Abstract
Introduction: Advanced paternal age (APA) is a reported risk factor for schizophrenia in the offspring. We performed a meta-analysis of this association, considering the effect of gender and study design. Methods: We identified articles by searching Pub Med, PsychInfo, ISI, and EMBASE, and the reference lists of identified studies. Previously unpublished data from the Northern Finland 1966 Birth Cohort (NFBC 1966) study were also included. Results: There were 6 cohort studies and 6 case–control studies that met the inclusion criteria. In both study designs, there was a significant increase in risk of schizophrenia in the offspring of older fathers (≥30) compared to a reference paternal age of 25–29, with no gender differences. The relative risk (RR) in the oldest fathers (≥50) was 1.66 [95% confidence interval (95% CI): 1.46–1.89, P < 0.01]. A significant increase in risk was also found for younger fathers (<25) in males (RR = 1.08, 95% CI: 1.02–1.14, P = 0.01) but not females (RR = 1.04, 95% CI: 0.97–1.14, P = 0.28). The population attributable risk percentage (PAR%) was 10% for paternal age ≥30 and 5% for paternal age <25. Discussion: Both APA (≥30) and younger paternal age (<25) increase the risk of schizophrenia; younger paternal age may be associated with an increased risk in males but not females. This risk factor increases the risk of schizophrenia as much as any single candidate gene of risk. The mechanism of these associations is not known and may differ for older and younger fathers.
Keywords: paternal age, schizophrenia, epidemiology, meta-analysis, gender differences
Introduction
There is an extensive literature on advanced paternal age (APA) as a risk factor for a wide variety of adverse health outcomes in the offspring that occur throughout the lifespan, including cleft lip and palate, cancer, congenital heart defects, and neuropsychiatric conditions such as autism, epilepsy, and bipolar disorder.1,2 APA has also been associated with poorer intellectual performance in the offspring.3,4 The literature suggests that for many disorders, there is no obvious cut-off point beyond which paternal age should be considered “advanced.”5–8 Instead, there is usually a linear increase in risk of a disorder with increasing paternal age.
APA has also been reported to increase the risk of schizophrenia in the offspring. Potentially confounding factors such as maternal age, parity of the mother, socioeconomic status, family history, social support, ethnicity, marital status, and geography have all been examined and do not appear to account for the effect.9–13 A majority of these studies, however, did not consider male and female offspring separately, although there is evidence that APA has a sexually dimorphic effect for other neuropsychiatric disorders. For instance, Reichenberg et al.14 found that the adjusted odds ratio for APA-associated risk of autism was about 3 times greater in females than in males. Within schizophrenia spectrum disorders, there is also evidence for another risk factor with a sexually dimorphic effect: maternal–fetal blood incompatibility may increase risk in males but not in females.15
We performed a meta-analysis in order to better estimate the effect size of this association and to determine whether there was a sexually dimorphic effect. We included data from the Northern Finland 1966 Birth Cohort (NFBC 1966) study, which has not previously been published, in the meta-analysis (see Supplemental material for details).
Methods
Study Design
Studies on paternal age and schizophrenia risk in the offspring were systematically searched using Medline (PubMed), EMBASE, PsycInfo (via Ovid), and ISI (Science and Social Science Citation Index) in May 2008 and again in December 2009. The search strategy used was “paternal age and (schizophrenia or psychotic disorders).” This search resulted in 38 citations from Medline, 55 from EMBASE, 34 from PsycInfo, and 35 from ISI. From these citations, as well as a manual review of their reference lists, we identified 27 potential studies (including previously unpublished data from the NFBC 1966) for inclusion in the meta-analysis, which are described in Table 1. We excluded 6 studies due to either (1) absence of a comparison group,16–18 (2) >20% of cases had a diagnosis of affective psychosis19 (2 of the 3 samples), or (3) significant overlap in the study population.20,21 In the 2 cases of overlap in the study population, we excluded the study with fewer cases of schizophrenia.
Table 1.
Characteristics of Studies of Paternal Age and Schizophrenia
| Study | Location | Type | Diagnosis | Cases (n) | Included | Comment |
| Johanson, 1958 | Sweden | Case–control | Schizophrenia | 138 | Yes | No comparison group |
| Gregory, 1959 | Canada | Case series | Schizophrenia | 453 | No | No comparison group |
| Farina, 1963 | US | Case series | Schizophrenia | 167 | No | No comparison group |
| Granville-Grossman, 1966 | UK | Case–control | Schizophrenia | 942 | Yes | |
| Bojanovsky and Gerylovová, 1967 | Case series | Schizophrenia | 221 | No | No comparison group | |
| Costello et al., 1968 | UK | Case–control | Schizophrenia | 29 | No | Summary data not available |
| Hare and Moran, 1979 | UK | Case–control | Schizophrenia | 1032 | No | Summary data not available |
| Gillberg, 1982 | Sweden | Case–control | Psychosisa | 30 | No | Summary data not available |
| Kinnell, 1983 | UK | Case–control | Schizophrenia | 320 | No | Summary data not available |
| Malama et al., 1988 | Greece | Case–control | Schizophrenia (Feighner criteria) | 221 | No | Summary data not available |
