Abstract
Until recently, the social cognitive impairment in schizophrenia has been underappreciated and remains essentially untreated. Deficits in emotional processing, social perception and knowledge, theory of mind, and attributional bias may contribute to functional social cognitive impairments in schizophrenia. The amygdala has been implicated as a key component of social cognitive circuitry in both animal and human studies. In addition, structural and functional studies of schizophrenia reproducibly demonstrate abnormalities in the amygdala and dopaminergic signaling. Finally, the neurohormone oxytocin plays an important role in multiple social behaviors in several mammals, including humans. We propose a model of social cognitive dysfunction in schizophrenia and discuss its therapeutic implications. The model comprises abnormalities in oxytocinergic and dopaminergic signaling in the amygdala that result in impaired emotional salience processing with consequent social cognitive deficits.
Keywords: social cognition, emotion perception, neuropeptide, hormone
While hallucinations and paranoid delusions often represent the public face of schizophrenia, the positive symptoms alone do not define the disorder or the devastation that results from its pathology. Persons with schizophrenia may be more handicapped by social cognitive deficits, which correlate with inhibited interpersonal functioning and vocational achievement.1 And though antipsychotic medications are not a panacea, they often provide some measure of relief from positive symptoms. However, social cognitive deficits remain relatively untouched by currently available medicines,2,3 though recent evidence suggests efficacy of some psychosocial treatments.4,5 Increasing attention to the impact of social cognitive impairment in schizophrenia has led to concerted efforts to parse this domain into subcategories more amenable to hypothesis-driven research6; these include theory of mind (ToM), social perception, social knowledge, attributional bias, and emotion processing.7
We suggest that the social cognitive deficits common to persons diagnosed with schizophrenia correspond to an underlying abnormality of emotional salience processing. The amygdala, as a seat of emotion processing and information relay, is a likely candidate for dysfunction in schizophrenia. It is hypothesized that aberrant interactions between dopaminergic reward systems, a dysfunctional amygdala, and the neurohormone oxytocin engender a neural milieu that improperly assigns emotional salience to environmental stimuli. This deficit in turn results in aberrant social cognition that may ultimately lead to misguided social responses, from withdrawal and isolation to suspicion and paranoia. This article will review the evidence for social cognitive and amygdalar deficits in schizophrenia, the role of amygdala and oxytocin in social cognition, and an integrated model that can account for a core functional impairment in the disorder. The proposed model is amenable to hypothesis-driven experimentation to better understand pathophysiologic interactions between brain areas and neurotransmitters that might be utilized to develop neurotherapeutics and more accurate measures of disease and recovery states in schizophrenia.
Social Cognitive Deficits in Schizophrenia
Reviews of the growing literature on cognitive function in schizophrenia consistently report deficits in multiple domains of social cognition,8–10 although the specific nature of these deficits has not been defined.11 A review of 110 studies of emotional-processing deficits in schizophrenia9 presented accumulated evidence that patients with schizophrenia have deficits in emotion expression, experience, and recognition in the verbal, acoustic, and facial domains. While these deficits also correlate with nonsocial cognitive domains, especially attention and vigilance, the relation between social and nonsocial cognition is uncertain.6 Yet, nonsocial cognition does not explain all of the variance in social functioning in schizophrenia patients12 and appears to rely on different neural circuits than social cognition.13
Facial emotion recognition is one of the best-studied emotional-processing domains in schizophrenia. Patients have difficulty in identifying emotional expressions in others’ faces, particularly when the emotions are negative or threatening. This seems especially true in chronic schizophrenia,14 despite controlling for baseline differences in discriminating between faces based on nonemotional characteristics. This deficit in emotion identification remains when persons with schizophrenia are compared with persons with affective psychoses15 or across cultures.16
Moreover, facial emotion recognition deficits seem to be compounded by an inability to appropriately consider the social context when assessing others’ emotions. Patients with schizophrenia who are asked to judge facial emotion in relation to contextual clues are unable to utilize emotional context to modify their judgments, particularly when judging negative emotions.17 Likewise, Penn et al18 found patients to be impaired on utilizing social contextual clues to sequence typical social behaviors or judge others’ anxiety levels, implicating aberrant contextual analysis in emotion-processing deficits that may contribute to social perception deficits as well.
Emotional memory is a fundamental component of emotional-processing systems that also appears to be dysfunctional in schizophrenia. Impairments in error monitoring and spatial working memory have been demonstrated to affect memory for faces in schizophrenia.19 Patients with schizophrenia also show a deficit in the capacity to enhance memories associated with emotionally salient cues, with such deficits in recall being most pronounced for arousing negative scenes.20 This evidence points to an alteration in storage and/or retrieval of emotional memories in schizophrenia that would appear to distort social perception by impairing the ability to utilize memory for nonverbal affective cues to assess social situations.
Though theories relating different social cognitive functions to one another in terms of psychologic constructs and neural underpinnings are largely untested,10 investigations such as those above suggest that impairments in bottom-up processes like emotion perception may generate impairments in more top-down cognitive processes that rely on prior bottom-up analyses. Impairment on ToM tasks has been shown repeatedly in patients with schizophrenia, although the correlation with symptom domains, neurocognitive deficits, and disease chronicity is not well established.21,22 Some studies suggest specific impairments in affective ToM tasks in which judgments about intentions or beliefs must be made based on emotional verbal cues and facial expressions.23 The tendency to interpret sarcastic statements as sincere also correlates with deficits in affective prosody and basic pitch perception.24 These findings suggest that an inability to analyze emotional communication may impede the ability of patients with schizophrenia to understand the emotional states of others in more complex ways.
The outward manifestations of these social cognitive deficits in schizophrenia may include negative symptoms.10 Negative symptoms of schizophrenia include blunted or incongruous emotional responses, anhedonia, social isolation, paucity of speech, diminished spontaneous movements or actions, apathy, and lack of motivation. While the clinical confluence of negative symptomatology and the social cognitive deficits of schizophrenia suggests shared aspects, their neuroanatomic and functional relationship is unclear.13 Difficulty in recognizing emotion and understanding social context could result in social withdrawal and isolative behavior; dysfunctional emotional expression could contribute to blunted or flat affect. Additionally, confusion with regard to negative emotions and threat perception could encourage misconstrual of benign interactions as threatening, known as hostile attributional bias, leading to paranoia and further isolation.25 In fact, studies show that aspects of negative symptomatology correlate with deficits in detection of faces showing negative emotions26 and identification of emotions and their intensities.27 Though negative symptoms seem to intersect with social cognition, their interaction requires further exploration28 because it is not understood whether social cognitive deficits cause negative symptoms or vice versa or if they are both effects of another causal process.
