Abstract
Learned irrelevance (LIrr) refers to a form of selective learning that develops as a result of prior noncorrelated exposures of the predicted and predictor stimuli. In learning situations that depend on the associative link between the predicted and predictor stimuli, LIrr is expressed as a retardation of learning. It represents a form of modulation of learning by selective attention. Given the relevance of selective attention impairment to both positive and cognitive schizophrenia symptoms, the question remains whether LIrr impairment represents a state (relating to symptom manifestation) or trait (relating to schizophrenia endophenotypes) marker of human psychosis. We examined this by evaluating the expression of LIrr in an associative learning paradigm in (1) asymptomatic first-degree relatives of schizophrenia patients (SZ-relatives) and in (2) individuals exhibiting prodromal signs of psychosis (“ultrahigh risk” [UHR] patients) in each case relative to demographically matched healthy control subjects. There was no evidence for aberrant LIrr in SZ-relatives, but LIrr as well as associative learning were attenuated in UHR patients. It is concluded that LIrr deficiency in conjunction with a learning impairment might be a useful state marker predictive of psychotic state but a relatively weak link to a potential schizophrenia endophenotype.
Keywords: latent inhibition, prodromal symptoms, selective attention, state marker, endophenotype, associative learning
Introduction
Deficits in selective attention have long been recognized as one of the core features relevant to positive as well as cognitive symptoms of schizophrenia.1,2 The inability to adequately process surrounding stimuli by selectively attending to stimuli relevant to current perception while ignoring irrelevant spurious cues is believed to contribute to the sensory overload characteristic of schizophrenia patients. One form of selective attention is controlled by stimulus history in which subjects learn to ignore specific stimuli with a history of irrelevance. A popular translational paradigm that has been extensively employed to study this form of selective attention is latent inhibition (LI).3 LI refers to the attentional control over learning in which inconsequential experience of a specific stimulus retards subsequent associative learning about that stimulus, eg, its predictiveness over another stimulus. LI can be readily demonstrated in humans and animals and is found to be attenuated in both unmedicated4,5 and medicated6,7 acute schizophrenia patients, although intact LI has also been reported in unmedicated acute schizophrenia patients.8 LI disruption reflects effective learning about the predictor stimulus in spite of the previous experience of its irrelevance as a predictive cue and therefore cannot be merely attributed to a general form of learning failure but rather can be viewed as accelerated learning under such a preexposed condition.
Observations in animals and human volunteers indicate that the expression of LI is highly sensitive to dopaminergic manipulation. For instance, the dopamine-releasing drug amphetamine readily disrupts LI9–11—a finding that is consistent with the hyperdopaminergia hypothesis of schizophrenia, suggesting that acute schizophrenia is associated with heightened mesolimbic dopaminergic neurotransmission.12 Treatment with antipsychotic drugs, which are efficient dopamine receptor antagonists, has been shown to be effective in reinstating LI, and normal LI has been identified in both acute and chronic medicated schizophrenia patients.8,13–16 LI expression in schizophrenia patients also appears to be influenced by the duration of illness, as substantiated by a report showing intact LI in unmedicated symptomatic chronic schizophrenia patients.4 Moreover, there are studies demonstrating enhanced LI in both drug-free6 and medicated6,17 chronic schizophrenia patients, suggesting that this form of aberrant LI expression may be related to the negative symptoms that are prevalent in chronic schizophrenia. The apparent inconsistency across LI studies in schizophrenia patients may indicate that changes in LI expression represent a state rather than a trait marker of schizophrenia,18 linking LI disruption and positive symptoms7,19 and persistent or potentiated LI to negative symptoms.6,13 However, demonstration of LI disruption among constantly medicated chronic schizophrenia patients14,20 as well as in healthy first-degree relatives of schizophrenia patients14 challenges this view and suggests instead LI disruption as a trait marker or even an endophenotype of the disease. An endophenotype refers to an internal and intermediate phenotype linking clinical symptoms to the genetic predisposition to the disease.21 Gottesman and Gould22 emphasize that endophenotypes are heritable state-independent phenotypes capable of indexing the underlying genetic vulnerability to the disease. In addition, endophenotypes should be associated with the disorder and observed in unaffected family members at a higher frequency than in the general population.22 Identification of such endophenotypes may offer a better understanding of the complex genetic architecture and related etiological and physiological mechanisms of the disease.23
The present study was designed to examine the presence of learned inattention deficits in 2 cohorts of subjects differing in terms of the nature of their susceptibility to developing schizophrenia and their present mental states. One group was asymptomatic first-degree relatives of confirmed cases of schizophrenia (SZ-relatives), who hence had a clear genetic risk but were devoid of any overt signs of psychosis. The other group of subjects were showing prodromal signs of schizophrenia in the form of attenuated positive symptoms, and although some might go on to recover completely, they were considered clinically at ultrahigh risk (UHR) of progressing into schizophrenic psychosis. Hence, both SZ-relatives and UHR subjects were regarded as being along the schizophrenia continuum, the former based solely on a genetic contribution and the latter solely on symptomatological grounds.