| Bertranpetit and Fananas, 1993 | Spain | Case–control | Schizophrenia | 120 | No | Summary data not available |
| Raschka, 1998 | Canada | Case series | Schizophrenia (DSM) | 574 | No | Summary data not available |
| Malaspina et al., 2001 | Israel | Birth cohort | Nonaffective psychosis (ICD-10) | 630 | Yes | |
| Brown et al., 2002 | US | Birth cohort | Schizophrenia spectrum disorders (ICD-9) | 71 | Yes | |
| Dalman and Allebeck, 2002 | Sweden | Case–control | Schizophrenia (ICD-8,9) | 420 | Yes | |
| Byrne et al., 2003 | Denmark | Case–control | Schizophrenia (ICD-8,10) | 7704 | Yes | For case–control analyses only |
| Zammit et al., 2003 | Sweden | Conscript cohort | Schizophrenia (ICD-8,9) | 337 | Yes | |
| El-Saadi et al., 2004 | Sweden | Case–control | Psychosis (ICD-8-10) | 134 | No | >20% of cases with affective psychosis |
| Australia | Case–control | Psychosis (DSM-III-R) | 117 | No | >20% of cases with affective psychosis | |
| Denmark | Birth cohort | Psychosis (ICD-10) | 11672 | No | Sample overlaps with Laursen et al., 2007 | |
| Pulver et al., 2004 | US | Case series | Schizophrenia and schizoaffective disorder | 376 | No | No comparison group |
| Sipos et al., 2004 | Sweden | Birth cohort | Schizophrenia (ICD-9,10) | 639 | Yes | |
| Ekeus et al., 2005 | Sweden | Birth cohort | Schizophrenia (ICD-9,10) | 366 | No | Sample overlaps with Sipos et al., 2004 |
| Tsuchiya et al., 2005 | Japan | Case–control | Schizophrenia (DSM-IV) | 99 | Yes | |
| Laursen et al., 2007 | Denmark | Birth cohort | Schizophrenia (ICD-8,10) | 13297 | Yes | |
| Torrey et al., 2009 | US | Birth cohort | Schizophrenia (DSM-IV) | 88 | Yes | |
| Lopez-Castroman et al., 2009 | Spain | Case–control | Schizophrenia spectrum disorders (ICD-10) | 356 | No | Summary data not available |
| Miller et al., 2009 (present study) | Finland | Birth cohort | Schizophrenia (DSM-III-R) | 100 | Yes | Northern Finland 1966 birth cohort |
Psychosis = infantile autism, other childhood psychosis, schizophrenia or schizophreniform psychosis, affective psychosis.
For the remaining 21 studies, we attempted to contact the authors to request summary data stratified by the same paternal age groups and gender. We were unable to consider paternal age as a continuous measure because a majority of authors were unable to share data at the level of the individual subject. The summary data included (1) the number of cases of schizophrenia and (2) the number of non-cases, for the following paternal age groups: <20, 20–24, 25–29, 30–34, 35–39, 40–44, 45–49, 50–54, and ≥55. The requested summary data were not obtained for 9 of these studies,22–29 as either (1) we were unable to contact the authors or (2) the authors were unable to provide the requested summary data.
The 12 studies are included in the meta-analysis,9–13,30–34 including 6 cohort studies and 5 case–controls studies are in bold in Table 1. In one of the cohort studies,31 data were provided in person-years for each 5-year paternal age group. We divided each of these values by 16.2—the mean duration of follow-up in this study—to estimate the number of cases of schizophrenia and the number of non-cases. With the exception of the study by Malaspina et al.,33 which included subjects with nonaffective psychosis, cases were restricted to a diagnosis of schizophrenia (either DSM or ICD criteria) for all studies. A flow chart summarizing the study selection process is presented in Figure 1. A detailed description of the Northern Finland 1966 Birth Cohort is available as Supplementary material.
Fig. 1.
Flow Chart of the Study Selection Process.
Statistical Analysis
Meta-analysis
We initially stratified paternal age into 9 age groups: <20, 20–24, 25–29, 30–34, 35–39, 40–44, 45–49, 50–54, and ≥55. However, because the upper and lower ends of this distribution led to relatively smaller cells sizes, we combined the ≤19 and 20–24 groups into a <25 age group and the 50–54 and ≥55 groups into a ≥50 age group. From the summary data, we calculated relative risk (RR) and 95% confidence intervals (95% CIs) for schizophrenia by paternal age group for each of the 12 individual studies, with risk set equal to 1.00 for the reference group (paternal age 25–29). We then performed a meta-analysis to estimate pooled effect sizes (and 95% CIs) for the risk of schizophrenia by 5-year paternal age groups, again with risk = 1.00 for the reference group (paternal age 25–29). Separate meta-analyses were performed for (1) all studies, (2) cohort studies, and (3) case–control studies. For the analysis of all studies, the study by Byrne et al.10 was excluded due to its substantial overlap with the larger sample from Laursen et al.32 In secondary meta-analyses, we considered males and females separately for (1) all studies, (2) cohort studies, and (3) case–control studies. For the analyses of case–control studies by gender, the study by Byrne et al.10 could not be included as gender-stratified data were unavailable. Fixed effects pooled estimates and 95% CIs were calculated using the method of Mantel and Haenszel. P-values were considered statistically significant at the α = 0.05 level. Random effects estimates were also calculated. Funnel plots were generated to assess for publication bias. The statistical analyses were performed using Stata 10.0 (StataCorp LP, College Station, TX).
Results
Meta-analysis
As described in Table 2, the cohort studies included a total of 3 000 729 subjects (53% male) and 14 568 cases of schizophrenia (66% male). The case–control studies included a total of 8733 cases of schizophrenia and 1 945 092 controls.
Table 2.
