Abstract
Cancer progression is commonly segregated into processes of primary tumour growth and secondary metastasis. Recent evidence suggests that a subpopulation of cancer cells, cancer stem cells (CSCs), is responsible for tumour growth in cancer. However, the role of CSCs in cancer metastasis is unclear. In this study, we found that the C terminus of CD44 contributes to sphere formation and survival in vitro via the CD44–SRC–integrin axis. In addition, nuclear CD44/acetylated-STAT3 is required for clonal formation in vitro and tumourigenicity in vivo. Nuclear CD44 binds to various promoters identified by chromatin immunoprecipitation-seq, including that of c-myc and Twist1, leading to cell fate change through transcriptional reprogramming. We propose that nuclear CD44/acetylated-STAT3 performs an unexpected tumour-progressing function by enhancing cell outgrowth into structures where cells with properties of CSCs can be generated from differentiated somatic cells in suspension culture, and then exhibit attributes of cells that have undergone an epithelial–mesenchymal transition, leading to tumour metastasis, and a resulting worse prognosis.
Keywords: cancer stem cell, CD44, epithelial–mesenchymal transition, side population, STAT3
Introduction
In 1994, John E Dick and colleagues first demonstrated that most human acute myeloid leukaemia cells have limited proliferative capacity, suggesting that the leukaemic clone may be maintained by a rare population of stem cells called cancer stem cells (CSCs) (Lapidot et al, 1994). Recently, solid tumours as well as haematopoietic cancers have been demonstrated to include a small population of cancer-initiating cells that are capable of regenerating the heterogeneous population of cancer cells in xenographic transplants (Dick, 2005; Trosko, 2006; O’Brien et al, 2007). Although stem cell activities can indeed be detected through serial passage and clonal analysis, the generation of spheres in and of itself is often taken as sufficient evidence for the existence of a stem cell. The sphere-forming culture (Reynolds and Weiss, 1992) rapidly emerged as the assay of choice and has since become a valuable tool for isolating—and understanding the biology of—embryonic, adult, and CSCs. However, the details of the mechanism involved in this morphological phenomenon are virtually unknown. It is a technical challenge to identify and characterize the cancer-initiating cells with CSC properties due to the rarity of CSCs in the tissue of origin and the lack of specific markers. Most studies have used putative stem cell markers or side populations to isolate unique subsets of cancer cells from different types of tumours.
CD44 is an important marker for a number of different CSC lineages, although its cellular function in CSCs is not clear. CD44 was used to isolate breast CSCs (Al-Hajj et al, 2003), prostate CSCs (Collins et al, 2005; Patrawala et al, 2006), pancreatic CSCs (Li et al, 2007), and colorectal CSCs (Dalerba et al, 2007b). However, why CD44 is a CSC marker is still unknown. We previously showed that CD44v and its physiological ligand osteopontin (OPN) are frequently overexpressed in human gastric cancer and that OPN-engaged CD44v ligation confers on cells increased matrix-derived survival through inducing lipid raft coalescence to facilitate the CD44–Src–integrin signalling axis (Lee et al, 2007, 2008). Furthermore, we demonstrated that CD44, once engaged, is internalized and translocated to the nucleus; there it binds to various promoters, including that of cyclin D1, leading to cell fate change through transcriptional reprogramming (Lee et al, 2009).
Among the most ominous properties of malignant cancer cells are their capacity to metastasize, that is, to move from their primary tissue of origin and seed in a different anatomical compartment, where they sustain the growth of a secondary tumour lesion. Because CSCs appear to be preferentially endowed with the capacity to self-renew, and thus to be responsible for the long-term maintenance of tumour growth, it has been predicted that they might be also primarily responsible for the formation of tumour metastases (Dalerba et al, 2007a). This assumption, however, has not yet been addressed experimentally, and the relationship between CSC and metastasis remains obscure. The epithelial–mesenchymal transition (EMT) is a key developmental programme that is often activated during cancer invasion and metastasis (Thiery, 2003). Recent studies also have showed that overexpression of these EMT-related transcription factors can also induce a CD44-high/CD24-low pattern on epithelial cells, which is associated with the somatic cells obtaining stem cell and CSC properties (Mani et al, 2008). Metastatic potential depends on multiple factors that determine overall tumour cell growth, survival, angiogenesis, and invasion. For epithelial malignancies, the EMT is considered to be a crucial event in the metastatic process, which involves disruption of epithelial cell homoeostasis and the acquisition of a migratory mesenchymal phenotype. The EMT appears to be controlled by canonical pathways such as the Wnt and transforming growth factor β pathways, both of which can be aberrantly activated during neoplasia. A recent report suggests that there may be a direct link between the EMT and acquisition of stem cell properties (Mani et al, 2008).
In this study, we found that many diverse lines of evidence suggest a possible link between CD44, CSCs, the sphere-forming culture, and tumour metastasis. During the process of tumour metastasis, lipid raft-associated CD44 is required for survival in the suspension condition, and then nuclear CD44/acetylated-STAT3 generates cells with properties of CSCs and the EMT phenotype by transcriptional reprogramming, leading to drug resistance, tumour metastasis, and a resulting poor prognosis. The observations in this study have important implications, as they propose to indicate that targeting of CD44 might be key to interfering with the formation and spread of cancers. This study, for the first time, will demonstrate the role of CD44 in tumour metastasis by using a model where multiple phenotypic cancer cell subpopulations coexist in a dynamic equilibrium and where the tumourigenic and metastatic properties of cell subsets, including CSCs, can be tested concurrently, trying to chart their functional and hierarchical relationships.
Results
CD44 leading to stable changes in cell ability and morphology after the suspension culture
In this study, we tested whether the transcriptional reprogramming led by nuclear CD44 has an active role in transforming cancer cells to a CSC-like phenotype. First, we analysed the expression patterns of the CSC surface marker CD44 using fluorescence-activated cell sorting (FACS) for the following six human colon cancer cell lines: COLO 205, COLO 320, HCT-116, HT29, DLD-1, and LoVo cells. As shown in Supplementary Figure S1, COLO 205, COLO 320, and HCT-116 cells showed a high level of CD44 expression, with up to 90% of cells expressing CD44, whereas HT29, DLD-1, and LoVo cells showed as little as 70% expression. We fractionated HT29 and DLD-1 cells by FACS into CD44+ and CD44− cell fractions. We also established HT29/CD44− and DLD-1/CD44− cell clones that express various CD44 mutants (HT29/CD44−/CD44-myc and DLD-1/CD44−/CD44-myc). As shown in Figure 1A, after in vitro culture for 6–12 days under sphere-forming conditions, HT29/CD44+ and HT29/CD44−/CD44-myc cells produced sphere colonies, whereas HT29/CD44−/Mock cells did not. Furthermore, CD44 transcripts eliminated in HT29/CD44+ cells by a lentivirus-based RNA interference technique (Lee et al, 2008) significantly inhibited sphere formation. To test the self-renewal capability of the sphere-forming cells, we dissociated the primary spheres into single cells and performed secondary sphere assays. Interestingly, we found ∼10 secondary spheres in HT29/CD44+ and HT29/CD44−/CD44-myc cells formed per 100 seeded cells (10%) but <3% formed in HT29/CD44−/Mock and HT29/CD44+/CD44-shRNA cells. Moreover, the proportion of sphere-forming cells isolated from already-formed spheres remained the same through subsequent serial passages (Figure 1B). This indicates that the sphere-forming cells are capable of self-renewing and that the conditions of sphere culture encourage an increase in and subsequent stable maintenance of the number of self-renewing cells among the larger population of CD44-expressing cells.
Figure 1.