In this study, a learned inattention paradigm related to LI—learned irrelevance (LIrr)—was employed.24 LIrr differs from LI in the critical stimulus preexposure phase in that LIrr involves the explicit exposure to uncorrelated presentations of both the predicted and the future predictive stimuli, whereas in LI, only the latter is preexposed. Theoretical accounts of the 2 phenomena are closely related.25–28 Similar to LI, LIrr disruption has been linked to schizophrenia, with reports of LIrr deficiency among both medicated and unmedicated first-episode schizophrenics as well as among medicated acute patients.29–31 The 2 existing studies of LIrr expression in chronic schizophrenia patients both demonstrated LIrr impairment30,31 (contrasting with some LI studies), although this was attributed to weak associative learning as such rather than to a specific failure in learning to ignore the preexposed stimuli. A within-subject LIrr paradigm was adopted here similar to that which has proven to be sensitive to first-episode schizophrenia.29
Methods
Subjects
The study was conducted at the University Hospital of Psychiatry, Bern, and at the Specialised Outpatient Service for Early Psychosis, Department of Psychiatry, Bruderholz, Switzerland. None of the subjects had previously been assessed for the expression of LI or LIrr. They were first informed with a complete description of the study and the test procedures, whereupon written consent was obtained. Additional informed parental consent was obtained for patients who were under the age of 18 years. The study had been approved by the ethics committees of the cantons of Bern and Basel, Switzerland.
First-Degree Relatives of Schizophrenia Patients
Twenty-three (13 females and 10 males) asymptomatic subjects who had one (parents: n = 8, siblings: n = 8, and children: n = 3) or more (n = 4) first-degree relatives with a Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV) diagnosis of a schizophrenia-spectrum disorder were recruited from the University Hospital of Psychiatry, Bern, and through support groups by advertisements. All participants were screened for psychiatric disorders according to DSM-IV, using the short check questionnaire of the diagnostic interview Diagnostisches Expertensystem für Psychische Störungen (DIA-X) questionnaire32 for axis I disorders and the Structured Clinical Interview for DSM Disorders questionnaire33 for axis II disorders. Subjects with any DSM-IV axis I or axis II disorder or a history of psychiatric conditions (eg, psychosis, depression, anxiety disorder, or substance abuse) were excluded. In addition, all subjects were required to complete the self-report Schizotypal Personality Questionnaire (SPQ).34
UHR Subjects
Seventeen patients (6 females and 11 males) with prodromal symptoms of schizophrenia were recruited through the Bruderholz Early Psychosis Service, Switzerland. Referrals to this outpatient service were through family physicians and other primary care services, school counseling services, psychiatrists, and psychologists in private practice, as well as family members and by self-referrals. All potential subjects were assessed using the Structured Interview for Prodromal Syndromes (SIPS)35 and the companion Scale of Prodromal Symptoms (SOPS). The SOPS covers 19 items divided into 4 subscales: positive, negative, disorganized, and general symptoms. Symptoms are rated on a 7-point scale from 0 = absent to 6 = severe and psychotic for positive symptoms, extreme for all other symptoms. The SIPS interview also contains a schizotypal rating scale and a research version of the Global Assessment of Functioning (GAF).36 Individuals who meet at least one of the following criteria are regarded to be at UHR of developing schizophrenia: (1) brief intermittent psychotic syndrome (SOPS score of 6 on one item of the positive symptom subscale for less than 1 wk), (2) attenuated positive prodromal syndrome (SOPS score of 3–5 on one item of the positive symptom subscale at least once per week over the past month; symptoms having begun or worsened in the last year), and (3) genetic risk and deterioration syndrome (family history of any psychotic disorder in first-degree relatives and/or schizotypal personality disorder in the identified patient and a drop in the GAF score during the last year). All the 17 patients met the UHR criterion of subsyndromal positive symptoms, scoring 3–5 on the positive symptom scale containing unusual thought content (n = 7), suspicion/paranoia (n = 3), grandiosity (n = 1), perceptual anomalies (n = 8), and disorganized communication (n = 1). Two (1 female and 1 male) UHR patients additionally had an identifiable family history of schizophrenia and were excluded from the UHR group. Thus, the UHR and SZ-relative groups were sufficiently distinguishable with respect to (1) positive prodromal syndrome (present in UHR subjects but not in SZ-relatives) and (2) identifiable hereditary (and hence genetic) risk (present in SZ-relatives but not UHR subjects) in a complementary manner.
Healthy Control Subjects
Control subjects were recruited through hospital staff and in schools by advertisement and verbal inquiries. Because the SZ-relatives and UHR patients differed substantially in age, 2 separate groups of age-matched healthy control subjects were recruited for the purpose of comparison (see table 1). The mean ± SD age of the healthy control group for the SZ-relative group (n = 33) was 49.4 ± 12.9 years (range = 19–65 y), whereas that of the healthy control group for the UHR patients (n = 17) was 19.1 ± 4.4 years (range = 16–33 y). In addition, gender ratio, education levels, and nicotine consumption were closely matched. The demographic and clinical data of all 4 groups of subjects are detailed in table 1.
Table 1.
Demographic and Clinical Data
| First-Degree Relatives | Controls (Relatives) | UHR Patients | Controls (UHR) | |
| N (female/male) | 23 (13/10) | 33 (20/13) | 15 (5/10) | 17 (8/9) |
| Mean age (y) ± SD (range) | 49.4 ± 12.9 (19–65) | 46.6 ± 14.5 (19–66) | 19.3 ± 4.7 (16–33) | 18.6 ± 2.9 (16–26) |
| SOPS positive symptoms total score ± SEM | — | — | 6.3 ± 0.6 | — |
| SPQ total score ± SEM | 13 ± 1.36 | 10.9 ± 1.38 | — | — |
Note: UHR = ultrahigh risk; SOPS = Scale of Prodromal Symptoms; SPQ = Schizotypal Personality Questionnaire.