Effect of Paternal Age on Schizophrenia Risk by Gender
| Study design | Gender | Paternal age group | Cases (n) | Total (N) | Risk | 95% CI | P-value | Heterogeneity |
I2 | |
| χ2 | P-value | |||||||||
| All | Male | <25 | 2223 | 322 272 | 1.08 | 1.02–1.14 | 0.01 | 3.31 | 0.97 | 0.0 |
| 25–29 | 3242 | 541 871 | 1.00 | Reference | ||||||
| 30–34 | 2463 | 403 014 | 1.03 | 0.97–1.08 | 0.35 | 11.75 | 0.30 | 14.9 | ||
| 35–39 | 1444 | 197 934 | 1.12 | 1.06–1.19 | <0.01 | 29.04 | <0.01 | 65.6 | ||
| 40–44 | 705 | 84 639 | 1.21 | 1.11–1.32 | <0.01 | 14.28 | 0.16 | 30.0 | ||
| 45–49 | 268 | 30 372 | 1.24 | 1.09–1.41 | <0.01 | 18.51 | 0.03 | 51.4 | ||
| ≥50 | 160 | 13 318 | 1.73 | 1.47–2.04 | <0.01 | 42.59 | <0.01 | 83.6 | ||
| Total | 10505 | 1 593 420 | ||||||||
| Female | <25 | 1131 | 292 431 | 1.04 | 0.97–1.12 | 0.28 | 3.55 | 0.90 | 0.0 | |
| 25–29 | 1747 | 490 458 | 1.00 | Reference | ||||||
| 30–34 | 1438 | 358 326 | 1.10 | 1.03–1.19 | <0.01 | 7.55 | 0.48 | 0.0 | ||
| 35–39 | 796 | 173 377 | 1.12 | 1.03–1.23 | 0.01 | 5.42 | 0.71 | 0.0 | ||
| 40–44 | 412 | 72 362 | 1.24 | 1.10–1.38 | <0.01 | 4.37 | 0.74 | 0.0 | ||
| 45–49 | 156 | 26 471 | 1.22 | 1.03–1.44 | 0.02 | 15.27 | <0.01 | 73.8 | ||
| ≥50 | 96 | 11 304 | 1.61 | 1.30–1.99 | <0.01 | 9.84 | 0.02 | 69.5 | ||
| Total | 5776 | 142 4729 | ||||||||
| Both | <25 | 3359 | 614 202 | 1.06 | 1.01–1.11 | 0.02 | 5.24 | 0.87 | 0.0 | |
| 25–29 | 4975 | 1 030 437 | 1.00 | Reference | ||||||
| 30–34 | 3876 | 759 384 | 1.06 | 1.01–1.10 | 0.02 | 9.52 | 0.48 | 0.0 | ||
| 35–39 | 2219 | 369 965 | 1.13 | 1.08–1.19 | <0.01 | 30.01 | <0.01 | 66.7 | ||
| 40–44 | 1106 | 156 254 | 1.22 | 1.14–1.30 | <0.01 | 10.34 | 0.41 | 3.3 | ||
| 45–49 | 417 | 56 543 | 1.21 | 1.09–1.34 | <0.01 | 28.03 | <0.01 | 67.9 | ||
| ≥50 | 252 | 24 481 | 1.66 | 1.46–1.89 | <0.01 | 31.56 | <0.01 | 77.8 | ||
| Total | 16 204 | 3 011 266 | ||||||||
| Cohort | Male | <25 | 2122 | 321 496 | 1.08 | 1.02–1.14 | 0.01 | 1.96 | 0.86 | 0.0 |
| 25–29 | 3025 | 539 437 | 1.00 | Reference | ||||||
| 30–34 | 2207 | 400 513 | 1.02 | 0.96–1.07 | 0.58 | 8.00 | 0.16 | 37.5 | ||
| 35–39 | 1261 | 196 274 | 1.10 | 1.03–1.18 | <0.01 | 22.63 | <0.01 | 77.9 | ||
| 40–44 | 613 | 83 734 | 1.19 | 1.09–1.31 | <0.01 | 9.97 | 0.08 | 49.7 | ||
| 45–49 | 226 | 29 994 | 1.21 | 1.06–1.39 | <0.01 | 7.00 | 0.22 | 28.6 | ||
| ≥50 | 138 | 13 126 | 1.83 | 1.54–2.18 | <0.01 | 28.36 | <0.01 | 85.9 | ||
| Total | 9592 | 1 584 574 | ||||||||
| Female | <25 | 1057 | 291 749 | 1.05 | 0.97–1.14 | 0.21 | 2.68 | 0.31 | 0.0 | |
| 25–29 | 1541 | 488 133 | 1.00 | Reference | ||||||
| 30–34 | 1219 | 355 888 | 1.13 | 1.04–1.21 | <0.01 | 2.05 | 0.73 | 0.0 | ||
| 35–39 | 643 | 171 757 | 1.14 | 1.03–1.25 | <0.01 | 2.54 | 0.64 | 0.0 | ||
| 40–44 | 321 | 71 447 | 1.28 | 1.13–1.44 | <0.01 | 1.36 | 0.85 | 0.0 | ||
| 45–49 | 124 | 26 075 | 1.35 | 1.13–1.62 | <0.01 | 0.51 | 0.78 | 0.0 | ||
| ≥50 | 71 | 11 107 | 1.86 | 1.48–2.35 | <0.01 | 0.10 | 0.75 | 0.0 | ||
| Total | 4976 | 1 416 156 | ||||||||
| Both | <25 | 3179 | 613 245 | 1.06 | 1.01–1.12 | 0.01 | 4.00 | 0.54 | 0.0 | |
| 25–29 | 4566 | 1 027 570 | 1.00 | Reference | ||||||
| 30–34 | 3426 | 756 401 | 1.05 | 1.01–1.10 | 0.03 | 5.01 | 0.42 | 0.0 | ||
| 35–39 | 1904 | 368 031 | 1.13 | 1.07–1.18 | <0.01 | 24.51 | <0.01 | 79.6 | ||
| 40–44 | 934 | 155 181 | 1.22 | 1.14–1.31 | <0.01 | 7.72 | 0.17 | 35.3 | ||
| 45–49 | 350 | 56 069 | 1.24 | 1.12–1.38 | <0.01 | 6.67 | 0.25 | 25.1 | ||