Amygdala and Social Cognition
A potential locus of dysfunctional emotional processing seen in schizophrenia, and perhaps manifested as social cognitive deficits, is the amygdala. An extensive literature documents the role of this limbic structure in multiple facets of emotion analysis (for review see reference29). The amygdala responds to many facial expressions, but especially to fear.30 The amygdala is activated by fearful stimuli prior to conscious awareness of the stimuli and has been shown to enhance cortical activation in response to emotional stimuli.29 Deficits in the interpretation of fearful faces after amygdala damage seem to relate to a lack of attention to the eyes.29
Other aspects of social cognition are affected by amygdala damage as well. Patients with amygdala lesions fail to impose social context on nonsocial stimuli, so that moving shapes typically interpreted as meaningful are simply described as shapes.29 When determining whether facial expressions signify fear or surprise, clues suggesting surprise decrease amygdala activity in control participants, whereas body movements consistent with fear increase amygdala activity.29 Persons with intact amygdala function respond to faces deemed “untrustworthy” or less approachable, whereas patients with amygdalar damage rate these faces as more trustworthy.29
The amygdala may not only process emotional stimuli but may also interact with dopaminergic systems to assign salience to particular stimuli.10,31 The amygdala is extensively connected to dopaminergic nuclei, including the nucleus accumbens and ventral tegmental area.31–33 Dopamine antagonism can prevent the memory-enhancing effects of emotionally arousing images,34 an enhancement likely mediated by the amygdala.35 The fact that most pharmacologic treatments for schizophrenia currently involve dopamine antagonism thus becomes problematic in that it may mean patients are sacrificing some social cognitive capacity for control of positive symptoms.
Phillips et al36 propose a differential impact of the basolateral vs the central nuclei of the amygdala on dopaminergic output. The authors suggest that the central amygdala affects tonic dopaminergic tone in the ventral tegmental area, thereby regulating dopaminergic tone in the nucleus accumbens and modulating the incentive value of environmental stimuli. The basolateral amygdala, in turn, affects transient increases in dopamine in the nucleus accumbens and medial prefrontal cortex in order to choose behavior sequences that are appropriate responses to incentivized environmental stimuli. Additional support for the effect of dopamine systems on amygdala function comes from reports that the dopaminergic state in patients with Parkinson's disease affects amygdala responsiveness during emotion perception tasks.37
In addition to a potential role in facilitating the assignment of salience to emotional stimuli via its connections to dopaminergic systems, the amygdala also exhibits functional connectivity with cortical areas implicated in higher-order social cognition.10,31 Cortical efferents to the amygdala contribute to regulation of emotional perceptions and reactions. Green and Phillips25 suggest that the amygdala and prefrontal networks are involved in a system of threat detection based on faces and social stimuli, wherein bidirectional amygdala and prefrontal processing of sensory perceptions may allow sensory and attentional resources to be directed to features suggestive of threat. Functional magnetic resonance imaging (fMRI) data show that dorsolateral prefrontal cortex modulation of amygdala responses to anticipated negative emotional stimuli parallels participants’ capacity to utilize cognitive reappraisal strategies.38 In a similar fashion, amygdala responses to emotional conflict appear to be influenced by interactions with the rostral anterior cingulate cortex in a neural “conflict resolution” circuit.39 Animal models further demonstrate prefrontal cortical inhibition of emotional learning in amygdala neurons.31
In addition to these cortical influences on amygdala function, growing evidence suggests that the interaction may be bidirectional, with the amygdala itself possibly modulating other neural circuits. Functional neuroimaging work shows a reliance on the amygdala's subcortical connections for “unconscious” responses to fearful stimuli, while conscious fear responses require neuronal interaction between the amygdala and the anterior cingulate cortex.40 Based on work showing the maturation of amygdalo-cortical connectivity in rats, Cunningham et al41 hypothesize that the development of these neuronal links may determine the maturation of emotion-processing acumen in the developing human brain. By this model, abnormalities of such amygdalo-cortical connections could account for much of the psychopathology rooted along the neurodevelopmental trajectory. In animal models, medial prefrontal cortex relies on efferents from the basolateral amygdala for the conveyance of emotionally salient learned associations,31 with recent work demonstrating developmental connectivity from basolateral amygdala efferents to rat prefrontal medial cortical gamma-aminobutyric acid (GABA) interneurons hypothesized to relate to deficits in schizophrenia.42,43 This same connectivity has recently been modeled in humans based on extensive fMRI data.44 Prefrontal areas have been implicated in a neural network that includes the amygdala, superior temporal sulcus, and fusiform gyrus and is involved in many ToM and social perception tasks.10,45 Given such wide-based connectivity with cortical areas devoted to emotion processing and regulation, the amygdala is well positioned to mediate the processes whereby emotional salience makes stimuli more memorable, directs attentional resources toward the salient stimuli, and shapes behavioral responses to these stimuli.
Amygdalar Abnormalities in Schizophrenia
Amygdalar dysfunction in schizophrenia may explain some of the social cognitive deficits associated with this disease. Structural magnetic resonance imaging (MRI) studies have found reduced volumes of the hippocampus and amygdala in schizophrenia.46 A meta-analysis of 58 studies that used structural MRI to investigate the brains of schizophrenia patients found a 6% volume reduction in the bilateral amygdale of patients compared with controls.47
Imaging advances have demonstrated functional amygdala abnormalities in the realm of social cognition in schizophrenia as well. While control subjects activate their amygdale in response to a sadness induction paradigm, subjects with schizophrenia do not.48 In simply viewing affective pictures during a positron emission tomography scan, persons with schizophrenia show less activity in the amygdala and prefrontal cortex, though they report similar emotional responses to the stimuli.49 This suggests that altered amygdalar tone may influence the responsiveness of interconnected brain areas to emotional stimuli.25
In an fMRI study of facial emotion identification,50 participants with schizophrenia showed equivalent task accuracy but with decreased activation in the involved network of amygdala, hippocampus, thalamus, fusiform gyrus, and frontal and visual association cortex. More surprisingly, control participants showed greater activation in the inferior frontal, orbitofrontal, and mesial amygdala regions for correct responses to fear and anger items, whereas participants with schizophrenia showed increased activity focused in the amygdale for incorrect responses. Activation of the thalamus, amygdala, and hippocampus of patients in response to fear expressions was also highly correlated with flat affect. Aberrant amygdala activity in patients with prominent negative symptoms may thus correlate with inaccurate emotion perceptions.
