Abstract
Receptor protein tyrosine phosphatase α (RPTPα)-mediated Src activation is required for survival of tested human colon and oestrogen receptor-negative breast cancer cell lines. To explore whether mutated RPTPα participates in human carcinogenesis, we sequenced RPTPα cDNAs from five types of human tumours and found splice mutants in ∼30% of colon, breast, and liver tumours. RPTPα245, a mutant expressed in all three tumour types, was studied further. Although it lacks any catalytic domain, RPTPα245 expression in the tumours correlated with Src tyrosine dephosphorylation, and its expression in rodent fibroblasts activated Src by a novel mechanism. This involved RPTPα245 binding to endogenous RPTPα (eRPTPα), which decreased eRPTPα–Grb2 binding and increased eRPTPα dephosphorylation of Src without increasing non-specific eRPTPα activity. RPTPα245–eRPTPα binding was blocked by Pro210 → Leu/Pro211 → Leu mutation, consistent with the involvement of the structural ‘wedge’ that contributes to eRPTPα homodimerization. RPTPα245-induced fibroblast transformation was blocked by either Src or eRPTPα RNAi, indicating that this required the dephosphorylation of Src by eRPTPα. The transformed cells were tumourigenic in nude mice, suggesting that RPTPα245-induced activation of Src in the human tumours may have contributed to carcinogenesis.
Keywords: alternative splicing, cancer, RPTPα, Src, protein tyrosine phosphatase
Introduction
Src protein tyrosine kinase specific activity is increased without mutation in many human cancers, suggesting that aberrant upstream regulation is involved (Yeatman, 2004; Wheeler et al, 2009). Src is primarily regulated by the phosphorylation state of Tyr530 (human Src numbering throughout). Phosphotyrosine (pTyr)530 binds the Src SH2 domain, thereby stabilizing a compact, inactive state; therefore, pTyr530 → Phe mutation or dephosphorylation activates Src (Hunter, 1987). PTyr530 dephosphorylation is often accompanied by autophosphorylation of pTyr419 within the activation loop, which results in the full ∼10-fold increase in kinase activity (Boggon and Eck, 2004). Activity is still increased ∼5-fold even when autophosphorylation is prevented by Tyr419 → Phe mutation (Kmiecik and Shalloway, 1987; Piwnica-Worms et al, 1987). The transmembrane receptor protein tyrosine phosphatase (RPTP)α (Pallen, 2003, for review) can dephosphorylate both pTyr530 and pTyr419 in vitro and activates Src ∼5-fold by dephosphorylating both residues in vivo (Zheng et al, 1992, 2000; den Hertog et al, 1993). In addition to activating Src, RPTPα overexpression can induce transformation (Zheng et al, 1992, 2000), induce neuronal differentiation and outgrowth (den Hertog et al, 1993; Yang et al, 2002), or modulate cell-substratum adhesion (Harder et al, 1998), depending on cell type. Conversely, pTyr530 phosphorylation is increased and Src activity, integrin-mediated signalling responses, cell spreading, and the transient mitotic activation of Src are decreased in RPTPα−/− cells (Ponniah et al, 1999; Su et al, 1999; Zheng and Shalloway, 2001; Zeng et al, 2003). In addition, RPTPα RNAi deactivates Src and induces apoptosis in human colon and oestrogen receptor-negative (ER−) breast cancer cell lines (Zheng et al, 2008). Thus, RPTPα appears to be an important physiological regulator of Src and might collaborate with it in some types of human carcinogenesis.
RPTPα's activation of Src is reduced by homodimerization at the cell surface, which is stabilized by interactions involving its extracellular, transmembrane, and D1 and D2 catalytic domains (Jiang et al, 1999, 2000; Tertoolen et al, 2001). The D1 domain is responsible for most catalytic activity (Wang and Pallen, 1991; den Hertog et al, 1993). The D2 domain is required for RPTPα-Src binding and might be involved in substrate presentation to the D1 region (Vacaru and den Hertog, 2010b). Src-dephosphorylating activity is also regulated by pTyr789 (Zheng et al, 2000), a Grb2-binding residue located near the RPTPα COOH terminus (den Hertog et al, 1994; Su et al, 1994): ‘Phosphotyrosine displacement’ by pTyr789 transiently replaces pTyr530 in the Src SH2 domain binding pocket, thereby deprotecting pTyr530 while increasing the ability of RPTPα to dephosphorylate Src in vitro (Zheng et al, 2000). Correspondingly, Tyr789 → Phe (Y789F) mutation impairs the abilities of overexpressed RPTPα to bind, dephosphorylate, and activate Src in vivo (Zheng et al, 2000), to transform NIH3T3 cells (Zheng et al, 2000), and to activate Fyn, a related Src family kinase, in T cells (Maksumova et al, 2007).
Regulation of Src-directed activity by the level of Tyr789 phosphorylation is quantitative, not absolute, and depends on the biological system (see Supplementary data): Y789F mutation reduces but does not eliminate RPTPα-Src binding (Vacaru and den Hertog, 2010a), and some activation of Src in vivo can be detected when RPTPα(Y789F) is retrovirally expressed at high levels (Kapp et al, 2007). Additionally, third-party proteins may act in lieu of pTyr789 in some situations. For example, an autophosphorylated pTyr in FAK can displace pTyr530 from the Src SH2 domain (Schaller et al, 1994; Zhao and Guan, 2009), and it has been suggested that this, along with integrin–RPTPα interactions, can replace both the phosphotyrosine displacement and colocalization functions of pTyr789 in integrin-activated cells (Chen et al, 2006). An analogous mechanism might explain the ability of a mutant lacking Tyr798 (the Tyr789 homologue in the neuronal form of RPTPα; see Supplementary data) to activate Src in neuronal cells (Yang et al, 2002).
About 20% of Tyr789 in RPTPα is phosphorylated in NIH3T3 cells, but most of this is bound by the Grb2 SH2 domain (den Hertog et al, 1994; Su et al, 1994), which blocks its ability to displace pTyr530 (Zheng et al, 2000). This is consistent with consensus binding data (Songyang and Cantley, 1995) and experimental measurements (Zheng et al, 2000), indicating that Grb2 SH2 has ∼10-fold higher binding affinity than Src SH2 for binding pTyr789 and its surrounding sequence. Thus, adding Grb2 SH2 blocks dephosphorylation of Src by RPTPα in vitro (Zheng et al, 2000) and is likely to have the same effect in vivo (Zheng and Shalloway, 2001). Since even a small decrease in Grb2–RPTPα binding causes a large fractional increase in the amount of Tyr789-phosphorylated/Grb2-unbound RPTPα that is available for phosphotyrosine displacement, this system can act as a signal amplifier.
The deactivation of Src and induction of apoptosis induced by RPTPα RNAi in human colon and ER– breast cancer lines (Zheng et al, 2008) suggested that altered RPTPα might activate Src in some human cancers of these types. To test this hypothesis, we sequenced RPTPα cDNAs from 36 human tumours of five types and found incorrectly spliced mutants in colon, breast, and liver tumours. One mutant, which was found in all three tumour types, was studied in detail and shown to induce Src dephosphorylation and activation, to transform Fisher rat embryo fibroblasts (REFs), and to form xenograft tumours in nude mice. It accomplishes this by an unexpected mechanism: it binds with endogenous RPTPα (eRPTPα), which reduces eRPTPα–Grb2 binding and coordinately increases eRPTPα's ability to activate Src, possibly by enhanced phosphotyrosine displacement. The splice mutant is generated by the inappropriate inclusion of an extremely conserved cryptic exon that has been found in a tissue-specific cDNA library.
Results
Identification of three RPTPα truncation mutants in human tumours
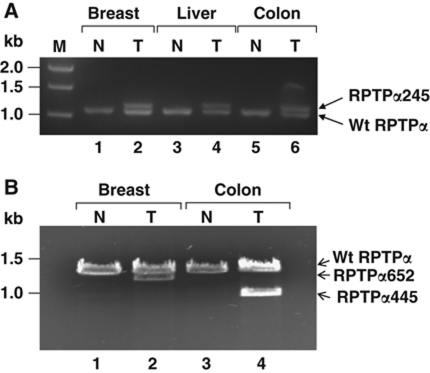
We used reverse transcription (RT)–PCR to amplify and sequence the coding region of oligo-dT primed RPTPα cDNAs from 36 tumours (and paired normal samples from surrounding tissue) of five tissue types from randomly selected patients of Chinese descent (Supplementary Table S1). Based on prior epidemiological and cancer cell line studies (Zheng et al, 2008; Wheeler et al, 2009), our a priori hypothesis was that mutations would be found in colon and breast tumours. Indeed, six altered-mobility cDNAs were amplified from 3/8 (mutated/total) colon, 2/9 breast, and also from 1/2 liver tumours, but only wild-type (wt)-mobility cDNAs were amplified from 7 lung and 10 thyroid tumours and from the 36 normal samples (Figure 1 and data not shown). The observed frequency-of-occurrence of mutants in the a priori-predicted pooled colon and breast tumour population was ∼30% with a Wilson confidence interval (α=0.05) of 13–53%.
Figure 1.
