Abstract
Ventricular enlargement and reduced prefrontal volume are consistent findings in schizophrenia. Both are present in first episode subjects and may be detectable before the onset of clinical disorder. Substance misuse is more common in people with schizophrenia and is associated with similar brain abnormalities. We employ a prospective cohort study with nested case control comparison design to investigate the association between substance misuse, brain abnormality, and subsequent schizophrenia. Substance misuse history, imaging data, and clinical information were collected on 147 subjects at high risk of schizophrenia and 36 controls. Regions exhibiting a significant relationship between level of use of alcohol, cannabis or tobacco, and structure volume were identified. Multivariate regression then elucidated the relationship between level of substance use and structure volumes while accounting for correlations between these variables and correcting for potential confounders. Finally, we established whether substance misuse was associated with later risk of schizophrenia. Increased ventricular volume was associated with alcohol and cannabis use in a dose-dependent manner. Alcohol consumption was associated with reduced frontal lobe volume. Multiple regression analyses found both alcohol and cannabis were significant predictors of these abnormalities when simultaneously entered into the statistical model. Alcohol and cannabis misuse were associated with an increased subsequent risk of schizophrenia. We provide prospective evidence that use of cannabis or alcohol by people at high genetic risk of schizophrenia is associated with brain abnormalities and later risk of psychosis. A family history of schizophrenia may render the brain particularly sensitive to the risk-modifying effects of these substances.
Keywords: alcohol, cannabis, familial, psychosis, imaging
Introduction
Schizophrenia is associated with structural brain abnormalities.1 Many of these abnormalities precede the development of illness in people who later become unwell and cannot simply be attributed to antipsychotic medication.2 However, besides familial factors,3 the reasons for the development of schizophrenia and its associated structural abnormalities are far from clear. Environmental factors account for a significant proportion of the risk for schizophrenia, may account for structural magnetic resonance imaging (MRI) changes, and their modification may offer opportunities for primary prevention.
Schizophrenia is strongly associated with substance use, the 3 most commonly abused substances being alcohol, tobacco, and cannabis.4,5 For some substances, this relationship may be causal, the evidence being most robust for cannabis.6–8 A causative link between alcohol and schizophrenia has also been proposed.9 There is however a dearth of prospective studies investigating this putative association, which could potentially be further obscured by the practice of characterizing those with a history of heavy drinking and a chronic psychosis as having alcoholic hallucinosis.
In common with schizophrenia, misuse of some substances has been associated with brain structural abnormalities. These have been best characterized for alcoholism; generalized brain tissue loss, lateral ventricular enlargement, and prefrontal cortex deficits have been repeatedly observed;10–12 and medial temporal lobe volume loss also reported.13–15 Evidence associating cannabis use with brain structural abnormalities in healthy control subjects is less convincing, 2 recent reviews finding no consistent structural imaging findings.16,17
In schizophrenia, the brain structural abnormalities may precede illness and be a marker of risk,2 whereas in alcoholism, they are generally regarded as being a consequence of repeated substance use.12,18 Despite these differences, comparison of the nature of the deficits observed in the 2 conditions reveals considerable overlap, only superior anterior temporal lobe volume loss being greater in schizophrenic than alcohol-dependent subjects.19 In keeping with this, individuals with both conditions exhibit compounded abnormalities, volume loss being greater in comorbid individuals than those with either condition alone.20 In contrast to the negative findings in healthy subjects, individuals with schizophrenia who use cannabis also exhibit exaggerated structural abnormalities, more pronounced gray matter loss being demonstrated when compared with noncannabis using schizophrenic control subjects.21,22
Thus, people with schizophrenia may have a particular sensitivity to brain tissue loss on exposure to either alcohol or cannabis. If this susceptibility is present prior to illness being diagnosed, then substance misuse by individuals at elevated risk of schizophrenia may be associated with detectable structural abnormalities. Indeed, this may be so even when the cumulative quantities of substances consumed is considerably less than in studies demonstrating the brain structural consequences of habitual substance use in nonschizophrenic populations.
Examining whether substance misuse is associated with structural brain abnormalities in people who are well but at risk of schizophrenia is, as yet, untested. If such a link were established, it could provide evidence that people at genetically high risk of schizophrenia are indeed particularly vulnerable to the brain structural consequences of substance misuse. This would have obvious ramifications for the interpretation of the structural imaging abnormalities observed in established schizophrenia. If, however, use of these substances was also associated with the subsequent development of schizophrenia, the implications of such findings could be potentially even further reaching. It would suggest that these brain structural changes actually reflected processes by which substance use increases the risk of subsequently developing of schizophrenia.
