Abstract
Background: One of the most influential cognitive models of auditory verbal hallucinations (AVH) suggests that a failure to adequately monitor the production of one’s own inner speech leads to verbal thought being misidentified as an alien voice. However, it is unclear whether this theory can explain the phenomenological complexity of AVH. We aimed to assess whether subjective perceptual and experiential characteristics may be linked to neural activation in the inner speech processing network. Methods: Twenty-two patients with schizophrenia and AVH underwent a 3-T functional magnetic resonance imaging scan, while performing a metrical stress evaluation task, which has been shown to activate both inner speech production and perception regions. Regions of interest (ROIs) comprising the putative inner speech network were defined using the Anatomical Automatic Labeling system. Correlations were calculated between scores on the “loudness” and “reality” subscales of the Auditory Hallucination Rating Scale (AHRS) and activation in these ROIs. Second, the AHRS subscales, and general AVH severity, indexed by the Positive and Negative Syndrome Scale, were correlated with a language lateralization index. Results: Louder AVH were associated with reduced task-related activity in bilateral angular gyrus, anterior cingulate gyrus, left inferior frontal gyrus, left insula, and left temporal cortex. This could potentially be due to a competition for shared neural resources. Reality on the other hand was found to be associated with reduced language lateralization. Conclusion: Strong activation of the inner speech processing network may contribute to the subjective loudness of AVH. However, a relatively increased contribution from right hemisphere language areas may be responsible for the more complex experiential characteristics, such as the nonself source or how real AVH are.
Keywords: fMRI, schizophrenia, language, cognition
Introduction
Auditory verbal hallucinations (AVH) are a prominent feature of schizophrenia. It has been estimated that 70% of all patients experience hallucinations at some point during the course of their illness.1 There is, however, considerable phenomenological variability in the experience of auditory hallucinations. In a seminal study, Nayani and David2 administered a semistructured questionnaire to 100 psychotic patients with a history of hallucinations in order to systematically characterize their AVH. They observed considerable interindividual variability in both form and content of the AVH. Given the evident heterogeneity of AVH, a finer subcategorization is conceivable and is likely to have important theoretical, clinical, and empirical implications. Different aspects of AVH may have differing cognitive and neural substrates, and a single-deficit theory is unlikely to be able to explain such a complex event as AVH.3 Most modern theories agree on AVH as internally generated events that are misattributed to an external source.4 One of the most influential cognitive models suggests that a failure to adequately monitor the production of one’s own inner speech may lead to a loss of agency, in which case verbal thought is misidentified as an alien voice. Inner speech, sometimes referred to as “verbal thoughts” or “verbal imagery,” has been defined as our ability to “talk” silently to ourselves.5 The neural correlates of inner speech have been relatively well studied using neuroimaging methods. Inner speech was found to recruit activation of traditional speech production areas of the language-dominant hemisphere, namely inferior frontal cortex, insula, and supplementary motor cortex.4 The monitoring of inner speech as well as auditory imagery was associated with activity in the superior temporal cortices and anterior cingulate.6 It thus seems that “inner” and “outer” speech processing rely on similar systems in the brain.7 In patients with schizophrenia, the inner speech network may be functionally altered compared with nonhallucinators and healthy controls. It is particularly the monitoring subpart of the network seems to be affected.6 Interestingly, neuroimaging studies of AVH-related activity have found that similar brain areas are involved during the perception of hallucinated voices.8–10 Consequently, it has been suggested that the processing of (inner) speech and the spontaneous generation of AVH may compete for the same neurophysiological resources.11 However, the elegant and parsimonious inner speech explanation has largely overlooked the question of whether AVH are phenomenologically consistent with inner speech.12 AVH have a distinct “auditory” quality that is typically lacking in normal inner speech. In addition, AVH sound like a real voice, ie, distinguishable from the own inner voice. To our knowledge, no studies to date have assessed whether and how these salient phenomenological characteristics of hallucinated voices relate to inner speech processing, although it has been suggested that in particular the aberrant activation of the posterior temporal subpart of the network, involved in speech perception, could lend AVH the “sensory” qualities that are ultimately so defining of the experience of a “voice.” We therefore aimed to assess whether these sensory/perceptual characteristics may be linked to activity in the inner speech network, during a behaviorally controlled inner speech production task. We chose to employ the commonly used Auditory Hallucination Rating Scale (AHRS), to document AVH characteristics, and focused on the 2 subscales relating to the perceptual quality of AVH, namely “loudness” and “reality.”13 From a phenomenological standpoint, the former could be construed as one of the most salient perceptual characteristics, distinguishing AVH from intrusive thoughts and normal verbal thought in a quantitative rather than qualitative way.14 Reality has been defined as a hallmark of AVH,15,16 and describes a high degree of conviction that the AVH resembles a real human voice. Loudness may be taken as a unidimensional and quite clearly defined sensory characteristic, whereas reality refers more to the experiential quality of the AVH as a true voice with a particular identity.