Paranoid symptoms also correlate with abnormal emotion perception. Arousal measured by skin conductance is hyperactive in persons with paranoid schizophrenia when viewing fearful faces, while amygdala and prefrontal activity was simultaneously reduced.51 Similarly, on a task of rating trustworthiness of faces, patients with paranoid schizophrenia showed reduced activation in the social cognitive network comprising the amygdala, fusiform gyrus, and prefrontal cortex,52 particularly when rating untrustworthy faces.53 Moreover, decreased activation of this neural network to judgments of untrustworthy faces correlated significantly with lower scores on the Social Functioning Scale.53
The above studies suggest that abnormalities of the amygdala as a component of a functional neural network may significantly impact social cognitive abilities in persons with schizophrenia and simultaneously contribute to other symptom domains as well. According to the model of Brunet-Gouet and Decety,54 fluctuations in abnormal dopaminergic flow during the disease course of schizophrenia may be responsible for periods of greater negative symptoms vs paranoid delusions. Aberrant dopaminergic tone during an acute exacerbation of schizophrenia coupled with emotional-processing dysfunction in the amygdala may interfere with cortical operations and result in abnormalities in threat perception, attributional bias, and ToM. Acutely, these may cause social anxiety, increased perception of threat, and ultimately paranoid delusions. Chronically, when abnormalities in the dopaminergic state may be partially compensated, abnormal emotional salience detection may engender a persistent state of sensitivity to threatening or negative social stimuli that discourage social interactions, perpetuating a cycle that leads to social withdrawal and blunted affect.
Oxytocin in Social Cognition
A growing body of evidence from animal experiments and, more recently, human studies implicates the nonapeptide hormone oxytocin in the emotion–processing dependent social cognitive functions described above. Behaviors that depend on recognition of conspecifics (members of the same species) and the formation of close bonds between mates or families may be animal correlates of behaviors dependent on social cognition. An infusion of oxytocin in the lateral septum or medial preoptic area in male rats prolongs the time that the rats will remember a familiar animal.55 Mice with the gene for the oxytocin receptor (OTR) knocked out have a deficit in recognizing familiar mice that can be recovered by infusion of oxytocin into the medial amygdala prior to exposure to the familiar mouse. The deficit is then recreated by injection of an oxytocin antagonist in the medial amygdala.56
Oxytocin not only seems to be necessary for social memory but also for behaviors dependent on social recognition. The administration of exogenous oxytocin can induce stereotypical maternal behaviors in virgin rats, whereas oxytocin antagonists can prevent the onset of maternal behavior.57 The amount of maternal care (licking and nursing) a rat pup receives correlates with an increase in OTRs as an adult in the brain areas necessary for oxytocin-mediated anxiolysis, stress relief, and maternal behaviors.58 Similarly, oxytocin antagonism or oxytocin infusion in female rat pups leads to decreased or increased maternal behaviors, respectively, when the pups themselves become mothers. Thus, the social and emotional support from the mother, engendered by oxytocin, results in the offspring having both increased coping mechanisms and, later, increased parental behavior. New mother rats given oxytocin showed the same increase in brain activation in the olfactory bulb, amygdala, prefrontal cortex, ventral tegmental area, nucleus accumbens, and insula that was produced by suckling. Oxytocin antagonists decreased activity in these areas59 suggesting that the role of oxytocin in social and emotional bonding is mediated by the amygdala, prefrontal cortex, and dopaminergic systems discussed above.
Research in vole species also lends support to a function for oxytocin in emotion and social cognition. While prairie voles are social, monogamous, and parental, montane voles are isolative and nonparental. Although both species have oxytocin, the receptor distribution is different, in that social prairie voles have OTRs in brain areas associated with reward, including the nucleus accumbens and prelimbic cortex.57 After mating, prairie voles demonstrate a “partner preference,” in that they will choose to spend more time with their mate. With cerebral oxytocin injections, this partner preference can be induced without mating. OTR antagonists likewise prevent the formation of pair bonds after mating, despite identical mating behaviors. After giving birth, the nonsocial montane voles temporarily become parental for several days prior to abandoning their pups. Remarkably, this period of parenting coincides with a change in the distribution of OTRs in the montane vole brain to resemble the OTR distribution typically found in prairie voles.
Where animal studies connect oxytocinergic systems with social memory, pair bonding, and parental behavior, human studies associate oxytocin with social behaviors including facial emotion perception, trust, and coping with stress. Thirty healthy men were shown pictures of people's eyes and asked to decide what the person shown might be feeling. Compared with placebo, participants who received intranasal oxytocin prior to the task performed significantly better, with a more pronounced difference for the more difficult task items60. This improved ability to infer another's emotional state from facial cues may underlie oxytocin's role in social bonding. That is, social relationships are facilitated by mutual understanding of shared, or unshared, feelings or thoughts. Oxytocin dysfunction may thus play a role in disorders involving social deficits, particularly those with neurodevelopmental origins such as autistic spectrum disorders.61 In support of this, single-dose infusion of oxytocin was found to improve processing and retention of socially relevant stimuli in individuals with autism or Asperger's disorder.62 Though both schizophrenia and autism are disorders with social deficits, whether there is a neural basis for this similarity remains untested and in fact some authors predict opposing etiologic processes to explain this parallel.63 Further, research utilizing oxytocin administration is limited by our inadequate knowledge of oxytocin transit across the blood–brain barrier, such that the central vs peripheral action of intravenous or intranasal oxytocin has not been established.
In a task designed to measure interpersonal trust, healthy participants were given a small amount of money and asked to decide how much to donate to an anonymous recipient.64 Recipients who received more money had higher plasma oxytocin levels, and the amount of money they gave back to the investor correlated with the amount received. In comparison to receiving an amount of money determined randomly, recipients’ oxytocin levels were significantly higher when receiving money from a human investor, even though the average amount of money transferred was the same.64 This suggests that oxytocinergic systems respond to gestures of trust, but only in a social context.
Oxytocin may thus be involved in prosocial behaviors such as understanding others’ emotions or establishing trust, as well as the supportive elements of social relationships. Healthy men underwent a social stress test after some combination of oxytocin, social support from a friend, or placebo, and salivary cortisol levels were measured as a reflection of physiologic stress. Cortisol levels were lower after social support, but participants achieved the lowest cortisol levels after social support plus oxytocin.65 In a fMRI paradigm comparing mothers viewing their infants vs persons viewing their romantic partners, there was significant overlap between areas activated by both types of attachment and with dopaminergic reward areas and brain regions associated with OTRs.66
Oxytocin, Dopamine, and the Amygdala
Given the overlap between the role of the amygdala and the influence of oxytocin on social cognition, we hypothesize that the effects of oxytocin on social behavior are mediated via interactions with the emotion-processing elements of the amygdala and its dopaminergic connections. Animal studies show OTRs localized in the central nucleus of the amygdala and to a lesser extent in the medial and basolateral nuclei, as well as in the nucleus accumbens and hippocampus, among other areas.67–72 While human and primate studies are very limited, there is some evidence for oxytocin binding in the striatum, substantia nigra, amygdala, and hippocampus.73–75 Further, oxytocin's anxiolytic effects may be mediated by enhancing GABAergic transmission in inhibitory interneurons in limbic structures.70,76–78 Interestingly, deficits in GABAergic mechanisms have prominent pathophysiologic consequences in schizophrenia,79 suggesting possible deficits in oxytocin-mediated effects on cognition in schizophrenia that involve GABA interneurons.