Investigation of these possibilities requires a prospective study of subjects who are scanned when well and then followed up until a sufficient proportion develop schizophrenia. Such a design enables exploration of the relationship between level of exposure to various substances and both volumes of structures of particular interest (namely, the ventricles, frontal lobes, amygdala-hippocampal complex [AHC], and thalami) and risk of subsequently developing schizophrenia. The Edinburgh High-Risk Study (EHRS) provides exactly this opportunity.
Methods
Participants and Assessments
Data were collected on people at elevated risk of schizophrenia as part of the EHRS. Details of this recruitment process have been described previously.23,24 In brief, individuals with schizophrenia, with a family history of schizophrenia, and with adolescent relatives were identified from psychiatric hospital case records. We then approached their relatives, and the 150 high-risk subjects aged 16–25 years who agreed to participate were given a detailed clinical, neuropsychological, and brain imaging assessment. A control group of 36 subjects, without a family history of schizophrenia, were also recruited from the same areas of the country as the high-risk subjects.
At entry into the study, as one of this battery of assessments, lifetime highest level of use of various drugs was ascertained by self-report in face-to-face interviews. Level of use was then categorized for each substance, category cutoffs being assigned as appropriate for each of the substances in question. These cutoffs are detailed in table 1 for alcohol, cannabis, and tobacco. For alcohol, a low-use category of ≤3 units/wk was chosen to enable separation of none-teetotal but occasional users from those consuming alcohol more regularly. As we speculated that high-risk individuals may be particularly susceptible to the brain structural consequences of alcohol, we believed separation of these 2 groups to be particularly important. The latter group could contain individuals using considerable quantities of alcohol, and this could be even more of an issue if there were any underreporting of alcohol consumption. In the case of ecstasy, amphetamines, and LSD, the categories of use employed were never, isolated (use on a maximum of 3 occasions), repeated (use >3 occasions but less than monthly), and frequent (use at a level greater than once a month). Members of the control group were also examined as above.
Table 1.
Demographic Details of High-Risk Subjects in Each of the Substance Exposure Categories
| Alcohol | ||||||
| Level of Exposurea | Teetotal | Occasional | Regular | Exceed Safe Limits | Dependence | P |
| Number of subjects (%) | 10 (7.1) | 28 (20) | 56 (40) | 37 (26) | 9 (6.4) | |
| Mean age in y (SD) | 20.9 (2.3) | 19.8 (3.2) | 21.4 (2.8) | 21.9 (2.8) | 21.2 (2.9) | .05 |
| Gender (M:F) | 4:6 | 14:14 | 29:27 | 18:19 | 7:2 | .53 |
| Handedness (R:L:both) | 10:0:0 | 23:4:1 | 51:2:3 | 32:2:1 | 7:0:2 | .16 |
| Mean IQ (SD) | 100.9 (16.9) | 104.0 (16.0) | 98.4 (12.2) | 94.9 (9.4) | 94.3 (8.9) | .06 |
| Cannabis | ||||||
| Level of Exposureb | Nil | Isolated | Occasional | Frequent | Most days | P |
| Number of subjects (%) | 50 (35.2) | 23 (16.2) | 26 (18.3) | 15 (10.6) | 28 (19.7) | |
| Mean age in y (SD) | 21.1 (3.0) | 21.5 (3.3) | 21.7 (2.5) | 20.6 (2.5) | 21.0 (2.9) | .77 |
| Gender (M:F) | 20:30 | 11:12 | 15:11 | 8:7 | 20:8 | .11 |
| Handedness (R:L:both) | 42:5:2 | 22:0:1 | 24:1:1 | 13:1:0 | 23:2:3 | .65 |
| Mean IQ (SD) | 98.4 (14.3) | 101.8 (13.9) | 100.1 (12.8) | 96.6 (7.0) | 95.3 (11.1) | .41 |
| Tobacco | ||||||
| Level of Exposurec | Nil | 0–10 | 11–20 | 21+ | P | |
| Number of subjects (%) | 62 (45.3) | 42 (30.7) | 20 (14.6) | 13 (9.5) | ||
| Mean age in y (SD) | 21.4 (3.1) | 20.6 (2.5) | 21.0 (3.0) | 22.5 (2.7) | .20 | |
| Gender (M:F) | 32:30 | 24:18 | 10:10 | 7:6 | .94 | |
| Handedness (R:L:both) | 55:5:2 | 37:3:1 | 16:1:2 | 12:0:1 | .72 | |
| Mean IQ (SD) | 102.1 (13.6) | 95.3 (9.3) | 101.6 (12.6) | 89.8 (8.4) | <.01 | |
Note: M, male; F, female; R, right; L, left.
Exposure categories, based on highest level of consumption of alcohol during period of maximal use, are as follows: teetotal = no history of alcohol use; occasional = use never exceeded approximately 3 U/wk; regular = regular use but not exceeding 14 U/wk for women or 21 U/wk for men; exceed safe limits = exceeding safe recommendations; dependence = history alcohol dependence.