We expected to find an attenuated response in the inner speech network, as a function of the perceptual quality and experiential complexity of the hallucinations, due to increased competition for shared neural resources. Based on literature review,17 we identified a bilateral set of regions of interest (ROIs) that comprise the putative inner speech processing network, including the opercular (IFGo; BA 44) and triangular (IFGt; BA 45) part of the inferior frontal gyrus (IFG), insula (BA 13/14), supplementary motor area (SMA; BA 6), superior temporal gyrus (STG; BA 22), and anterior cingulate cortex (ACC; BA 24), as well as additional areas thought to be instrumental mediators in language processing, namely middle temporal gyrus (MTG; BA 21), angular gyrus (BA 39), and supramarginal gyrus (BA 40). Second, we were interested in a potential link between AVH characteristics and language lateralization. Anatomical and functional studies have provided evidence of a reduction in the normal cerebral asymmetry in schizophrenia compared with healthy controls (for a meta-analysis, see Sommer et al.18). Particularly, general severity of AVH has been related to reduced lateralization of language functions.19 We sought to investigate whether gradations of lateralization in linguistic processing could be linked to variability in important features of AVH, such as loudness and perceived reality.
We selected a patient sample that was relatively homogeneous with regard to clinically rated general severity of AVH. In each of the subjects, AVH were rated as moderately severe to quite severe (a score of 4, 5, or 6 on the hallucination item of the Positive and Negative Syndrome Scale; PANSS20), all patients were considered to be chronically ill, and medication resistant with regard to AVH presence. However, as suggested by the literature, phenomenologically, there was considerable variability in the actual subjective perceptual and experiential AVH features, which was our major research interest.
Methods
Subjects
Twenty-two right-handed patients (11 males) meeting Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria (APA, 1994) for schizophrenia were recruited from inpatient as well as outpatient facilities of the University Medical Center Groningen and mental health clinics in the provinces of Drenthe, Friesland, and Groningen. Diagnosis was confirmed by a trained rater, using the Schedules for the Clinical Assessment in Neuropsychiatry.21 Age ranged from 18 to 60, with a mean of 36.18 (SD = 12.31). All but one of the patients was on stable doses of antipsychotic medication at the time of participation. Nineteen patients received atypical antipsychotic medication, 2 patients received typical antipsychotic medication, 2 received a combination of both, and one patient was unmedicated. All patients were classified as medication resistant with regard to AVH, defined by the persistence of AVH in face of at least 2 trials, consisting of at least 6 weeks of treatment at adequate dosage, with different antipsychotic medications. Detailed demographical and clinical data are presented in table 1. After complete oral and written description of the study to the subjects, written informed consent was obtained. In the week prior to participating in the functional magnetic resonance imaging (fMRI) experiment, psychotic symptoms were assessed with the Dutch version of the PANSS. Phenomenological characteristics of the AVH were assessed with the AHRS, with particular interest in the subscales loudness and reality. Loudness was rated on a scale from 1 (a whisper) to 5 (shouting), and reality is rated from 0 to 5 as the extent to which the perception of the most prominent voice is alike to a real human voice.
Table 1.