| ≥50 | 209 | 24 232 | 1.79 | 1.56–2.06 | <0.01 | 14.75 | <0.01 | 72.9 | ||
| Total | 14 568 | 3 000 729 | ||||||||
| Case–control | Male | <25 | 101 | 776 | 1.04 | 0.78–1.37 | 0.80 | 1.30 | 0.86 | 0.0 |
| 25–29 | 217 | 2434 | 1.00 | Reference | ||||||
| 30–34 | 256 | 2501 | 1.19 | 0.96–1.47 | 0.11 | 1.72 | 0.79 | 0.0 | ||
| 35–39 | 183 | 1660 | 1.44 | 1.14–1.83 | <0.01 | 1.84 | 0.77 | 0.0 | ||
| 40–44 | 92 | 905 | 1.41 | 1.04–1.90 | 0.03 | 3.26 | 0.52 | 0.0 | ||
| 45–49 | 42 | 378 | 1.49 | 0.99–2.24 | 0.06 | 10.64 | 0.01 | 71.8 | ||
| ≥50 | 22 | 192 | 1.06 | 0.63–1.78 | 0.83 | 10.57 | <0.01 | 81.1 | ||
| Total | 913 | 8846 | ||||||||
| Female | <25 | 74 | 682 | 0.92 | 0.67–1.27 | 0.62 | 0.27 | 0.97 | 0.0 | |
| 25–29 | 206 | 2325 | 1.00 | Reference | ||||||
| 30–34 | 219 | 2438 | 0.91 | 0.72–1.15 | 0.43 | 2.71 | 0.44 | 0.0 | ||
| 35–39 | 153 | 1620 | 1.02 | 0.78–1.34 | 0.88 | 2.33 | 0.51 | 0.0 | ||
| 40–44 | 91 | 915 | 0.96 | 0.69–1.34 | 0.82 | 0.53 | 0.77 | 0.0 | ||
| 45–49 | 32 | 396 | 0.54 | 0.33–0.89 | 0.02 | 2.95 | 0.09 | 66.0 | ||
| ≥50 | 25 | 197 | 0.73 | 0.42–1.25 | 0.25 | 0.00 | 0.99 | 0.0 | ||
| Total | 800 | 8573 | ||||||||
| Both | <25 | 1781 | 42 218 | 1.06 | 1.00–1.13 | 0.06 | 1.29 | 0.94 | 0.0 | |
| 25–29 | 2666 | 65 172 | 1.00 | Reference | ||||||
| 30–34 | 2104 | 46 108 | 1.06 | 1.00–1.13 | 0.07 | 4.62 | 0.46 | 0.0 | ||
| 35–39 | 1185 | 24 480 | 1.09 | 1.02–1.18 | 0.02 | 6.82 | 0.24 | 26.6 | ||
| 40–44 | 621 | 11 125 | 1.22 | 1.10–1.34 | <0.01 | 2.66 | 0.75 | 0.0 | ||
| 45–49 | 236 | 4268 | 1.17 | 1.02–1.35 | 0.03 | 20.69 | <0.01 | 80.7 | ||
| ≥50 | 140 | 1721 | 1.57 | 1.31–1.89 | <0.01 | 15.88 | <0.01 | 81.1 | ||
| Total | 8733 | 195 092 | ||||||||
Values in bold were statistically significant at the P < 0.05 level.
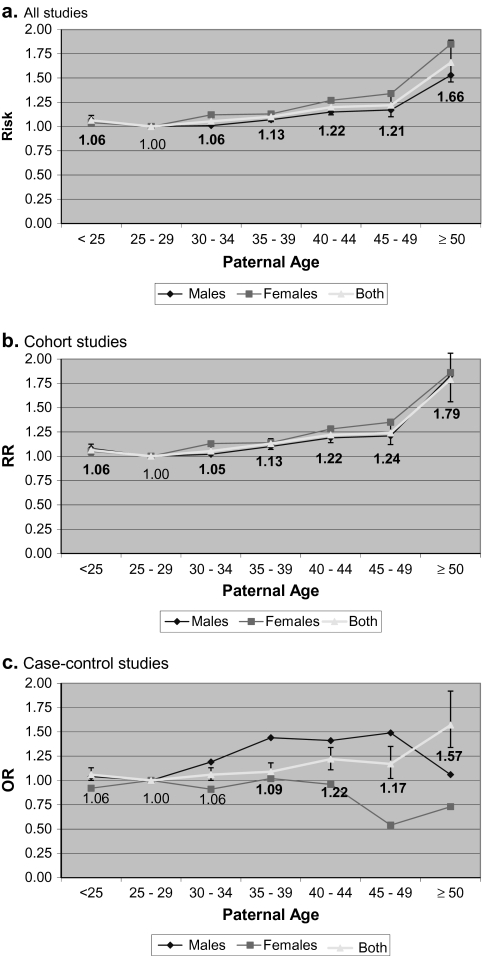
Table 2 and Figure 2 present the estimates of effect sizes with 95% CIs from the meta-analysis, compared to a reference paternal age of 25–29, for each paternal age group, by study design. In cohort, case–control, and all studies, we found (1) an increase in risk of schizophrenia in the offspring with increasing paternal age (>30 years of age), as well as a significant increase in risk of schizophrenia in the offspring of younger fathers (<25 years of age). Effect sizes were similar for cohort and case–control studies. The results of all meta-analyses were similar when repeated using a random effects model (data not shown). Funnel plots showed no evidence of publication bias (see Supplemental Figure 1). In all studies, there was significant heterogeneity between the studies in 3 paternal age classes: (1) 35–39, heterogeneity χ2 = 29.95, P < 0.01, I2 (variation in effect size attributable to heterogeneity) = 70.0%, (2) 45–49, heterogeneity χ2 = 27.02, P < 0.01, I2 = 70.4%, and (3) ≥50, heterogeneity χ2 = 31.56, P < 0.01, I2 = 77.8%. In a sensitivity analysis, the heterogeneity in the paternal age 35–39 class was no longer significant after removing the study by Laursen et al.,32 the heterogeneity in the paternal age 45–49 class was no longer significant after removing the study by Granville-Grossman,29 and the heterogeneity in the paternal age ≥50 was no longer significant after removing 2 studies (Granville-Grossman30 and Laursen et al.32; data not shown). For each of these 3 paternal age classes, the effect size estimates remained significant.