Exposure categories, based on highest level of exposure to cannabis during period of maximal use, are as follows: nil = never used cannabis; isolated = used on maximum of 3 occasions; occasional = regular use but less than monthly; frequently = use of cannabis monthly or greater; most days = use of cannabis more than 3 days a week.
Level of tobacco use: number of cigarettes (or equivalent) per day.
As described previously,25 both the high-risk and control subjects were followed up at regular intervals during the course of the study, which ran until 2004. Present State Examination ratings were obtained at each point of follow-up and after diagnosis in subjects who became ill. This report utilizes only the baseline imaging and clinical data, together with rates of development of schizophrenia during the entire study.
MRI Scanning and Analysis
Each participant underwent MRI scanning on a 1-T Siemens (Erlangen, Germany) Magnetom scanner. Details of image acquisition and processing have been given elsewhere.26
Image processing used the software package Analyze (Mayo Foundation, Rochester, MN) to outline neuroanatomical structures and ascertain their volumes.26 The volumes of structures with preexisting evidence of structural abnormalities secondary to substance use were included in this analysis. Structures selected were the lateral ventricles, third ventricle, fourth ventricle, right and left prefrontal lobes, AHC, and thalamic nuclei. The prefrontal, temporal, and AHC volumes were defined according to standard criteria,27,28 and the remaining regions of interest were outlined by naturalistic boundaries, described in previous studies.26,29
Volumetric image processing was done by 3 investigators, who examined all the brain regions above on 5 brains to ensure reliability between raters (mean correlation coefficient = 0.94 [range = 0.78–0.99]).
Statistical Analysis
Statistical testing was conducted with the Statistical Program for the Social Sciences 14. Demographics were compared between high-risk subgroups with different levels of exposure to the various substances with analysis of variance and the χ2 test.
The relationship between substance use and structure volumes was explored in 2 steps: firstly, investigation of a dose-dependent relationship and subsequently by multiple regression analysis. This 2-step analysis was required because of the potential collinearity between both the levels of different substances used by individuals and the effects of the various substances on structure volumes. Multiple regression has the potential to clarify which relationships do exist, while accounting for potential confounders. The multiple regression analysis focused on structures that had already demonstrated a significant dose-response relationship between volume and level of substance use.
As level of substance use was ascertained using ordinal measures, Spearman's test was used for the first step, with separate analyses being performed for each substance and each region of interest. These analyses were undertaken for both high-risk and control subjects. The significance of differences between R values in the high-risk and control groups was then determined by Fisher's Z test.
Multiple regression analysis employed a backward elimination model, with the independent variables of interest allotted to 6 separate blocks. These blocks were gender, age, whole-brain volume, alcohol use, cannabis use, and tobacco use. Gender was entered as a binary variable and age and whole-brain volume as continuous variables. In the blocks representing exposure to each of the substances, levels of use were represented as dummy variables in relation to a reference group. In the case of cannabis and cigarettes, the reference group was no use ever. As there were only 10 teetotal subjects (and their characteristics were not in keeping with the general trends seen with increasing levels of alcohol use), for alcohol the reference group was occasional use. Separate multiple regression analyses were performed for each brain region that had demonstrated a significant dose response relationship with at least one substance. In each case, the brain structure was entered as the dependent variable. Analysis was repeated with current IQ (measured using the Wechsler Adult Intelligence Scale Revised)30 as an additional block. Due to low subject numbers, the multiple regression analyses were not undertaken in the control group.
A number of the high-risk subjects had used illicit drugs other than cannabis. Regression analysis was therefore repeated again, excluding any individuals with a history of dependence on any substance other than alcohol, cannabis, or tobacco. As excluding subjects with any level of exposure to illicit drugs other than cannabis would have resulted in too great a loss of statistical power, it was not feasible to repeat regression analysis without these subjects. A supplementary regression analysis was therefore run including level of past use of ecstasy, amphetamines, and LSD as additional regression blocks.
In common with other studies, the distribution of ventricular volumes showed a right sided skew.31 These data were therefore log transformed prior to the regression analysis. Subsequent standardized residuals were checked for normality.
The χ2 test or likelihood ratios (where sample size was small) were used to compare numbers developing schizophrenia in high- and low-exposure groups. Where significant differences were found, odds ratios (ORs) were calculated.
Results
One hundred forty-seven high-risk and 36 healthy control subjects had usable scans. For the vast majority of individuals, full information on past and present use of each of the 3 substances of primary interest was available. However, information on alcohol use was not available for 7 high-risk subjects, cannabis use for 5, and tobacco use for 10. Demographics, organized by level of use of each of the 3 substances, are shown for high-risk subjects in table 1. There is a (nonsignificant) trend for more males in the highest exposure groups to both cannabis and alcohol. There is also a significant difference in age across the alcohol exposure groups, occasional users tending to be younger. Additionally, there is a significant difference in IQ across the tobacco exposure groups, the heaviest smokers having the lowest IQ. For demographic/drug use data in the control subjects, see supplementary information.