Summarizes the Demographical and Clinical Characteristics of the Patient Sample (n = 22)
| Mean | SD | Minimum | Maximum | |
| Age (years) | 36.18 | 12.31 | 19 | 60 |
| Education level (years) | 14.00 | 1.89 | 12 | 18 |
| Illness duration (months) | 151.86 | 140.04 | 17 | 480 |
| Age at onset (years) | 23.82 | 9.56 | 9 | 51 |
| Number of hospital admissions | 3.56 | 2.53 | 1 | 10 |
| Medication (Chlorpromazine equivalents) | 580 | 466 | 0 | 1400 |
| Auditory Hallucination Rating Scale | ||||
| Loudness | 2.95 | 1.05 | 1 | 5 |
| Reality | 3.77 | 1.54 | 0 | 5 |
| Total | 25.68 | 5.88 | 12 | 36 |
| Positive and Negative Syndrome Scale | ||||
| P3 (hallucinations) | 4.77 | 0.61 | 4 | 6 |
| Positive scale | 16.18 | 4.18 | 10 | 23 |
| Negative scale | 14.45 | 4.37 | 7 | 24 |
| General psychopathology | 30.64 | 8.40 | 17 | 49 |
Procedure
The metrical stress evaluation task consisted of 2 conditions and a baseline comparison in an interleaved block design. The baseline condition consisted of a 30-s display of a centrally placed fixation cross. In the active task conditions, bisyllabic Dutch nouns were visually presented in the middle of the screen, in white letters on black background. Each stimulus was presented for 2.5 s, followed by a 2.5-s display of a fixation cross. Each block consisted of 12 stimuli, whose order was randomized within blocks. During the “semantic” condition, subjects were simply required to decide with a button press whether the word represented a positive or negative concept. Examples of positive items are “peace,” “summer” etc. Negative items on the other hand were, eg, “sadness,” “loss.” Words were matched for length and frequency. However, of interest for the current investigation is the “phonology” condition, during which subjects judged the metrical stress of the same visually presented words. Metrical stress was on the first syllable in half of the stimuli. Subjects indicated with a button press whether the stress was on the first or the second syllable. The phonological task requires active generation of the appropriate phonological code from memory and has been shown to activate both inner speech production and perception regions in healthy controls.22 A total of 2 runs were presented, each consisting of 8 active and 8 resting blocks.
Image Acquisition
fMRI scanning was performed on a 3-T Philips Intera Scanner (Best, the Netherlands). Functional T2*-weighted images were acquired using gradient-echo echo planar imaging (echo time [TE]: 30 ms; repetition time [TR]: 2500 ms; flip angle: 80°; field of view: 224.0, 136.5, 224.0). Images were acquired in 39 contiguous 3.5-mm slices. Functional images were acquired during 2 runs of 11 min (320 volumes per run). A high-resolution T1-weighted turbo field echo structural scan was also acquired for each participant.
Data Analysis
Descriptive data for the demographic and clinical variables are given in table 1. Data processing of the fMRI data was carried out with SPM5 (The Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk/spm/). Functional images were first realigned and then coregistered to the same virtual space with the anatomical data. Images were transformed to standard Montreal Neurological Institute space23 and smoothed with a 10-mm Gaussian Kernel. ROIs were defined according the Anatomical Automatic Labeling library24 and included bilateral IFG, opercular and triangular parts, and bilateral STG; MTG, insula, SMA, angular and supramarginal gyrus, and ACC. Individual subject images were analyzed at the first level to produce estimates for the contrast of interest (phonology condition minus baseline). The phonology condition was contrasted to the baseline condition, rather than the active semantic control condition, in order to maximize the potential to identify inner speech-related activity in the predefined language-related ROI’s (see figure 2) (The phonological processing load was a priori thought to be stronger in the phonology condition compared with the semantics condition. However, the possibility that subjects performed some form of inner speech during the semantics condition cannot be excluded even if this was not explicitly requested. Observing the resulting contrast (subtraction of semantics from phonology conditions) therefore did not reveal robust language-related activity at the group level in the predefined inner speech processing regions.). Estimates of the signal change for the contrast of interest were extracted using the Marsbar toolbox for SPM. Nonparametric correlations (Kendall’s tau) were calculated between scores on the AHRS subscales loudness and reality on the one hand and the extracted contrast values on the other hand. Second, a language lateralization index was computed by aggregating the data from the left and right hemisphere ROIs encompassing the language processing network. In order to achieve a general index of language lateralization. The lateralization index was computed according the method used by Sommer et al.25, ie, the difference in signal change for the contrast of interest for the left hemispheric ROIs vs the right hemispheric ROIs divided by the total sum of the signal change. Correlations were assessed between, on the one hand, loudness and reality, frequency of occurrence, and general hallucination severity, measured with the hallucination item (P3) of the PANSS and the language lateralization index on the other hand. In order to correct the calculation of multiple correlations, False Discovery Rate correction at α = .05 was applied, according to the method described by Benjamini and Hochberg figure 1.26
Fig. 2.
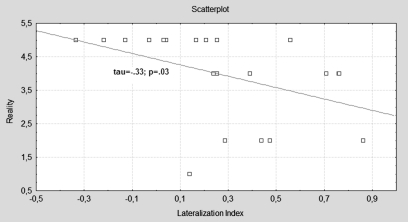
Shows the Scatterplot of the Negative Correlation between the Language Lateralization Index and Scale “Reality of AVH,” Which Indexes the Global Experiential Sense of How Real the Hallucinated Voices Appear to the Subject, as Indicated on a Scale from 0 (Not Real at All) to 5 (Very Real).