Research on functional interactions between oxytocin and dopaminergic systems suggests a bidirectional system. In rats, oxytocin agonism in dopaminergic centers is necessary for the onset of maternal behaviors.80 Cocaine is a potent antagonist of the dopamine transporter and cocaine administration in rats results in decreased intracerebral oxytocin, upregulation of OTRs, and decreased OTR affinity in the amygdala81; similarly, OTR expression and capacity for anxiolysis in the central amygdala are subject to dopaminergic control.82 Immunohistochemical experiments in rat brains suggest that dopaminergic fibers may even regulate oxytocin release from the paraventricular nucleus of the hypothalamus.83 Oxytocin's ability to induce penile erections in male rats is dependent on glutamatergic transmission in the ventral tegmental area and dopaminergic transmission in the nucleus accumbens, suggesting a role for oxytocin in facilitating the rewarding aspect of sexual behavior.84
Interactions between amygdala and oxytocin are supported by work in which 15 healthy participants received intranasal oxytocin before performing a matching task with angry or fearful faces and scenes or neutral shapes.85 fMRI showed significantly less amygdala activity to angry and fearful stimuli after oxytocin compared with placebo, with a more pronounced difference for the facial stimuli compared with scenes. This suggests that the social relevance of emotional stimuli modifies the degree of oxytocinergic input to the amygdala. Furthermore, after viewing angry or fearful stimuli, oxytocin significantly reduced participants’ amygdalar communication with brainstem areas that activate autonomic fear responses. Oxytocin also decreased amygdala responses to fearful, angry, and happy faces in comparison with neutral faces.86
Baumgartner et al87 used the interpersonal trust paradigm to assay neural activity changes associated with the “investment” decisions in social vs nonsocial conditions. Control subjects utilized feedback to reduce their subsequent giving based on how often recipients failed to reciprocate the investors’ financial generosity. Participants who received intranasal oxytocin continued giving just as generously, despite such feedback. This lack of responsiveness to perceived “betrayal” in the oxytocin group was accompanied by diminished activity in amygdala and brainstem effector regions implicated in fear responses. Together, the above investigations suggest a link between oxytocin and amygdalar emotion processing through which oxytocin may dampen reactions to emotional stimuli, resulting in facilitation of prosocial behaviors.
A Systems Model of Social Cognition and its Therapeutic Implications for Schizophrenia
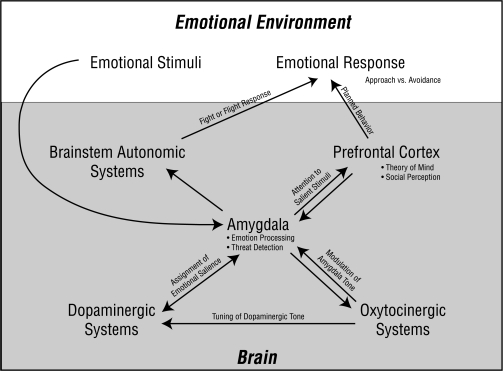
Integrating the findings related to social cognition, several systems appear to interact to register, evaluate, assign priority to, and react to social stimuli (figure 1). The amygdala exerts an important role in emotion recognition, particularly with negatively valenced stimuli and in social situations. The amygdala is also well connected to dopaminergic networks and is itself affected by dopaminergic signaling. In combination with dopaminergic interconnections, the amygdala may filter emotional input in such a way that particular stimuli are deemed salient, while other stimuli are less robustly encoded.31 Other social cognitive capacities may then depend on the amygdala's emotion-processing and salience assignment. Studies show differential amygdala activity in response to emotional stimuli even prior to conscious awareness of the stimuli, in addition to amygdalar alterations of cortical processing of relevant stimuli. Thus, cortical and subcortical systems may rely on interactions with the amygdala to dictate further processing of and behavioral reactions to emotional stimuli. For example, visual cortex may rely on amygdalar assignment of salience to direct visual attention to particular stimuli. Prefrontal cortical feedback from the amygdala may allow ToM processing to occur based on the evaluation of salient emotional features of others’ facial expressions and affective prosody. Midbrain autonomic centers may react to amygdalar input by activating fear responses, such as elevated heart rate or respiratory rate, muscle tension, or perspiration.
Fig. 1.
The Amygdala Serves as the Central Integrative Unit in this Portion of the Emotional-Processing System. Emotional stimuli evoke emotional responses mediated via the pictured neurocircuitry. Emotional stimuli activate amygdala efferents based on the stimuli's valence. Threatening stimuli may activate connections with brainstem autonomic systems that initiate sympathetic and hypothalamic-pituitary-adrenal axis activity associated with the fear response. In contrast, received prosocial or trust behaviors may activate efferent output from the amygdala to oxytocinergic (OT) systems. The OT systems in turn feedback through the amygdala to dampen the brainstem autonomic response in addition to acting on dopaminergic systems (eg, ventral tegmental area, ventral striatum, and nucleus accumbens) to assign salience to perceived trust behaviors that might cumulatively facilitate a response of prosocial behaviors and bonding. Amygdala interactions with the prefrontal cortex may both direct cortical activity, such as allowing visual attention to be focused on emotionally salient features of the environment, and comprise a feedback loop that allows theory of mind and social perception to occur. The higher cortical regions plan and carry out the behavioral response. In persons with schizophrenia, the system is changed by functional alterations in the amygdala and prefrontal cortex as well as in oxytocinergic and dopaminergic systems. Aberrant amygdala input to OT centers may result in diminished OT inhibition of brainstem autonomic activity via nuclei in the amygdala. Altered OT input to dopamine systems coupled with dopaminergic overactivity may cause assignment of emotional salience to inappropriate stimuli. This may prevent the integrated signaling that comprises the feedback loop between the amygdala and the prefrontal cortex, thus making appropriate direction of attention, social perception, and theory of mind difficult to achieve. The resultant emotional response will vary with illness severity and subtype but may include multiple deficits such as social withdrawal, blunted affect, incongruous emotional responses, and paranoia.
Animal models show a clear role for oxytocin in social interactions that depend upon basic “emotion processing,” including social recognition and memory, parental behavior, and pair bonding. Behavioral studies in humans demonstrate a role for oxytocin in emotion perception, trust, and social coping mechanisms. Neuroimaging in humans supports activity in oxytocin-rich areas during romantic partner or infant viewing, as well as effects of exogenous oxytocin on emotion-stimulated amygdala activity and connectivity. Oxytocin has been anatomically and functionally linked to the amygdala and dopaminergic systems in several animal and human studies. We hypothesize that oxytocinergic input to amygdalar and dopaminergic emotion-processing systems is essential for the processing of emotional stimuli in a social context, thus allowing the initiation of prosocial behaviors and mediating the effects of social bonds on coping mechanisms.