Some high-risk subjects had a history of use of illicit drugs other than cannabis. In only 3 subjects, with a history of opiate addiction, did level of past use constitute dependence. Data on levels of use of cocaine, ecstasy, amphetamines, and LSD are given in table 2.
Table 2.
Highest Levels of Use of Cocaine, Ecstasy, Amphetamines, and LSD by the High-Risk Subjects
| Drug | Level of Use: N (% of All High-Risk Subjects With That Level of Use) |
|||
| Never | Isolated | Repeated | Frequent | |
| Cocaine | 140 (94.6) | 0 | 2 (1.4) | 0 |
| Ecstasy | 111 (75.0) | 9 (6.1) | 9 (6.1) | 13 (8.8) |
| Amphetamine | 96 (64.9) | 21 (14.2) | 13 (8.8) | 12 (8.1) |
| LSD | 103 (69.6) | 14 (9.5) | 14 (9.5) | 11 (7.4) |
Dose-Response Relationships
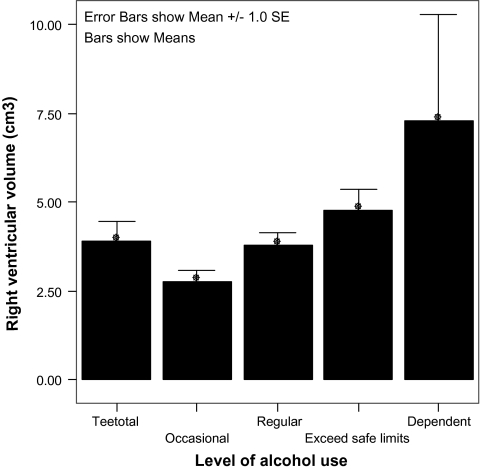
Structures with significant or near-significant relationships between level of exposure to a substance and raw volume in high-risk subjects are shown in table 3. Level of alcohol consumption correlated significantly and positively with volume of the left and right lateral ventricles (r = 0.192, P = .023, and r = 0.174, P = .005, respectively, right lateral ventricle association shown graphically in figure 1) and third ventricle (r = 0.183, P = .031). Conversely, the level of alcohol consumption showed a negative correlation with volume of the left and right prefrontal lobes (r = −0.194, P = .022, and r = −0.166, P = .049, respectively).
Table 3.
Mean (SD) Volumes (in Cubic Centimeters) of Regions of Interest in High-Risk Subjects With Histories of Increasing Exposure to Alcohol, Cannabis, and Tobacco
| Brain Region | Level of Exposure to Alcohol |
|||||
| Teetotal | Occasional | Regular | Exceed Safe Limits | Dependent | Correlation Analysis | |
| Whole-brain volume | 1334.48 (90.99) | 1384.96 (118.75) | 1326.41 (136.61) | 1324.84 (125.59) | 1391.75 (66.98) | r = −0.062, P= .465 |
| Left lateral ventricle | 4.97 (2.77) | 2.89 (2.00) | 4.07 (2.20) | 5.37 (4.12) | 7.06 (8.78) | r = 0.192, P= .023 |
| Right lateral ventricle | 3.92 (1.70) | 2.75 (0.77) | 3.78 (2.74) | 4.79 (3.44) | 7.31 (8.89) | r = 0.174, P= .005 |
| Third ventricle | 0.38 (0.21) | 0.29 (0.14) | 0.39 (0.24) | 0.47 (0.29) | 0.50 (0.42) | r = 0.183, P= .031 |
| Left frontal lobe | 77.6 (7.26) | 81.51 (14.18) | 75.00 (1.12) | 72.15 (10.47) | 78.38 (10.32) | r = −0.194, P= .022 |
| Right frontal lobe | 78.65 (7.79) | 84.65 (1.25) | 79.31 (13.40) | 75.76 (12.13) | 79.84 (11.19) | r = −0.166, P= .049 |
| Level of Exposure to Cannabis |
||||||
| Nil | Isolated | Regular | Frequent | Most Days | Correlation Analysis | |
| Whole-brain volume | 1327.04 (135.51) | 1341.76 (132.33) | 1349.0 (139.26) | 1317.06 (81.63) | 1387.49 (109.09) | r = 0.129, P= .126 |
| Left lateral ventricle | 3.45 (2.21) | 4.78 (3.11) | 4.52 (3.20) | 4.51 (4.00) | 5.83 (5.70) | r = 0.208, P= .013 |
| Right lateral ventricle | 3.08 (1.81) | 4.44 (4.09) | 4.19 (2.53) | 4.30 (2.63) | 5.23 (5.70) | r = 0.226, P= .007 |
| Third ventricle | 0.31 (0.19) | 0.34 (0.18) | 0.45 (0.32) | 0.53 (0.25) | 0.47 (0.31) | r = 0.271, P= .001 |
| Left frontal lobe | 76.94 (12.37) | 72.31 (13.46) | 75.67 (11.59) | 76.58 (10.40) | 78.70 (10.77) | r= 0.044, P= .603 |
| Right frontal lobe | 79.44 (12.52) | 77.60 (14.68) | 79.52 (12.92) | 79.97 (10.29) | 81.38 (12.57) | r= 0.058, P = .494 |
| Level of Exposure to Tobacco |
||||||
| Smoking | Nil | 0–10 | 10–20 | 20+ | Correlation Analysis | |
| Whole-brain volume | 1357.37 (135.56) | 1332.05 (121.08) | 1319.07 (124.60) | 1352.07 (109.29) | r = −0.062, P= .173 | |
