 (0%) | Abdominal pseudocyst | 1 (3%) |
| Blood culture in VA shunt | 1 (3%) | Blood culture in VA shunt | 0 (0%) |
|
| |||
| Surgical approach to treatment | |||
| Full removal | 30 (97%) | Full removal | 27 (87%) |
| Externalization | 1 (3%) | Externalization | 4 (13%) |
|
| |||
| Shunt location after infection | |||
| Ventriculoperitoneal | 21 (68%) | Ventriculoperitoneal | 18 (58%) |
| Ventriculoatrial | 6 (19%) | Ventriculoatrial | 5 (16%) |
| Multiple ventriculoperitoneal | 4 (13%) | Multiple ventriculoperitoneal | 7 (23%) |
| Shunt removal | 0 (0%) | Shunt removal | 1 (3%) |
|
| |||
| Duration of externalization/EVD placement*, Median (Range) | 16 days (7–116) | 15 days (3 to 52) | |
The child with externalization of 116 days was discharged with no EVD in place and returned for CSF shunt replacement 71 days later
Of the 31 children with SI-2, 18 developed SI-3 and 8 developed SI-4. The reinfection rate following SI-2 was 60% (95% CI: 42–75%, n=18/30) and following SI-3 was 47% (95% CI: 26–69%, n=8/17); at the time of treatment for SI-2 and SI-3 one child in each group had their shunt permanently removed. In contrast, the initial infection rate for the entire cohort using a per-patient denominator was 21% (95% CI: 18–25%, n=123/579), and the second shunt infection rate was 25% (95% CI: 18–34%, n=31/123).
Infection-free survival curves are shown in Figure 2. The median time to SI-3 was 477 days (range 5–828) and to SI-4 was 2137 days (range 9–2137), compared to a median of 65 days (range 3–2326) to SI-2 following SI-1.
Microbiology of repeated shunt infections
Figure 3 lists the microorganisms isolated at SI-1 and SI-2 for each child. At the time of diagnosis of SI-2, 27 organisms grew from the CSF of 26 children. The vast majority of organisms at SI-2 were Gram-positive (93%, 25/27), including primarily coagulase negative Staphylococcus species (56%, 15/27), followed by S. aureus (19%, 5/27) and S. viridans (11%, 3/27); Gram-negative organisms comprised the remainder (7%, 2/7). Three (10%, 3/31) of the SI-2 infection episodes were polymicrobial (patients E, O, and BB).
As shown in Figure 3, more than half of the children with SI-2 (18/31, 58%, 95% CI: 41–74%) demonstrated Gram-stain concordance between SI-1 and SI-2, whereas one-third of the children (10/31, 32%, 95% CI: 19–50%) demonstrated Gram-stain discordance at SI-2. Three children had infection diagnosed by visible hardware and are not considered. The median time between SI-1 and SI-2 was 83 days (range 5–2326 days) for the concordant group and 79 days (range 3–1456 days) for the discordant group (log rank test p =0.5).
We looked for evidence of emerging antimicrobial resistance among the 18 children with Gram-stain concordance in CSF cultures between SI-1 and SI-2. Three of SI-2 organisms in CSF (17%, 95% CI: 6–39%) were resistant to antibiotics used to treat SI-1; these organisms included S. viridans (child P) and S. epidermidis (children BB and EE).
Nine of the children (29%, 95% CI: 16–47%) had identical organisms grown in either CSF and/or wound cultures at SI-2 to those in their initial shunt infection. Three infections (10%, 95% CI: 3–25%) were of the same organism within one month of completion of therapy for the first infection, meeting “relapse” criteria used by previous authors. [10]
For the 18 children who developed SI-3, Figure 4 lists the microorganisms isolated from subsequent infection episodes by child. At SI-3, Gram-positive organisms comprised the vast majority of organisms (94%, 16/17), including primarily S. aureus (47%, 8/17), followed by coagulase negative Staphylococcus species (24%, 4/17) and S. viridans (12%, 2/17); Gram-negative organisms comprised the remainder (6%, 1/17). Most children demonstrated Gram-stain concordance between SI-2 and SI-3 (14/18, 78%, 95% CI: 55–91%). One child (1/18, 6%, 95% CI: 1–26%) demonstrated Gram-stain discordance. Seven children (7/18, 36%, 95% CI: 20–61%) had identical organisms grown in CSF cultures in both SI-2 and SI-3 episodes; one of the infections (6%, 95% CI: 1–26%) was within one month of completion of therapy for the first infection.
As shown in Figure 4, after SI-4, Gram-negative organisms comprised an increasing proportion of the small numbers of CSF specimens.