Persons with schizophrenia often manifest prominent symptoms related to aberrant social interactions and emotional tone, including social withdrawal, blunted affect, and lack of motivation. The well-studied structural and functional alterations in the amygdale and dopaminergic systems in schizophrenia, as well as the schizophrenia-related emotion-processing deficits, suggest that dysfunction in the amygdala-dopamino-oxytocinergic circuitry may underlie some of the most disabling aspects of the disease. Because oxytocin appears to have physiologic effects at early stages of development in both animal and human models, there is potential for oxytocin dysregulation to result in neurodevelopmental deficits that could contribute to schizophrenia. Several animal models of schizophrenia demonstrate reversal of social deficits by targeted oxytocin administration88,89 or altered OTR expression in areas implicated in social cognitive functions.90 To date, several studies have measured oxytocin in schizophrenia in serum and cerebrospinal fluid, with most studies showing higher baseline levels of oxytocin or its binding protein.91–94 A recent small study found plasma oxytocin levels correlated with schizophrenic patients’ accuracy in categorizing facial affects.95
Several older studies began to examine the therapeutic potential of oxytocin in schizophrenia. In an open label study, patients were reported as clinically improved following oxytocin administered daily for 6–10 days.96 In a small double-blind study, treatment-refractory patients demonstrated negative symptoms improvement following oxytocin treatment.97 While the therapeutic potential of oxytocin in schizophrenia remains uncertain, these early studies are suggestive of clinical benefit.
Future Research Directions
Future research on oxytocinergic function in schizophrenia is warranted, particularly in relation to identified social cognitive deficits. Neuroanatomic and functional brain activity correlates with behavioral paradigms that assess specific domains of social cognition can help define potential oxytocinergic system dysfunction, as well as the interplay between oxytocin, dopamine, and the amygdala. Just as schizophrenia represents a heterogeneous clinical phenotype and social cognition involves complex capacities, so the underlying mechanisms are likely to involve interactions among multiple complex biological systems. Thus, while oxytocinergic dysfunction may contribute to social cognitive deficits, the role of oxytocin is unlikely to be explained by a simple change in abundance of the hormone. Nor is oxytocinergic dysfunction likely to explain all or even most of the variation across disparate tasks and patient subsets.
Perhaps more likely is that oxytocin may modulate a key social cognitive process that is then utilized in a multitude of social functions. At this time, the level and manner in which oxytocin affects the social–emotional processing stream is unclear—if oxytocin facilitates emotion perception, this could then contribute to other social cognitive abilities such as social perception or ToM; alternatively, oxytocin could affect social motivation or hedonia thus driving effort in social contexts.
Challenges in paradigms to assay the role of oxytocin in social cognition include the difficulty of controlling for task complexity. Social context often lends added layers of complexity that complicate analysis of results in a population typically heavily affected by increased demands on cognitive load, attention, and other executive functions. Furthermore, social cognition relies for most tasks on nonsocial cognition. Face emotion recognition relies on face recognition, which according to the particular task may depend on visual capacities, attention, vigilance, and working memory.
The specific role of oxytocin in social cognition could be better understood with paradigms measuring performance and neural activity on well-validated tasks that contrast single domains of social cognition, both in healthy control participants and in patients with schizophrenia. For example, an initial experiment could compare schizophrenia patients with healthy control participants using fMRI and behavioral measures with a face emotion identification task with placebo and oxytocin conditions. This could draw into focus the effects of oxytocin on the amygdala during a particular facet of social cognitive processing. Comparing to nonemotive tasks involving facial judgment (eg, assigning gender or age) would help to differentiate social vs nonsocial cognitive performance.
Similar experiments could be designed with trustworthiness or theory of mind tasks, though neural activity during these tasks in patients with schizophrenia is less well delineated. Judgments of trustworthiness or behavior in economic trust games may demonstrate the aberrant assignment of emotional salience that is hypothesized here to contribute to schizophrenia symptomatology. Including volumetric analysis of the amygdala might give in vivo clues to the correlation between pathologic changes in the limbic system and social cognitive deficits. Controlled experiments with dopamine agonists and antagonists in patients in conjunction with oxytocin manipulations might elucidate social cognitive neural circuits. Care must be taken to account for clinical variables (level of symptoms), medication regimens (particularly dopamine antagonists), and gender (given the lack of clarity regarding the effects of oxytocin in men vs women98) in any such experiments.
The present model is predominantly aimed at understanding neurobiologic underpinnings of social cognitive deficits in schizophrenia to take advantage of neuroimaging advances as tools to investigate the neural changes in social cognitive processing relevant to oxytocinergic systems. Because this area of inquiry has been largely motivated and informed by psychologic constructs,13 including our present model, integrating psychologic models into neurobiologic assays will be important to understand oxytocin's role in social cognitive deficits. The interplay between, eg, metacognition as it relates to patients’ insight and propensity to interpret environmental stimuli in a biased manner99 that may result in social deficits, and the role of oxytocin in diminishing social deficits by perhaps facilitating prosocial biases or cognitive interpretations, exemplifies the importance of a biopsychologic approach to attaining a holistic understanding of social cognition in schizophrenia.
Conclusion
Social cognitive dysfunction in persons with schizophrenia is increasingly recognized as a significant realm of impairment. What is known regarding amygdala and dopaminergic alterations in schizophrenia combined with data on the role of oxytocin in prosocial behavior suggest a neural circuit that may contribute to aberrant emotional salience processing. Although social deficits in schizophrenia are unlikely to be manifestations of a simple oxytocin imbalance, targeted administration of oxytocin may prove useful as a probe for pathophysiologic circuitry. Furthermore, given the lack of therapeutic efficacy of antipsychotic medications for social cognitive deficits, the current review provides a rational basis for better understanding the therapeutic potential of oxytocin in schizophrenia, perhaps in combination with developing psychosocial treatment approaches.
Funding
Lieber Center for Schizophrenia Research and Treatment to J.A.L.; National Institutes of Health (RC1 MH089084 to L.F.J.).
Acknowledgments
We would like to thank Dr Edward Smith for his helpful discussions and critical review of the manuscript. We also thank Ms Eve Vagg for her assistance with preparation of the figure. Financial disclosures: A.J.R. reports no competing interests. L.F.J. has served as a consultant for AstraZeneca. J.A.L. serves as a consultant and/or advisor for AstraZeneca, Eli Lilly, Forest Laboratories, GlaxoSmithKline, Pfizer, and Wyeth. He does not receive financial compensation or salary support for his participation as a consultant or as a member of a board; he holds a patent from Repligen.