All potential control subjects were screened for axis I disorders by the DIA-X questionnaire.32 Control subjects who were matched to the SZ-relatives were additionally asked to complete the SPQ. The SPQ total scores did not differ significantly between groups (t = 0.69, df = 55, not significant [ns]). Subjects with a personal history of a DSM-IV axis I disorder and/or a family history of schizophrenia-spectrum disorders were excluded. Additional exclusion criteria applied to all groups (patients and control subjects) were traumatic brain injury, substance abuse (except nicotine), epilepsy, or other neurological disorder and serious medical conditions that might be suspected to affect information processing or cognitive function.
The LIrr Test
The procedure for the demonstration and assessment of the LIrr effect has been fully described before.29,37 It was implemented here using a personal computer running a C++ program. The paradigm was based on a computerized visual target detecting task in which the subjects were instructed to press the space bar of the computer keyboard as soon as the target stimulus (the letter X) appeared on the screen. In addition to the target stimulus, 10 other capital Latin letters were used as nontarget stimuli: the 5 vowels (A, E, I, O, and U) and a set of 5 consonants (B, D, T, Y, and Z). All stimuli were presented in yellow against a blue background in the center of the screen, one at a time, at a 1-second rate with no interstimulus interval. There were a total of 450 stimulus presentations, from which 75 were targets and the remaining 375 nontargets, with a test session therefore lasting 450 seconds (7.5 min).
The test comprised 15 blocks of stimulus presentation, with successive blocks varying among 3 possible types of test condition: random (R), preexposed (PE), and non-preexposed (NPE). The blocks were arranged in a predetermined sequence with the restriction that no 2 consecutive blocks belonged to the same condition. Each condition was presented 5 times (ie, 5 blocks) in total. Regardless of test condition, each block contained a sequence of 30 stimulus presentations: 5 targets (X), 5 potentially predictive nontarget letters by virtue of the fact that they immediately preceded the target, and 20 nonpredictive filler letters taken from the consonants set (B, D, T, Y, and Z). The filler letters (between 1 and 8 in succession, randomized in sequence) served to “fill” the intervals between consecutive predictor-target pairs.
The critical difference between the conditions R, PE, and NPE was the predictability of the target by the pretarget letter. In the R blocks, the 5 pretarget letters varied randomly among the 5 PE letters (ie, B, D, T, Y, and Z), each serving only once as the pretarget letter and hence giving no prediction as to whether the next stimulus would be the target or not (ie, zero contingency). In PE blocks, the pretarget letters also consisted of the consonants (ie, B, D, T, Y, or Z), with the difference to the R blocks that the same consonant was used throughout a block and each of the 5 consonants was used as pretarget letter in one particular PE block. Given that the set of consonants acted as filler letters in all blocks and occasionally as pretarget letters in PE blocks, they were effectively preexposed throughout the test and perceived as poor target-predictor letters. The NPE blocks were essentially configured in the same manner as the PE blocks, except that the pretarget letters were taken from the set of vowels (A, E, I, O, U) each serving as target predictor in one particular NPE block. The vowels were therefore reliable target-predictor letters.
The reaction time (RT) of target detection, defined as the time measured between the target onset and key press, was taken as the key dependent variable (accurate to 1 ms) as a function of test conditions (R vs PE vs NPE). Low RT was expected to reflect good predictability of the target, and the RT was therefore expected to be the lowest in NPE and the highest in R, with the RTs obtained in PE being intermediate. Significantly higher RTs in PE relative to NPE reflect the LIrr effect.
In addition to the RTs, the numbers of target misses and false alarms were recorded and separately analyzed.
For the analysis of the LIrr effect, only the last 3 target RTs (ie, third, fourth, and fifth) within each block were taken into account on the assumption that the first 2 predictor-target pairings generated an association enabling anticipations of the subsequent target presentations.
Data Analysis
The expression of LIrr in the UHR patients and the SZ-relatives was separately examined in comparison with their respective control groups. Preliminary analyses confirmed that the expression of LIrr differed significantly between the 2 healthy control groups, and thus, separate analyses were necessary for the effective assessment of LIrr in the 2 patient groups.
To better conform to the distributional and homoscedasticity assumptions of parametric analysis of variance (ANOVA), the average RT (in milliseconds) per block calculated for each subject was first subjected to natural logarithmic transformation before being submitted to a 2 × 3 × 5 (group × condition × block) split-plot ANOVA. Subsequent significant effects were further examined by pairwise comparisons or restricted ANOVAs whenever appropriate to assist interpretation. The number of target misses and false alarms were analyzed separately by a 2 × 3 (group × condition) ANOVA.
All statistical analyses were performed using SPSS 15 statistical software. A type 1 error rate of 0.05 or below was accepted as showing statistical significance. The precise P value as well as the associated effect size as indexed by partial eta-squared ( of significant effects were reported.
Results
Response Characteristics
SZ-Relatives.
Analysis of the number of target misses failed to reveal any significant difference between groups (F1,54 = 0.09, ns) or across test conditions (F2,108 = 0.8, ns). The interaction between groups and conditions was also not significant (F2,108 = 0.39, ns). Similarly, no difference between groups was detected in terms of the number of false alarms (F1,54 = 0.05, ns), which however did vary across test conditions (F2,108 = 7.08, P < .005, ). This was attributed to a lower false alarm rate in PE (0.46 ± 0.09 SEM) compared with R (0.89 ± 0.14 SEM) or NPE (1.11 ± 0.16 SEM). Importantly, the group-by-condition interaction was not significant (F2,108 = 0.11, ns), suggesting that this pattern across conditions did not significantly differ between SZ relatives and control subjects.