| Left lateral ventricle | 4.36 (2.83) | 4.19 (3.11) | 4.32 (2.47) | 6.94 (7.83) | r= 0.040, P = .639 | |
| Right lateral ventricle | 3.81 (2.68) | 4.07 (2.87) | 4.01 (3.00) | 6.04 (7.80) | r = 0.079, P= .361 | |
| Third ventricle | 0.37 (0.27) | 0.42 (0.26) | 0.40 (0.15) | 0.49 (0.35) | r = 0.142, P= .099 | |
| Left frontal lobe | 77.33 (11.58) | 75.81 (12.55) | 73.34 (11.96) | 75.80 (12.47) | r = −0.146, P= .089 | |
| Right frontal lobe | 80.80 (12.37) | 80.63 (11.84) | 76.04 (15.77) | 75.34 (11.29) | r = −0.151, P= .079 | |
Note: Data are displayed only for the whole brain and those brain regions in which a significant correlation was observed between volume and level of exposure to at least one substance.
Fig. 1.
Bar Graph Illustrating Increased Volume of the Right Lateral Ventricle (SE) in Association With Increasing Levels of Exposure to Alcohol.
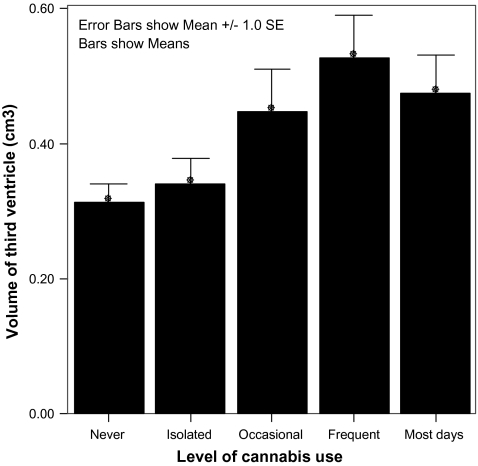
Level of cannabis use also correlated significantly and positively with volume of the left and right lateral ventricles (r = 0.208, P = .013, and r = 0.226, P = .007, respectively) and third ventricle (r = 0.271, P = .001, association shown graphically in figure 2). No significant associations were found between tobacco use and volumes of any of the structures of interest. The associations between substance use and structure volumes outlined above were not apparent in the control group (see supplementary information); though power to detect such an association was admittedly lower, it was absent even at trend level. Additionally, on application of Fisher's Z test, there were significant interactions between group membership and the structural consequences of both alcohol and cannabis use. For alcohol, this was significant in the right prefrontal lobe (Z = −2.5, P = .02) and third ventricle (Z = 3.45, P = <.001), while for cannabis it was significant in the third ventricle (Z = 3.05, P = .002).
Fig. 2.
Bar Graph Illustrating Increased Volume of the Third Ventricle (SE) in Association With Increasing Levels of Exposure to Cannabis.
A striking feature of alcohol structure volume/exposure relationships is the existence of a “J-shaped curve”; it seems that despite a clear tendency for a history of greater alcohol consumption to be associated with larger ventricular volume, third, fourth, and lateral ventricular volumes in lifetime abstainers are greater than in those with a history of occasional use (eg, figure 1). A complementary relationship is seen in the frontal lobes. This pattern is not seen with tobacco and cannabis (eg, figure 2).
Multiple Regression Analysis
Multiple regression analyses were performed for the lateral and third ventricles and the frontal lobes. Output from these analyses is shown in table 4. With a single exception (discussed below), the inclusion of IQ as an additional factor in the model did not significantly alter the results. Several findings are particularly noteworthy. First, as expected from the dose-response analysis above, level of alcohol consumption was a significant predictor of both right and left ventricular volumes. As well as higher levels of alcohol consumption being positively correlated with ventricular volume however, even relatively modest consumption of alcohol (at levels within government recommendations) was correlated with ventricular enlargement. Interestingly, lifetime abstinence also positively correlated with lateral ventricle volume.