RPTPα cDNAs from human tumour and control specimens. Total RNA was extracted from frozen tumour (T) and normal (N) tissue samples (see reference numbers listed in Supplementary Table S1: (A) #58, #69, and #97; (B) #128 and #223) and double-stranded cDNAs, which together spanned the entire RPTPα-coding regions, were produced from 5 μg total RNA by RT–PCR (see Supplementary Table S3 for primers) and analysed by 1% agarose gel electrophoresis and ethidium bromide staining. (A) The 5′-proximal cDNAs, which include wt codons 1–361, are shown. (B) The 3′-proximal cDNAs, which include wt codons 344–793, are shown. The positions of molecular weight standards (M) are indicated in kilobase pairs.
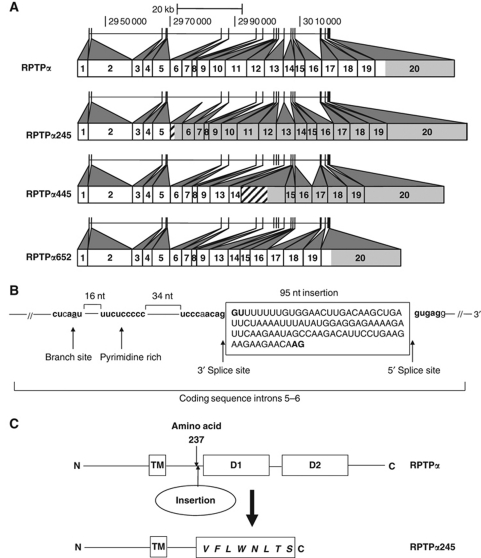
Multiple independent cDNA plasmid clones were generated from each sample and sequenced. All wt-mobility cDNAs had the wt sequence while each altered-mobility cDNA had one of three sequences encoding truncated proteins containing 245, 445, or 652 residues (designated RPTPα245, RPTPα445, or RPTPα652) and lacking the D1 domain. RPTPα245 and RPTPα445 also lacked the D2 domain and contained additional spurious residues (Figure 2A; Table I). Comparison with the genomic sequence showed that RPTPα445 and RPTPα652 resulted from incorrect splice donor–acceptor pairing, while RPTPα245 contained a 95-nucleotide (nt) cryptic exon having U2-type splice acceptor/donor sites (Sharp and Burge, 1997) within coding sequence introns 5–6 (Figure 2B).
Figure 2.
Wild-type and splice mutant RPTPα sequence structures. (A) RPTPα: The numbered boxes represent the coding sequence (CDS) exons of the ubiquitously expressed short (793 amino acid) isoform RPTPα mRNAs (Vega PTPRA-001 and -002). (See Figure 7 for a map of all exons.) Shading indicates non-coding regions. (Shading of the first eight non-coding nucleotides of CDS exon 1 cannot be distinguished at this scale.) The corresponding locations of the wt exons in human chromosome 20 are indicated by the short vertical bars in the horizontal line above (assembly GRCh37/hg19, February 2009). RPTPα245: the inserted 95 nt cryptic exon following CDS exon 5 introduces new coding sequence (hatching) and a stop codon that makes all downstream exons non-coding (shaded). RPTPα445: CDS exons 10–12 are deleted without affecting the reading frame; the inclusion of the intron between CDS exons 14 and 15 introduces new coding sequence and a stop codon. RPTPα652: Exons 10–12 are deleted. See Table I for additional details. (B) The cryptic exon in RPTPα245 matches nucleotides 2 979 977–2 980 071 of chromosome 20 and uses cryptic splice sites that are flanked by consensus U2-type splicing sequences (bold), an upstream pyrimidine-rich region (bold), and a splice branch site (underlined) (Sharp and Burge, 1997; Zhang, 1998). (C) RPTPα245 differs from wt RPTPα by the insertion after amino acid 237 of eight amino acids (bold italic) followed by a premature stop codon. TM: transmembrane domain; D1 and D2: catalytic domains.
Table 1. RPTPα mutants observed in human tumour samples.
| Mutant | Occurrence | Splicing error | Protein mutation | Deleted catalytic regions |
|---|---|---|---|---|
| RPTPα245 | 1/9 Breast | New cryptic exon within | Δ(238–793); +8 spurious | D1, D2 |
| 2/8 Colon | CDS introns 5–6 | COOH amino acids | ||
| 1/2 Liver | ||||
| 0/7 Lung | ||||
| 0/10 Thyroid | ||||
| RPTPα445 | 0/9 Breast | Deleted CDS exons 10–12; | Δ(339–479); Δ(561–793); | D1, D2 |
| 1/8 Colon | CDS introns 14–15 not excised | +26 spurious COOH amino acids | ||
| 0/2 Liver | ||||
| 0/7 Lung | ||||
| 0/10 Thyroid | ||||
| RPTPα652 | 1/9 Breast | Deleted CDS exons 10–12 | Δ(339–479) | D1 |
| 0/8 Colon | ||||
| 0/2 Liver | ||||
| 0/7 Lung | ||||
| 0/10 Thyroid | ||||
| Exons and introns are numbered according to the wt RPTPα short isoform sequence starting with 1 at the first coding sequence exon (this is exon 4 in Vega transcript PTPRA-001 and exon 5 in PTPRA-002). Protein sequences are numbered according to the wt sequence starting with 1 at the N-terminal Met. CDS: coding sequence. | ||||
All mutant-containing RT–PCR products contained approximately equal amounts of wt and mutant amplicons (Figure 1), indicating, since the PCRs were competitive (Zentilin and Giacca, 2007), that mutant mRNAs were present in significant amounts. This is notable since the RPTPα245 and RPTPα445 mRNAs have premature termination codons predicted to induce nonsense-mediated decay (Isken and Maquat, 2008). Thus, defects in this regulatory mechanism may be involved.
Expression of RPTPα245 and tyrosine dephosphorylation of Src in human tumour cells
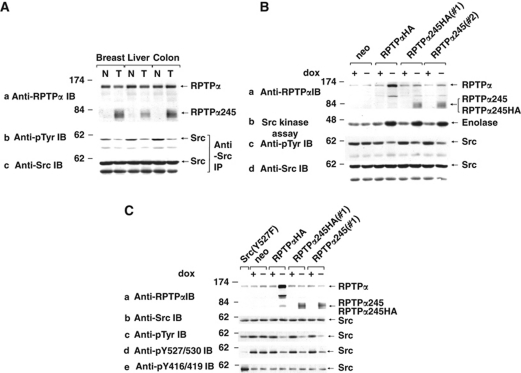
Because it occurred in two colon, one breast, and one liver tumours, RPTPα245 was selected for further study. Immunoblotting human tissue lysates (Figure 3A, panel a) revealed diffuse ∼80 kDa RPTPα-immunoreactive bands (consistent with a 245 amino-acid protein glycosylated to the same extent as wt RPTPα) in the colon, breast, and liver tumours and not in the paired normal tissues at roughly the same level as eRPTPα. Tumour cell eRPTPα levels were ⩾80% of normal cell levels, suggesting that splice mutant expression was not at the expense of wt expression. Anti-phosphotyrosine (pTyr) and anti-Src immunoblots of anti-Src immunoprecipitates showed that Src tyrosine phosphorylation was decreased by 50–65% in the tumours relative to normal tissues (panels b and c). (There was insufficient tumour tissue for additional measurements.) Therefore, phosphorylation of pTyr530 was decreased by at least this amount, and by an even larger amount if pTyr419 was autophosphorylated in a compensating manner. In either case, this implies that Src was activated in the tumour cells.
Figure 3.
Dephosphorylation and activation of Src induced by RPTPα245 in human tumours and REF overexpressors. (A) Lysates from human patient-matched tumour (T) and normal (N) tissues were (a) immunoblotted with anti-RPTPα antibody or (b, c) immunoprecipitated with anti-Src monoclonal antibody and then immunoblotted with either anti-pTyr or anti-Src antibody. The diffuse electrophoretic mobility of RPTPα245, probably from heterogeneous glycosylation, is not observed for wt RPTPα because of mobility compression in the higher molecular weight gel region. (B) REFs overexpressing the indicated RPTPα variants (or the neo-negative control line expressing no exogenous RPTPα) under Tet-off control were incubated either in the presence (+) or absence (−) of dox, and cell lysate aliquots were (a, c, d) immunoblotted with the indicated antibodies or (b) immunoprecipitated with anti-Src monoclonal antibody and incubated with [γ-32P]ATP and acid-denatured enolase for in vitro kinase assay. Molecular weight markers are indicated in kDa. (Control experiments (not shown) indicate that the reduced number of anti-RPTPα antibody epitopes reduces RPTPα245 band intensities by ∼20%. All quantitative analyses accounted for this.) (C) Induced and uninduced REFs expressing the RPTPα variants, the neo-negative control line, and a NIH3T3-derived line expressing chicken Src(Y527F) (which is constitutively phosphorylated at pTyr416) were immunoblotted with anti-Src and the indicated generic and phospho-specific anti-pTyr antibodies. IP: immunoprecipitate; IB: immunoblot.