UHR Patients.
The response analysis again failed to reveal any difference between groups (F1,30 = 1.4, ns), across conditions (F2,60 = 0.04, ns), or a group-by-condition interaction (F2,60 = 3.05, ns). A similar pattern of null effects was also obtained in terms of false alarms (group: F1,30 = 3.9, ns; condition: F2,60 = 1.85, ns; group-by-condition interaction: F2,60 = 1.4, ns). It is concluded that response accuracy was essentially unaffected in the UHR patient group in comparison with its matched control group.
Learned Irrelevance
SZ-Relatives.
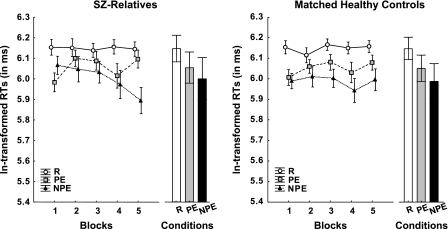
As expected, the RTs in R were the highest, while NPE yielded the lowest average RTs with PE condition inbetween these 2 extremes. This pattern was observed in both the SZ-relatives and the corresponding control group (see figure 1). However, there was clear evidence that this overall pattern progressively emerged over the course of the 5 blocks of the test, especially with respect to the difference between PE and NPE and especially in the SZ-relatives. In comparison, RTs in R were stably high across blocks. These impressions were confirmed by a split-plot ANOVA of RT, which yielded a main effect of condition (F2,108 = 31.8, P < .000001, ) and a significant condition-by-block interaction (F8,432 = 3.6, P < .0005, ). The analysis also did not reveal any significant effect involving group. Pairwise comparisons suggested that overall performance in R significantly differed from NPE (P < .0005) and PE (P < .05). Although pairwise comparison between the latter 2 conditions did not yield a significant difference (P = .2) when performance was collapsed across blocks and groups, the presence of the condition-by-block interaction might indicate also that the expression of LIrr as defined by attenuated performance in the PE condition relative to the NPE condition was dependent on testing blocks. This was evaluated by an additional 3-way ANOVA restricted to the PE and NPE conditions only, which again yielded a condition-by-block interaction (F4,216 = 3.6, P = .001, ). Post hoc comparison confirmed that RT (collapsed across groups) was significantly longer in the PE than in the NPE conditions at each of the final 3 blocks (P’s < .05), lending support for an emergence of LIrr in the final 3 blocks of the test. Again, neither the main effect of group nor its interaction terms achieved statistical significance, suggesting that the block-dependent profile of LIrr expression did not differ between groups.
Fig. 1.
Reaction Time to Target per Condition (Random [R], Preexposed [PE], and Non-preexposed [NPE]) and Block (1–5) in Asymptomatic First-Degree Relatives of Schizophrenia Patients (SZ-Relatives) and Matched Healthy Control Subjects. A significant condition-by-block interaction indicates that the learned irrelevance (LIrr) effect (reflected by longer RTs in PE relative to NPE) as well as associative learning (indicated by higher RTs in NPE/PE relative to R) progressively emerge with blocks. The lack of an interaction involving group suggests a similar performance in SZ-relatives and healthy control subjects. Data are presented as In-transformed mean RTs ± standard error of the mean.
UHR Patients.
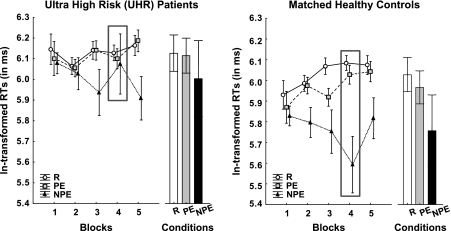
Performance across the 3 conditions again conformed to the expected direction: The highest RTs were observed in R, followed by PE and then NPE. Such a trend was seen in both UHR patients and their matched control subjects (see figure 2). The main effect of condition was highly significant with a similar effect size as in the SZ-relatives (F2,60 = 13.3, P < .00005, ). Again, a block-dependent difference among the 3 conditions was evident, which was more pronounced in the control subjects and differed somewhat from the UHR patients. This resulted in the emergence of a group × condition × block interaction (F8,240 = 2.3, P < .05, ). Examination of figure 2 readily suggests that while LIrr was distinctly expressed in block 4 in the healthy control subjects, it was clearly absent in the UHR patients. Restricted ANOVAs confined to each block revealed a significant group-by-condition interaction only at block 4 (F2,60 = 4.77, P < .05, ) but not at any other block. This stemmed primarily from the UHR patients’ increase in RT in NPE relative to control subjects (P < .05). Indeed, the overall ANOVA also yielded a significant effect of group (F1,30 = 7.01, P < .05, ), although the group-by-condition interaction failed to attain statistical significance (F2,60 = 1.71, ns).
Fig. 2.