Discussion
We assembled the largest group of children with multiple shunt infections, and the first to distinguish first, second, and subsequent infections, to date. Treatment usually included removal of infected CSF shunt and EVD placement followed by new shunt placement after about two weeks of IV antibiotic treatment. After second CSF shunt infection, the re-infection rate was 60%, and the median time to subsequent infections was prolonged. Over half of children demonstrated Gram-stain concordance with initial shunt infection (58%) and with subsequent infection (78%). A minority (17%) demonstrate an emergence of antimicrobial resistance. Approximately one third of infections had identical organisms with initial infection (29%) and with subsequent infections (36%), but a minority (10% and 6% respectively) occurred within one month of completion of therapy from the prior infection (relapse). Among children with multiple CSF shunt infections, Gram-positive organisms predominated (93% of SI-2, 94% of SI-3) but after SI-4, Gram-negative organisms comprised an increasing proportion of the CSF bacterial species.
The study population was predominantly under the age of 6 months at the time of initial CSF shunt placement, male, Caucasian, with ventriculoperitoneal shunt placement. Treatment of CSF shunt infections was consistent in the population. The vast majority underwent shunt removal, and the duration of externalization/EVD placement was consistently about two weeks. Future studies within this cohort will examine differences between children who only develop initial shunt infection and those who develop recurrent shunt infections.
The rates of initial infection and second infection in our study cohort are consistent with reports in the literature using per-patient denominators. [1–7] In contrast, the re-infection rate following second shunt infection of 60% is a new finding that is much higher than previous reports of CSF shunt infection. However, no prior work has distinguished between second and subsequent CSF shunt infections. On the other hand, this work did not consider intervening surgeries for non-infectious causes, so subsequent infections cannot be directly attributed the management of the second shunt infection. Nonetheless, this finding is consistent with common observations in clinical practice that a subset of children with CSF shunt infection develops multiple subsequent re-infections. Once a child has developed a second CSF shunt infection, they are clearly at substantially higher risk for repeated infections, and warrant close monitoring.
It also appears that after second shunt infection, development of additional infections occur far later than their first and second infections. We observed a median time to re-infection of 65 days (range 3–2326) in this group; similarly, Kulkarni et al. reported that the risk of second shunt infection plateaued in children after 90 days. [13]
Over half of children displayed Gram-stain concordance with their initial shunt infection. However, the majority of SI-2 would qualify as re-infections rather than relapses according to previously published criteria. [10] While Gram-stain concordance should be taken under consideration when selecting treatment regimens for second CSF shunt infection, the initial empiric antibiotic coverage should include a broad spectrum of efficacy until the causative microorganism is recovered.
We considered the possibility of emerging antimicrobial resistance, as prior antimicrobial exposure is a risk factor in the development of resistance genes. In children, this is best described in children with recurrent urinary tract infection [18] and in nasal carriage of S. pneumoniae [18, 19]. Development of antimicrobial resistance did not appear between first and second CSF shunt infection, but should continue to be monitored in future studies.
This study includes several limitations. This work did not systematically consider revisions following second shunt infection. From 2001 to 2002 antibiotic impregnated shunt tubing may have been used; these data are not feasible to capture, and may have affected recovery of organisms from CSF. We were also unable to capture and consider antibiotic use between infection episodes in both outpatient and inpatient settings. However, this study has the strengths of a large cohort of children with rich information about both initial CSF shunt infection management and subsequent organisms. Subsequent work will capitalize on the availability of this information to systematically examine the timing of shunt revision surgeries and risk factors for development of SI-2.
Children with second CSF shunt infections experience high subsequent reinfection rates and longer time to re-infection. Despite data about Gram-stain concordance data, broad empiric antimicrobial coverage should still be used initially when treating repeated CSF shunt infections until the infecting organism is identified. Improved understanding of risk factors underlying multiple shunt infection is critical as these children experience a high degree of morbidity.
Acknowledgments
We are indebted to Marcie Langley for her diligent work assembling the original cohort, as well as her invaluable support for the conduct of this project. We thank the Division of Inpatient Medicine at PCMC for additional support for this work, including Gena Fletcher, Flory Nkoy, and Chris Maloney. Finally, we thank Carrie Byington and Mandy Allison for their mentorship of Dr. Tuan in the University of Utah Mentored Program in Pediatric Research. This work was presented in part by Dr. Tuan at the Pediatric Academic Societies meeting in May 2010 in Vancouver, Canada and by Dr. Simon at the pediatric section of the American Association of Neurological Surgeons in December 2010 in Cleveland, Ohio.
Financial Disclosure: TDS is supported by Award K23NS062900 from the National Institute of Neurological Disorders And Stroke, the Child Health Corporation of America via the Pediatric Research in Inpatient Setting Network Executive Council, and Seattle Children’s Center for Clinical and Translational Research. This publication was supported by a PCMC Innovative Research Grant, the Children’s Health Research Center at the University of Utah, Seattle Children’s Center for Clinical and Translational Research, and CTSA Grant Number ULI RR025014 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). None of the sponsors participated in design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH.
Abbreviations
- CI
confidence intervals
- CSF
cerebrospinal fluid
- EVD
external ventricular drain
- NCRR
National Center for Research Resources
- NIH
National Institutes of Health
- PCMC
Primary Children’s Medical Center
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- SI
shunt infection
Footnotes
Conflicts of Interest: None of the authors have potential financial or personal conflicts of interest to disclose.