Dephosphorylation and activation of Src by expression of RPTPα245 in REFs
Coding sequences for RPTPα245 and RPTPα245HA (which had a carboxyl-terminal haemagglutinin (HA)-tag) were cloned into a Tet-off inducible expression vector and co-transfected into REFs with neo as a coselectable marker. Multiple inducible cell lines of each type were cloned. Lines expressing HA-tagged human wt RPTPα (RPTPαHA) or no exogenous RPTPα (neo) were used as controls. All RPTPα variants were inducibly expressed in the absence of doxycyline (dox) at 4−5 × the eRPTPα level, reduced Src tyrosine phosphorylation by 60–70%, and increased Src-specific activity for dephosphorylation of enolase by ∼4-fold (Figure 3B). Immunoblots with phospho-specific antibodies showed that RPTPα245 expression reduced pTyr530 phosphorylation without associated increase in pTyr419 phosphorylation (Figure 3C). This corresponded to the effect of wt RPTPα and agrees with previous results, showing that RPTPα dephosphorylates both pTyr419 and pTyr530 (Zheng et al, 1992, 2000) and activates Src when both residues are dephosphorylated (Zheng et al, 1992; den Hertog et al, 1993).
Transformation of REFs and in vivo tumourigenicity by RPTPα245
Wt RPTPα overexpression has previously been shown to induce focus formation in monolayer culture, anchorage-independent growth, and tumours in nude mice (Zheng et al, 1992, 2000). The comparative activities of RPTPα245 were determined using the same assays. REFs expressing either RPTPαHA, RPTPα245HA, or RPTPα245 had similar focus forming efficiencies when mixed with normal REFS; these ranged from 10 to 16% (Supplementary Table S2). Concordantly, all formed colonies in soft agarose with similar efficiencies ranging from 11 to 17% (Figure 4A; Supplementary Figure S1; Supplementary Table S2). Two independent cell lines expressing RPTPα245 and two expressing RPTPα245HA were tested for in vivo tumourigenicity by subcutaneous injection into nude mice (Table II). All were tumourigenic and formed tumours that rapidly grew at the same rate as those induced by wt RPTPα.
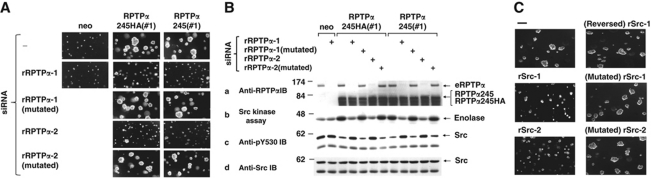
Figure 4.
Transformation by RPTPα245 requires both eRPTPα and Src. (A) REFs expressing no exogenous RPTPα (neo), RPTPα245HA, or RPTPα245 were transfected with the indicated rat siRNAs, suspended in media containing 0.3% soft agarose without dox, and grown for 21 days. (B) REFs expressing RPTPα245HA, RPTPα245, or only neo were grown without dox for 16 h and transfected without or with rat RPTPα siRNAs that targeted wt RPTPα but not RPTPα245 (Supplementary Table S3) or mutated control siRNAs. After further growth without dox for 12 h, lysate aliquots were (a, c, d) immunoblotted with the indicated antibodies or (b) immunoprecipitated with anti-Src monoclonal antibody and incubated with [γ-32P] ATP and acid-denatured enolase for in vitro kinase assay. (C) REFs expressing RPTPα245 (line #1) were transfected with the indicated rat Src siRNAs and tested for growth in soft agarose for 21 days.
Table 2. Tumourigenicity in nude mice.
| Protein | Cell line | #Tumours#injected | Latency to tumour formation (days) |
|---|---|---|---|
| Neo | REF(pTet-Splice)#1 | 0/5 | — |
| RPTPαHA | REF(RPTPHAα)#1 | 4/4 | 4–7 |
| RPTPα245HA | REF(RPTPα245HA)#1 | 4/4 | 5–6 |
| RPTPα245HA | REF(RPTPα245HA)#2 | 4/4 | 4–7 |
| RPTPα245 | REF(RPTPα245)#1 | 4/4 | 5–7 |
| RPTPα245 | REF(RPTPα245)#2 | 4/4 | 5–8 |
| Eight-week-old female BALB/cASlac-nu (athymic nude) mice were each injected subcutaneously at one site on the back with 2.5 × 105 cells of the indicated types and were monitored for 34 days. All tumours appeared by 8 days and grew to ∼1 cm diameter by 27 days. The cells differed only in the exogenous gene expressed along with the neomycin-resistance coselectable marker; neo cells expressed only the resistance marker. | |||
Transformation by RPTPα245 requires both eRPTPα and Src
These results were surprising since RPTPα245 lacks both catalytic domains (Figure 2C). We reasoned that it might activate Src and transform by stimulating eRPTPα. To test this hypothesis, RPTPα245- and RPTPα245HA-expressing REFs were treated with two siRNAs (and mutated control siRNAs) targeted against regions of the rat RPTPα mRNA that were deleted in the RPTPα245 expression plasmids (Supplementary Table S3) and subjected to biochemical and colony-forming assays. Both siRNAs, but not the controls, suppressed eRPTPα expression by 80–90% without affecting RPTPα245 or Src expression and eliminated the increase in Src kinase activity and decrease in Src Tyr530 phosphorylation induced by RPTPα245 or RPTPα245HA expression (Figure 4B). Moreover, RPTPα RNAi almost completely suppressed colony formation (Figure 4A; Supplementary Table S2). In addition, two siRNAs against rat Src (Supplementary Table S3) each suppressed Src expression by 85–95% (not shown) and reduced the induction of colony formation by RPTPα245 by ∼80% when measured 21 days after plating (Figure 4C). Mutated control siRNAs had no detectable effect. This indicates that both eRPTPα and Src are required for RPTPα245-induced transformation and suggests that transformation requires increased eRPTPα-mediated dephosphorylation of Src pTyr530; though additional actions on other substrates are not excluded.
Non-covalent binding of RPTPα245 with eRPTPα and the associated PTP activity
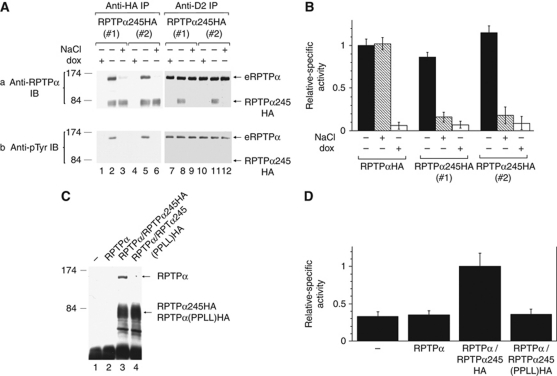
We hypothesized that RPTPα245-induced activation of Src involved activation of eRPTPα via RPTPα245–eRPTPα heterodimerization. To test this in non-saturating conditions, anti-HA immunoprecipitates from cell lines expressing RPTPα245HA at about half the eRPTPα level were immunoblotted with anti-RPTPα antibody (Figure 5A, panel a, lanes 1–6). Only slightly less eRPTPα than RPTPα245HA was detected, suggesting that the two proteins heterodimerized. In addition, only ∼15% of the eRPTPα remained associated with RPTPα245HA after a single 0.5 M NaCl wash (lanes 3 and 6), indicating that binding was non-covalent. Confirmatory results were obtained in the converse assay using anti-RPTPα D2 antibody, which binds eRPTPα and not RPTPα245, for immunoprecipitation and anti-RPTPα antibody for immunoblotting (lanes 7–12). Expression of RPTPα245 did not noticeably affect the level of overall eRPTPα Tyr789 phosphorylation (Figure 5A, panel b, cf lanes 7 and 10 with 8 and 11). Conversely, since the ratios of the eRPTPα band intensities in the anti-pTyr and anti-RPTPα immunoblots are very similar in the anti-HA immunoprecipitates, which detected eRPTPα bound by RPTPα245HA, and in the anti-D2 immunoprecipitates, which detected total eRPTPα, we conclude that binding of RPTPα245HA does not depend on the phosphorylation state of Tyr789. The NaCl wash did not detectably affect the amount of eRPTPα in parallel co-immunoprecipitates with RPTPαHA (not shown), probably because the single wash did not dissociate these molecules, which are expected to bind more tightly because of additional interactions involving the D1 and D2 domains (Jiang et al, 2000; Tertoolen et al, 2001).
Figure 5.
Non-covalent association of RPTPα245 with eRPTPα and PTP activities. (A) RPTPαHA and RPTPα245HA were immunoprecipitated with anti-HA antibody from uninduced (dox+) or induced (−) expressor cell lines and washed with lysis buffer not containing (−) or containing (+) 0.5 M NaCl to dissociate non-covalently bound proteins. Aliquots of the washed immunoprecipitates were immunoblotted with either anti-RPTPα or anti-pTyr antibodies. (PTyr789 is the only pTyr in RPTPα detected by this antibody (Zheng et al, 2000). Anti-RPTPα immunoblots of the lysates (not shown) indicated that the induced cells contained about half as much RPTPα245HA as eRPTPα.) (B) Portions of the immunoprecipitates from (A) and a parallel immunoprecipitate from RPTPαHA-expressing cells (not shown) were incubated with [32P]Tyr-phosphorylated MBP for 5 min and the amount of 32P released was measured and normalized to the amount of RPTPα plus RPTPαHA in the dox–/NaCl– immunoprecipitate in the corresponding cell line (s.d.'s; n=2). Negligible 32P was released in experiments using control neo cells that expressed no exogenous RPTPα (not shown). (C) 293T cells were transiently transfected with (lane 1) empty vector pXJ41 or co-transfected with plasmid p41RPTPα and (lane 2) pXJ41, (lane 3) p41RPTPα245HA, or (lane 4) p41RPTPα245(PPLL)HA, which exogenously expressed the indicated RPTPα variants. After 16 h growth, anti-HA immunoprecipitates were prepared and immunoblotted with anti-RPTPα antibody. (D) Equal portions of the anti-HA immunoprecipitates from two independent experiments like that shown in (C) and parallel immunoprecipitates from RPTPαHA-expressing cells (not shown) were used in a [32P]Tyr-MBP dephosphorylation assay as in (B). Relative amounts of released 32P are shown (s.d.'s; n=2).