CD44 allows outgrowth of cells into spheres, leading to stable changes in cell proliferative ability and morphology after the suspension culture. (A) Microscopic analysis of spheres cultivated in suspension for 6 and 12 days. (Top panel) HT29/CD44− cells were transfected with plasmid encoding CD44s (CD44-myc) or control vector (Mock). (Bottom panel) HT29/CD44+ cells were infected by lentivirus-encoding shRNA targeting CD44 (CD44-shRNA) or control scrambled shRNA (Cont-shRNA). Bars, 50 μm. (B) In vitro quantification of spheres formed by cells described in (A) during four serial passages (p1–p4). (C, D) The C terminus of CD44 is required for sphere formation over four serial passages. (Top) Schematic representation of the transcripts encoding wild-type CD44s and its in-frame C-terminal (in C)/N-terminal (in D) deletion mutants. TM, transmembrane domain; ICD, intracellular domain. (Bottom) In vitro quantification of spheres formed by stable HT29/CD44− clones expressing C-terminal deletion mutants of CD44s. (E) The sphere-forming culture triggers stable changes in cell morphology. Higher-power views are shown in the bottom panels. Bars, 50 μm. (F, G) The sphere-forming culture triggers stable changes in cell ability. HT29/CD44− stable clones (in F) and HT29/CD44+ cells (in G) (described in E) were plated at 105 cells per six-well dish in 1% FBS RPMI medium. Total viable cell number was determined. Data in (B–D, F, G) were derived from three independent experiments and are presented as mean values±s.d. *P<0.05; **P<0.01; ***P<0.005 (t-test). AD (in E–G): monolayer cell culture grown in tissue culture plates; SPH (in E–G): cultured under sphere-forming conditions; SPH → AD (in E–G): all cells in the spheres migrated back onto the plate to reform a monolayer.
To further define the regions of CD44 that are involved in sphere formation, a series of C-terminal deletion mutants were generated from the wild-type and from the cysteine mutant. Figure 1C shows that CD44sΔ67, CD44sΔ67C286A, CD44sΔ61C286,295A and CD44sΔ61C286,295A/KA failed to promote sphere formation. This is consistent with our previous observation that CD44 promotes matrix-derived survival through the CD44–Src–integrin axis in lipid rafts (Lee et al, 2008). Wild-type CD44s, CD44sC286,295A, and CD44sΔ37C286,295A can translocate into lipid rafts, associate with Src, trigger integrin activation, and then provide survival signalling. Taken together, a one-to-one relationship may exist between sphere formation and CD44-elicited survival signalling. In contrast, the N terminus of CD44 did not contribute to sphere formation (Figure 1D).
The spheres formed from CD44-expressing cells could be maintained in suspension for months. During this period, they formed a central cavity and increased in size. When the spheres were transferred back to adhesive tissue culture plates, they migrated back onto plates, reforming a monolayer (Figure 1E). Surprisingly, all of the cells in these monolayers differed from HT29 and DLD-1 before sphere formation—they were morphologically heterogeneous and smaller (compare SPH → AD with AD). These monolayer cells derived from HT29/CD44+ (Figure 1E) and DLD-1/CD44+ (data not shown) spheres were smaller in size and generated a morphologically distinct cell type, demonstrating a stable morphological transition. When the cells became confluent in adhesive tissue culture plates, they began to form 3D colonies or mounds of cells. Subsequently, outgrowth of cells in these 3D colonies led to detachment from the culture plate and formation of spheres in suspension after 24 days. Because cells in the spheres underwent a stable morphological transition that may reflect reprogramming, we performed cell proliferation assays to examine the proliferative ability and behaviour of cells derived from spheres after 12 days in suspension culture (SPH → AD), and compared these results with cells cultured (monolayer) in tissue culture plates (AD). Surprisingly, the cells derived from spheres expressing wild-type CD44 (HT29/CD44−/CD44-myc cells in Figure 1E and HT29/CD44+/parental and HT29/CD44+/Cont-shRNA cells in Figure 1G) exhibited increased proliferation (there was no difference in cell viability). In contrast, cells expressing CD44sΔ61C286,295A/KA in HT29/CD44− (Figure 1F) or CD44 transcripts eliminated in HT29/CD44+ cells by a lentivirus-based RNA interference technique (Figure 1G) did not promote proliferation after the sphere-forming culture. The similar results were also shown in DLD-1 cells (Supplementary Figure S2). Taken together, the above results suggest that wild-type CD44 is required for sphere formation and forces cells derived from spheres to stable changes in cell morphology and ability.
C terminus of CD44 contributes to anoikis resistance through the CD44–Src–integrin axis in lipid rafts
Loss of extracellular matrix adhesion induces normal cells to undergo apoptosis—a process known as anoikis. By contrast, oncogenically transformed cells are relatively resistant to anoikis (Frisch and Ruoslahti, 1997). In addition to facilitating the initial expansion of tumours, resistance to anoikis is key to metastatic dissemination, as tumour cells must survive in several different foreign microenvironments before they can colonize distant organs. In particular, the condition of sphere-forming culture in vitro is very similar to the suspension in blood vessels in vivo. Therefore, the sphere-forming culture may be a useful model in tumour progression, especially when evaluating tumour migration and suspension in blood vessels in vivo. When a monolayer of HT29/CD44− cells was trypsinized and the resulting cells were cultured in sphere-forming conditions, spheres did not form, and the suspension cells began to die after 48–72 h (data not shown). However, when HT29/CD44− cells that ectopically expressed CD44s were trypsinized, the cells were resistant to anoikis and they formed spheres in suspension. In our previous study (Lee et al, 2008), we found that the CD44–Src–integrin axis in lipid rafts was crucial for CD44-elicited survival signalling. To examine whether the formation of the intact signal axis is required for CD44-mediated survival against anoikis, cells were treated with lipid raft-destabilizing drugs (methyl-β-cyclodextrin (MβCD) or nystatin) and the Src inhibitor PP2 before the suspension culture. Our data showed that inhibition of raft formation by MβCD or nystatin and inhibition of Src activity abolished CD44-mediated survival (Figure 2A). To further define the regions of CD44 involved in the resistance to anoikis, a series of C-terminal deletion mutants were generated from the wild-type and from the cysteine mutant. Consistent with the results of Figure 1C, CD44sΔ67, CD44sΔ67C286A, CD44sΔ61C286,295A, and CD44sΔ61C286,295A/KA underwent anoikis (Figure 2B). Wild-type CD44s, CD44sC286,295A, and CD44sΔ37C286,295A survived after 120 h in suspension. Moreover, the N terminus of CD44 also did not contribute to anoikis resistance (Figure 2C).
Figure 2.
C terminus of CD44 leads to increased resistance to anoikis during the sphere-forming culture through the CD44–SRC–integrin axis in lipid rafts. (A) Cells after treatment were then cultured in suspension for 120 h before apoptosis assays by flow cytometric analyses of sub-G1 fractions. (B, C) Stable HT29/CD44− clones expressing C-terminal deletion mutants of CD44s (in B) or N-terminal deletion mutants of CD44s (in C) were cultured in suspension for 120 h before apoptosis assays by flow cytometric analyses of sub-G1 fractions. (D, E) HT29/CD44+ (in D) and HT29/CD44−/CD44-myc (in E) cells were cultured (monolayer) in tissue culture plates (AD, left panel) or in sphere-forming culture (SPH, right panel). Triton X-100-insoluble raft fractions were isolated by sucrose gradient fractionation, pooled, and analysed by western blotting for the individual proteins indicated. (F) Stable clones were cultured (monolayer) in tissue culture plates (AD, left panel) or in sphere-forming culture (SPH, right panel). The lipid rafts were isolated by sucrose gradient fractionation. An equal volume of each fraction was analysed by western blotting for the proteins indicated. The relative intensities of the bands were densitometrically quantified and normalized to Cont-shRNA (CD44+ cells) and Mock (CD44− cells). The red asterisks in upper part: CD44 proteins with expected sizes. (G) HT29/CD44+ cells were treated with control IgG or blocking Ab against integrin β1 or infected with a lentivirus encoding a shRNA targeting Src (Src-shRNA) or a control scrambled shRNA (Cont-shRNA), followed by cultured in suspension for 120 h, and apoptosis was measured by flow cytometric analysis of sub-G1 fractions. (H) In vitro quantification of spheres formed by cells described in (G) during four serial passages (p4). Data in (A–C, G, H) were derived from three independent experiments and are presented as mean values±s.d. *P<0.05; **P<0.01; ***P<0.005 (t-test).