Reaction Time to Target per Condition (Random [R], Preexposed [PE], and Non-preexposed [NPE]) and Block (1–5) in the Ultrahigh Risk (UHR) Patients and Their Matched Healthy Control Subjects. As in the asymptomatic first-degree relatives of schizophrenia patients (SZ-relatives), the difference between conditions was block dependent. The relationship between condition and block differed between groups as indicated by the significant group × condition × block interaction; a performance difference was especially evident in block 4, where healthy control subjects show intact learned irrelevance (LIrr, reflected by higher RTs in PE relative to NPE) as well as associative learning in NPE (indicated by higher RTs in NPE relative to R), while such differences were absent in the UHR patients. Further analyses revealed that UHR patients were deficient in LIrr as well as associative learning across blocks compared with the healthy control subjects. Data are presented as In-transformed mean RTs ± standard error of the mean.
The source of the significant group × condition × block interaction was further examined from 2 a priori perspectives using restricted ANOVAs. On the one hand, learning as such was examined by focusing on the contrast between R and NPE, and on the other, LIrr was investigated by contrasting PE and NPE. The restricted ANOVAs revealed a significant and a close to significant, respectively, 3-way interaction (PE vs NPE: F4,120 = 3.07, P < .05, R vs NPE: F4,120 = 2.34, P = .058, ), which provides statistical support to the interpretations that the expression of associative learning and LIrr across blocks was impaired in the UHR patients.
Discussion
The present study represents the first attempt to examine the expression of LIrr in asymptomatic first-degree relatives of schizophrenia patients, evaluating its status as a potential endophenotype of information processing deficits in schizophrenia. The parallel study measuring LIrr in UHR patients, who were identified by the overt presence of attenuated positive symptoms, has further enabled us to evaluate if performance deficiency in the present LIrr test might be considered as a state marker of psychosis. The outcome in the SZ-relatives suggests that LIrr was intact in asymptomatic subjects bearing genetic risk to schizophrenia. Although the overall magnitude of LIrr appeared limited, closer examination of the final 3 blocks of the test revealed clear LIrr expression in both SZ-relatives and their matched control subjects (see figure 1) when RTs in PE condition became longer than the NPE condition—satisfying the operational definition of LIrr. Hence, it is unlikely that the failure to detect a deficit in LIrr in SZ-relatives could be solely attributed to the overall weakness of the LIrr effect when the 5 blocks of tests were considered altogether. This outcome contradicts the hypothesis that deficiency in learned inattention as assessed by LIrr and related translational paradigms, such as LI, constitutes a test of an endophenotype of schizophrenia.14 On the other hand, the study involving the UHR patients provided suggestion of an LIrr deficiency, although this deficit appears to depend critically on blocks (see figure 2). This block dependency suggests that the present test design is prone to the effect of training. As evidenced already in the SZ-relatives, associative learning (as indicated by the shorter RT in the NPE relative to R) was susceptible to a training effect. This may not be attributed to procedural or perceptual training as no such tendency was observed in the R condition. One possibility is that generalization from one NPE target predictor to another might be expected on the basis that they all belonged to the category of vowels. Because the assessment of LIrr depends on the level of learning exhibited in NPE, it comes as little surprise therefore that the LIrr effect also exhibited sensitivity to a training (block) effect. LIrr was absent in both UHR patients and their control subjects in the first block and emerged progressively especially in the control subjects (figure 2). It was against this background that the UHR patients showed a different profile than the control subjects, as indicated by the emergence of a 3-way (group × condition × block) interaction in the absence of a group-by-condition interaction. As it turned out, the most pronounced evidence for a disruption of LIrr in UHR patients was restricted to the fourth block, with a similar but weaker tendency in the second block. We have no explanation as to why the fourth block appeared to be more sensitive to this effect, but it is interesting to note that the UHR patients’ performance in PE in this particular block also showed a tendency of shorter RT. This adds to the possibility that this might be linked to some deficiency in inattention to the preexposed stimulus. Nonetheless, it is clear that the contribution of the resulting attenuation of LIrr in the UHR patients was largely accounted for by a deficiency in associative learning (as indexed by a relatively small performance difference between NPE and R). This outcome is not dissimilar to that obtained in first-episode schizophrenia patients29 and also to that reported by Martins et al14 in both schizotypal as well as nonschizotypal first-degree relatives of schizophrenia patients. Hence, while the null finding in the SZ-relatives here is more directly at variance with the results reported by Martins et al,14 the present finding of LIrr disruption in UHR patients is still in keeping with their results obtained in schizotypal relatives. Similar to report of Martins et al,14 the attenuation of LIrr in the UHR patients was severely confounded by a deficit in associative learning as such.
To date, the only studies in LI and LIrr in first-degree relatives are by Martins et al14 and this present study. However, other techniques sensitive to deficient information processing have been applied, such as prepulse inhibition (PPI) of the acoustic startle reflex and auditory P50-evoked potential. Both PPI38,39 and P5040,41 (similar to LI) have been shown to be impaired in healthy first-degree relatives, indicating that deficits in preattentive information processing might serve as an endophenotype of schizophrenia.
In order to be an endophenotype, these deficits must be present along the entire schizophrenia continuum. Indeed, modest impairments in P50 in subjects at risk without an affected family member and profound deficits in at-risk subjects with an affected first-degree relative have consistently been found.42 Furthermore, a significant PPI deficit has been found in prodromal subjects.43 In contrast, LIrr deficiency does not appear to represent a schizophrenia endophenotype but rather a state marker for psychotic symptoms.