Fig. 2.
Effect of Paternal Age on Schizophrenia Risk in 5-Year Groups. Error bars represent confidence interval boundaries for the estimates for males and females combined (“Both”). (a) All studies; (b) Cohort studies; (c) Case–control studies. Note that the study by Byrne et al.10 could not be included in the curves for males and females as gender-stratified data were unavailable.
Table 2 and Figure 2 also present the estimates of effect sizes with 95% CIs from the meta-analyses with males and females considered separately. In all studies and in cohort studies, the association between younger fathers (<25) and schizophrenia risk was significant in males (RR = 1.08, 95% CI: 1.02–1.14, P = 0.01) but not females (RR = 1.04, 95% CI: 0.97–1.12, P = 0.28), although the point estimates were similar. In case–control studies, the estimate for male offspring of younger fathers was similar to cohort studies but was not statistically significant (OR = 1.04, 95% CI: 0.78–1.37, P = 0.80). In all studies, estimates for males and females were similar for all paternal age classes ≥30. The results of all-secondary meta-analyses were similar when repeated using a random effects model (data not shown).
Using the summary data provided by each of the authors, we also estimated the population attributable risk percentage (PAR%) for paternal age. The PAR% is the incidence of outcome (schizophrenia) in the total population (defined here as paternal age either <30 or ≥25), minus the incidence of outcome among the unexposed (defined here as the reference paternal age group of 25–29), divided by the incidence of outcome in the total population, and multiplied by 100%. For paternal age <25, the PAR% was 6%, 1%, and 5% in cohort, case–control, and all studies, respectively. For paternal age ≥30, the PAR% was 7%, 10%, and 10% in cohort, case–control, and all studies, respectively. These estimates did not differ significantly for males and females. For paternal age <25, the PAR% was 5% for males and 3% for females in all studies. For paternal age ≥30, the PAR% was 8% for males and 13% for females in all studies.
Discussion
We found a significant increase in risk of schizophrenia in the offspring increasing paternal age (≥30 years of age). We also found a significant increase in risk of schizophrenia in the offspring of younger fathers (<25), which may also be associated with an increased risk in males but not females. We did not find evidence for gender effects on the relationship between older paternal age (≥30) and schizophrenia risk in the offspring. The PAR% was 10% for paternal age ≥30 and 5% for paternal age <25 in all studies.
Strengths and Limitations
An important strength of our study is that we included data from all published (and one unpublished) cohort studies of this association. Two previous meta-analyses35,36 found that APA was associated with an increased risk of schizophrenia. Our analysis differed from these analyses in several ways. First, we considered the effect of gender on the association, which was not previously examined. By requesting summary data from the authors, we were able to use the same age classes across all studies, which enabled us (1) to use a consistent reference group, (2) to estimate risks by 5-year age groups, as opposed to looking for a threshold age of increased risk, 3) to incorporate data from several studies not included in the previous meta-analyses (Laursen et al.,32 Tsuchiya et al.34 and previously unpublished data from the NFBC 1966), 4) to calculate the PAR% for paternal age, and 5) to test for an association in younger fathers, which was not previously examined. Last, for every study in our primary meta-analysis except Malaspina et al.,33 which included subjects with nonaffective psychosis, cases were restricted to a diagnosis of schizophrenia (by DSM and ICD criteria).
An important limitation of the present study was that effect sizes were calculated as crude risks by paternal age groups. We were not able to control for potential confounding factors such as maternal age and socioeconomic status in the analysis. However, it is reassuring that within most individual studies included in the meta-analysis, the effect of increasing paternal age remained significant and was often greater,9,13,33 after adjusting for maternal age.
Study Heterogeneity
A vast majority of studies in the meta-analysis used national birth registries to determine paternal age, although no studies confirmed biological paternity, which could contribute to the heterogeneity of the results. Two studies, Granville-Grossman30 and Laursen et al.,32 made the largest contribution to the heterogeneity in the results of the sensitivity analysis for all studies. The controls in the study by Granville-Grossman30 were siblings of the schizophrenia probands. The author also standardized parental ages to a reference population in order to exclude the effect of sibship size, meaning that actual parental ages were not used. Thus, selection bias may have contributed to the heterogeneity of the results. The study by Laursen et al. 32 was the largest single study in the meta-analysis, comprising 82% (n = 13 297) of all subjects with schizophrenia in the meta-analysis. The estimates for paternal age groups ≥30 were generally among the lowest for this study compared with others (see Supplemental Figure 2), which may have contributed to the observed heterogeneity.
Younger Fathers
An important finding from our analysis was a significant association between younger fathers (<25) and risk of schizophrenia, and this risk may be greater in male than in female offspring. Several previous studies,10–12,20 as well as our data from the NFBC 1966, found a non-significant increased risk in the offspring of younger fathers, after adjustment for potential confounders. In one of these studies,10 the effect was greater in males than in females. One possibility is that the association with younger fathers is due to confounding factors such as maternal age, for which we were not able to statistically control, or selection bias.