We next examined the distribution of CD44 in lipid rafts after the sphere-forming culture by solubilizing HT29/CD44+ (Figure 2D) and HT29/CD44−/CD44-myc (Figure 2E) cells in 1% cold Triton X-100 solution followed by sucrose gradient centrifugation. The lipid rafts were recovered from the low-density buoyant fractions (fractions 2–4), as indicated by the presence of caveolin-1 and flotillin-2, whereas the Triton X-100-soluble cellular components were distributed over fractions 7–10. As shown, the sphere-forming culture (SPH, right panels) induced lipid raft coalescence and promoted the enrichment of CD44, Src, and integrin β1 into lipid rafts in HT29/CD44+ (Figure 2D) and HT29/CD44−/CD44-myc (Figure 2E) cells. We further showed that after the sphere-forming culture (SPH, right panel), CD44 mutants such as CD44sΔ67C286A, CD44sΔ61C286,295A/KA (which is defective in association with lipid rafts and in inducing lipid raft reorganization) (Lee et al, 2008), CD44sΔ67C286A, CD44sΔ67, CD44sΔ61C286,295A, and CD44sΔ61C286,295A/KA (which is defective in Src interaction and fails to promote Src translocation into lipid rafts and activation) (Lee et al, 2008) failed to induce integrin activation (Figure 2F), resistance to anoikis (Figure 2B), and sphere formation (Figure 1C). The similar results were also shown in HCT-116 and DLD-1 cells (Supplementary Figure S3A). To further substantiate the role of integrin and Src in CD44-elicited functions (resistant to anoikis and consequent sphere formation), pretreatment of cells with blocking Ab against integrin β1 or Src transcription was eliminated in HT29 cells by a lentivirus-based RNA interference technique. As shown, pretreatment of cells with blocking Ab against integrin β1 or the introduction of shRNA against Src significantly abolished CD44-mediated survival (Figure 2G) and subsequent sphere-forming abilities (Figure 2H). The similar results were also shown in DLD-1 cells (Supplementary Figure S3B and C).
Nuclear CD44/acetylated-STAT3 is required for sphere growth in vitro in sphere-forming cells
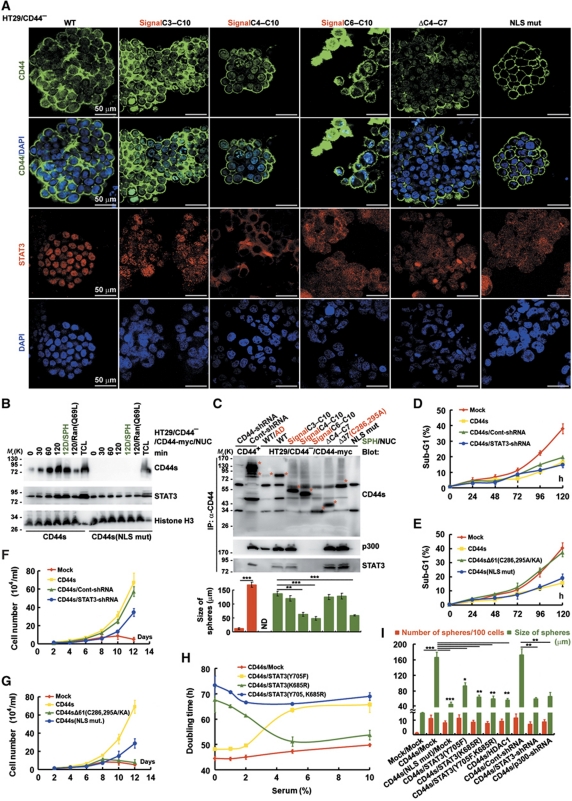
Even though a majority of HT29/CD44− cells that express one of a series of N-terminal deletion mutants (especially CD44sC4–C10 (constant exons 4–10 in CD44s) and CD44sC6–C10) retained sphere formation potential, the size of the resultant spheres was significantly reduced in the cells expressing CD44sC4–C10 and CD44sC6–C10 (Figure 3A and bottom panel of 3C), suggesting that the N-terminal region encoded by C3 in CD44 is required for CD44-elicited sphere growth. In our previous study, we demonstrate that CD44, once engaged, is internalized and translocated to the nucleus, where it binds to various promoters leading to cell fate change (accelerating cell proliferation) through transcriptional reprogramming (Lee et al, 2009). As shown in Figure 3A, ectopic expression of wild-type CD44s but not the CD44(NLS) mutant (which cannot translocate into the nucleus, as proved by immunofluorescence staining) conferred increased sphere size, suggesting that localization of CD44 in the nucleus is an important aspect of its growth-stimulating function. Moreover, in the nucleus, the acetylated-STAT3 dimer remains associated with CD44 through the N-terminal region encoded by C3 and binds to various promoters, leading to increased cell proliferation (Lee et al, 2009). Consistent with our previous report, HT29/CD44−/CD44sC4–C10 (62.5±3.5 μm) and HT29/CD44−/CD44sC6–C10 (54.1±5.3 μm) (which are defective in association with STAT3) significantly decreased the size of spheres in comparison to spheres expressing wild-type CD44s (137.2±6.1 μm), CD44sC3–C10 (128±4.9 μm) and CD44sΔC4–C7 (132.9±6.7 μm) (Figure 3A), demonstrating that nuclear CD44/STAT3 is crucial for sphere growth. Next, we further tested whether cells cultured in suspension could enhance CD44 and STAT3 translocation into the nucleus. As shown in Figure 3B, cells cultured in suspension promoted CD44 and STAT3 nuclear translocation in a time-dependent manner. In contrast, the CD44(NLS) mutant failed to enter the nucleus. To corroborate that the bipartite NLS is essential for the nuclear localization of CD44 protein, a Ran GTPase mutant Ran(Q69L) (Macara, 2001) was ectopically expressed in HT29/CD44−/CD44-myc cells. Figure 3B shows that overexpression of Ran(Q69L) completely blocked CD44 and STAT3 nuclear import after the suspension culture. According to western blotting, all of the CD44 N-terminal deletion mutants in HT29/CD44−/CD44-myc spheres could translocate into the nucleus except for the CD44s(NLS) mutant, and the amount of nuclear CD44 in spheres was higher than in cells maintained as subconfluent monolayers (WT/AD) (Figure 3C). Co-immunoprecipitation further revealed that STAT3 and p300 were in complex with CD44 in HT29/CD44−/CD44-myc spheres (Figure 3C). The similar results were also shown in HCT-116 and DLD-1 cells (Supplementary Figure S4). The N-terminal region encoded by C3 in CD44 was important for their interaction (as STAT3 and p300 did not appear in co-immunoprecipitations with N-terminal deletions of CD44 that did not contain C3 (CD44sC4–C10 and CD44sC6–C10)). Figure 3D–G shows that a nuclear CD44/STAT3 signal was required for sphere growth (the CD44s(NLS) mutant and CD44s/STAT3-shRNA had no effect on anoikis resistance, but significantly blocked CD44-mediated cell proliferation), whereas the CD44–Src–integrin axis in lipid rafts was crucial for survival (CD44sΔ61C286,295A/KA completely abolished CD44-elicited survival signalling). In suspension culture (in serum-free medium), STAT3 acetylation at lysine 685 was more important for sphere growth, as cells expressing CD44s/STAT3(K685R) cultured in low-serum medium had a longer doubling time (Figure 3H). Moreover, the nuclear CD44 (as proved by CD44s(NLS mut)/Mock) and the acetylated-STAT3 (by p300, as proved by CD44s/STAT3(K685R), CD44s/STAT3(Y705F, K685R), CD44s/HDAC1, CD44s/STAT3-shRNA, and CD44s/p300-shRNA) had no effect on sphere formation but was required for sphere growth (Figure 3I).
Figure 3.