Table 4.
Association of Variables of Interest With Regional Brain Volumes as Determined by the Primary Regression Analysis of Data From People at High Risk of Schizophrenia
| Dependent Variable | Independent Variables | β | t (df) | P Value | Adjusted R2 (Complete Model) | Impact of Including IQ in Model |
| Left lateral ventricle | Teetotal | .23 | 2.59 (134) | .011 | 0.11 | Dependent use becomes significant (t = 1.85, P = .066) |
| Regular use of alcohol | .21 | 2.06 (134) | .042 | |||
| Exceed safe limits of alcohol | .33 | 3.32 (134) | .001 | |||
| Use of cannabis most days | .20 | 2.31 (134) | .022 | |||
| Right lateral ventricle | Whole-brain volume | .26 | 3.19 (134) | .002 | 0.14 | Nil |
| Teetotal | .19 | 2.13 (134) | .035 | |||
| Regular use of alcohol | .26 | 2.34 (134) | .021 | |||
| Exceed safe limits of alcohol | .40 | 3.74 (134) | <.001 | |||
| Alcohol dependence | .29 | 3.20 (134) | .002 | |||
| Left frontal lobe | Whole-brain volume | .69 | 12.00 (134) | <.001 | 0.58 | Nil |
| Exceed safe limits of alcohol | −.12 | −2.18 (134) | .031 | |||
| Isolated use of cannabis | −.13 | −2.31 (134) | .023 | |||
| Age (y) | −.15 | −2.64 (134) | .009 | |||
| Right frontal lobe | Whole-brain volume | .67 | 11.1 (134) | <.001 | 0.54 | Exceed safe limits of alcohol becomes significant (t = −1.99, P= .049) |
| Exceed safe limits of alcohol | −.10 | −1.6 (134) | .106 | |||
| >20 cigarettes a day | −.13 | −2.2 (134) | .032 | |||
| Age (y) | −.14 | −2.3 (134) | .020 | |||
| Third ventricle | Frequent use of cannabis | .20 | 2.6 (134) | .012 | 0.15 | Nil |
| Gender | −.33 | −4.1 (134) | <.001 | |||
| Age (y) | .15 | 1.9 (134) | .065 |
Alcohol use exceeding UK government recommendations (more than 14 units/wk for women or 21 units/wk for men) correlated with reduced left frontal lobe volume. This correlation was significant on the right side only with inclusion of IQ in the model (R2 = 0.54, P = .049). No level of alcohol consumption was correlated with third ventricle volume (despite the strong dose-response relationship).
Third ventricular volume did however correlate positively with frequent use of cannabis. Left ventricular volume also correlated positively with use of cannabis most days. Though isolated use of cannabis correlated negatively with volume of the left frontal lobe, this was not within the context of a dose-response relationship. Tobacco consumption of more than 20 cigarettes a day correlated negatively with volume of the right frontal lobe; again the dose-response relationship between tobacco use and volume of the right frontal lobe had not reached significance.
Rerunning the regression analysis excluding the 3 subjects with a history of opiate dependence did not alter the above findings. However, rerunning the regression analysis with inclusion of highest use of amphetamines, ecstasy, and LSD as additional regression blocks did. Although alcohol use exceeding safe recommendations remained a significant predictor of left lateral ventricle and left frontal lobe volumes, it was no longer a significant predictor of right lateral ventricle volume. Frequent cannabis use remained a significant predictor of third ventricular volume but not left lateral ventricle volume. Most notably, however, in this expanded regression model, a history of frequent (highest level of use greater than or equal to monthly) and repeated (highest level of use several times a year) amphetamine use was predictor of increased right and left lateral ventricular volume (R2 = 0.125, P = .005, and R2 = 0.226, P = .011, respectively), and repeated use of ecstasy was a predictor of increased left lateral ventricle volume (R2 = 0.226, P = .011).
Despite the dose-response relationships between alcohol and cannabis and structure volumes, it was not always the highest drug exposure level that came out of the regression model as being significant predictors. For this reason, the regression analysis was repeated, combining the 2 highest exposure groups to each drug to create a single dummy variable representing all subjects with a history of substantial exposure to either substance. The combined cannabis exposure category correlated significantly with third ventricular volume, this being the case both with and without the inclusion of other illicit drugs in the regression model. Similarly on combining the 2 highest alcohol exposure groups, the resulting variable correlated significantly with left and right lateral ventricular and left frontal lobe volume.