References
- 1.Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophr Bull. 2006;32(suppl 1):S44–S63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sergi MJ, Green MF, Widmark C, et al. Social cognition [corrected] and neurocognition: effects of risperidone, olanzapine, and haloperidol. Am J Psychiatry. 2007;164:1585–1592. doi: 10.1176/appi.ajp.2007.06091515. [DOI] [PubMed] [Google Scholar]
- 3.Harvey PD, Patterson TL, Potter LS, Zhong K, Brecher M. Improvement in social competence with short-term atypical antipsychotic treatment: a randomized, double-blind comparison of quetiapine versus risperidone for social competence, social cognition, and neuropsychological functioning. Am J Psychiatry. 2006;163:1918–1925. doi: 10.1176/ajp.2006.163.11.1918. [DOI] [PubMed] [Google Scholar]
- 4.Horan W, Kern RS, Green M, Penn DL. Social cognition training for individuals with schizophrenia: emerging evidence. Am J Psychiatr Rehabil. 2008;11:205–252. [Google Scholar]
- 5.Horan WP, Kern RS, Shokat-Fadai K, Sergi MJ, Wynn JK, Green MF. Social cognitive skills training in schizophrenia: an initial efficacy study of stabilized outpatients. Schizophr Res. 2009;107(1):47–54. doi: 10.1016/j.schres.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green MF, Olivier B, Crawley JN, Penn DL, Silverstein S. Social cognition in schizophrenia: recommendations from the measurement and treatment research to improve cognition in schizophrenia new approaches conference. Schizophr Bull. 2005;31:882–887. doi: 10.1093/schbul/sbi049. [DOI] [PubMed] [Google Scholar]
- 7.Green MF, Penn DL, Bentall R, et al. Social cognition in schizophrenia: an NIMH workshop on definitions, assessment, and research opportunities. Schizophr Bull. 2008;34:1211–1220. doi: 10.1093/schbul/sbm145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueser KT, Penn DL, Blanchard JJ, Bellack AS. Affect recognition in schizophrenia: a synthesis of findings across three studies. Psychiatry. 1997;60:301–308. doi: 10.1080/00332747.1997.11024808. [DOI] [PubMed] [Google Scholar]
- 9.Tremeau F. A review of emotion deficits in schizophrenia. Dialogues Clin Neurosci. 2006;8(1):59–70. doi: 10.31887/DCNS.2006.8.1/ftremeau. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ochsner KN. The social-emotional processing stream: five core constructs and their translational potential for schizophrenia and beyond. Biol Psychiatry. 2008;64(1):48–61. doi: 10.1016/j.biopsych.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards J, Jackson HJ, Pattison PE. Emotion recognition via facial expression and affective prosody in schizophrenia: a methodological review. Clin Psychol Rev. 2002;22:789–832. doi: 10.1016/s0272-7358(02)00130-7. [DOI] [PubMed] [Google Scholar]
- 12.Penn DL, Corrigan PW, Bentall RP, Racenstein JM, Newman L. Social cognition in schizophrenia. Psychol Bull. 1997;121(1):114–132. doi: 10.1037/0033-2909.121.1.114. [DOI] [PubMed] [Google Scholar]
- 13.Penn DL, Sanna LJ, Roberts DL. Social cognition in schizophrenia: an overview. Schizophr Bull. 2008;34:408–411. doi: 10.1093/schbul/sbn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kucharska-Pietura K, David AS, Masiak M, Phillips ML. Perception of facial and vocal affect by people with schizophrenia in early and late stages of illness. Br J Psychiatry. 2005;187:523–528. doi: 10.1192/bjp.187.6.523. [DOI] [PubMed] [Google Scholar]
- 15.Edwards J, Pattison PE, Jackson HJ, Wales RJ. Facial affect and affective prosody recognition in first-episode schizophrenia. Schizophr Res. 2001;48:235–253. doi: 10.1016/s0920-9964(00)00099-2. [DOI] [PubMed] [Google Scholar]
- 16.Leppanen JM, Niehaus DJ, Koen L, Du Toit E, Schoeman R, Emsley R. Emotional face processing deficit in schizophrenia: a replication study in a South African Xhosa population. Schizophr Res. 2006;84:323–330. doi: 10.1016/j.schres.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Green MJ, Waldron JH, Coltheart M. Emotional context processing is impaired in schizophrenia. Cogn Neuropsychiatry. 2007;12:259–280. doi: 10.1080/13546800601051847. [DOI] [PubMed] [Google Scholar]
- 18.Penn DL, Ritchie M, Francis J, Combs D, Martin J. Social perception in schizophrenia: the role of context. Psychiatry Res. 2002;109:149–159. doi: 10.1016/s0165-1781(02)00004-5. [DOI] [PubMed] [Google Scholar]
- 19.Silver H, Goodman C, Bilker W, et al. Impaired error monitoring contributes to face recognition deficit in schizophrenia patients. Schizophr Res. 2006;85(1–3):151–161. doi: 10.1016/j.schres.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 20.Hall J, Harris JM, McKirdy JW, Johnstone EC, Lawrie SM. Emotional memory in schizophrenia. Neuropsychologia. 2007;45:1152–1159. doi: 10.1016/j.neuropsychologia.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Brune M. “Theory of mind” in schizophrenia: a review of the literature. Schizophr Bull. 2005;31(1):21–42. doi: 10.1093/schbul/sbi002. [DOI] [PubMed] [Google Scholar]
- 22.Bora E, Yucel M, Pantelis C. Theory of mind impairment in schizophrenia: meta-analysis. Schizophr Res. 2009;109(1–3):1–9. doi: 10.1016/j.schres.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 23.Shamay-Tsoory SG, Aharon-Peretz J, Levkovitz Y. The neuroanatomical basis of affective mentalizing in schizophrenia: comparison of patients with schizophrenia and patients with localized prefrontal lesions. Schizophr Res. 2007;90(1–3):274–283. doi: 10.1016/j.schres.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 24.Leitman DI, Ziwich R, Pasternak R, Javitt DC. Theory of Mind (ToM) and counterfactuality deficits in schizophrenia: misperception or misinterpretation? Psychol Med. 2006;36:1075–1083. doi: 10.1017/S0033291706007653. [DOI] [PubMed] [Google Scholar]
- 25.Green MJ, Phillips ML. Social threat perception and the evolution of paranoia. Neurosci Biobehav Rev. 2004;28:333–342. doi: 10.1016/j.neubiorev.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Suslow T, Roestel C, Ohrmann P, Arolt V. Detection of facial expressions of emotions in schizophrenia. Schizophr Res. 2003;64:137–145. doi: 10.1016/s0920-9964(03)00061-6. [DOI] [PubMed] [Google Scholar]
- 27.Gur RE, Kohler CG, Ragland JD, et al. Flat affect in schizophrenia: relation to emotion processing and neurocognitive\ measures. Schizophr Bull. 2006;32:279–287. doi: 10.1093/schbul/sbj041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green MF, Penn DL, Bentall R, et al. Social cognition in schizophrenia: an NIMH workshop on definitions, assessment, and research opportunities. Schizophr Bull. 2008;34(6):1211–1220. doi: 10.1093/schbul/sbm145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 30.Morris JS, Frith CD, Perrett DI, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- 31.Laviolette SR. Dopamine modulation of emotional processing in cortical and subcortical neural circuits: evidence for a final common pathway in schizophrenia? Schizophr Bull. 2007;33:971–981. doi: 10.1093/schbul/sbm048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reynolds GP. Increased concentrations and lateral asymmetry of amygdala dopamine in schizophrenia. Nature. 1983;305:527–529. doi: 10.1038/305527a0. [DOI] [PubMed] [Google Scholar]