Nuclear CD44/acetylated-STAT3 is required for sphere growth in vitro in sphere-forming cells. (A) HT29/CD44− (expressing CD44s mutants) stable clones were cultured in sphere-forming conditions for 12 days, immunostained for CD44 (green, top panel) and counterstained with DAPI (blue, second panel); immunostained for STAT3 (red, third panel) and counterstained with DAPI (blue, bottom panel). Representative images taken by confocal laser microscopy are shown. Bars, 50 μm. (B) Nuclear extracts were prepared from stable HT29/CD44− clones expressing wild-type CD44s or the NLS mutant that were cultured in suspension for the indicated times. 12D/SPH: cells were cultured in sphere-forming conditions for 12 days; 120/Ran(Q69L): cells were transfected with plasmids encoding Ran(Q69L) and then cultured in suspension for 120 h; TCL, total cell lysate. (C) (Top panel) Nuclear extracts were prepared from spheres of HT29 stable clones and then immunoprecipitated using anti-CD44 followed by western blotting. (Bottom panel) The diameter of spheres was measured after 12 days in sphere-forming culture. The red asterisks in upper part: CD44 proteins with expected sizes. ND, not determined. (D, E) Stable HT29/CD44− clones infected with a lentivirus encoding a shRNA targeting STAT3 or a control scrambled shRNA (in D) and expressing wild-type (in D, E) or mutant (in E) CD44s were cultured in suspension for the designated times before apoptosis assays by flow cytometric analyses of sub-G1 fractions. (F, G) The cells described in (D, E) were cultured in sphere-forming conditions for the indicated times; the total viable cell number was determined. (H) Stable clones were cultured in suspension with the indicated concentrations of serum for 12 days; the doubling time was determined. (I) Stable clones were cultured in sphere-forming conditions for 12 days. The number of spheres/100 cells and the size of spheres were determined. Data in (C–I) were derived from three independent experiments and are presented as mean values±s.d. *P<0.05; **P<0.01; ***P<0.005 (t-test). WT/AD (in C): HT29/CD44− cells expressing wild-type CD44s were cultured (monolayer) in tissue culture plates. SPH (in B, C): cultured in sphere-forming conditions.
Nuclear CD44/STAT3 signalling is crucial for reprogramming of cancer cells to a CSC phenotype via transcriptional regulation of c-myc expression
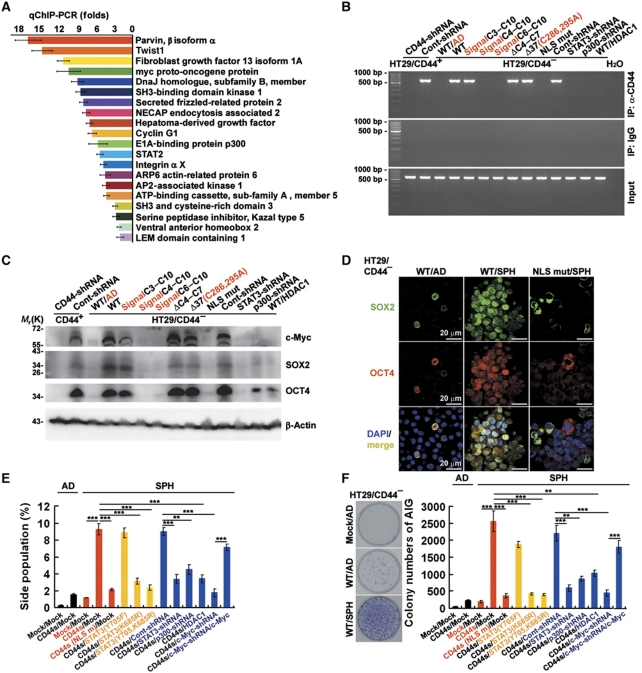
To better understand the roles of nuclear CD44 in self-renewal and transcriptional reprogramming, chromatin immunoprecipitation (ChIP) was performed to search for DNA sequences bound by nuclear CD44 complexes in HT29/CD44+ spheres. A total of 82 clones were obtained from the DNA fragments pulled down by anti-CD44 mAb. A National Center for Biotechnology Information basic local alignment search tool search indicated that they contain sequences corresponding to the promoters of several genes, including c-myc and Twist1 (data not shown). We further validated the association of nuclear CD44 with a subset of the genomic loci identified in our ChIP experiments with anti-CD44 mAb by employing qChIP-PCR in three independent experiments. The 20 highest-scoring genes selected are shown in Figure 4A. ChIP assays also showed that nuclear CD44 was bound to the promoter region of c-myc only in the spheres that showed acetylated-STAT3 dimer association with nuclear CD44, including those expressing wild-type, C3–C10, ΔC4–C7, and Δ37(C286,295A) of CD44s and Cont-shRNA in comparison to cells expressing wild-type CD44s maintained as subconfluent monolayers (WT/AD) (Figure 4B). Consistent with this, the expression level of c-myc transcripts (data not shown) and proteins (Figure 4C) significantly increased. Because cells in the spheres undergo a stable morphological transition that may reflect reprogramming (Figure 1E), we used western blotting to examine the expression of stem cell-related genes in cells derived from spheres after 12 days in sphere-forming culture and compared these results with cells cultured (monolayer) in tissue culture plates (WT/AD). These proteins included c-Myc, SOX2, and OCT4, whose expression is related with reprogramming of somatic cells to a stem cell phenotype. Expression of c-Myc, SOX2, and OCT4 in cells expressing wild-type, C3–C10, ΔC4–C7, and Δ37(C286,295A) of CD44s and Cont-shRNA increased during sphere formation in suspension culture (Figure 4C). The similar results were also shown in HCT-116 and DLD-1 cells (Supplementary Figure S5A). To confirm protein expression, spheres were immunostained for SOX2 and OCT4. After 12 days in suspension, we observed only low-level cytoplasmic staining for SOX2 and OCT4 in cells expressing wild-type CD44s maintained as subconfluent monolayers (WT/AD) and spheres expressing the CD44s(NLS) mutant (NLS mut/SPH) (Figure 4D). In contrast, nuclear immunostaining for SOX2 and OCT4 became evident in spheres expressing wild-type CD44s (WT/SPH). This nuclear immunostaining was also found in spheres expressing wild-type, C3–C10, ΔC4–C7, and Δ37(C286,295A) of CD44s and Cont-shRNA (data not shown).
Figure 4.
CD44-expressing cells are reprogrammed into stem-like cells after the suspension culture. (A) Validation by qChIP-PCR of putative nuclear CD44 target genes in HT29/CD44+ spheres. A ChIP assay was performed with chromatin from HT29/CD44+ spheres using anti-CD44 mAb. The immunoprecipitated DNA was amplified by qPCR. Equal amounts of anti-CD44 ChIP DNA and total input DNA were used for qPCR employing SYBR Green detection with an ABI7900HT system. (B) Nuclear extracts were prepared from spheres of HT29/CD44+ infected with a lentivirus encoding a shRNA targeting CD44 or a control scrambled shRNA and those of the HT29/CD44−/CD44-myc mutants infected with a lentivirus encoding a shRNA targeting STAT3 or p300, a control scrambled shRNA, or expressing HDAC1. ChIP was performed using anti-CD44 mAb or control IgG. PCR amplification of the designated regions within the c-myc promoter was performed. (C) Total cell lysates were prepared from the spheres described in (B). The expression of c-Myc, SOX2, and OCT4 was assessed by western blotting. β-Actin was used as a loading control. (D) Immunostaining for SOX2 and OCT4 is shown in spheres of HT29/CD44− expressing wild-type CD44s or the NLS mut. (E) Cells derived from spheres described in Figure 3I were stained with Hoechst 33342. Side-population cells excluding Hoechst 33342 were determined. (F) Anchorage-independent assays (AIGs) were performed on cells derived from spheres described in Figure 3I. Data in (A, E, F) were derived from three independent experiments and are presented as mean values±s.d. **P<0.01; ***P<0.005 (t-test). WT/AD (in B–D and F): HT29/CD44− cells expressing wild-type CD44s were cultured (monolayer) in tissue culture plates. AD (in E, F): monolayer culture in tissue culture plates; SPH (in D–F): cultured under sphere-forming conditions.