Portions of the unwashed and washed anti-HA immunoprecipitates were incubated with [32P]Tyr-phosphorylated myelin basic protein (MBP) for 5 or 10 min (to verify reaction linearity) to measure their activities on unprotected substrates (Figure 5B). In all cases, PTP activity was proportional to the amount of full-length protein present (i.e., eRPTPα plus RPTPαHA), consistent with the hypothesis that all PTP activity associated with RPTPα245HA was catalysed by bound eRPTPα. Moreover, the specific activities (relative to the amount of full-length protein) were the same in the RPTPαHA and RPTPα245HA immunoprecipitates. We conclude that RPTPα245–eRPTPα binding accounts for the RPTPα245-associated PTP activity and that the specific activity of eRPTPα for unprotected substrates is the same in heterodimers and homodimers.
Endogenous RPTPα homodimerization involves binding of a helix-turn-helix ‘wedge’ (amino acids ∼200–240) to the dyad-related D1 region; this can be weakened by Pro210 → Leu/Pro211 → Leu mutation within the wedge (Jiang et al, 1999, 2000). To see if the wedge was involved in RPTPα245–eRPTPα binding, we co-transfected transient expression plasmids for RPTPα, RPTPα245HA, or (double-mutant) RPTPα245(PPLL)HA into 293T cells and assayed the amounts of co-precipitated wt RPTPα in anti-HA immunoprecipitates (Figure 5C). RPTPα was co-precipitated only from cells co-transfected with the RPTPα- and RPTPα245HA-expressing plasmids and not from cells co-transfected with RPTPα and RPTPα245(PPLL)HA. Concordantly, using portions of the Figure 5C immunoprecipitates in the PTP assay with [32P]MBP (Figure 5D) we found that the anti-HA immunoprecipitate from cells co-transfected with RPTPα and RPTPα245HA, but not from cells co-transfected with RPTPα and RPTPα245(PPLL)HA, had above-background PTP activity. This supports the hypotheses that RPTPα245–eRPTPα binding involves the wedge and that all RPTPα245HA-associated PTP activity comes from bound eRPTPα.
Binding to RPTPα245 suppresses eRPTPα binding to Grb2
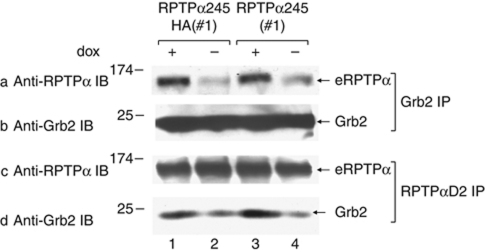
Since RPTPα245 expression increased eRPTPα's ability to dephosphorylate Src but not its ability to dephosphorylate MBP, we reasoned that RPTPα245–eRPTPα binding might act by enhancing phosphotyrosine displacement. While expression of RPTPα245HA does not affect the eRPTPα Tyr789 phosphorylation level (Figure 5A, panel b), it might decrease the amount of interfering pTyr789-Grb2 binding. To test this hypothesis, lysates from uninduced and induced RPTPα245 and RPTPα245HA expressor cells were immunoprecipitated with either anti-Grb2 antibody or anti-D2 antibody, which binds eRPTPα but not RPTPα245, and then immunoblotted with either anti-RPTPα or anti-Grb2 antibody (Figure 6). This co-immunoprecipitation experiment showed that expression of either RPTPα245 or RPTPα245HA reduced the Grb2–eRPTPα binding by 3–5-fold. Since some eRPTPα may not have been bound to RPTPα245HA, this might reflect an even larger reduction in Grb2 binding by the mutant-bound eRPTPα fraction.
Figure 6.
Effect of RPTPα245 expression on eRPTPα–Grb2 binding. Lysates from uninduced (dox+) and induced (−) RPTPα245 and RPTPα245HA overexpressor cells were immunoprecipitated with anti-Grb2 (a, b) or anti-RPTPαD2 (c, d) polyclonal antibodies. The immunoprecipitates were then immunoblotted with anti-RPTPα 7-091 antibody or monoclonal anti-Grb2 antibody. When induced, RPTPα245 and RPTPα245HA were expressed at ∼2 × endogenous level (not shown).
Extreme conservation of the cryptic exon in RPTPα245
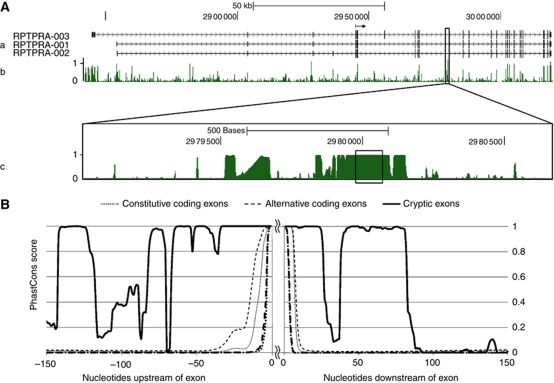
The only defect in the RPTPα245 mRNA is the inclusion of the 95-nt cryptic exon (cx95). Figure 7A shows that cx95 is strongly conserved across placental mammals: its phastCons (Siepel et al, 2005) probability of being in a conserved region is 1.000 and its FASTA (Pearson and Lipman, 1988) alignment between human and mouse has an E-value (Pearson, 1998) of ∼10−21. The implication that it may have a normal function is further supported by the very high conservation of its flanking regions (Sorek and Ast, 2003; Kaufmann et al, 2004; Yeo et al, 2005; Sugnet et al, 2006): cx95 lies within a region of ∼150 contiguous nts having phastCons scores ⩾0.98 and within a region of ∼300 nt that is highly conserved except for some small intervals (Figure 7B).
Figure 7.
Extreme conservation of cx95, the 95-nt cryptic exon in RPTPα245, and its flanking region in placental mammals. (A) Evolutionary conservation in the RPTPα-coding region. (a) The locations of the exons in the three predominant RPTPα transcripts (Vega PTPRA-001, -002, -003) relative to the human genomic sequence (assembly GRCh37/hg19, February, 2009) as shown on the UCSC Genome Browser (Kent et al, 2002) are indicated by vertical bars on the three corresponding horizontal lines. (Numbers correspond to chromosome 20 locations.) The coding region begins at the position indicted by the horizontal arrow at 2 944 924. (b) The probability that each nucleotide is located within a region that is evolutionarily conserved within 33 placental mammals (computed by the hidden Markov model phastCons algorithm (Siepel et al, 2005) using the vertebrate Multiz alignment (Blanchette et al, 2004)) is displayed as shown in the UCSC Genome Browser. The boxed region surrounds the 95-nt cryptic exon incorporated into the RPTPα245 mRNA. (c) The boxed region from (b) is expanded and the 95-nt cryptic exon is boxed. (B) Evolutionary conservation in the cx95 flanking regions. Placental mammal PhastCons conservation probabilities were computed for the nucleotides flanking cx95, 32 572 human alternative coding exons, and 171 060 human constitutive coding exons. The solid line displays the probabilities in the regions flanking cx95, the heavy dashed (dotted) line displays the median probabilities in the regions flanking the alternative (constitutive) coding exons, and the light dashed (dotted) lines display the upper third quartile limits of the probabilities for the alternative (constitutive) coding sequences. (The heavy dotted line displaying median probabilities for the constitutive coding exons is mostly obscured.)
Discussion
The ability of RPTPα245 to bind eRPTPα, decrease eRPTPα–Grb2 binding, and increase eRPTPα activation of Src reveals a new mechanism by which RPTPα can be activated to transform cells. Src activation is not explained by a generic increase in eRPTPα activity, since eRPTPα's ability to dephosphorylate the unprotected pTyrs in MBP is not increased. The Src-specific activation can be explained by the associated decreased eRPTPα–Grb2 binding without decreased pTyr789 phosphorylation. Since most pTyr789 is bound by Grb2 (den Hertog et al, 1994; Su et al, 1994), even a small decrease in Grb2 binding will cause a large fractional increase in the amount of unbound pTyr789, thereby enhancing phosphotyrosine displacement and Src dephosphorylation. However, the mechanism by which RPTPα245–eRPTPα binding decreases Grb2 binding is not known.