In this respect, uniquely state-dependent or symptom-driven translational markers are also valuable. These allow the correlation with pathophysiological mechanisms directly underlying symptom production, and when applied to selective breeding or genetic engineered animals, the identification of inheritable genetic variations that are truly not state-dependent. But a question that arises is what is it that distinguishes LIrr from other related translational paradigms, including LI, PPI, and P50, which seem to be trait sensitive? Although PPI and P50 are more related to early preattentive sensory gating mechanisms,44 LI is more closely related to LIrr. Indeed, Hall and colleagues27 assert that LIrr is not dissimilar to LI in the sense that the resulting weaker learning was attributable to reduced salience to the predictive stimulus (conditioned stimulus; CS). The observation that LIrr is typically stronger than LI, and even the sum of LI and the unconditioned stimulus (US)-preexposure effect, was explained by the fact that such stimulus preexposure effects (especially LI) are heavily dependent on contextual consistency. Given that LIrr is conducted with the predicted stimulus (US) being present in both the preexposure and the conditioning stage, this facilitates or promotes the LI effect. Theoretically, Hall’s45 theoretical account of LIrr is therefore essentially not different from his explanation of LI. In contrast, Mackintosh and colleagues46–48 suggest that LIrr involves unique psychological processes that are distinct from and operates in addition to the modulation of attention by preexposed CS and preexposed US alone. Mackintosh and colleagues46–48 postulate that the animals learn during the noncorrelative (random) CS/US preexposure the lack of predictive relationship between this particular pair of stimuli, and this as such is responsible at least in part for the measured LIrr effect (in the form of retarded learning). In this sense, the mere sum of the CS preexposure alone and US preexposure is insufficient to capture this element of learning about the specific lack of CS-US relationship. Given that we failed to obtain evidence in support of impaired LIrr in SZ-relatives, who are expected to exhibit LI impairment,14 is an inconsistency readily accommodated by Mackintosh’s theory but poses some surprise to Hall’s position. The latter should predict that SZ-relatives are similarly affected in LI and LIrr because their emergence essentially involves similar psychological processes. If dysfunction of these processes is responsible for the LI deficit, then a similar outcome ought to be present also when they were evaluated using the LIrr paradigm. Hence, intact LIrr demonstrated in SZ-relatives here would appear to support the interpretation by Mackintosh and colleagues.46–48 The LIrr paradigm employed might likely capture the particular form of perceptual learning that retards learning of a specific predictor-target association and therefore remained insensitive to SZ-relatives. This hypothesis would need to be directly tested in SZ-relatives by obtaining current indices of LI and LIrr in the same subjects. In addition, novel test paradigms of LIrr would be instrumental for testing if the present LIrr test design might be associated with unique features.
Future studies would profit from the inclusion of the matching schizophrenia patients akin to the SZ-relatives, thereby extending the power of interpretation. Ideally, the recruitment of discordant monozygotic twins would offer a perfect setup to extend the investigation of schizophrenia endophenotypes. Although the findings of LIrr and associative learning impairment in UHR patients are of obvious interest, a follow-up study allowing the distinction between UHR patients that would eventually develop full-blown psychosis or remained symptomatically stable would be tremendously useful. One would then be able to compare if they could be similarly distinguished on the basis of the present dataset. In conclusion, the present study raises critical questions as to whether LI and LIrr are equivalent in terms of their relevance to schizophrenia endophenotypes and suggests the interesting possibility that LIrr deficits may represent a state-dependent marker. Support of this possibility is also derived from a study in healthy subjects showing that LIrr was only disrupted in subjects with high levels of schizotypy, who showed normal LI after the same number of preexposures, while both LIrr and LI were intact in subjects with low schizotypy scores.49 Taken together, the relative trait and state sensitivities of acquired inattention as exemplified by the LIrr and LI paradigms remain to be further evaluated.
Acknowledgments
The authors are especially grateful to Pietro Ballinari for his assistance in statistical matters and to Stephen Petty for his editorial input.
References
- 1.Nuechterlein KH, Dawson ME, Green MF. Information-processing abnormalities as neuropsychological vulnerability indicators for schizophrenia. Acta Psychiatr Scand Suppl. 1994;384:71–79. doi: 10.1111/j.1600-0447.1994.tb05894.x. [DOI] [PubMed] [Google Scholar]