Multipotential stem cells have been characterized as a side population based on their properties compared with the main population of cells present (Challen and Little, 2006). Also, these side-population properties are shared by a small subset of cells in tumours, referred to as CSCs, that can recapitulate the tumour heterogeneity in xenographic transplants (Challen and Little, 2006). Because of the reexpression of stem cell-related genes (Figure 4D) and the stable changes in morphology seen in sphere-derived cells (Figure 1E), we wondered if sphere formation might be reprogramming the CD44-expressing cancer cells to generate side-population cells with properties of CSCs. We began by examining HT29/CD44−/CD44-myc cells maintained as subconfluent monolayers (AD) and cells derived from spheres (SPH) in Hoechst dye–exclusion assays. We found that CD44s/Mock maintained as subconfluent monolayers (AD) and cells derived from nuclear CD44s/STAT3 signalling-defective spheres (CD44s(NLS mut)/Mock, CD44s/STAT3(K685R), CD44s/STAT3(Y705F, K685R), CD44s/STAT3-shRNA, CD44s/p300-shRNA, and CD44s/HDAC1) did not exclude Hoechst dye (Figure 4E). However, ∼10% of sphere cells expressing CD44s/Mock, CD44s/STAT3(Y705F), and CD44s/Cont-shRNA were Hoechst negative. The similar results were also shown in HCT-116 and DLD-1 cells (Supplementary Figure S5B–D). Indeed, properties such as anchorage independence, which are thought to be a hallmark of transformed cells, have recently been described as a property of stem cells (Reynolds and Weiss, 1996; Weiss et al, 1996; Dontu et al, 2003). Equally important, the reprogrammed cells containing a high percentage of side-population cells formed ∼10-fold more colonies in soft agar suspension culture (anchorage-independent growth, AIG) than did the control, CD44s/Mock/AD, or Mock/Mock/SPH cells (Figure 4F). The similar results were also shown in HCT-116 and DLD-1 cells (Supplementary Figure S5E–G). To further substantiate the role of c-Myc in CD44-mediated reprogramming to CSCs, c-myc transcription was eliminated in HT29 and DLD-1 cells by a lentivirus-based RNA interference technique. As shown in Figure 4E and F and Supplementary Figure S5C–G, the introduction of shRNA against c-myc significantly abolished CD44-mediated generating cells with properties of CSCs (side population and AIG). In contrast, Twist1 was not crucial for CD44-elicited reprogramming to a CSC phenotype. Consistent with this, overexpression CD44 in HT29/CD44− cells after the suspension culture significantly increased the expression of all c-myc, STAT3, SOX2, and OCT4 mRNA (Supplementary Figure S6A). Knockdown of c-myc transcripts in HT29/CD44+ cells after the suspension culture significantly abolished all genes upregulation (Supplementary Figure S6B). Furthermore, CD44 and STAT3 transcripts were enhanced by c-Myc via a positive feedback loop (Supplementary Figure S6B). Taken together, this indicates that intact nuclear CD44/STAT3 signalling is crucial for reprogramming of cancer cells to a CSC phenotype via transcriptional regulation of c-myc expression and subsequent self-renewal of CSCs.
CD44-expressing cells after the suspension culture exhibit attributes of cells that have undergone an EMT
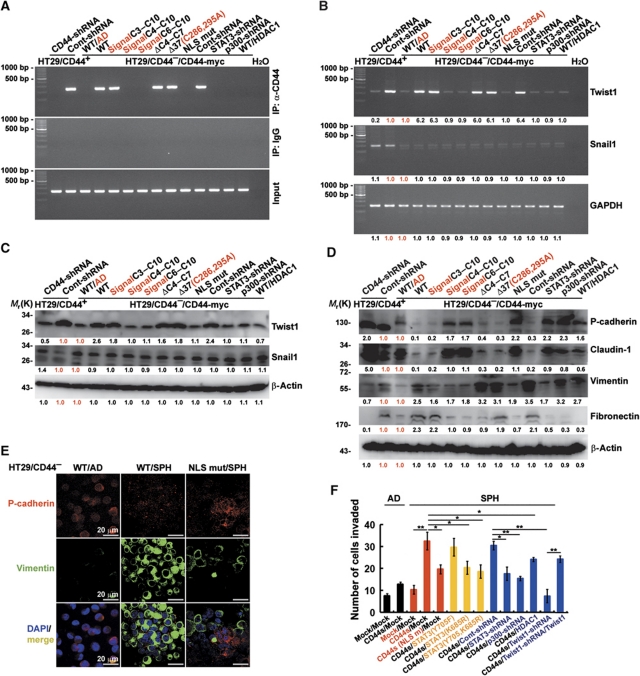
The EMT is a key developmental programme that is often activated during cancer invasion and metastasis (Thiery, 2003). Recent studies also have showed that overexpression of these EMT-related transcription factors can also induce a CD44-high/CD24-low pattern on epithelial cells, which is associated with the somatic cells obtaining stem cell and CSC properties (Mani et al, 2008). Interestingly, the promoter of Twist1, an E box-binding transcriptional repressor that represses E-cadherin and leads to EMT, was isolated by ChIP assays performed to search for DNA sequences bound by nuclear CD44 complexes (Figure 4A). Therefore, we wondered whether expression of these EMT transcription factors was induced in the spheres expressing CD44. To better understand the roles of nuclear CD44 in self-renewal and transdifferentiation programmes in cancer stem-like cells, ChIP assays were performed. ChIP assays showed that nuclear CD44 was bound to the promoter region of Twist1 only in the spheres that showed acetylated-STAT3 dimer association with nuclear CD44, including those expressing wild-type, C3–C10, ΔC4–C7, and Δ37(C286,295A) of CD44s and Cont-shRNA in comparison to cells expressing wild-type CD44s maintained as subconfluent monolayers (WT/AD) (Figure 5A). In agreement, the expression level of Twist1 transcripts (Figure 5B) and proteins (Figure 5C) significantly increased in spheres expressing wild-type, C3–C10, ΔC4–C7, and Δ37(C286,295A) of CD44s and Cont-shRNA. The similar results were also shown in HCT-116 and DLD-1 cells (Supplementary Figure S7A). Individual members of a group of six to eight transcription factors are capable of orchestrating EMT programmes during embryonic development and in cancer (Peinado et al, 2007; Moreno-Bueno et al, 2008). Furthermore, some of these transcription factors, including Twist1, have a part in overcoming cellular senescence (Ansieau et al, 2008) and in generating tumourigenic CSCs (Mani et al, 2008). As anticipated, the resulting cells acquired mesenchymal appearance, downregulated the expression of proteins encoding epithelial markers (such as P-cadherin and claudin-1), and upregulated proteins encoding mesenchymal markers (such as Vimentin and Fibronectin) (Figure 5D). The similar results were also shown in HCT-116 and DLD-1 cells (Supplementary Figure S7B). To confirm protein expression, spheres were immunostained for P-cadherin and Vimentin. Indeed, we found that the expression levels of these EMT-associated genes in cells derived from HT29/CD44−/CD44-myc spheres (WT/SPH) resembled the levels seen in cells that have undergone EMT, whereas the expression levels of these genes in the HT29/CD44−/CD44-myc cells maintained as subconfluent monolayers (WT/AD) and cells derived from HT29/CD44−/CD44s(NLS mut) spheres (NLS mut/SPH) did not. Specifically, relative to levels in the WT/AD and NLS mut/SPH cells, the WT/SPH cells exhibited a strong reduction in the P-cadherin protein and significantly higher expression of Vimentin (Figure 5E).
Figure 5.
CD44-expressing cells after the suspension culture exhibit attributes of cells that have undergone an EMT. (A) Nuclear extracts were prepared from spheres described in Figure 4B. ChIP was performed using anti-CD44 or control IgG. PCR amplification of the designated regions within the Twist1 promoter was performed. (B) mRNAs were prepared from the spheres described in Figure 4B. The expression of Twist1 and Snail1 was evaluated by RT–PCR. (C) Total cell lysates were prepared from the spheres described in Figure 4B. The expression of Twist1 and Snail1 was measured by western blotting. (D) Total cell lysates were prepared from the spheres described in Figure 4B. The expression of epithelial markers (P-cadherin and claudin-1) and mesenchymal markers (Vimentin and Fibronectin) was measured by western blotting. (E) Immunostaining for P-cadherin and Vimentin was shown in spheres of HT29/CD44− expressing wild-type CD44s or the NLS mut. (F) Boyden chamber assays were performed on cells derived from spheres described in Figure 2I to assess the invasive activity. Data were derived from three independent experiments and are presented as mean values±s.d. *P<0.05; **P<0.01 (t-test). The relative intensities of the bands (in B–D) were densitometrically quantified and normalized to Cont-shRNA (CD44+ cells) and WT/AD (CD44− cells). WT/AD (in A–E): HT29/CD44− cells expressing wild-type CD44s were cultured (monolayer) in tissue culture plates. AD (in F): monolayer culture in tissue culture plates; SPH (in F): cultured under sphere-forming conditions.