A simple structural possibility is that the binding of one Grb2 molecule, which involves binding of its SH2 domain to pTyr789 and binding of its C-terminal SH3 domain to the N-terminal region of the D1 domain (den Hertog and Hunter, 1996; Su et al, 1996), involves both eRPTPα molecules in the homodimer: one binding Grb-SH2 and one binding Grb2-C-SH3 (Supplementary Figure S2a). If this were true, then displacement of eRPTPα by RPTPα245 would form an RPTPα245–eRPTPα heterodimer that would bind Grb2 poorly, since RPTPα245, which lacks D1, would not provide the Grb2-SH3 binding site needed to pair with the eRPTPα Grb2-SH2 binding site (Supplementary Figure S2b). Alternatively, other processes such as altered Ser phosphorylation or D2-Src binding (see below) could be involved.
Reduced RPTPα–Grb2 binding without altered Tyr789 phosphorylation also occurs during mitosis (Zheng and Shalloway, 2001) and might be related to the RPTPα245-induced process. For example, the mitotic reduction might involve disruption of RPTPα homodimers by a different mechanism, and/or the RPTPα245-induced reduction might involve changes in Ser180 or Ser204 phosphorylation, two primary Ser phosphorylation sites in RPTPα (Tracy et al, 1995) that have a role during mitosis (Zheng et al, 2002; Vacaru and den Hertog, 2010a). Thus, it will be interesting to investigate the possibility that RPTPα homodimerization changes during mitosis or that Grb2–RPTPα binding changes in response to other factors known to modulate the RPTPα homodimerization (e.g., disulphide linkages, mutation, and oxidative stress; Jiang et al, 1999; Jiang et al, 2000; Tertoolen et al, 2001; Blanchetot et al, 2002).
Jiang et al (1999, 2000) showed that RPTPα homodimerization is inhibited by a Pro210 → Leu/ Pro211 → Leu mutation within the wedge, presumably by disrupting wedge interaction with the dyadically related D1 region (Bilwes et al, 1996). Similarly, Pro210 → Leu/Pro211 → Leu mutation in RPTPα245HA strongly inhibits its binding to eRPTPα (Figure 5C), suggesting that RPTPα245 and eRPTPα form a heterodimer having the same wedge–D1 interaction (Supplementary Figure S2b). Insertion of the wedge into the paired D1 region is predicted to sterically inhibit RPTPα catalytic activity (Bilwes et al, 1996), but binding of RPTPα245HA to RPTPα does not change RPTPα's ability to dephosphorylate MBP (Figure 5B). This could indicate that the RPTPα245–RPTPα wedge–D1 interaction simply replaces the same interaction in the RPTPα homodimer leaving substrate accessibility unchanged. However, we do not exclude the alternative possibility that the wedge might bind the D2 domain, since wedge–D2 binding can be detected in a yeast two-hybrid assay (Blanchetot and den Hertog, 2000). The D2 domain participates both in RPTPα homodimerization (Jiang et al, 2000; Tertoolen et al, 2001) and in Src binding and activation (Vacaru and den Hertog, 2010b), so it is possible that RPTPα245 binding-induced effects on D2 might participate in its activation of Src-specific RPTPα activity. Additional structural studies are needed to clarify these issues. Regardless, quantitative consideration of the co-immunoprecipitation experiments (Figure 5) indicates that RPTPα245HA–RPTPα245HA homodimerization is very weak relative to RPTPα245HA–eRPTPα binding; otherwise, competitive homodimerization would not permit the extensive RPTPα245HA–eRPTPα binding that is observed. This suggests that interaction of the RPTPα245 wedge with the eRPTPα catalytic domain(s) significantly increases the background binding free energy that is provided by interactions of the extracellular and transmembrane domains.
The RPTPα juxtamembrane domain (residues 175–240) also binds the D2 regions of other receptor PTPs (RPTPσ, RPTPδ, and LAR) in a yeast two-hybrid system (Blanchetot and den Hertog, 2000). The juxtamembrane is almost identical to the intracellular region of RPTPα245 (Table I), so it is possible that RPTPα245 also interacts with other receptor PTPs. However, most (⩾70%) RPTPα245HA binds eRPTPα (Figure 5A), so at most only a small fraction could bind other RPTPs. The RPTPα RNAi experiments (Figure 4) show that such interactions, if they exist at all, are not sufficient for RPTPα245-induced Src activation or transformation, but the possibility that they contribute is not excluded.
Barr et al (2009) have suggested that a steric clash of D2 domains would prevent the wedge–D1 interaction in the RPTPα homodimer, suggesting that the ‘head-to-toe’ conformation found in RPTPγ involving D1–D2 contacts is more likely than the ‘head-to-head’ conformation, involving D1–D1 and D2–D2 contacts, implied by the RPTPα D1 homodimer structure (Bilwes et al, 1996). However, the RPTPα rotational changes observed following oxidative stress suggest that the D1–D2 linkage is not rigid (Blanchetot et al, 2002; van der Wijk et al, 2003) and would allow such a clash to be relieved. Moreover, the head-to-toe model does not explain the dependence of RPTPα–RPTPα homodimerization on Pro210 → Leu/Pro211 → Leu mutation, since it places the wedge facing away from both the D1 and D2 regions of the bound molecule. While we, therefore, think it more likely that RPTPα homodimerizes in the head-to-head conformation, the head-to-toe conformation is not incompatible with any of the possible explanations for the effect of RPTPα245–eRPTPα association on Grb2 binding suggested above.
The mechanism accounting for the tumour-specific presence of the splice mutants is another interesting open question. Bioinformatic analysis suggests the intriguing possibility that cx95, the 95-nt cryptic exon included in RPTPα245, may have a specialized role as an alternative RPTPα exon in some normal situations: its excellent flanking U2 splice signals (Figure 2B), the extremely high conservation of cx95 and its flanking regions (Figure 7), the presence of cx95 in an RPTPα transcript found in a screen of full-length cDNAs from normal human brain (FLJ Human cDNA Database (http://flj.lifesciencedb.jp) FLJ33424; GenBank AK090743.1), ExonScan analysis (Wang et al, 2004), and RankVista analysis (Frazer et al, 2004; Prabhakar et al, 2006; Wang et al, 2007; http://genome.lbl.gov/vista/index.shtml) suggest that it might be expressed in developing tissues or be used to regulate RPTPα expression by diverting mRNA processing into futile end points that are subject to nonsense-mediated decay (see Supplementary data). Thus, it will be interesting to screen for tissue-specific or transient expression of cx95-containing mRNAs; identifying environmental or biochemical factors that regulate its normal expression may provide clues as to its expression in tumours. This may also help to illuminate the reasons accounting for the survival of the RPTPα245 mRNA, which satisfies the rules for predicting targets of nonsense-mediated decay (Isken and Maquat, 2008).
The relevance of these results to human carcinogenesis requires more investigation. Alternative splicing has been implicated in cancer (Cooper et al, 2009; van Alphen et al, 2009; Ward and Cooper, 2010) with misregulation of many splicing factors correlated with tumours. Although in most cases assessing the causative role of splice variants has remained elusive, at least one has been identified as oncogenic (Karni et al, 2007; Grosso et al, 2008). While the mutants might only be splicing epiphenomena (Nilsen and Graveley, 2010; Luco et al, 2011) with no carcinogenic role, it is notable that they were found primarily in colon and breast cancers, the two subtypes that were a priori identified from the Src and RPTPα RNAi experiments (Zheng et al, 2008) that motivated this screen: Mutants were present in 5/17 of the colon and breast tumours (13–53% Wilson confidence interval; α=0.05), but only in 1/19 of tumours of other types (0.93–025% Wilson confidence interval; α=0.05). Moreover, Src was tyrosine dephosphorylated, and thus presumably activated, in the tumour cells that expressed RPTPα245 and not in the adjacent normal cells that did not express it (Figure 3A). Given that RPTPα245 activates Src when expressed in REFs, it is likely that it was responsible for activation of Src in the tumour cells. However, it will be necessary to study human tumour lines expressing RPTPα245 to verify this and to determine whether RPTPα245-induced activation of Src contributes to transformation in the human tumours. In the same vein, studies with transgenic mice expressing the mutant will be needed to extend the demonstration that RPTPα245-transformed REFs are tumourigenic in nude mice.
Given these caveats, these results bolster previous evidence, suggesting that RPTPα has a role in some colon and ER− breast cancers. Tabiti et al (1995) have shown that high-level RPTPα expression is associated with late-stage colon carcinoma, which might be related to the correlation between elevated Src activity and poor clinical prognosis in colorectal cancer (Aligayer et al, 2002). This could explain why continued RPTPα expression is needed to maintain elevated Src activity and suppress apoptosis in tested human colon cancer cell lines (Zheng et al, 2008). RPTPα expression is also required for Src activation in and the survival of tested ER−, but not ER+, breast cancer lines (Zheng et al, 2008), suggesting that its role in breast cancer depends on ER status (or possibly correlated factors). For example, in contrast with tested ER− breast cancer lines, the ER+ breast cancer line MCF7 does not require RPTPα for survival (Zheng et al, 2008); instead, RPTPα overexpression in this line inhibits growth (Ardini et al, 2000). Thus, the observed correlation of RPTPα expression with low breast cancer tumour grade (Ardini et al, 2000) may only describe a property of the ER+ breast cancer subset, since these are four times more prevalent than ER− breast cancers in Caucasian and Asian populations (Hausauer et al, 2007). The ER status of the breast tumours provided for this study was not known, so it will be important to determine this in future, expanded tumour screens. In addition, since all tumours in this study were from Chinese patients, it will be important to screen tumours from non-Chinese patients in the same way to test for potential dependencies on genetic background.