Fig. 1.
Shows the Pattern of Activation Derived during the Phonological Task Condition of the Metrical Stress Evaluation Task, at the Group Level (n = 22). Group results are displayed for illustrative purposes, as for the ROI analysis, individual subject images were analyzed at the first level to produce estimates for the contrast of interest (P < .001, uncorrected). The phonology condition was contrasted with the resting baseline, rather than the active semantic control condition, in order to maximize the potential to identify linguistic activity in the predefined language-related ROI’s. Estimates of the signal change for the contrast of interest were then extracted using the MarsBaR toolbox for SPM.
Results
Behavioral Results
Reaction times (RTs) and percentage correct were obtained on the phonological condition to ensure that subjects performed the task adequately. Although the patients’ performance level was relatively suppressed compared with an average 92% correct reported for healthy controls,22 the mean accuracy was well above chance level at 75.41% correct, with a SD of 17.27%. The mean RT was 1586 ms, with a standard deviation of 366.72 ms, which is comparable with the findings in healthy controls (1677 ± 171 ms22).
fMRI Results
Contrasting the phonology condition with the baseline, significant negative correlations were observed between loudness and activation of the left and right angular gyrus (respectively, τ = −.56; P = .0003, τ = −.56; P = .0002), left and right ACC (τ = −.42; P = .006, τ = −.44; P = .004), and left triangular IFG (τ = −.43; P = .005), as well as the left opercular part of the IFG (τ = −.40; P = .009), left MTG (τ = −.50; P = .001), and left insula (τ = −.38; P =.01). No significant correlations were observed for the reality subscale. This scale however did correlate negatively with the language lateralization index (τ = −.33; P = .03), whereas loudness did not.
No significant correlations were observed for frequency of occurrence of hallucinations, as assessed by the relevant item of the AHRS, or item P3 of the PANSS, which assesses general severity of hallucinations.
In order to assess the specificity of these findings, we performed an additional analysis employing the semantic control task, by contrasting this condition with the baseline, similar to the phonology minus baseline contrast reported above. We calculated nonparametric correlations between the estimates of the signal change for that contrast (again extracted using the MarsBaR toolbox for SPM) and scores on the AHRS subscales loudness and reality. None of the correlations were found to be significant at P < .05 figure 2.
Discussion
In this study, we investigated the association between phenomenological variability in the perceptual characteristics of hallucinated voices and activation of the inner speech processing network. Subjective loudness of AVH was found to correlate negatively with task-related phonological activation in a distributed network of areas involved in inner speech processes. The sense of reality associated with the voice hearing experience on the other hand was linked to reduced language lateralization.
The inner speech network may be divided into functional subparts, subserving different aspects of the inner speech experience. The IFG and the insula are 2 regions implicated in the production of inner speech (ie, the “inner voice”) and are known to be activated when people imagine performing mental auditory imagery. Temporoparietal structures, such as the angular gyrus, which is part of Wernicke’s area, are more involved in the receptive aspect of inner speech. The MTG then has been implicated in imagining hearing others’ speech and probably relates to the monitoring of inner speech as it is produced.11 Each of these areas showed reduced activation during the phonological processing task, as the perceived loudness of the AVH increased. It has previously been suggested that AVH and inner speech may compete for the same neurophysiological resources.27 The negative correlation observed with perceived loudness of AVH therefore seems to indicate that as AVH become more perceptually salient (i.e. louder), they take up more resources involved in the processing of inner speech, resulting in a reduction of task-related activity. Thus, inner speech production and the presumably subsequent perception/evaluation are involved in developing the sensory quality of the AVH experience. AVH may thus be a form or inner speech that acquired perceptual characteristics through aberrant activation of auditory and speech processing pathways. Activation in regions employed in the metacognitive act of monitoring inner speech, ie, the MTG and the anterior cingulate, was reduced as well with increased AVH loudness. Shergill et al. 28 showed that parametric variations in the rate of inner speech production linearly influenced neuronal response in these monitoring regions. This fits with our observation that as the perceptual salience of the AVH increases, fewer resources seem available to monitor processing during the phonological task. Interestingly, previous research has shown that the neural response at the level of the auditory cortex appears to be linked to subjective loudness (the perception of the stimulus) rather than the physical properties (the actual physical intensity).29 This fits with the findings of the current investigation in the sense that the neural response to the imagined stimuli also appears to vary with the subjectively experienced loudness. In contrast to the Langers et al. study, our findings related not to the primary auditory regions but revealed a link with brain regions involved in producing and perceiving internally generated auditory stimuli.