Risk of Developing Schizophrenia
None of the control subjects developed schizophrenia. The proportion of high-risk subjects developing schizophrenia was greater in those with a history of alcohol dependence than subjects with all other levels of alcohol exposure combined (P = .017, OR = 6.35; 95% confidence interval [CI] = 1.53–26.26). Similarly, when all subjects with cannabis exposure greater than isolated use were compared with those below this cutoff, the former had an elevated rate of developing schizophrenia (P = .029, OR = 3.18; 95% CI = 1.08–9.36). Use of ecstasy and amphetamines did not predict development of schizophrenia. Given the lower numbers of subjects exposed to these drugs however, this may be attributable to a lack of power.
Discussion
These are the first findings to demonstrate that within a population at high risk of schizophrenia for genetic reasons, but clinically well, those who abuse alcohol and/or cannabis have structural imaging findings distinguishing them from those who do not. Results from supplementary regression analysis suggest that use of amphetamines and ecstasy by this population is also associated with structural abnormalities. Additionally, both alcohol dependence and regular cannabis use were associated with the subsequent development of schizophrenia. Use of these substances by people not at elevated risk of schizophrenia was not associated with comparable imaging or clinical findings. We believe that the most likely explanation for these findings is that people at high risk of schizophrenia are more vulnerable to the effects of these substances on the brain, but alternative explanations will also be considered.
Self-medication
Though increasingly questioned (eg, by Smit et al32 and Moore et al8 in relation to cannabis; and Drake and Mueser33 in relation to alcohol), the idea that the association between substance misuse and schizophrenia is driven by self-medication for psychotic symptoms remains pervasive.34 For self-medication to be a feasible explanation for the current findings, it would require that those with greater structural abnormalities were more unwell and consumed more substances. Individuals in this study did not have schizophrenia at the time of assessment. Though many had experienced partial or transient psychotic symptoms, these were not related to brain structural abnormalities.29 Self-medication is thus not a tenable explanation for the associations observed in this study. Additionally, the fact that structural abnormalities are detectable in association with substance misuse in a still well population provides important insights that can be extrapolated more generally: Brain abnormalities potentially precipitated by substance misuse are detectable prior to the development of illness.
Brain Structural Abnormalities Predispose to Substance Misuse
A potential explanation for the observed association between brain structural abnormalities and substance misuse is that, independent of any associations with schizophrenia, these abnormalities predispose to substance misuse. The compatibility of this explanation with Chambers’ theory that cortical and hippocampal dysfunctions in schizophrenia are responsible for the greater reinforcing properties of drugs of misuse (and hence development of drug problems) in this population is evident.35 Given the cross-sectional nature of this imaging data, we cannot here address this model as an explanation for the observed findings. When considered together with longitudinal data demonstrating the progressive nature of brain structural abnormalities with ongoing alcohol use however,18 this explanation is again untenable as a complete model. Though structural abnormalities may well predispose to substance misuse, this is clearly not the whole story; it is indeed the case that alcohol misuse does impact on brain structure.
The Observed Findings Are an Expected Consequence of Substance Use
This hinges on the structural abnormalities observed being a “normal” consequence of the reported levels of drug use. Studies identifying the characteristic structural abnormalities associated with alcoholism were discussed in the “Introduction”; these were generally undertaken in subjects much older than the study population, with a long history of very heavy alcohol use. Structural abnormalities identified in younger alcoholics have been much more subtle. The only significant findings in a case-control comparison of alcohol-dependent and light-drinking men with a mean age of 30 years were reduced cortical gray matter in the prefrontal cortex.12 A few studies have investigated structural imaging findings consequent to alcohol abuse/dependence in late adolescence. These initially reported volume reductions in the hippocampus, but not in other regions, (including the lateral ventricles).36 More recently, reduced prefrontal cortex volume has also been reported,37 though a subsequent study identified this only in female subjects.38
The subjects in the current study have an average age of 21 years; none have been treated for alcohol dependence, and few of even the heaviest users have consumed total quantities of alcohol even approaching that in the studies outlined above. Thus, though subtle structural changes, possibly including a degree of frontal lobe volume loss, may be observable in “uncomplicated” alcoholism in this age range, the gross structural abnormalities we observed would not be expected in a healthy population.
As discussed above, in the case of cannabis, there is little evidence for brain structural abnormalities even in chronic users.16,17 Studies comparing ecstasy users to control subjects also fail to identify notable volumetric structural abnormalities.39 More marked abnormalities have been reported in association with methamphetamine use. Specifically, in a cross-sectional study comparing subjects with a history of long-standing methamphetamine dependence to nonusing control subjects, hippocampal and right-sided cingulate gray matter loss accompanied by right frontal ventricular enlargement were reported.40 Reduced cortical gray matter has been reported in several subsequent reports, together with enlargement of the corpus striatum (reviewed in Berman et al41).