Younger paternal age is also associated with preterm birth,37 congenital abnormalities,38–41 and Type 1 diabetes42 after adjustment for potential confounders, which indirectly supports the plausibility of this association. Although the effect size was small, our findings raise the interesting possibility of different causal mechanisms for schizophrenia between the offspring of younger and older fathers.
Population Attributable Risk
We found a PAR% in all studies of 10% for paternal age ≥30, compared to a reference paternal age of 25–29. Because the PAR% varies with both the risk (RR or OR) associated with an exposure (eg, paternal age ≥30) and its prevalence, caution must be exercised in the interpretation of this result. The PAR% refers to a family of concepts. Greenland and Robins43 distinguished between the etiologic and excess fraction. The etiologic fraction is the proportion of cases that the exposure had played a causal role in its development. The excess fraction is the proportion of cases among the exposed population that is in excess in comparison with the unexposed. Our results describe the excess fraction for paternal age because it is not possible to establish the causality of this association.
Our estimate of PAR is lower than the attributable risk for APA of 26.6% reported by Malaspina et al.,33 the only other study to report this measure. Effect size estimates for each paternal age group in the study by Malaspina et al.33 were consistently higher than those for all studies in this meta-analysis (see Supplemental Figure 2), which is reflected in a higher PAR% than what we found. This difference also raises the question of possible ethnic differences because the study of Malaspina et al.33 was conducted in a sample that was overwhelmingly Ashkenazi Jewish, while many of the other studies may have been less ethnically homogenous. The effect of paternal age may depend on the genetic background in which it exerts its influence. Another potential factor contributing to the higher PAR% in the study by Malaspina et al.33 is that this study included subjects with nonaffective psychosis. It is possible that the paternal age effect may be greater when extended beyond schizophrenia to include all nonaffective psychosis.
Conclusions
The increased risk associated with paternal age <25 raises the possibility that the mechanisms of abnormal development associated with this very young group may differ from those associated with paternal age ≥30. The risk factor of paternal age increases the risk for schizophrenia as much as any single candidate gene.44
Whether either of the associations that emerged from this analysis is due to biological or psychosocial factors, or both, remains unclear. There is some evidence for an increased rate of de novo mutations with APA.45 Immature spermatids and/or low activity of DNA repair or antioxidant enzymes have also been proposed as mechanisms for increased de novo genetic disorders in the offspring of younger fathers.46 It is possible that the association with APA is due to delayed childbearing by fathers with schizophrenia and related disorders. However, one study47 found an association between APA and sporadic (versus familial) schizophrenia, but there is a failure to replicate.19 Although the possibility of epigenetic changes, such as imprinting, DNA methylation, or histone acetylation, has also been proposed, this mechanism has not been thoroughly investigated,48 and both mutations and epigenetic changes may contribute. Paternal age may also be associated with an adverse psychosocial environment for offspring, thereby increasing risk of schizophrenia. Both younger and older fathers may be associated with increased unwantedness of pregnancy, which is a potential risk factor for schizophrenia.49
An understanding of this risk factor has substantial public health potential, as average paternal ages are increasing1 and APA is common, has widespread effects, and is a potentially modifiable risk factor. Public awareness of the potential health hazards associated with older fatherhood may decrease the delay in having children. Accounting for the APA effect as a potential confounding factor may also increase the signal-to-noise ratio in other epidemiological and genetic analyses in schizophrenia. Subsequent studies will be important to clarifying the pathophysiology of a determinant of schizophrenia.
Supplementary Material
Supplementary material and Figures 1 and 2 are available at http://schizophreniabulletin.oxfordjournals.org.
Funding
Academy of Finland (110143 and 212848 to M.I., 125853, to J.M.); Sigrid Juselius Foundation to M.I. and A.A.; Oy H. Lundbeck Ab to B.J.M.; University of Oulu to B.J.M.
Supplementary Material
Acknowledgments
The authors wish to thank Drs Alan Brown, Majella Byrne, Christina Dalman, David Kern, Thomas Laursen, Dolores Malaspina, Mary Perrin, Per Tynelius, Kenji Tsuchiya, and Stanley Zammit for sharing data.
Conflict of Interest: Drs Miller, Messias, Miettunen, Alaräisänen, Järvelin, Koponen, and Dr. Räsänen have nothing to disclose. Dr Isohanni received consulting and/or speaking fees from AstraZeneca and Janssen-Cilag. Dr Kirkpatrick received consulting and/or speaking fees from Pfizer, Organon, AstraZeneca, Wyeth, Bristol Myers Squibb, Cephalon, Lilly, and Solvay.
Contributors: Drs Miller and Kirkpatrick designed the study. Dr Miller managed the literature searches. Drs Messias, Miettunen, and Miller managed the analyses. Drs Miller and Kirkpatrick wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Previous presentation: This manuscript was previously presented as a poster at the 12th International Congress on Schizophrenia Research, March 28–April 1, 2009, San Diego, California.