Inner speech theory possibly fails to account for other, potentially clinically relevant characteristics of AVH. Perceived reality, the extent to which the AVH resembles a real voice, did not relate linearly with inner speech production, perception, or monitoring activity. This also seems to suggest that the sense of how real a voice is, does not immediately relate to the simple perceptual quality of the experience, such as loudness. Arguably, the AVH may be experienced as quite real, even though it is perceived as a mere whisper, and vice versa may not sound much like a human voice even though it is loud and salient. It has indeed been argued that “something can count as a voice, without being experienced as audition-like, or mistaken for sensory perception of another’s speech.”30 Inner speech theory therefore seems insufficient to explain the full richness of this type of experience. It seems more fitting to conceive of the “voice experience” as a multidimensional and highly individually determined event. It is likely that the experience of AVH entails an involved multiple-step process, in which activation of inner speech areas represent just one aspect, namely, the relay of perceptual qualities to aberrantly activated linguistic material. It is conceivable that higher order attribution processes are involved in giving meaning to the anomalous sensory experience. An externalizing bias may lead to the (mis-) attribution of an internal event to a nonself source. In fact, one theory31 states that AVH are in fact unintentional activations of episodic memories. The failure to inhibit thoughts of a memory after deciding it is irrelevant has been shown to result in intrusive thoughts. The loss of contextual information may lead to memory representations being confused with ongoing reality. Secondarily, linking delusional beliefs to the experience may lead to a more elaborate development of voice’s identity and sense of realness. Specifically, in the case of AVH, the source could be memories of verbal thoughts or of conversations both with others and with the self. Our data suggest that aberrant re-activation of the inner speech processing network may lend the intrusive verbal thought its typical sensory quality.
Interestingly, we found that the sense of reality was related to reduced lateralization of activation during the language task. Language processing in healthy right-handers is typically subserved by the left hemisphere, whereas evidence from structural and functional neuroimaging studies indicates that patients with schizophrenia may be characterized by a reduction in language lateralization, both in first episode and in more chronic variations of the illness.32–35 More specifically, associations have been reported between reduced lateralization and the positive symptom domain or with AVH in particular during language tasks such as verb generation,19 verbal fluency,36 and single-word reading.37 Interestingly, one study38 showed that AVH are associated with cerebral activity arising from the right rather than the left inferior frontal speech production regions, whereas covert speech in the same subjects originated from the usual left hemispheric speech production region. The right hemisphere does have a limited capacity for the production of short phrases of low-linguistic complexity, often with a negative emotional content39, and in general, language functions of the right hemisphere are typically related to more pragmatic (and less linguistic) aspects of language, eg, prosody (speech melody). Thus, when inner speech originates from activity in right hemisphere homologues of language regions, it is more likely to have a distinctly simple and negative content, as well as particular prosodic features. In addition, self-produced linguistic activity normally leads to inhibition of language perception areas. This mechanism may be more prone to failure when the activity is derived from an unusual site (ie, the right hemisphere), which would in turn cause verbal thought to be misattributed to an external agent. This fits with our finding of an increased sense of reality of AVH with decreased lateralization, as enhanced contribution of the right hemisphere to during linguistic processing may enrich the language experience with nonlinguistic detail such as prosodic information and emotional salience, making it harder to distinguish the final inner percept from external speech.