- 2.McGhie A, Chapman J. Disorders of attention and perception in early schizophrenia. Br J Med Psychol. 1961;34:103–116. doi: 10.1111/j.2044-8341.1961.tb00936.x. [DOI] [PubMed] [Google Scholar]
- 3.Lubow RE, Moore AU. Latent inhibition: the effect of nonreinforced pre-exposure to the conditional stimulus. J Comp Physiol Psychol. 1959;52:415–419. doi: 10.1037/h0046700. [DOI] [PubMed] [Google Scholar]
- 4.Gray NS, Pilowsky LS, Gray JA, Kerwin RW. Latent inhibition in drug naive schizophrenics: relationship to duration of illness and dopamine D2 binding using SPET. Schizophr Res. 1995;17:95–107. doi: 10.1016/0920-9964(95)00034-j. [DOI] [PubMed] [Google Scholar]
- 5.Vaitl D, Lipp O, Bauer U, et al. Latent inhibition and schizophrenia: Pavlovian conditioning of autonomic responses. Schizophr Res. 2002;55:147–158. doi: 10.1016/s0920-9964(01)00250-x. [DOI] [PubMed] [Google Scholar]
- 6.Rascle C, Mazas O, Vaiva G, et al. Clinical features of latent inhibition in schizophrenia. Schizophr Res. 2001;51:149–161. doi: 10.1016/s0920-9964(00)00162-6. [DOI] [PubMed] [Google Scholar]
- 7.Baruch I, Hemsley DR, Gray JA. Differential performance of acute and chronic schizophrenics in a latent inhibition task. J Nerv Ment Dis. 1988;176:598–606. doi: 10.1097/00005053-198810000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Williams JH, Wellman NA, Geaney DP, Cowen PJ, Feldon J, Rawlins JN. Reduced latent inhibition in people with schizophrenia: an effect of psychosis or of its treatment. Br J Psychiatry. 1998;172:243–249. doi: 10.1192/bjp.172.3.243. [DOI] [PubMed] [Google Scholar]
- 9.Gray NS, Pickering AD, Hemsley DR, Dawling S, Gray JA. Abolition of latent inhibition by a single 5 mg dose of d-amphetamine in man. Psychopharmacology (Berl) 1992;107:425–430. doi: 10.1007/BF02245170. [DOI] [PubMed] [Google Scholar]
- 10.Thornton JC, Dawe S, Lee C, et al. Effects of nicotine and amphetamine on latent inhibition in human subjects. Psychopharmacology (Berl) 1996;127:164–173. doi: 10.1007/BF02805990. [DOI] [PubMed] [Google Scholar]
- 11.Kumari V, Cotter PA, Mulligan OF, et al. Effects of d-amphetamine and haloperidol on latent inhibition in healthy male volunteers. J Psychopharmacol. 1999;13:398–405. doi: 10.1177/026988119901300411. [DOI] [PubMed] [Google Scholar]
- 12.Swerdlow NR, Koob GF. Dopamine, schizophrenia, mania, and depression: toward a unified hypothesis of cortico-striato-pallido-thalamic function. Behav Brain Sci. 1987;10:197–245. [Google Scholar]
- 13.Cohen E, Sereni N, Kaplan O, et al. The relation between latent inhibition and symptom-types in young schizophrenics. Behav Brain Res. 2004;149:113–122. doi: 10.1016/s0166-4328(03)00221-3. [DOI] [PubMed] [Google Scholar]
- 14.Martins SA, Jones SH, Toone B, Gray JA. Impaired associative learning in chronic schizophrenics and their first-degree relatives: a study of latent inhibition and the Kamin blocking effect. Schizophr Res. 2001;48:273–289. doi: 10.1016/s0920-9964(00)00141-9. [DOI] [PubMed] [Google Scholar]
- 15.Leumann L, Feldon J, Vollenweider F, Ludewig K. Effects of typical and atypical antipsychotics on prepulse inhibition and latent inhibition in chronic schizophrenia. Biol Psychiatry. 2002;52:729. doi: 10.1016/s0006-3223(02)01344-6. [DOI] [PubMed] [Google Scholar]
- 16.Swerdlow NR, Braff DL, Hartston H, Perry W, Geyer MA. Latent inhibition in schizophrenia. Schizophr Res. 1996;20:91–103. doi: 10.1016/0920-9964(95)00097-6. [DOI] [PubMed] [Google Scholar]
- 17.Gal G, Barnea Y, Biran L, et al. Enhancement of latent inhibition in patients with chronic schizophrenia. Behav Brain Res. 2009;197:1–8. doi: 10.1016/j.bbr.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 18.Swerdlow NR, Stephany N, Wasserman LC, Talledo J, Sharp R, Auerbach PP. Dopamine agonists disrupt visual latent inhibition in normal males using a within-subject paradigm. Psychopharmacology (Berl) 2003;169:314–320. doi: 10.1007/s00213-002-1325-6. [DOI] [PubMed] [Google Scholar]
- 19.Weiner I. The “two-headed” latent inhibition model of schizophrenia: modeling positive and negative symptoms and their treatment. Psychopharmacology (Berl) 2003;169:257–297. doi: 10.1007/s00213-002-1313-x. [DOI] [PubMed] [Google Scholar]
- 20.Vaitl D, Lipp OV. Latent inhibition and autonomic responses: a psychophysiological approach. Behav Brain Res. 1997;88:85–93. doi: 10.1016/s0166-4328(97)02310-3. [DOI] [PubMed] [Google Scholar]
- 21.Gottesman II, Shields J. Genetic theorizing and schizophrenia. Br J Psychiatry. 1973;122:15–30. doi: 10.1192/bjp.122.1.15. [DOI] [PubMed] [Google Scholar]
- 22.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 23.Braff DL, Freedman R, Schork NJ, Gottesman II. Deconstructing schizophrenia: an overview of the use of endophenotypes in order to understand a complex disorder. Schizophr Bull. 2007;33:21–32. doi: 10.1093/schbul/sbl049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orosz A, Cattapan-Ludewig K, Gal G, Feldon J. Latent inhibition and learned irrelevance paradigms: a convenient way to assess information processing deficits in schizophrenia. In: Almann KV, editor. Schizophrenia Research Trends. New York, NY: Nova Science Publishers, Inc; 2008. pp. 137–161. [Google Scholar]