The Academy of Finland, Sigrid Juselius Foundation, and University of Oulu had no further role in study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
References
- 1.Bray I, Gunnell D, Davey Smith G. Advanced paternal age: how old is too old? J Epidemiol Community Health. 2006;60:851–853. doi: 10.1136/jech.2005.045179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cannon M. Contrasting effects of maternal and paternal age on offspring intelligence: the clock ticks for men too. PLoS Med. 2009;6:e42. doi: 10.1371/journal.pmed.1000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malaspina D, Reichenberg A, Weiser M, et al. Paternal age and intelligence: implications for age-related genomic changes in male germ cells. Psychiatr Genet. 2005;15:117–125. doi: 10.1097/00041444-200506000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Saha S, Barnett AG, Foldi C, et al. Advanced paternal age is associated with impaired neurocognitive outcomes during infancy and childhood. PLoS Med. 2009;6:e40. doi: 10.1371/journal.pmed.1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bingley PJ, Douek IF, Rogers CA, Gale EA. Influence of maternal age at delivery and birth order on risk of type 1 diabetes in childhood: prospective population based family study. Bart's-Oxford Family Study Group. BMJ. 2000;321:420–4. doi: 10.1136/bmj.321.7258.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dockerty JD, Draper G, Vincent T, Rowan SD, Bunch KJ. Case-control study of parental age, parity and socioeconomic level in relation to childhood cancers. Int J Epidemiol. 2001;30:1428–1437. doi: 10.1093/ije/30.6.1428. [DOI] [PubMed] [Google Scholar]
- 7.Frans EM, Sandin S, Reichenberg A, Lichtenstein P, Långström N, Hultman CM. Advancing paternal age and bipolar disorder. Arch Gen Psychiatry. 2008;65:1034–1040. doi: 10.1001/archpsyc.65.9.1034. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Cupples LA, Rosenberg L, Colton T, Kreger BE. Parental ages at birth in relation to a daughter's risk of breast cancer among female participants in the Framingham Study (United States) Cancer Causes Control. 1995;6:23–29. doi: 10.1007/BF00051677. [DOI] [PubMed] [Google Scholar]
- 9.Brown AS, Schaefer CA, Wyatt RJ, et al. Paternal age and risk of schizophrenia in adult offspring. Am J Psychiatry. 2002;159:1528–1533. doi: 10.1176/appi.ajp.159.9.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrne M, Agerbo E, Ewald H, Eaton WW, Mortensen PB. Parental age and risk of schizophrenia: a case-control study. Arch Gen Psychiatry. 2003;60:673–678. doi: 10.1001/archpsyc.60.7.673. [DOI] [PubMed] [Google Scholar]
- 11.Dalman C, Allebeck P. Paternal age and schizophrenia: further support for an association. Am J Psychiatry. 2002;159:1591–1592. doi: 10.1176/appi.ajp.159.9.1591. [DOI] [PubMed] [Google Scholar]
- 12.Sipos A, Rasmussen F, Harrison G, et al. Paternal age and schizophrenia: a population based cohort study. BMJ. 2004;329:1070. doi: 10.1136/bmj.38243.672396.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zammit S, Allebeck P, Dalman C, et al. Paternal age and risk for schizophrenia. Br J Psychiatry. 2003;183:405–408. doi: 10.1192/bjp.183.5.405. [DOI] [PubMed] [Google Scholar]
- 14.Reichenberg A, Gross R, Weiser M, et al. Advancing paternal age and autism. Arch Gen Psychiatry. 2006;63:1026–1032. doi: 10.1001/archpsyc.63.9.1026. [DOI] [PubMed] [Google Scholar]
- 15.Insel BJ, Brown AS, Bresnahan MA, Schaefer CA, Susser ES. Maternal-fetal blood incompatibility and the risk of schizophrenia in offspring. Schizophr Res. 2005;80:331–342. doi: 10.1016/j.schres.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Gregory I. An analysis of familial data on psychiatric patients: parental age, family size, birth order, and ordinal position. Br J Prev Soc Med. 1959;(12):42–59. doi: 10.1136/jech.12.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farina A, Storrs C, Barry H, Garmezy N. Birth order of recovered and nonrecovered schizophrenics. Arch Gen Psychiatry. 1963;(9):224–228. doi: 10.1001/archpsyc.1963.01720150034005. [DOI] [PubMed] [Google Scholar]
- 18.Bojanovsky J, Gerylovova A. Schizophrenia and the parental age of patients. Preliminary report. Cesk Psychiatr. 1967;(63):85–87. [PubMed] [Google Scholar]
- 19.Pulver AE, McGrath JA, Liang KY, Lasseter VK, Nestadt G, Wolyniec PS. An indirect test of the new mutation hypothesis associating advanced paternal age with the etiology of schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2004;124:6–9. doi: 10.1002/ajmg.b.20066. [DOI] [PubMed] [Google Scholar]
- 20.El-Saadi O, Pedersen CB, McNeil TF, et al. Paternal and maternal age as risk factors for psychosis: findings from Denmark, Sweden and Australia. Schizophr Res. 2004;67:227–236. doi: 10.1016/S0920-9964(03)00100-2. [DOI] [PubMed] [Google Scholar]
- 21.Ekéus C, Olausson PO, Hjern A. Psychiatric morbidity is related to parental age: a national cohort study. Psychol Med. 2006;36:269–276. doi: 10.1017/S0033291705006549. [DOI] [PubMed] [Google Scholar]
- 22.Costello AJ, Gunn JC, Dominian J. Aetiological factors in young schizophrenic men. Br J Psychiatry. 1968;114:433–441. doi: 10.1192/bjp.114.509.433. [DOI] [PubMed] [Google Scholar]
- 23.Hare EH, Moran PA. Raised parental age in psychiatric patients: evidence for the constitutional hypothesis. Br J Psychiatry. 1979;134:169–177. doi: 10.1192/bjp.134.2.169. [DOI] [PubMed] [Google Scholar]