This study has a number of limitations, and future research may seek to address these issues requiring further clarification. First, we specifically chose to focus on specific perceptual aspects of AVH, namely loudness and reality, as they relate to the inner speech theory. However, other variations in the phenomenological experience (eg, spatial location, specific content, emotional valence, etc.), that may hold additional clinical relevance, might be linked to specific cognitive and neural processes. In fact, the uniformity of AVH has previously been called into question. It has been suggested that some of the phenomenological characteristics will be associated with differential neural substrates.40 In that vein, Plaze et al.41 recently showed structural variability in the STG region in relation to the experience of internally or externally localized AVH. In addition, future studies employing inner speech as a proxy for AVH may seek to employ stimulus material closer in form and content to spontaneous inner speech and/or AVH (eg, sentences with emotional content rather than single words). In fact, the question as to what extent experimentally induced “inner speech processes” emulate spontaneous inner speech remains open (eg, see Stephane et al.42). In addition, although perceptual characteristics are defining for hallucinations,1 recent research has shown that AVHs are clearly distinguished from inner speech by patients, but that this distinction is made by patients more often on the basis of the lack of control, nonself speaking voice, and the distinctive verbal content of rather than sensory characteristics.43 Therefore, more research is necessary to clarify the full complexity of interrelated perceptual, linguistic, and other cognitive processes involved in symptoms such as AVH and their neural substrates.44 Second, we did not include a control group of healthy subjects or nonhallucinating patients, as we were primarily interested in the quantitative relationship between symptom expression and activation of specific brain areas during inner speech processing. We can therefore not comment directly on whether task performance on a neural or behavioral level was “abnormal” in some way. However, the aim of this study was to provide a quantitative assessment with regard to the relationship between specific phenomenological AVH characteristics and the neural response during linguistic processing, rather than investigating the qualitative difference from nonhallucinating subjects. Third, unlike previous reports, we did not find a correlation between general hallucination severity, as indexed by the PANSS hallucination item and language lateralization. This is most likely due to our study design, namely the fact that the group was relatively small and was selected to be homogeneous with regard to hallucination severity as defined by clinical standards. All subjects scored a 4, 5, or 6 on the PANSS P3 item. This selected subject pool may affect the generalizability of the results to the general population of schizophrenia patients. However, even though AVH were rated as severe, individual variations in loudness and reality still existed in this subgroup of chronic, medication-resistant patients. The fact that we were able to link this variability in the phenomenological domain to variations in neural response means that the findings may nevertheless offer insight into the neurobiological underpinnings of AVH, which may be of relevance to the population in general.
In sum, these results point to a link between inner speech processes, including the generation and monitoring of inner speech and the perceptual salience of AVH in terms of perceived loudness. However, inner speech theory seems insufficient to explain the full phenomenological complexity of AVH. Additional abnormalities within the language processing system and post hoc attributions are probably required to produce the complex and rich experience of AVH. In that vein, it was shown that reduced language lateralization contributes to the more general sense of reality of the experienced voices.
Funding
Ubbo Emmius Grant of the University of Groningen, the Netherlands (180/800514 to A.V.).
Acknowledgments
The authors would like to acknowledge Marjolijn Hoekert, Marte Swart, Edith Liemburg, Lisette van der Meer, and Hanneke Geugies for their assistance with patient interviews, Dr Hanneke Westenbroek for the in-patient care and recruitment of patients, and Cees Slooff, Lex Wunderink, Hans Klein, and Jack Jenner for their assistance in the recruitment of patients.
References
- 1.Aleman A, Laroi F. Hallucinations: The Science of Idiosyncratic Perception. Washington, DC: American Psychological Association; 2008. [Google Scholar]