In summary, binding of RPTPα245 to eRPTPα activates its Src-specific, but not its generic, dephosphorylating activity and results in fibroblast transformation. This is accompanied by decreased RPTPα–Grb2 binding without decreased Tyr789 phosphorylation and so may be explained simply by changes in phosphotyrosine displacement. The structure of the RPTPα245–eRPTPα complex, the mechanisms accounting for the reduced Grb2 binding and altered splicing, and the possibility that RPTPα245 and the other splice mutants have roles in human carcinogenesis merit further study.
Materials and methods
Reagents
Anti-RPTPα (7-091), anti-RPTPαD2 (7-054) rabbit polyclonal antibodies, and anti-Src mAb327 monoclonal antibody have been described (Lipsich et al, 1983; Zheng et al, 2000). Anti-HA rabbit polyclonal antibody (Y-11) was from Santa Cruz Biotechnology, anti-phosphotyrosine mouse monoclonal (pTyr-100; #9411), anti-pTyr527 rabbit polyclonal (#2105; used to detect pTyr530), and anti-pTyr416 (D49G4; #6943; used to detect pTyr419) rabbit polyclonal antibodies were from Cell Signaling Technology, HRP-linked secondary antibodies for immunoblots were from Jackson ImmunoResearch, and Protein A-sepharose beads (used with polyclonal antibodies) and GammaBind sepharose (used with monoclonal antibodies) were from Amersham Biosciences. The relative binding of anti-RPTPα (7-091) to wt RPTPα and RPTPα245 was determined by immunoblotting lysates from cells expressing RPTPαHA and RPTPα245HA with both anti-RPTPα (7-091) and anti-HA antibodies and quantitatively comparing band intensities at different titrations. NIH(pcsrc527/focus)C cells, which express chicken Src(Y527F), have been described (Kmiecik and Shalloway, 1987).
Human tissue lysate preparation
Human tumour (T) and normal (N; from distant parts of the excised tissue) samples weighing 20–40 mg each were frozen in liquid nitrogen within 10 min of surgical removal. They were later thawed, homogenized twice (20 s each) with a Power Gen Model 125 homogenizer (Fisher Scientific) on ice in 0.5 ml RIPA buffer (1% (vol/vol) Triton X-100/0.5% (wt/vol) Na deoxycholate/0.1% Na dodecyl sulphate/0.15 M NaCl/0.01 M NaH2PO4, pH 7.2) supplemented with 60 mM Na4P2O7, 25 mM NaF, 2 mM EDTA, 1 mM Na3VO4, 1 mM phenylmethylsulphonyl fluoride, aprotinin (100 kallikrein inhibitor units/ml), leupeptin (10 μg/ml), and 1 mM Na2MoO4, centrifuged at 15 000 r.p.m. for 30 min, and the supernatants were frozen at −80°C for subsequent use.
RT–PCR cloning and sequencing
Thawed tumour or normal tissue samples weighing ∼30 mg were homogenized twice on ice for 20 s with a Power Gen Model 125 in 1.5 ml microcentrifuge tubes containing 0.5 ml TRIzol RNA isolation reagent (Invitrogen) according to the manufacturer's instructions. cDNAs were reverse transcribed from 5 μg total RNA using Superscript II (Invitrogen) and oligonucleotide (dT) primers. Overlapping DNAs that spanned the complete RPTPα-coding region were amplified in two separate reactions using high fidelity Taq polymerase (Invitrogen) and two sets of RPTPα-specific primers (Supplementary Table S3). After an initial cycle of 2 min at 95°C, the reaction was cycled 1 min, 95°C; 1 min, 55°C; 2 min, 72°C for 30 times. PCR products were separated by 1% agarose gel electrophoresis, stained with ethidium bromide, and the amplified cDNAs bands were purified using QIAquick Gel Extraction kits (Qiagen). These were cloned into pCR2.1-TOPO using the TOPO TA Cloning Kit (Invitrogen) as described by the manufacturer. Ten independent clones were generated from each tumour and five from each paired normal tissue; all were sequenced using primers binding in the flanking vector regions (Supplementary Table S3) on an Applied Biosystems AB13730XL sequencer by Invitrogen (Shanghai). (Two apparent point mutations in clones from normal-mobility amplicons appeared in the first round of sequencing. However, these did not reappear in reamplified cDNAs, indicating that they were PCR artefacts.) The (identical) plasmids containing the RPTPα245-coding sequence were designated pCR2.1RPTPα245.
Expression plasmid construction
Plasmid pTPTPα, which contains the human short isoform RPTPα sequence with an HA tag, has been described (Zheng et al, 2000). DNAs containing the entire RPTPα245-coding sequence with or without an appended HA tag and flanked by HindIII sites were generated from plasmid pCR2.1PTPα245 by PCR with Taq polymerase (Invitrogen) and the RPTPα cloning primers listed in Supplementary Table S3. (The nucleotides upstream of the AUG starting codon were changed to match the Kozak consensus sequence (Kozak, 1987) by the PCR primer.) These DNAs were cleaved with HindIII and ligated into pTet-Splice (Clontech) to make plasmids pNTPTPα245 and pTPTPα245, which inducibly express RPTPα245 and HA-tagged RPTPα245HA. These plasmids do not contain any RPTPα-derived sequence outside the RPTPα245-coding region; in particular, they lack the regions targeted by the RPTPα siRNAs used in the RNAi experiments.
Empty vector pXJ41 (identical to pXJ40 (Xiao et al, 1991) except that the multiple cloning site module is reversed) and human RPTPα-expressing plasmid p41PTPα (Zheng et al, 1992) have been described. p41PTPα245HA, for transiently expressing RPTPα245HA, was constructed by cloning the 0.73-kb HindIII-HindIII DNA fragment from pTPTPα245HA containing the RPTPα245HA-coding sequence into pXJ41. Plasmid p41PTPα245(PPLL)HA for expressing the mutant containing Pro210 → Leu/Pro211 → Leu mutations was constructed by point mutagenesis of p41PTPα245HA using the GeneEditor In Vitro Site-Directed Mutagenesis System (#Q9280, Promega), as instructed by the manufacturer and the mutating DNA oligomer shown in Supplementary Table S3.
Generation and growth of inducible RPTPα-overexpressing REFs
G418-resistant cells were cloned and tested for inducible expression of RPTPα following co-transfection of REFs with pSV2neo (Southern and Berg, 1982), pTet-TAK (Clontech; for Tet-off inducible expression) and one of the RPTPα expression plasmids described above. G418-resistant colonies were cloned and tested for inducible expression of RPTPα as described (Zheng et al, 2000). A cell line (neo) co-transfected with pSV2neo, pTet-TAK, and the empty pTet-Splice vector was used as a negative control. Cells were maintained in Dulbecco's modified Eagle medium (DMEM; GIBCO) with 300 μg/ml penicillin, 100 μg/ml streptomycin, 10% calf serum, and 5 ng/ml dox (to suppress RPTPα expression). Expression was induced by growth without dox for 16–18 h before experiments.
Transient expression of RPTPα variants in 293T cells
293T cells were grown in 100 mm tissue culture dishes in DMEM without antibiotics for 24 h (reaching 60–70% confluence) and then co-transfected for 4 h with either 8 μg pXJ41 or 4 μg p41RPTPα mixed with 4 μg of either pXJ41, p41PTPα245HA, or p41PTPα245(PPLL)HA using 30 μl Lipofectamine (Invitrogen) as described by the manufacturer. Cells were then fed with fresh media with serum and antibiotics and incubated for 24 h before assay.
Biological assays
Assays for focus formation by cell mixing (Johnson et al, 1985) and for colony formation in 0.3% soft agarose in the absence of dox (Zheng et al, 2000) were performed as described. BALB/cASlac-nu mice were from The Shanghai Slac Laboratory Animal Center. SiRNA transfection was as in Zheng et al (2008) and cells were assayed for colony formation in 0.3% soft agarose in the absence of dox as described.
Biochemical assays
For immunoblots and immunoprecipitations, cells were lysed in binding buffer (50 mM Tris–HCl pH 7.2, 50 mM NaCl, 2 mM EDTA, 25 mM NaF, 1% NP-40, 1 mM Na3VO4, 10 μg/ml aprotinin, and 10 μg/ml leupeptin); the same buffer but with 10% glycerol was used for co-immunoprecipitations. Clarified lysates containing either 50 μg (human) or 30 μg (rat) total cell protein were used for direct immunoblots; either 300 μg (human) or 500 μg (rat) total cell protein were used for immunoprecipitations and co-immunoprecipitations. Immunoprecipitates were prepared using either 2 μg of anti-HA polyclonal antibody or 2 μl of anti-D2 antiserum with incubation with inversion for 2 h at 4°C with inversion. Complexes were bound to protein A-sepharose beads for another 1 h at 4°C. Immunoprecipitates were washed (1 min with hand inversion) with 0.8 ml lysis buffer, as indicated once with binding buffer raised to 0.5 M NaCl (0.8 ml), and twice with binding buffer. Subsequent kinase or PTPase assays were performed as described (Zheng et al, 2000) using 10% of the immunoprecipitates; the remainder being divided equally between the immunoblots (Zheng et al, 2000).