- 24.Gillberg C. Parental age in child psychiatric clinic attenders. Acta Psychiatr Scand. 1982;66:471–478. [PubMed] [Google Scholar]
- 25.Kinnell H. Parental age in schizophrenia. Br J Psychiatry. 1983;142:204. doi: 10.1192/bjp.142.2.204a. [DOI] [PubMed] [Google Scholar]
- 26.Malama IM, Papaioannou DJ, Kaklamani EP, Katsouyanni KM, Koumantaki IG, Trichopoulos DV. Birth order sibship size and socio-economic factors in risk of schizophrenia in Greece. Br J Psychiatry. 1988;152:482–486. doi: 10.1192/bjp.152.4.482. [DOI] [PubMed] [Google Scholar]
- 27.Bertranpetit J, Fananas L. Parental age in schizophrenia in a case-controlled study. Br J Psychiatry. 1993;162:574. doi: 10.1192/bjp.162.4.574. [DOI] [PubMed] [Google Scholar]
- 28.Raschka LB. Parental age and schizophrenia. Magyar Andrologica. 1998;2:47–50. [Google Scholar]
- 29.Lopez-Castroman J, Gomez DD, Bellosos JJC, et al. Differences in maternal and paternal age between schizophrenia and other psychiatric disorders. Schizophr Res. 2010;116:184–190. doi: 10.1016/j.schres.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Granville-Grossman KL. Parental age and schizophrenia. Br J Psychiatry. 1966;112:899–905. doi: 10.1192/bjp.112.490.899. [DOI] [PubMed] [Google Scholar]
- 31.Johanson E. A study of schizophrenia in the male. Acta Psychiatr Scand. 1958;33(suppl 125):7–107. [PubMed] [Google Scholar]
- 32.Laursen TM, Munk-Olsen T, Nordentoft M, Bo Mortensen P. A comparison of selected risk factors for unipolar depressive disorder, bipolar affective disorder, schizoaffective disorder, and schizophrenia from a danish population-based cohort. J Clin Psychiatry. 2007;68:1673–1681. doi: 10.4088/jcp.v68n1106. [DOI] [PubMed] [Google Scholar]
- 33.Malaspina D, Harlap S, Fennig S, et al. Advancing paternal age and the risk of schizophrenia. Arch Gen Psychiatry. 2001;58:361–367. doi: 10.1001/archpsyc.58.4.361. [DOI] [PubMed] [Google Scholar]
- 34.Tsuchiya KJ, Takagai S, Kawai M, et al. Advanced paternal age associated with an elevated risk for schizophrenia in offspring in a Japanese population. Schizophr Res. 2005;76:337–342. doi: 10.1016/j.schres.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Torrey EF, Buka S, Cannon TD, et al. Paternal age as a risk factor for schizophrenia: how important is it? Schizophr Res. 2009;114:1–5. doi: 10.1016/j.schres.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 36.Wohl M, Gorwood P. Paternal ages below or above 35 years old are associated with a different risk of schizophrenia in the offspring. Eur Psychiatry. 2007;22:22–26. doi: 10.1016/j.eurpsy.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Abel EL, Kruger M, Burd L. Effects of maternal and paternal age on Caucasian and Native American preterm births and birth weights. Am J Perinatol. 2002;19:49–54. doi: 10.1055/s-2002-20173. [DOI] [PubMed] [Google Scholar]
- 38.Archer NP, Langlois PH, Suarez L, Brender J, Shanmugam R. Association of paternal age with prevalence of selected birth defects. Birth Defects Res A Clin Mol Teratol. 2007;79:27–34. doi: 10.1002/bdra.20316. [DOI] [PubMed] [Google Scholar]
- 39.Kazaura M, Lie RT, Skjaerven R. Paternal age and the risk of birth defects in Norway. Ann Epidemiol. 2004;14:566–570. doi: 10.1016/j.annepidem.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Mclntosh GC, Olshan AF, Baird PA. Paternal age and the risk of birth defects in offspring. Epidemiology. 1995;6:282–288. doi: 10.1097/00001648-199505000-00016. [DOI] [PubMed] [Google Scholar]
- 41.Olshan AF, Teschke K, Baird PA. Paternal occupation and congenital anomalies in offspring. Am J Ind Med. 1991;20:447–475. doi: 10.1002/ajim.4700200403. [DOI] [PubMed] [Google Scholar]
- 42.Tai TY, Wang CY, Lin LL, Lee LT, Tsai ST, Chen CJ. A case-control study on risk factors for type 1 diabetes in Taipei City. Diabetes Res Clin Pract. 1998;42:197–203. doi: 10.1016/s0168-8227(98)00105-3. [DOI] [PubMed] [Google Scholar]
- 43.Greenland S, Robins JM. Conceptual problems in the definition and interpretation of attributable fraction. Am J Epidemiol. 1988;128:1185–1197. doi: 10.1093/oxfordjournals.aje.a115073. [DOI] [PubMed] [Google Scholar]
- 44.Allen NC, Bagade S, McQueen MB, et al. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet. 2008;40:827–834. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- 45.Crow JF. The high spontaneous mutation rate: is it a health risk? Proc Natl Acad Sci USA. 1997;94:8380–8386. doi: 10.1073/pnas.94.16.8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malaspina D. Paternal factors and schizophrenia risk: de novo mutations and imprinting. Schizophr Bull. 2001;27:379–393. doi: 10.1093/oxfordjournals.schbul.a006882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malaspina D, Brown A, Goetz D, et al. Schizophrenia risk and paternal age: a potential role for de novo mutations in schizophrenia vulnerability genes. CNS Spectr. 2002;7:26–29. doi: 10.1017/s1092852900022239. [DOI] [PubMed] [Google Scholar]
- 48.Perrin MC, Brown AS, Malaspina D. Aberrant epigenetic regulation could explain the relationship of paternal age to schizophrenia. Schizophr Bull. 2007;33:1270–1273. doi: 10.1093/schbul/sbm093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herman DB, Brown AS, Opler MG, et al. Does unwantedness of pregnancy predict schizophrenia in the offspring? Findings from a prospective birth cohort study. Soc Psychiatry Psychiatr Epidemiol. 2006;41:605–610. doi: 10.1007/s00127-006-0078-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.