- 2.Nayani TH, David AS. The auditory hallucination: a phenomenological survey. Psychol Med. 1996;26:177–189. doi: 10.1017/s003329170003381x. [DOI] [PubMed] [Google Scholar]
- 3.Waters FA, Badcock JC, Michie PT, Maybery MT. Auditory hallucinations in schizophrenia: intrusive thoughts and forgotten memories. Cogn Neuropsychiatry. 2006;11:65–83. doi: 10.1080/13546800444000191. [DOI] [PubMed] [Google Scholar]
- 4.Allen P, Aleman A, McGuire PK. Inner speech models of auditory verbal hallucinations: evidence from behavioural and neuroimaging studies. Int Rev Psychiatry. 2007;19:407–415. doi: 10.1080/09540260701486498. [DOI] [PubMed] [Google Scholar]
- 5.Stephens LG, Graham G. When Self-consciousness Breaks: Alien Voices and Inserted Thoughts. Cambridge, MA: MIT Press; 2000. [Google Scholar]
- 6.Shergill SS, Bullmore ET, Brammer MJ, Williams SC, Murray RM, McGuire PK. A functional study of auditory verbal imagery. Psychol Med. 2001;31:241–253. doi: 10.1017/s003329170100335x. [DOI] [PubMed] [Google Scholar]
- 7.Aleman A, Van't Wout M. Subvocalization in auditory-verbal imagery: just a form of motor imagery? Cogn Process. 2004;5:228–231. [Google Scholar]
- 8.McGuire PK, Shah GM, Murray RM. Increased blood flow in Broca's area during auditory hallucinations in schizophrenia. Lancet. 1993;342:703–706. doi: 10.1016/0140-6736(93)91707-s. [DOI] [PubMed] [Google Scholar]
- 9.Dierks T, Linden DE, Jandl M, et al. Activation of Heschl's gyrus during auditory hallucinations. Neuron. 1999;22:615–621. doi: 10.1016/s0896-6273(00)80715-1. [DOI] [PubMed] [Google Scholar]
- 10.Shergill SS, Bullmore E, Simmons A, Murray R, McGuire P. Functional anatomy of auditory verbal imagery in schizophrenic patients with auditory hallucinations. Am J Psychiatry. 2000;157:1691–1693. doi: 10.1176/appi.ajp.157.10.1691. [DOI] [PubMed] [Google Scholar]
- 11.McGuire PK, Silbersweig DA, Wright I, et al. Abnormal monitoring of inner speech: a physiological basis for auditory hallucinations. Lancet. 1995;346:596–600. doi: 10.1016/s0140-6736(95)91435-8. [DOI] [PubMed] [Google Scholar]
- 12.Jones SR. Do we need multiple models of auditory verbal hallucinations? Examining the phenomenological fit of cognitive and neurological models. Schizophr Bull. 2010;36:566–575. doi: 10.1093/schbul/sbn129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman RE, Hawkins KA, Gueorguieva R, et al. Transcranial magnetic stimulation of left temporoparietal cortex and medication-resistant auditory hallucinations. Arch Gen Psychiatry. 2003;60:49–56. doi: 10.1001/archpsyc.60.1.49. [DOI] [PubMed] [Google Scholar]
- 14.Moritz S, Laroi F. Differences and similarities in the sensory and cognitive signatures of voice-hearing, intrusions and thoughts. Schizophr Res. 2008;102:96–107. doi: 10.1016/j.schres.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Bleuler EP. Dementia Praecox oder Gruppe der Schizophrenien/Dementia Praecox or the Group of Schizophrenias. New York: International Universities Press; 1950. (original 1911) [Google Scholar]
- 16.Aggernaes A. The experienced reality of hallucinations and other psychological phenomena. An empirical analysis. Acta Psychiatr Scand. 1972;48:220–238. doi: 10.1111/j.1600-0447.1972.tb04364.x. [DOI] [PubMed] [Google Scholar]
- 17.Allen P, Laroi F, McGuire PK, Aleman A. The hallucinating brain: a review of structural and functional neuroimaging studies of hallucinations. Neurosci Biobehav Rev. 2008;32:175–191. doi: 10.1016/j.neubiorev.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Sommer I, Ramsey N, Kahn R, Aleman A, Bouma A. Handedness, language lateralisation and anatomical asymmetry in schizophrenia: meta-analysis. Br J Psychiatry. 2001;178:344–351. doi: 10.1192/bjp.178.4.344. [DOI] [PubMed] [Google Scholar]
- 19.Sommer IE, Ramsey NF, Kahn RS. Language lateralization in schizophrenia, an fMRI study. Schizophr Res. 2001;52:57–67. doi: 10.1016/s0920-9964(00)00180-8. [DOI] [PubMed] [Google Scholar]
- 20.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 21.Giel R, Nienhuis FJ. SCAN-2.1: Schedules for Clinical Assessment in Neuropsychiatry (in Dutch) Groningen, the Netherlands: WHO; 1996. [Google Scholar]
- 22.Aleman A, Formisano E, Koppenhagen H, Hagoort P, de Haan EH, Kahn RS. The functional neuroanatomy of metrical stress evaluation of perceived and imagined spoken words. Cereb Cortex. 2005;15:221–228. doi: 10.1093/cercor/bhh124. [DOI] [PubMed] [Google Scholar]
- 23.Evans AC, Collins DL, Milner B. An MRI-based stereotactic brain atlas from 300 young normal subjects. In: Proceedings of the 22nd Symposium of the Society for Neuroscience, Anaheim, CA; 1992; p. 408. [Google Scholar]
- 24.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 25.Sommer IE, Diederen KM, Blom JD, et al. Auditory verbal hallucinations predominantly activate the right inferior frontal area. Brain. 2008;131:3169–3177. doi: 10.1093/brain/awn251. [DOI] [PubMed] [Google Scholar]