Conservation of cx95
The phastCons placental mammal data were transferred from the UCSC Genome Browser (http://genome.ucsc.edu/cgi-bin/hgTrackUi?hgsid=162304396&c=chr20&g=cons46way) after computation using a parameter set (expected length=45, target coverage=0.3, ρ=0.31) that produced 5% conserved elements in the genome for the vertebrate conservation measurement.
Analysis of constitutive and alternative coding exon flanking regions
In all, 319 685 RefSeq human coding exons (http://genome.ucsc.edu/cgi-bin/hgTables; table:refGene; track:RefSeq Genes; group:Genes and Gene Prediction Tracks) and 92 222 alternative splicing events (table:knownAlt; track:Alt Events; group:Genes and Gene Prediction Tracks) were transferred from the UCSC Genome Table Browser (assembly GRCh37/hg19) to Galaxy (Taylor et al, 2007; Blankenberg et al, 2010) (http://g2.bx.psu.edu). The unique coding exons that did not overlap any alternative splicing events were designated constitutive coding exons; the exons in the complementary unique subset were designated alternative coding exons. The constitutive and alternative sets were then divided into plus and minus strand sequences (using Galaxy's Filter and Sort/Filter tool). The 150-nt upstream and downstream flanking sequences were then isolated (using Galaxy's Text Manipulation/Compute tool). These sequences were converted from BED to interval format (using Galaxy's Get Genomic Scores/Wiggle-to-Interval converter). Placental mammalian phastCons probabilities were transferred to Galaxy from the UCSC Genome Browser (http://hgdownload.cse.ucsc.edu/goldenPath/hg19/phastCons46way/placentalMammals/) and then merged with the flanking sequence intervals (using Galaxy's Get Genomic Scores/Aggregate datapoints tool). (It was necessary to use Edit Attributes to spoof the database build attribute to hg18 to process the data.) The resulting output was parsed using a customized script to obtain phastCons scores for the flanking nucleotides.
ExonScan analysis
The P-value of the ExonScan prediction was calculated from the rank of the score of the 95-nt insert (with 150 flanking nts on each side) relative to 10 000 sequences randomly sampled from 8 081 956 non-overlapping intron-derived sequences, each of which had a core 50–250 nt in length (typical of exons) having the canonical U2 splice site AG and GU flanking dinucleotides plus 150 flanking nts on each side.
Supplementary Material
Acknowledgments
We thank Hongying Yang and Wenhong Sun for expert technical assistance, Ross Resnick for manuscript critique, and Jeff Pleiss and Charles Danko for helpful discussions. This study was supported by Shanghai Pujiang Talent Fund (2005Z10), the Natural Science Foundation of Shanghai (09ZR1416400), and the National Natural Science Foundation of China (31071228).
Author contributions: JH and LY did all RT–PCR and cloning; RX did all RNA and protein extractions; HW and MW collected tumour samples and performed clinical and pathological analysis; BSW performed all bioinformatics studies and assisted in writing the paper; DS designed experiments, performed all statistical analyses, and co-wrote the paper; XZ made the cell lines, performed the biological experiments, designed experiments and co-wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Aligayer H, Boyd DD, Heiss MM, Abdalla EK, Curley SA, Gallick GE (2002) Activation of Src kinase in primary colorectal carcinoma: an indicator of poor clinical prognosis. Cancer 94: 344–351 [DOI] [PubMed] [Google Scholar]
- Ardini E, Agresti R, Tagliabue E, Greco M, Aiello P, Yang LT, Menard S, Sap J (2000) Expression of protein tyrosine phosphatase α (RPTPα) in human breast cancer correlates with low tumor grade, and inhibits tumor cell growth in vitro and in vivo. Oncogene 19: 4979–4987 [DOI] [PubMed] [Google Scholar]
- Barr AJ, Ugochukwu E, Lee WH, King ON, Filippakopoulos P, Alfano I, Savitsky P, Burgess-Brown NA, Muller S, Knapp S (2009) Large-scale structural analysis of the classical human protein tyrosine phosphatome. Cell 136: 352–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilwes AM, den Hertog J, Hunter T, Noel JP (1996) Structural basis for inhibition of receptor protein-tyrosine phosphatase-α by dimerization. Nature 382: 555–559 [DOI] [PubMed] [Google Scholar]
- Blanchetot C, den Hertog J (2000) Multiple interactions between receptor protein-tyrosine phosphatase (RPTP) α and membrane-distal protein-tyrosine phosphatase domains of various RPTPs. J Biol Chem 275: 12446–12452 [DOI] [PubMed] [Google Scholar]
- Blanchetot C, Tertoolen LG, den Hertog J (2002) Regulation of receptor protein-tyrosine phosphatase alpha by oxidative stress. EMBO J 21: 493–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchette M, Kent WJ, Riemer C, Elnitski L, Smit AF, Roskin KM, Baertsch R, Rosenbloom K, Clawson H, Green ED, Haussler D, Miller W (2004) Aligning multiple genomic sequences with the threaded blockset aligner. Genome Res 14: 708–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenberg D, Von Kuster G, Coraor N, Ananda G, Lazarus R, Mangan M, Nekrutenko A, Taylor J (2010) Galaxy: a web-based genome analysis tool for experimentalists. Curr Protoc Mol Biol Chapter 19: Unit 19.10.1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggon TJ, Eck MJ (2004) Structure and regulation of Src family kinases. Oncogene 23: 7918–7927 [DOI] [PubMed] [Google Scholar]
- Chen M, Chen SC, Pallen CJ (2006) Integrin-induced tyrosine phosphorylation of protein-tyrosine phosphatase-α is required for cytoskeletal reorganization and cell migration. J Biol Chem 281: 11972–11980 [DOI] [PubMed] [Google Scholar]
- Cooper TA, Wan L, Dreyfuss G (2009) RNA and disease. Cell 136: 777–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hertog J, Pals CE, Peppelenbosch MP, Tertoolen LG, de Laat SW, Kruijer W (1993) Receptor protein tyrosine phosphatase α activates pp60c-src and is involved in neuronal differentiation. EMBO J 12: 3789–3798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hertog J, Hunter T (1996) Tight association of GRB2 with receptor protein-tyrosine phosphatase a is mediated by the SH2 and C-terminal SH3 domains. EMBO J 15: 3016–3027 [PMC free article] [PubMed] [Google Scholar]
- den Hertog J, Tracy S, Hunter T (1994) Phosphorylation of receptor protein-tyrosine phosphatase α on Tyr789, a binding site for the SH3-SH2-SH3 adaptor protein GRB-2 in vivo. EMBO J 13: 3020–3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I (2004) VISTA: computational tools for comparative genomics. Nucleic Acids Res 32: W273–W279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosso AR, Martins S, Carmo-Fonseca M (2008) The emerging role of splicing factors in cancer. EMBO Rep 9: 1087–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder KW, Moller NP, Peacock JW, Jirik FR (1998) Protein-tyrosine phosphatase α regulates Src family kinases and alters cell-substratum adhesion. J Biol Chem 273: 31890–31900 [DOI] [PubMed] [Google Scholar]
- Hausauer AK, Keegan TH, Chang ET, Clarke CA (2007) Recent breast cancer trends among Asian/Pacific Islander, Hispanic, and African-American women in the US: changes by tumor subtype. Breast Cancer Res 9: R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T (1987) A tail of two src's: mutatis mutandis. Cell 49: 1–4 [DOI] [PubMed] [Google Scholar]
- Isken O, Maquat LE (2008) The multiple lives of NMD factors: balancing roles in gene and genome regulation. Nat Rev Genet 9: 699–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G, den Hertog J, Hunter T (2000) Receptor-like protein tyrosine phosphatase α homodimerizes on the cell surface. Mol Cell Biol 20: 5917–5929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G, den Hertog J, Su J, Noel J, Sap J, Hunter T (1999) Dimerization inhibits the activity of receptor-like protein-tyrosine phosphatase-α. Nature 401: 606–610 [DOI] [PubMed] [Google Scholar]
- Johnson PJ, Coussens PM, Danko AV, Shalloway D (1985) Overexpressed pp60c-src can induce focus formation without complete transformation of NIH 3T3 cells. Mol Cell Biol 5: 1073–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp K, Siemens J, Weyrich P, Schulz JB, Haring HU, Lammers R (2007) Extracellular domain splice variants of a transforming protein tyrosine phosphatase α mutant differentially activate Src-kinase dependent focus formation. Genes Cells 12: 63–73 [DOI] [PubMed] [Google Scholar]
- Karni R, de Stanchina E, Lowe SW, Sinha R, Mu D, Krainer AR (2007) The gene encoding the splicing factor SF2/ASF is a proto-oncogene. Nat Struc Mol Biol 14: 185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann D, Kenner O, Nurnberg P, Vogel W, Bartelt B (2004) In NF1, CFTR, PER3, CARS and SYT7, alternatively included exons show higher conservation of surrounding intron sequences than constitutive exons. Eur J Hum Genet 12: 139–149 [DOI] [PubMed] [Google Scholar]
- Kent WG, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D (2002) The human genome browser at UCSC. Genome Res 12: 996–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmiecik TE, Shalloway D (1987) Activation and suppression of pp60c-src transforming ability by mutation of its primary sites of tyrosine phosphorylation. Cell 49: 65–73 [DOI] [PubMed] [Google Scholar]