Numerous observations support the idea that EMT has a central role in tumour progression. During progression to metastatic competence, carcinoma cells acquire mesenchymal gene–expression patterns and properties. This results in changed adhesive properties and the activation of proteolysis and motility, which allows the tumour cells to metastasize and establish secondary tumours at distant sites (Tarin et al, 2005). We began by examining HT29/CD44−/CD44-myc cells maintained as subconfluent monolayers (AD), and cells derived from spheres (SPH) for invasion assays. We found that CD44s/Mock maintained as subconfluent monolayers (AD) and cells derived from nuclear CD44/STAT3 signalling-defective spheres (CD44s(NLS mut)/Mock, CD44s/STAT3(K685R), CD44s/STAT3(Y705F, K685R), CD44s/STAT3-shRNA, CD44s/p300-shRNA, and CD44s/HDAC1) had lower invasion abilities (Figure 5F). However, cells derived from spheres expressing CD44s/Mock, CD44s/STAT3(Y705F), and CD44s/Cont-shRNA were more invasive. The similar results were also shown in HCT-116 and DLD-1 cells (Supplementary Figure S7C). To further substantiate the role of Twist1 in CD44-mediated transforming to an EMT phenotype, Twist1 transcription was eliminated in HT29 and DLD-1 cells by a lentivirus-based RNA interference technique. As shown in Figure 5F and Supplementary Figure S7D and E, the introduction of shRNA against Twist1 significantly abolished CD44-mediated transforming to an EMT phenotype. In contrast, c-Myc was not crucial for CD44-elicited EMT transforming.
Expression of CD44/c-Myc enhances tumourigenesis and metastasis to the lung in experimental animal models
For in vivo tumourigenicity assay, mice were injected subcutaneously with 103–104 cells, which were derived from HT29/CD44+ and HCT-116 spheres infected by lentivirus-encoding shRNA targeting CD44 or HT29/CD44− spheres expressing various CD44 mutants. As shown in Table I, HT29/CD44−/CD44(WT), HT29/CD44+/Cont-shRNA, and HCT-116/Cont-shRNA cells that can form spheres and subsequently reprogramme into stem-like cells after the suspension culture in vitro elicited high tumourigenecity in vivo. In a dose response of HT29 and HCT-116 cells cultured in tissue culture plates (AD; 103–104 cells) injected per mouse, no tumour growth was evident at 16 weeks unless at least 106 cells were injected, where four of six mice developed tumours (data not shown). Injection of 103 cells derived from HT29/CD44−/CD44(WT) spheres formed tumours (4 of 6 animals), whereas no tumours were observed with cells derived from CD44Δ61(C286,295/KA) and CD44(NLS mut) spheres. Similar results were obtained with cells derived from HT29/CD44+/Cont-shRNA and HCT-116/CD44+/Cont-shRNA spheres showing the highest tumourigenic potential, with 6 of 6 and 4 of 6 animals developing tumours when injected with as few as 103 cells. The cells derived from spheres with the highest tumourigenic potential were those cells expressing CD44 and c-Myc, where 4–6 of 6 animals injected with 103 cells formed tumours, and cells negative for expression of these proteins did not develop any tumours. For experimental metastasis assays, cells (106, cultured in monolayer) in 100 μl PBS were injected into the tail vein. Mice were killed 3 weeks after injection, the left lung lobes were embedded. As shown in Table I, CD44-expressing cells injected into the tail vein of severe combined immunodeficient (SCID) mice displayed a higher ability to disseminate and form metastases in the lungs of mice. These tumours were positive for villin (a marker for intestinal cells), confirming that they were derived from the injected cells. Histologic analysis of such micrometastases confirmed that the metastatic lesions replaced large areas of the lung parenchyma, suggesting that cells retaining CD44/c-Myc/Twist1 axis gained extensive metastasis ability.
Table 1. In vivo tumourigenesis and metastasis assays.
| Tumourigenesis (SC) |
Metastasis (IV) | ||
|---|---|---|---|
| 104 Cells | 103 Cells | 106 Cells | |
| HT29/CD44 − | |||
| Mock | 0/6 | 0/6 | |
| CD44(WT) | 5/6 | 4/6 | 5/6 |
| CD44Δ61(C286,295A/KA) | 0/6 | 0/6 | |
| CD44(NLS mut) | 0/6 | 0/6 | |
| HT29/CD44 + | |||
| Cont-shRNA | 6/6 | 6/6 | 5/6 |
| CD44-shRNA | 0/6 | 0/6 | |
| STAT3-shRNA | 0/6 | 0/6 | |
| c-Myc-shRNA | 0/6 | 0/6 | |
| Twist1-shRNA | 5/6 | 4/6 | 0/6 |
| HCT-116 | |||
| Cont-shRNA | 5/6 | 4/6 | 4/6 |
| CD44-shRNA | 0/6 | 0/6 | |
| NOTE: For in vivo tumourigenicity assay, mice were injected subcutaneously with 103–104 cells, the cells derived from spheres (SPH). Tumourigenecity was evaluated at 6 weeks after transplantation. For experimental metastasis assays, HT29 and HCT-116 cells (106, AD) in 100 μl PBS were injected into the tail vein. Mice were killed 3 weeks after injection, the left lung lobes were embedded. | |||
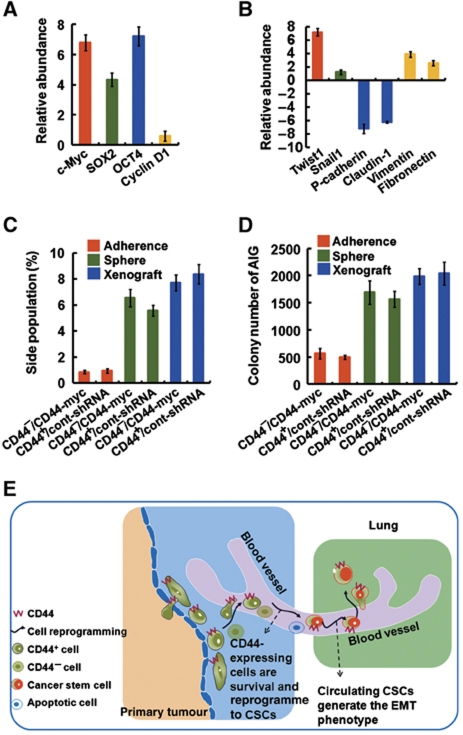
To test if the highly metastatic cancer cells derived from the xenografts (described in Table I) also exhibited the properties of CSCs and an EMT phenotype, the resultant tumours were analysed. The pattern of CSC-related genes (Figure 6A), and EMT-related genes (Figure 6B) expression evident in the secondary tumours in vivo was similar to that which was observed in vitro (Figures 4 and 5). Furthermore, the highly metastatic cancer cells derived from the xenografts indeed also possessed more side-population (Figure 6C) and clonal formation abilities (Figure 6D). In this study, we found that many diverse lines of evidence suggested a possible link between CD44, CSCs, the sphere-forming culture, and tumour progression. During the process of tumour metastasis, lipid raft-associated CD44 is required for survival in the suspension condition, and then nuclear CD44/acetylated-STAT3 generates cells with properties of CSCs and an EMT phenotype by transcriptional reprogramming, leading to tumour metastasis and resultant worse prognosis (Figure 6E).
Figure 6.