- 26.Benjamini Y, Hochberg Y. On the adaptive control of the false discovery fate in multiple testing with independent statistics. J Educ Behav Stat. 2000;25:60–83. [Google Scholar]
- 27.Woodruff PW, Wright IC, Bullmore ET, et al. Auditory hallucinations and the temporal cortical response to speech in schizophrenia: a functional magnetic resonance imaging study. Am J Psychiatry. 1997;154:1676–1682. doi: 10.1176/ajp.154.12.1676. [DOI] [PubMed] [Google Scholar]
- 28.Shergill SS, Brammer MJ, Fukuda R, Williams SC, Murray RM, McGuire PK. Engagement of brain areas implicated in processing inner speech in people with auditory hallucinations. Br J Psychiatry. 2003;182:525–531. doi: 10.1192/bjp.182.6.525. [DOI] [PubMed] [Google Scholar]
- 29.Langers D, van Dijk P, Schoenmaker ES, Backes WH. fMRI activation in relation to sound intensity and loudness. Neuroimage. 2007;35:709–718. doi: 10.1016/j.neuroimage.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 30.Stephens GL, Graham G. Reconceiving delusion. Int Rev Psychiatry. 2004;16:236–241. doi: 10.1080/09540260400003982. [DOI] [PubMed] [Google Scholar]
- 31.Waters FA, Badcock JC, Michie PT, Maybery MT. Auditory hallucinations in schizophrenia: intrusive thoughts and forgotten memories. Cogn Neuropsychiatry. 2006;11:65–83. doi: 10.1080/13546800444000191. [DOI] [PubMed] [Google Scholar]
- 32.Bleich-Cohen M, Hendler T, Kotler M, Strous RD. Reduced language lateralization in first-episode schizophrenia: an fMRI index of functional asymmetry. Psychiatry Res. 2009;171:82–93. doi: 10.1016/j.pscychresns.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Branch CA, Ardekani BA, Bertisch H, Hicks C, DeLisi LE. fMRI study of language activation in schizophrenia, schizoaffective disorder and in individuals genetically at high risk. Schizophr Res. 2007;96:14–24. doi: 10.1016/j.schres.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang ZJ, Shi JB, Yuan YG, Hao GF, Yao ZJ, Chen N. Relationship of auditory verbal hallucinations with cerebral asymmetry in patients with schizophrenia: an event-related fMRI study. J Psychiatr Res. 2008;42:477–486. doi: 10.1016/j.jpsychires.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Crow TJ, Done DJ, Sacker A. Cerebral lateralization is delayed in children who later develop schizophrenia. Schizophr Res. 1996;22:181–185. doi: 10.1016/s0920-9964(96)00068-0. [DOI] [PubMed] [Google Scholar]
- 36.Weiss EM, Hofer A, Golaszewski S, Siedentopf C, Felber S, Fleischhacker WW. Language lateralization in unmedicated patients during an acute episode of schizophrenia: a functional MRI study. Psychiatry Res. 2006;146:185–190. doi: 10.1016/j.pscychresns.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Stephane M, Hagen MC, Lee JT, et al. About the mechanisms of auditory verbal hallucinations: a positron emission tomographic study. J Psychiatry Neurosci. 2006;31:396–405. [PMC free article] [PubMed] [Google Scholar]
- 38.Sommer IE, Diederen KM, Blom JD, et al. Auditory verbal hallucinations predominantly activate the right inferior frontal cortex [published online ahead of print October 13, 2008] Brain. 131:3169–3177. doi: 10.1093/brain/awn251. doi: 10.1093/brain/awn251. [DOI] [PubMed] [Google Scholar]
- 39.Van Lancker D, Cummings JL. Expletives: neurolinguistic and neurobehavioral perspectives on swearing. Brain Res Brain Res Rev. 1999;31(1):83–104. doi: 10.1016/s0165-0173(99)00060-0. [DOI] [PubMed] [Google Scholar]
- 40.Crow T. Schneider's nuclear symptoms as the key to the structure of language. Neurol, Neurol Psychiatr Brain Res. 2007;14:87–94. [Google Scholar]
- 41.Plaze M, Paillère-Martinot ML, Penttilä J, et al. Where do auditory hallucinations come from? A brain morphometry study of schizophrenia patients with inner or outer space hallucinations [published online ahead of print August 7, 2009] Schizophr Bull. doi: 10.1093/schbul/sbp081. doi: 10.1093/schbul/sbp081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stephane M, Thuras P, Narallah H, Georgopoulos AP. The internal structure of the phenomenology of auditory verbal hallucinations. Schizophr Res. 2003;61:248–259. doi: 10.1016/s0920-9964(03)00013-6. [DOI] [PubMed] [Google Scholar]
- 43.Jones SR, Fernyhough C. Neural correlates of inner speech and auditory verbal hallucinations: a critical review and theoretical integration. Clin Psychol Rev. 2007;27:140–154. doi: 10.1016/j.cpr.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Hoffman RE, Varanko M, Gilmore J, Mishara AL. Experiential features used by patients to differentiate voices from ordinary verbal thought. Psychol Med. 2008;38:1167–1176. doi: 10.1017/S0033291707002395. [DOI] [PubMed] [Google Scholar]