References
- 1.Vinchon M, Dhellemmes P. Cerebrospinal fluid shunt infection: risk factors and long-term follow-up. Childs Nerv Syst. 2006;22(7):692–7. doi: 10.1007/s00381-005-0037-8. [DOI] [PubMed] [Google Scholar]
- 2.Frykberg T, Olden L. Infection as a cause of peritoneal catheter dysfunction in ventriculo-peritoneal shunting in children. Z Kinderchir. 1983;38(Suppl 2):84–6. doi: 10.1055/s-2008-1063084. [DOI] [PubMed] [Google Scholar]
- 3.Amacher AL, Wellington J. Infantile hydrocephalus: long-term results of surgical therapy. Childs Brain. 1984;11(4):217–29. doi: 10.1159/000120180. [DOI] [PubMed] [Google Scholar]
- 4.Cochrane DD, Kestle JR. The influence of surgical operative experience on the duration of first ventriculoperitoneal shunt function and infection. Pediatr Neurosurg. 2003;38(6):295–301. doi: 10.1159/000070413. [DOI] [PubMed] [Google Scholar]
- 5.Kestle J, et al. Long-term follow-up data from the Shunt Design Trial. Pediatr Neurosurg. 2000;33(5):230–236. doi: 10.1159/000055960. [DOI] [PubMed] [Google Scholar]
- 6.Borgbjerg BM, et al. Risk of infection after cerebrospinal fluid shunt: an analysis of 884 first-time shunts. Acta Neurochir (Wien) 1995;136(1–2):1–7. doi: 10.1007/BF01411427. [DOI] [PubMed] [Google Scholar]
- 7.Di Rocco C, Marchese E, Velardi F. A survey of the first complication of newly implanted CSF shunt devices for the treatment of nontumoral hydrocephalus. Cooperative survey of the 1991–1992 Education Committee of the ISPN. Childs Nerv Syst. 1994;10(5):321–7. doi: 10.1007/BF00335171. [DOI] [PubMed] [Google Scholar]
- 8.Simon TD, et al. Infection rates following initial cerebrospinal fluid shunt placement across pediatric hospitals in the United States. Clinical article. J Neurosurg Pediatr. 2009;4(2):156–65. doi: 10.3171/2009.3.PEDS08215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGirt MJ, et al. Risk factors for pediatric ventriculoperitoneal shunt infection and predictors of infectious pathogens. Clin Infect Dis. 2003;36(7):858–62. doi: 10.1086/368191. [DOI] [PubMed] [Google Scholar]
- 10.Odio C, McCracken GH, Jr, Nelson JD. CSF shunt infections in pediatrics. A seven-year experience. Am J Dis Child. 1984;138(12):1103–8. doi: 10.1001/archpedi.1984.02140500009004. [DOI] [PubMed] [Google Scholar]
- 11.Walters BC, et al. Cerebrospinal fluid shunt infection. Influences on initial management and subsequent outcome. J Neurosurg. 1984;60(5):1014–21. doi: 10.3171/jns.1984.60.5.1014. [DOI] [PubMed] [Google Scholar]
- 12.Kestle JR, et al. Management of shunt infections: a multicenter pilotstudy. J Neurosurg. 2006;105(3 Suppl):177–81. doi: 10.3171/ped.2006.105.3.177. [DOI] [PubMed] [Google Scholar]
- 13.Kulkarni AV, et al. Repeat cerebrospinal fluid shunt infection in children. Pediatr Neurosurg. 2001;35(2):66–71. doi: 10.1159/000050393. [DOI] [PubMed] [Google Scholar]
- 14.Whitehead WE, Kestle JR. The treatment of cerebrospinal fluid shunt infections. Results from a practice survey of the American Society of Pediatric Neurosurgeons. Pediatr Neurosurg. 2001;35(4):205–10. doi: 10.1159/000050422. [DOI] [PubMed] [Google Scholar]
- 15.Simon TD, et al. Reinfection following initial cerebrospinal fluid shunt infection. J Neurosurg Pediatr. 2010;6(3):277–85. doi: 10.3171/2010.5.PEDS09457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kestle J, et al. Standardized Protocol to Reduce CSF Shunt Infection: The HCRN Quality Improvement Initiative. Journal of Neurosurgery: Pediatrics. 2011 doi: 10.3171/2011.4.PEDS10551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17(8):857–72. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 18.Cheng CH, et al. Antibiotic resistance patterns of community-acquired urinary tract infections in children with vesicoureteral reflux receiving prophylactic antibiotic therapy. Pediatrics. 2008;122(6):1212–7. doi: 10.1542/peds.2007-2926. [DOI] [PubMed] [Google Scholar]
- 19.Samore MH, et al. High rates of multiple antibiotic resistance in Streptococcus pneumoniae from healthy children living in isolated rural communities: association with cephalosporin use and intrafamilial transmission. Pediatrics. 2001;108(4):856–65. doi: 10.1542/peds.108.4.856. [DOI] [PubMed] [Google Scholar]