- 33.Parkinson JA, Cardinal RN, Everitt BJ. Limbic cortical-ventral striatal systems underlying appetitive conditioning. Prog Brain Res. 2000;126:263–285. doi: 10.1016/S0079-6123(00)26019-6. [DOI] [PubMed] [Google Scholar]
- 34.Gibbs AA, Naudts KH, Spencer EP, David AS. The role of dopamine in attentional and memory biases for emotional information. Am J Psychiatry. 2007;164:1603–1609. doi: 10.1176/appi.ajp.2007.06081241. quiz 1624. [DOI] [PubMed] [Google Scholar]
- 35.Hamann SB, Ely TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nature Neurosci. 1999;2:289–293. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- 36.Phillips AG, Ahn S, Howland JG. Amygdalar control of the mesocorticolimbic dopamine system: parallel pathways to motivated behavior. Neurosci Biobehav Rev. 2003;27:543–554. doi: 10.1016/j.neubiorev.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Tessitore A, Hariri AR, Fera F, et al. Dopamine modulates the response of the human amygdala: a study in Parkinson's disease. J Neurosci. 2002;22:9099–9103. doi: 10.1523/JNEUROSCI.22-20-09099.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herwig U, Baumgartner T, Kaffenberger T, et al. Modulation of anticipatory emotion and perception processing by cognitive control. Neuroimage. 2007;37:652–662. doi: 10.1016/j.neuroimage.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 39.Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 40.Williams LM, Das P, Liddell BJ, Kemp AH, Rennie CJ, Gordon E. Mode of functional connectivity in amygdala pathways dissociates level of awareness for signals of fear. J Neurosci. 2006;26:9264–9271. doi: 10.1523/JNEUROSCI.1016-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453:116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- 42.Cunningham MG, Bhattacharyya S, Benes FM. Increasing Interaction of amygdalar afferents with GABAergic interneurons between birth and adulthood. Cereb Cortex. 2008;18:1529–1535. doi: 10.1093/cercor/bhm183. [DOI] [PubMed] [Google Scholar]
- 43.Benes FM. Emerging principles of altered neural circuitry in schizophrenia. Brain Res Brain Res Rev. 2000;31(2–3):251–269. doi: 10.1016/s0165-0173(99)00041-7. [DOI] [PubMed] [Google Scholar]
- 44.Stein JL, Wiedholz LM, Bassett DS, et al. A validated network of effective amygdala connectivity. Neuroimage. 2007;36:736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 45.Pinkham AE, Penn DL, Perkins DO, Lieberman J. Implications for the neural basis of social cognition for the study of schizophrenia. Am J Psychiatry. 2003;160:815–824. doi: 10.1176/appi.ajp.160.5.815. [DOI] [PubMed] [Google Scholar]
- 46.Nelson MD, Saykin AJ, Flashman LA, Riordan HJ. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch Gen Psychiatry. 1998;55:433–440. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- 47.Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157(1):16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 48.Schneider F, Weiss U, Kessler C, et al. Differential amygdala activation in schizophrenia during sadness. Schizophr Res. 1998;34:133–142. doi: 10.1016/s0920-9964(98)00085-1. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi H, Koeda M, Oda K, et al. An fMRI study of differential neural response to affective pictures in schizophrenia. Neuroimage. 2004;22:1247–1254. doi: 10.1016/j.neuroimage.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 50.Gur RE, Loughead J, Kohler CG, et al. Limbic activation associated with misidentification of fearful faces and flat affect in schizophrenia. Arch Gen Psychiatry. 2007;64:1356–1366. doi: 10.1001/archpsyc.64.12.1356. [DOI] [PubMed] [Google Scholar]
- 51.Williams LM, Das P, Harris AW, et al. Dysregulation of arousal and amygdala-prefrontal systems in paranoid schizophrenia. Am J Psychiatry. 2004;161:480–489. doi: 10.1176/appi.ajp.161.3.480. [DOI] [PubMed] [Google Scholar]
- 52.Pinkham AE, Hopfinger JB, Pelphrey KA, Piven J, Penn DL. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophr Res. 2008;99(1–3):164–175. doi: 10.1016/j.schres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pinkham AE, Hopfinger JB, Ruparel K, Penn DL. An investigation of the relationship between activation of a social cognitive neural network and social functioning. Schizophr Bull. 2008;34:688–697. doi: 10.1093/schbul/sbn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brunet-Gouet E, Decety J. Social brain dysfunctions in schizophrenia: a review of neuroimaging studies. Psychiatry Res. 2006;148:75–92. doi: 10.1016/j.pscychresns.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 55.Hammock EA, Young LJ. Oxytocin, vasopressin and pair bonding: implications for autism. Philos Trans R Soc Lond B Biol Sci. 2006;361:2187–2198. doi: 10.1098/rstb.2006.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Insel TR. A neurobiological basis of social attachment. Am J Psychiatry. 1997;154:726–735. doi: 10.1176/ajp.154.6.726. [DOI] [PubMed] [Google Scholar]
- 58.Pedersen CA, Boccia ML. Oxytocin links mothering received, mothering bestowed and adult stress responses. Stress. 2002;5:259–267. doi: 10.1080/1025389021000037586. [DOI] [PubMed] [Google Scholar]
- 59.Febo M, Numan M, Ferris CF. Functional magnetic resonance imaging shows oxytocin activates brain regions associated with mother-pup bonding during suckling. J Neurosci. 2005;25:11637–11644. doi: 10.1523/JNEUROSCI.3604-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biol Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 61.Bartz JA, Hollander E. The neuroscience of affiliation: forging links between basic and clinical research on neuropeptides and social behavior. Horm Behav. 2006;50:518–528. doi: 10.1016/j.yhbeh.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 62.Hollander E, Bartz J, Chaplin W, et al. Oxytocin increases retention of social cognition in autism. Biol Psychiatry. 2007;61:498–503. doi: 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 63.Crespi B. Genomic imprinting in the development and evolution of psychotic spectrum conditions. Biol Rev Camb Philos Soc. 2008;83:441–493. doi: 10.1111/j.1469-185X.2008.00050.x. [DOI] [PubMed] [Google Scholar]
- 64.Zak PJ, Kurzban R, Matzner WT. Oxytocin is associated with human trustworthiness. Horm Behav. 2005;48:522–527. doi: 10.1016/j.yhbeh.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 65.Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- 66.Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 67.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 68.Elands J, Barberis C, Jard S. [3H]-[Thr4, Gly7]OT: a highly selective ligand for central and peripheral OT receptors. Am J Physiol. 1988;254(1 Pt 1):E31–E38. doi: 10.1152/ajpendo.1988.254.1.E31. [DOI] [PubMed] [Google Scholar]