- 25.Baker AG, Mackintosh NJ. Preexposure to the CS alone, US alone, or CS and US uncorrelated: latent inhibition, blocking by context, or learned irrelevance? Learn Motiv. 1979;10:278–294. [Google Scholar]
- 26.Baker AG, Mackintosh NJ. Excitatory and inhibitory conditions following uncorrelated presentations of CS and US. Anim Learn Behav. 1977;5:315–319. [Google Scholar]
- 27.Bonardi C, Hall G. Learned irrelevance: no more than the sum of CS and US preexposure effects? J Exp Psychol Anim Behav Process. 1996;22:183–191. [Google Scholar]
- 28.Bonardi C, Yann OS. Learned irrelevance: a contemporary overview. Q J Exp Psychol B. 2003;56:80–89. doi: 10.1080/02724990244000188. [DOI] [PubMed] [Google Scholar]
- 29.Orosz AT, Feldon J, Gal G, Simon AE, Cattapan-Ludewig K. Deficient associative learning in drug-naive first-episode schizophrenia: results obtained using a new visual within-subjects learned irrelevance paradigm. Behav Brain Res. 2008;193:101–107. doi: 10.1016/j.bbr.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 30.Young AM, Kumari V, Mehrotra R, et al. Disruption of learned irrelevance in acute schizophrenia in a novel continuous within-subject paradigm suitable for fMRI. Behav Brain Res. 2005;156:277–288. doi: 10.1016/j.bbr.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 31.Gal G, Mendlovic S, Bloch Y, et al. Learned irrelevance is disrupted in first-episode but not chronic schizophrenia patients. Behav Brain Res. 2005;159:267–275. doi: 10.1016/j.bbr.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 32.Stieglitz RD. Diagnostik und Klassifikation psychischer Störungen. Göttingen, Germany: Hogrefe; 1998. [Google Scholar]
- 33.Wittchen HU, Fydrich T. Strukturiertes Klinisches Interview für DSM-IV. Manual zum SKID-I und SKID-II. Göttingen, Germany: Hogrefe; 1997. [Google Scholar]
- 34.Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Bull. 1991;17:555–564. doi: 10.1093/schbul/17.4.555. [DOI] [PubMed] [Google Scholar]
- 35.Miller TJ, McGlashan TH, Woods SW, et al. Symptom assessment in schizophrenic prodromal states. Psychiatr Q. 1999;70:273–287. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- 36.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 37.Orosz A, Feldon J, Gal G, Simon A, Cattapan-Ludewig K. Repeated measurements of learned irrelevance by a novel within-subject paradigm in humans. Behav Brain Res. 2007;180:1–3. doi: 10.1016/j.bbr.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Cadenhead KS, Swerdlow NR, Shafer KM, Diaz M, Braff DL. Modulation of the startle response and startle laterality in relatives of schizophrenic patients and in subjects with schizotypal personality disorder: evidence of inhibitory deficits. Am J Psychiatry. 2000;157:1660–1668. doi: 10.1176/appi.ajp.157.10.1660. [DOI] [PubMed] [Google Scholar]
- 39.Kumari V, Das M, Zachariah E, Ettinger U, Sharma T. Reduced prepulse inhibition in unaffected siblings of schizophrenia patients. Psychophysiology. 2005;42:588–594. doi: 10.1111/j.1469-8986.2005.00346.x. [DOI] [PubMed] [Google Scholar]
- 40.Price GW, Michie PT, Johnston J, et al. A multivariate electrophysiological endophenotype, from a unitary cohort, shows greater research utility than any single feature in the Western Australian family study of schizophrenia. Biol Psychiatry. 2006;60:1–10. doi: 10.1016/j.biopsych.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Louchart-de la CS, Nkam I, Houy E, et al. A concordance study of three electrophysiological measures in schizophrenia. Am J Psychiatry. 2005;162:466–474. doi: 10.1176/appi.ajp.162.3.466. [DOI] [PubMed] [Google Scholar]
- 42.Cadenhead KS, Light GA, Shafer KM, Braff DL. P50 suppression in individuals at risk for schizophrenia: the convergence of clinical, familial, and vulnerability marker risk assessment. Biol Psychiatry. 2005;57:1504–1509. doi: 10.1016/j.biopsych.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Quednow BB, Frommann I, Berning J, Kuhn KU, Maier W, Wagner M. Impaired sensorimotor gating of the acoustic startle response in the prodrome of schizophrenia. Biol Psychiatry. 2008;64:766–773. doi: 10.1016/j.biopsych.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 44.Braff DL. Information processing and attention dysfunctions in schizophrenia. Schizophr Bull. 1993;19:233–259. doi: 10.1093/schbul/19.2.233. [DOI] [PubMed] [Google Scholar]
- 45.Pearce JM, Hall G. A model for Pavlovian learning: variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychol Rev. 1980;87:532–552. [PubMed] [Google Scholar]
- 46.Bennett CH, Maldonado A, Mackintosh NJ. Learned irrelevance is not the sum of exposure to CS and US. Q J Exp Psychol B. 1995;48:117–128. [PubMed] [Google Scholar]
- 47.Mackintosh NJ. A theory of attention: variations in the associability of stimuli with reinforcement. Psychol Rev. 1975;82:276–298. [Google Scholar]
- 48.Mackintosh NJ. Stimulus selection: learning to ignore stimuli that predict no change in reinforcement. In: Hinde RA, Hinde JS, editors. Constraints on Learning. London, UK: Academic Press; 1973. pp. 75–96. [Google Scholar]
- 49.Schmidt-Hansen M, Killcross AS, Honey RC. Latent inhibition, learned irrelevance, and schizotypy: assessing their relationship. Cogn Neuropsychiatry. 2009;14:11–29. doi: 10.1080/13546800802664539. [DOI] [PubMed] [Google Scholar]