- Kozak M (1987) An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res 15: 8125–8148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsich LA, Lewis AJ, Brugge JS (1983) Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J Virol 48: 352–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luco RF, Allo M, Schor IE, Kornblihtt AR, Misteli T (2011) Epigenetics in alternative pre-mRNA splicing. Cell 144: 16–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksumova L, Wang Y, Wong NK, Le HT, Pallen CJ, Johnson P (2007) Differential function of PTPα and PTPα Y789F in T cells and regulation of PTPα phosphorylation at Tyr-789 by CD45. J Biol Chem 282: 20925–20932 [DOI] [PubMed] [Google Scholar]
- Nilsen TW, Graveley BR (2010) Expansion of the eukaryotic proteome by alternative splicing. Nature 463: 457–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallen CJ (2003) Protein tyrosine phosphatase α (PTPα): a Src family kinase activator and mediator of multiple biological effects. Curr Top Med Chem 3: 821–835 [DOI] [PubMed] [Google Scholar]
- Pearson WR, Lipman DJ (1988) Improved tools for biological sequence comparison. Proc Natl Acad Sci USA 85: 2444–2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson WR (1998) Empirical statistical estimates for sequence similarity searches. J Mol Biol 276: 71–84 [DOI] [PubMed] [Google Scholar]
- Piwnica-Worms H, Saunders KB, Roberts TM, Smith AE, Cheng SH (1987) Tyrosine phosphorylation regulates the biochemical and biological properties of pp60c-src. Cell 49: 75–82 [DOI] [PubMed] [Google Scholar]
- Ponniah S, Wang DZ, Lim KL, Pallen CJ (1999) Targeted disruption of the tyrosine phosphatase PTPα leads to constitutive downregulation of the kinases Src and Fyn. Curr Biol 9: 535–538 [DOI] [PubMed] [Google Scholar]
- Prabhakar S, Poulin F, Shoukry M, Afzal V, Rubin EM, Couronne O, Pennacchio LA (2006) Close sequence comparisons are sufficient to identify human cis-regulatory elements. Genome Res 16: 855–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT (1994) Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol 14: 1680–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PA, Burge CB (1997) Classification of introns: U2-type or U12-type. Cell 91: 875–879 [DOI] [PubMed] [Google Scholar]
- Siepel A, Bejerano G, Pedersen JS, Hinrichs AS, Hou M, Rosenbloom K, Clawson H, Spieth J, Hillier LW, Richards S, Weinstock GM, Wilson RK, Gibbs RA, Kent WJ, Miller W, Haussler D (2005) Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res 15: 1034–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z, Cantley LC (1995) SH2 domain specificity determination using oriented phosphopeptide library. Methods Enzymol 254: 523–535 [DOI] [PubMed] [Google Scholar]
- Sorek R, Ast G (2003) Intronic sequences flanking alternatively spliced exons are conserved between human and mouse. Genome Res 13: 1631–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern PJ, Berg P (1982) Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet 1: 327–341 [PubMed] [Google Scholar]
- Su J, Batzer A, Sap J (1994) Receptor tyrosine phosphatase R-PTP-α is tyrosine-phosphorylated and associated with the adaptor protein Grb2. J Biol Chem 269: 18731–18734 [PubMed] [Google Scholar]
- Su J, Muranjan M, Sap J (1999) Receptor protein tyrosine phosphatase α activates Src-family kinases and controls integrin-mediated responses in fibroblasts. Curr Biol 9: 505–511 [DOI] [PubMed] [Google Scholar]
- Su J, Yang LT, Sap J (1996) Association between receptor protein-tyrosine phosphatase RPTPα and the Grb2 adaptor. Dual Src homology (SH) 2/SH3 domain requirement and functional consequences. J Biol Chem 271: 28086–28096 [DOI] [PubMed] [Google Scholar]
- Sugnet CW, Srinivasan K, Clark TA, O'Brien G, Cline MS, Wang H, Williams A, Kulp D, Blume JE, Haussler D, Ares M Jr (2006) Unusual intron conservation near tissue-regulated exons found by splicing microarrays. PLoS Comput Biol 2: e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabiti K, Smith DR, Goh HS, Pallen CJ (1995) Increased mRNA expression of the receptor-like protein tyrosine phosphatase α in late stage colon carcinomas. Cancer Lett 93: 239–248 [DOI] [PubMed] [Google Scholar]
- Taylor J, Schenck I, Blankenberg D, Nekrutenko A (2007) Using Galaxy to perform large-scale interactive data analyses. Curr Protoc Bioinformatics Chapter 10: 10.5.1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tertoolen LG, Blanchetot C, Jiang G, Overvoorde J, Gadella TW Jr, Hunter T, den Hertog J (2001) Dimerization of receptor protein-tyrosine phosphatase alpha in living cells. BMC Cell Biol 2: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy S, van der Geer P, Hunter T (1995) The receptor-like protein-tyrosine phosphatase, RPTPα, is phosphorylated by protein kinase C on two serines close to the inner face of the plasma membrane. J Biol Chem 270: 10587–10594 [DOI] [PubMed] [Google Scholar]
- Vacaru AM, den Hertog J (2010a) Serine dephosphorylation of receptor protein tyrosine phosphatase α in mitosis induces Src binding and activation. Mol Cell Biol 30: 2850–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacaru AM, den Hertog J (2010b) Catalytically active membrane-distal phosphatase domain of receptor protein-tyrosine phosphatase α is required for Src activation. FEBS J 277: 1562–1570 [DOI] [PubMed] [Google Scholar]
- van Alphen RJ, Wiemer EA, Burger H, Eskens FA (2009) The spliceosome as target for anticancer treatment. Br J Cancer 100: 228–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wijk T, Blanchetot C, Overvoorde J, den Hertog J (2003) Redox-regulated rotational coupling of receptor protein-tyrosine phosphatase α dimers. J Biol Chem 278: 13968–13974 [DOI] [PubMed] [Google Scholar]
- Wang QF, Prabhakar S, Chanan S, Cheng JF, Rubin EM, Boffelli D (2007) Detection of weakly conserved ancestral mammalian regulatory sequences by primate comparisons. Genome Biol 8: R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Pallen CJ (1991) The receptor-like protein tyrosine phosphatase HPTPα has two active catalytic domains with distinct substrate specificities. EMBO J 10: 3231–3237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Rolish ME, Yeo G, Tung V, Mawson M, Burge CB (2004) Systematic identification and analysis of exonic splicing silencers. Cell 119: 831–845 [DOI] [PubMed] [Google Scholar]
- Ward AJ, Cooper TA (2010) The pathobiology of splicing. J Pathol 220: 152–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DL, Iida M, Dunn EF (2009) The role of Src in solid tumors. Oncologist 14: 667–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao JH, Davidson I, Matthes H, Garnier JM, Chambon P (1991) Cloning, expression, and transcriptional properties of the human enhancer factor TEF-1. Cell 65: 551–568 [DOI] [PubMed] [Google Scholar]
- Yang LT, Alexandropoulos K, Sap J (2002) c-SRC mediates neurite outgrowth through recruitment of Crk to the scaffolding protein Sin/Efs without altering the kinetics of ERK activation. J Biol Chem 277: 17406–17414 [DOI] [PubMed] [Google Scholar]
- Yeatman TJ (2004) A renaissance for SRC. Nat Rev Cancer 4: 470–480 [DOI] [PubMed] [Google Scholar]
- Yeo GW, Van Nostrand E, Holste D, Poggio T, Burge CB (2005) Identification and analysis of alternative splicing events conserved in human and mouse. Proc Natl Acad Sci USA 102: 2850–2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Si X, Yu WP, Le HT, Ng KP, Teng RM, Ryan K, Wang DZ, Ponniah S, Pallen CJ (2003) PTPα regulates integrin-stimulated FAK autophosphorylation and cytoskeletal rearrangement in cell spreading and migration. J Cell Biol 160: 137–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentilin L, Giacca M (2007) Competitive PCR for precise nucleic acid quantification. Nat Protoc 2: 2092–2104 [DOI] [PubMed] [Google Scholar]
- Zhang MQ (1998) Statistical features of human exons and their flanking regions. Hum Mol Genet 7: 919–932 [DOI] [PubMed] [Google Scholar]
- Zhao J, Guan JL (2009) Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev 28: 35–49 [DOI] [PubMed] [Google Scholar]
- Zheng XM, Resnick RJ, Shalloway D (2000) A phosphotyrosine displacement mechanism for activation of Src by PTPα. EMBO J 19: 964–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng XM, Resnick RJ, Shalloway D (2002) Mitotic activation of protein-tyrosine phosphatase α and regulation of its Src-mediated transforming activity by its sites of protein kinase C phosphorylation. J Biol Chem 277: 21922–21929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng XM, Resnick RJ, Shalloway D (2008) Apoptosis of estrogen-receptor negative breast cancer and colon cancer cell lines by PTPα and Src RNAi. Int J Cancer 122: 1999–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng XM, Shalloway D (2001) Two mechanisms activate PTPα during mitosis. EMBO J 20: 6037–6049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng XM, Wang Y, Pallen CJ (1992) Cell transformation and activation of pp60c-src by overexpression of a protein tyrosine phosphatase. Nature 359: 336–339 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.