- 69.Elands J, Barberis C, Jard S, et al. 125I-d(CH2)5[Tyr(Me)2, Tyr(NH2)9]AVP: iodination and binding characteristics of a vasopressin receptor ligand. FEBS lett. 1988;229:251–255. doi: 10.1016/0014-5793(88)81135-9. [DOI] [PubMed] [Google Scholar]
- 70.Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- 71.Neumann ID. Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J Neuroendocrinol. 2008;20:858–865. doi: 10.1111/j.1365-2826.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- 72.Insel TR. Oxytocin—a neuropeptide for affiliation: evidence from behavioral, receptor autoradiographic, and comparative studies. Psychoneuroendocrinology. 1992;17(1):3–35. doi: 10.1016/0306-4530(92)90073-g. [DOI] [PubMed] [Google Scholar]
- 73.Sofroniew MV, Weindl A, Schrell U, Wetzstein R. Immunohistochemistry of vasopressin, oxytocin and neurophysin in the hypothalamus and extrahypothalamic regions of the human and primate brain. Acta Histochem Suppl. 1981;24:79–95. [PubMed] [Google Scholar]
- 74.Loup F, Tribollet E, Dubois-Dauphin M, Dreifuss JJ. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Res. 1991;555:220–232. doi: 10.1016/0006-8993(91)90345-v. [DOI] [PubMed] [Google Scholar]
- 75.Fliers E, Guldenaar SE, van de Wal N, Swaab DF. Extrahypothalamic vasopressin and oxytocin in the human brain; presence of vasopressin cells in the bed nucleus of the stria terminalis. Brain Res. 1986;375:363–367. doi: 10.1016/0006-8993(86)90759-6. [DOI] [PubMed] [Google Scholar]
- 76.Muhlethaler M, Charpak S, Dreifuss JJ. Contrasting effects of neurohypophysial peptides on pyramidal and non-pyramidal neurones in the rat hippocampus. Brain Res. 1984;308(1):97–107. doi: 10.1016/0006-8993(84)90921-1. [DOI] [PubMed] [Google Scholar]
- 77.Viviani D, Terrettaz T, Magara F, Stoop R. Oxytocin enhances the inhibitory effects of diazepam in the rat central medial amygdala. Neuropharmacology. 2010;58(1):62–68. doi: 10.1016/j.neuropharm.2009.06.039. [DOI] [PubMed] [Google Scholar]
- 78.Terenzi MG, Ingram CD. Oxytocin-induced excitation of neurones in the rat central and medial amygdaloid nuclei. Neuroscience. 2005;134(1):345–354. doi: 10.1016/j.neuroscience.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 79.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 80.Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav Neurosci. 1994;108:1163–1171. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- 81.Jarrett TM, McMurray MS, Walker CH, Johns JM. Cocaine treatment alters oxytocin receptor binding but not mRNA production in postpartum rat dams. Neuropeptides. 2006;40:161–167. doi: 10.1016/j.npep.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM. CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J Neurosci. 2001;21:2546–2552. doi: 10.1523/JNEUROSCI.21-07-02546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lindvall O, Bjorklund A, Skagerberg G. Selective histochemical demonstration of dopamine terminal systems in rat di- and telencephalon: new evidence for dopaminergic innervation of hypothalamic neurosecretory nuclei. Brain Res. 1984;306(1–2):19–30. doi: 10.1016/0006-8993(84)90352-4. [DOI] [PubMed] [Google Scholar]
- 84.Melis MR, Succu S, Sanna F, Boi A, Argiolas A. Oxytocin injected into the ventral subiculum or the posteromedial cortical nucleus of the amygdala induces penile erection and increases extracellular dopamine levels in the nucleus accumbens of male rats. Eur J Neurosci. 2009;30:1349–1357. doi: 10.1111/j.1460-9568.2009.06912.x. [DOI] [PubMed] [Google Scholar]
- 85.Kirsch P, Esslinger C, Chen Q, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007;62(10):1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 87.Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58:639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 88.Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI. Social interaction deficits caused by chronic phencyclidine administration are reversed by oxytocin. Neuropsychopharmacology. 2005;30:1883–1894. doi: 10.1038/sj.npp.1300722. [DOI] [PubMed] [Google Scholar]
- 89.Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI. Prenatal stress generates deficits in rat social behavior: reversal by oxytocin. Brain Res. 2007;1156:152–167. doi: 10.1016/j.brainres.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu W, Pappas GD, Carter CS. Oxytocin receptors in brain cortical regions are reduced in haploinsufficient (+/−) reeler mice. Neurol Res. 2005;27:339–345. doi: 10.1179/016164105X35602. [DOI] [PubMed] [Google Scholar]
- 91.Beckmann H, Lang RE, Gattaz WF. Vasopressin—oxytocin in cerebrospinal fluid of schizophrenic patients and normal controls. Psychoneuroendocrinology. 1985;10:187–191. doi: 10.1016/0306-4530(85)90056-3. [DOI] [PubMed] [Google Scholar]
- 92.Legros JJ, Gazzotti C, Carvelli T, et al. Apomorphine stimulation of vasopressin- and oxytocin-neurophysins. Evidence for increased oxytocinergic and decreased vasopressinergic function in schizophrenics. Psychoneuroendocrinology. 1992;17:611–617. doi: 10.1016/0306-4530(92)90019-4. [DOI] [PubMed] [Google Scholar]
- 93.Linkowski P, Geenen V, Kerkhofs M, Mendlewicz J, Legros JJ. Cerebrospinal fluid neurophysins in affective illness and in schizophrenia. Eur Arch Psychiatry Neurol Sci. 1984;234:162–165. doi: 10.1007/BF00461555. [DOI] [PubMed] [Google Scholar]
- 94.Glovinsky D, Kalogeras KT, Kirch DG, Suddath R, Wyatt RJ. Cerebrospinal fluid oxytocin concentration in schizophrenic patients does not differ from control subjects and is not changed by neuroleptic medication. Schizophr Res. 1994;11:273–276. doi: 10.1016/0920-9964(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 95.Goldman M, Marlow-O'Connor M, Torres I, Carter CS. Diminished plasma oxytocin in schizophrenic patients with neuroendocrine dysfunction and emotional deficits. Schizophr Res. 2008;98(1–3):247–255. doi: 10.1016/j.schres.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bujanow W. Hormones in the treatment of psychoses. Br Med J. 1972;4:298. doi: 10.1136/bmj.4.5835.298-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bakharev VD, Tikhomirov SM, Lozhkina TK. [Psychotropic properties of oxytocin] Problemy endokrinologii. 1984;30(2):37–41. [PubMed] [Google Scholar]
- 98.Domes G, Lischke A, Berger C, et al. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2009;35(1):83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 99.Koren D, Seidman LJ, Poyurovsky M, et al. The neuropsychological basis of insight in first-episode schizophrenia: a pilot metacognitive study. Schizophr Res. 2004;70:195–202. doi: 10.1016/j.schres.2004.02.004. [DOI] [PubMed] [Google Scholar]