Characterization of the highly metastatic cancer cells derived from the xenografts. (A, B) Real-time PCR showing expression of mRNAs for the CSCs-related genes (in A) and EMT-related genes (in B) in the highly metastatic cancer cells derived from the xenografts (HT29/CD44+/Cont-shRNA). The comparison is to cells in subconfluent monolayers. (C) Cells derived from the xenografts described in (A) were stained with Hoechst 33342. Side-population cells excluding Hoechst 33342 were determined. (D) Anchorage-independent assays (AIGs) were performed on cells derived from the xenografts described in (A). (E) Model proposing a pathway for generation of cells with properties of CSCs from differentiated somatic cells in the suspension condition, and then exhibit attributes of cells that have undergone an EMT, leading to tumour metastasis. See text for discussion. Data in (A–D) were derived from three independent experiments and are presented as mean values±s.d.
Discussion
Solid tumours as well as haematopoietic cancers (Lapidot et al, 1994) have been shown to contain a small population of cancer-initiating cells (Dick, 2005; Trosko, 2006; O’Brien et al, 2007). Identification of markers for the prospective isolation of CSCs has been made for breast (CD44+ CD24−/low) (Al-Hajj et al, 2003), CNS (CD133+) (Singh et al, 2004), multiple myeloma (CD138–) (Matsui et al, 2004), melanoma (CD20+) (Fang et al, 2005), prostate (CD44+ α2β1+ CD133+) (Collins et al, 2005; Patrawala et al, 2006), HNSCC (CD44+) (Prince et al, 2007), colon (CD133+) (O’Brien et al, 2007; Ricci-Vitiani et al, 2007), colon (CD44+ EpCam+ CD166+) (Dalerba et al, 2007b), and pancreas (CD44+ EpCam+ CD24+) (Li et al, 2007). Despite these cell surface markers being widely used for isolating CSCs, the functional aspects of maintaining CSCs have yet to be determined. Clarification of this question and a better understanding of the properties of CSCs may be useful to develop better therapeutic approaches to fight cancer.
In our laboratory, we found that cell surface markers (CD44 in primary breast, prostate, and colon tumour specimens; CD133 in primary CNS tumour specimens; EpCam in primary pancreas tumour specimens) are functionally important to CSCs for sphere formation (data not shown). CD44 (Lee et al, 2009; Janiszewska et al, 2010), CD133, and EpCam (Maetzel et al, 2009) are known to migrate to the nucleus as an intact polypeptide or a proteolytic fragment with or without their corresponding ligands. These nuclear localized receptors have been shown to act as transcription factors for genes like cyclin D1. We previously showed that CD44, once engaged, is internalized and translocated to the nucleus; there it binds to various promoters leading to cell fate change through transcriptional reprogramming (Lee et al, 2009). In this study, we also demonstrated that the transcriptional reprogramming led by nuclear CD44 has an active role in transforming cancer cells to a CSC-like phenotype. The nuclear translocation of cell surface receptors may relate to their role as a CSC marker.
It is widely accepted that the metastatic process is comprised of a series of complex and sequential steps, including escape from the primary tumour (intravasation), dissemination via the blood or lymphatic system, survival within the circulation, arrest and extravasation into a secondary site, initiation of growth into micrometastases, and maintenance of growth as vascularized, clinically detectable macrometastases (Chambers et al, 2002; Pantel and Brakenhoff, 2004). However, the details of molecular mechanisms remain poorly understood. In this study, we found that CD44 has an important role in tumour metastasis. When tumour cells under sphere-forming conditions in vitro, C terminus of CD44 contributes to anoikis resistance through the CD44–Src–integrin axis in lipid rafts. Furthermore, nuclear CD44/STAT3 signalling is crucial for reprogramming of cancer cells to a CSC phenotype via transcriptional regulation of c-myc expression. We propose that nuclear CD44/acetylated-STAT3 performs an unexpected tumour-progressing function by enhancing cell outgrowth into structures where cells with properties of CSCs can be generated from differentiated somatic cells in the suspension condition, and then exhibit attributes of cells that have undergone an EMT, leading to tumour metastasis, and a resulting worse prognosis.
If the growth of cancers were driven by CSCs, it would have profound implications for cancer therapy. For many years, however, it has been recognized that small numbers of disseminated cancer cells can be detected at sites distant from primary tumours in patients that never manifest metastatic disease. One possibility is that most cancer cells lack the ability to form a new tumour such that only the dissemination of rare CSCs can lead to metastatic disease. If so, the goal of therapy must be to identify and kill this CSC population. If CSCs can be identified prospectively and isolated, then we should be able to identify more efficiently new diagnostic markers and therapeutic targets expressed by CSCs.
The study of circulating CSCs metastasized from solid tumours in patients has recently raised great interest, as these cells have been proposed as surrogate markers for more than a dozen pathologies, including detecting cancer metastasis. In addition, anticancer therapy as well as therapeutic metastasis-preventing treatments may directly and immediately affect circulating CSCs because of their direct exposure to the infused agent. Validating a biomarker (e.g. CD44) for anticancer or metastasis-preventing treatment agents is critically important for the development of these emerging therapies. Here, we propose a protocol for phenotypic identification and enumeration of circulating CSCs in human blood using the surface marker (CD44) and nuclear markers (CD44, STAT3, c-Myc, SOX2, and OCT4). This method allows further phenotypic analyses to explore the biology of these cells. In addition, it offers a platform for longitudinal studies of these cells in patients with different pathologies.
Materials and methods
Constructs
The wild-type CD44s and CD44s mutants with C-terminal deletions (CD44sΔ67) mutants were gifts from Ursula Günthert (Mielgo et al, 2007) (University of Basel, Petersplatz, Basel, Switzerland). The CD44s mutants with C-terminal deletions (Δ37 and Δ61) and N-terminal deletions (C3–C10, C4–C10, C6–C10, and ΔC4–C7) were generated by PCR amplification of the corresponding cDNA fragments using the wild-type CD44s as a template. The cysteine mutants (C286A and/or C295A), the lysine-to-alanine (KA) mutant, and the NLS mutant of CD44 were constructed by site-directed mutagenesis using the wild-type CD44s cDNA template. Wild-type STAT3 was purchased from Genediscovery Biotechnology. The STAT3 mutants with deletions (STAT3(1–134), STAT3(1–317), and STAT3(318–770)) were generated by PCR amplification of the corresponding cDNA fragments using the wild-type STAT3 as a template. The tyrosine mutants (STAT3(Y705F) and STAT3(K685R)) were constructed by site-directed mutagenesis using the wild-type STAT3 cDNA template.
Immunoblot analysis
Western blotting was performed as previously described (Lee et al, 2004). Images were recorded using the luminescent image analyser (FUSION SL; Vilber Lourmat), and the intensities of the bands were quantitated by densitometry using Bio-1D and Bio-Gene software (Vilber Lourmat).
Immunocytostaining
For immunofluorescence, cells were fixed in 4% paraformaldehyde, incubated with primary antibody and Alexa Fluor 594- or Alexa Fluor 488-conjugated secondary antibody, counterstained with DAPI, and examined under a laser-scanning confocal system (Radiance 2100; Bio-Rad Laboratories) with Plan-Apochromat oil immersion × 60 or × 100 NA 1.4 objective lenses (Nikon) using the standard analysis software (Lasersharp 2000; Bio-Rad Laboratories) as described (Lee et al, 2009). The images were arranged and labelled using Photoshop software (Adobe).
ChIP assay
ChIP assay was performed as previously described (Lee et al, 2009). Extracted DNA was analysed by PCR using primers spanning the proximal (nucleotide positions –498/+20) promoter regions of c-myc or (–204/–5) promoter regions of Twist1. Following 30 cycles of amplification, PCR products were run on a 1.5% agarose gel and analysed by ethidium bromide staining.
The sphere-forming culture
Cells (1000/ml) were grown in suspension culture using ultra-low-attachment plates (Corning) and serum-free RPMI (ATCC) supplemented with B27 (Invitrogen), 20 ng/ml EGF, and 10 ng/ml bFGF (BD Biosciences). The spheres with diameter >30 μm were then counted. For serial passages, spheres were harvested and dissociated to single cells with trypsin, and 100 dissociated cells were replated in a 96-well plate (a ultra-low-attachment plate) and cultured for 12 days. The spheres were then counted again. The individual spheres were found to be derived from single cells (Mani et al, 2008).
Identification and isolation of side-population cells
Cells were suspended in prewarmed RPMI containing 2% FBS and 10 mM HEPES, and stained with 5 μg/ml Hoechst 33342 dye (Molecular Probes) for 90 min at 37°C with or without 100 μM reserpine, which is an inhibitor of some ATP-binding cassette transporters. Cells were then washed and resuspended in HBSS containing 2% FBS and 10 mM HEPES. Before cell sorting, 0.25 μg/ml 7-AAD (Sigma) was added to exclude nonviable cells. The concentration of Hoechst 33342 and the incubation times were initially identified using samples that provided the highest frequency of side-population cells with the lowest cytotoxicity determined by 7-AAD staining. Side-population cells were analysed on a FACSAria (BD Biosciences) flow cytometer equipped with 424/44 nm band pass and 670 nm long pass optical filters (Omega Optical).
Animal experiments
SCID CB17 female mice (6 weeks old) were obtained from the animal centre (the College of Medicine, National Taiwan University, Taipei, Taiwan) maintained in a specific pathogen-free environment and all the experiments were carried out with the approval of the local authorities. For in vivo tumourigenicity assay, mice were injected subcutaneously with 103–104 cells, the cells derived from spheres in 100 μl of a mixture of DMEM/Matrigel (1:1). Tumourigenecity was evaluated at 6 weeks after transplantation. For experimental metastasis assays, HT29 cells (106) in 100 μl PBS were injected into the tail vein. Mice were killed 3 weeks after injection, the left lung lobes were embedded, and one section every 100 μm was stained with H&E according to the standard protocols. Metastasis was defined as a group of more than five enterocytes. The intestinal origin of cells was confirmed by villin immunostaining.
Statistical analysis
Statistical analysis of data was performed by Student’s t-test using SigmaPlot software. Difference was considered to be statistically significant at P<0.05.
Supplementary Material
Acknowledgments
This work was supported by funds from the National Science Council (99-2314-B-007-001 and 99-2321-B-007-004), Taiwan.
Author contributions: YJS and HML designed, performed, and analysed the experiments; YWC and GYC assisted in the interpretation of results; JLL wrote and edited the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 100: 3983–3988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansieau S, Bastid J, Doreau A, Morel AP, Bouchet BP, Thomas C, Fauvet F, Puisieux I, Doglioni C, Piccinin S, Maestro R, Voeltzel T, Selmi A, Valsesia-Wittmann S, Caron de Fromentel C, Puisieux A (2008) Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell 14: 79–89 [DOI] [PubMed] [Google Scholar]
- Challen GA, Little MH (2006) A side order of stem cells: the SP phenotype. Stem Cells 24: 3–12 [DOI] [PubMed] [Google Scholar]
- Chambers AF, Groom AC, MacDonald IC (2002) Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2: 563–572 [DOI] [PubMed] [Google Scholar]
- Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ (2005) Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res 65: 10946–10951 [DOI] [PubMed] [Google Scholar]
- Dalerba P, Cho RW, Clarke MF (2007a) Cancer stem cells: models and concepts. Annu Rev Med 58: 267–284 [DOI] [PubMed] [Google Scholar]
- Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA, Parmiani G, Castelli C, Clarke MF (2007b) Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA 104: 10158–10163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick JE (2005) Acute myeloid leukemia stem cells. Ann N Y Acad Sci 1044: 1–5 [DOI] [PubMed] [Google Scholar]
- Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS (2003) In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev 17: 1253–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE, Herlyn M (2005) A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res 65: 9328–9337 [DOI] [PubMed] [Google Scholar]
- Frisch SM, Ruoslahti E (1997) Integrins and anoikis. Curr Opin Cell Biol 9: 701–706 [DOI] [PubMed] [Google Scholar]
- Janiszewska M, De Vito C, Le Bitoux MA, Fusco C, Stamenkovic I (2010) Transportin regulates nuclear import of CD44. J Biol Chem 285: 30548–30557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE (1994) A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367: 645–648 [DOI] [PubMed] [Google Scholar]
- Lee JL, Lin CT, Chueh LL, Chang CJ (2004) Autocrine/paracrine secreted Frizzled-related protein 2 induces cellular resistance to apoptosis: a possible mechanism of mammary tumorigenesis. J Biol Chem 279: 14602–14609 [DOI] [PubMed] [Google Scholar]
- Lee JL, Wang MJ, Chen JY (2009) Acetylation and activation of STAT3 mediated by nuclear translocation of CD44. J Cell Biol 185: 949–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Wang MJ, Sudhir PR, Chen GD, Chi CW, Chen JY (2007) Osteopontin promotes integrin activation through outside-in and inside-out mechanisms: OPN-CD44V interaction enhances survival in gastrointestinal cancer cells. Cancer Res 67: 2089–2097 [DOI] [PubMed] [Google Scholar]
- Lee JL, Wang MJ, Sudhir PR, Chen JY (2008) CD44 engagement promotes matrix-derived survival through the CD44-SRC-integrin axis in lipid rafts. Mol Cell Biol 28: 5710–5723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM (2007) Identification of pancreatic cancer stem cells. Cancer Res 67: 1030–1037 [DOI] [PubMed] [Google Scholar]
- Macara IG (2001) Transport into and out of the nucleus. Microbiol Mol Biol Rev 65: 570–594, table of contents [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maetzel D, Denzel S, Mack B, Canis M, Went P, Benk M, Kieu C, Papior P, Baeuerle PA, Munz M, Gires O (2009) Nuclear signalling by tumour-associated antigen EpCAM. Nat Cell Biol 11: 162–171 [DOI] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133: 704–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui W, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y, Smith BD, Civin CI, Jones RJ (2004) Characterization of clonogenic multiple myeloma cells. Blood 103: 2332–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielgo A, Brondani V, Landmann L, Glaser-Ruhm A, Erb P, Stupack D, Gunthert U (2007) The CD44 standard/ezrin complex regulates Fas-mediated apoptosis in Jurkat cells. Apoptosis 12: 2051–2061 [DOI] [PubMed] [Google Scholar]
- Moreno-Bueno G, Portillo F, Cano A (2008) Transcriptional regulation of cell polarity in EMT and cancer. Oncogene 27: 6958–6969 [DOI] [PubMed] [Google Scholar]
- O’Brien CA, Pollett A, Gallinger S, Dick JE (2007) A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 445: 106–110 [DOI] [PubMed] [Google Scholar]
- Pantel K, Brakenhoff RH (2004) Dissecting the metastatic cascade. Nat Rev Cancer 4: 448–456 [DOI] [PubMed] [Google Scholar]
- Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra D, Zhou J, Claypool K, Coghlan L, Tang DG (2006) Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene 25: 1696–1708 [DOI] [PubMed] [Google Scholar]
- Peinado H, Olmeda D, Cano A (2007) Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 7: 415–428 [DOI] [PubMed] [Google Scholar]
- Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE (2007) Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA 104: 973–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S (1992) Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255: 1707–1710 [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S (1996) Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev Biol 175: 1–13 [DOI] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R (2007) Identification and expansion of human colon-cancer-initiating cells. Nature 445: 111–115 [DOI] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB (2004) Identification of human brain tumour initiating cells. Nature 432: 396–401 [DOI] [PubMed] [Google Scholar]
- Tarin D, Thompson EW, Newgreen DF (2005) The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res 65: 5996–6000; discussion 6000--5991 [DOI] [PubMed] [Google Scholar]
- Thiery JP (2003) Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol 15: 740–746 [DOI] [PubMed] [Google Scholar]
- Trosko JE (2006) From adult stem cells to cancer stem cells: Oct-4 Gene, cell-cell communication, and hormones during tumor promotion. Ann N Y Acad Sci 1089: 36–58 [DOI] [PubMed] [Google Scholar]
- Weiss S, Reynolds BA, Vescovi AL, Morshead C, Craig CG, van der Kooy D (1996) Is there a neural stem cell in the mammalian forebrain? Trends Neurosci 19: 387–393 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.