In summary, though there is evidence for gross volumetric abnormalities in individuals with long-standing, heavy use of amphetamines, there is no evidence for such sequelae in otherwise healthy individuals following even substantial use of the other illicit drugs discussed. None of the subjects in this study met criteria for dependence on amphetamines or ecstasy, and use was at markedly lower levels than in the studies outlined above. It thus again seems highly unlikely that structural abnormalities of the magnitude observed in the high-risk population can be regarded as an expected consequence of reported levels of substance use.
This is further supported by the absence of any comparable relationship between volumetric measurements and either alcohol or illicit substance use in the control group. Though numbers in this group are admittedly small, raising the possibility that it is underpowered to detect such relationships, there is no suggestion that they are even present at trend level. Indeed, Fischer Z test (which accounts for the relative size of each group) demonstrated a significant difference between high-risk and control subjects in the effect of alcohol on the right prefrontal lobe and cannabis on the third ventricle.
People at Elevated Risk of Schizophrenia Are Particularly Vulnerable to the Brain Structural Consequences of Substance Use
As discussed above, the brain structural abnormalities observed in this population in association with use of alcohol, cannabis, amphetamine, and ecstasy are all of greater magnitude than would be expected in healthy individuals with a comparable level of exposure. This is in keeping with previous evidence that brain volume loss is more pronounced in individuals with established schizophrenia who use either alcohol or cannabis,20,42 findings that suggested that individuals with schizophrenia have a particular sensitivity to brain volume loss on exposure to these substances. The current study builds on this work, being the first to provide structural imaging evidence that such a process may occur in a population at high risk of schizophrenia but currently clinically well. This provides biologically plausible evidence of how use of these substances may be contributing to the etiology of schizophrenia and clearly underlines the potential hazards of a high-risk population using them.
Cannabis and Alcohol in the Etiology of Schizophrenia
The proposal that cannabis may be contributing to the etiology of schizophrenia is not new, and the dangers of its use by people vulnerable to psychosis have previously been emphasized.6 These risks are reflected in this cohort, cannabis use again being associated with an increased rate of development of schizophrenia. More controversially, however, follow-up of this population also revealed a markedly elevated rate of schizophrenia in subjects with a history of alcohol dependence. Taken together with the pronounced structural abnormalities associated with alcohol consumption in this group, this raises the possibility that alcohol consumption could itself be interacting with vulnerability factors for schizophrenia to increase some individuals’ risk of developing the condition.
Though overshadowed by cannabis research in recent years, consideration of the possibility that alcohol use may play a role in the development of schizophrenia is not entirely novel. Indeed, such speculation has long been supported by the observation that chronic alcohol consumption may occasionally result in the generally self-limiting psychotic disorder known as alcoholic hallucinosis. In this condition, auditory hallucinations and delusions of reference and persecution are prominent, though other schizophrenic symptoms such as thought disorder and passivity are generally absent.27 Though this indicates that alcohol consumption can result in psychotic symptoms, the fact that family and genetic studies failed to demonstrate a greater prevalence of schizophrenia in relatives of patients with alcoholic hallucinosis led to genetic predisposition for the 2 conditions being regarded as independent.43 This appeared to reduce interest in the potential of alcoholic hallucinosis to shed light on the pathophysiology of schizophrenia, and there has been comparatively little research into the condition in recent years. There seems no good reason however that the results of these family studies should preclude the possibility that alcohol can potentially play a role in the etiology of schizophrenia.
A role for alcohol (and other substances under investigation in this study) in the development of schizophrenia is easily conceptualized within the framework of the stress vulnerability theory of schizophrenia.44 Within this model, alcohol interacts with other risk factors for schizophrenia to increase an individual's vulnerability to developing the condition. In this context, the finding that those with alcoholic hallucinosis have no family history of schizophrenia is not necessarily dissonant. It may be expected that as a consequence of heavy alcohol consumption some individuals (likely those with fewer susceptibility genes for schizophrenia) develop alcoholic hallucinosis, generally a temporary and self-limiting phenomena; those with a greater predisposition for schizophrenia, however, on exposure to similar or even lower levels of alcohol, may go on to develop a condition indistinguishable from classical schizophrenia.45 Given that they were selected on the basis of genetic loading for schizophrenia, the subjects in this study are heavily weighted toward the latter group. As would be expected from this theory, they exhibit prominent structural brain abnormalities in association with a history of heavy alcohol consumption, and alcohol dependence is associated with a significantly elevated risk of developing schizophrenia.
Relevance of Other Findings From the EHRS
Previous findings from the EHRS have shown that those subjects who develop schizophrenia exhibit significantly greater losses of gray matter density than those who remain well,46 these measures having utility in predicting schizophrenia.47 The current findings add to this, demonstrating that in individuals at elevated genetic risk of schizophrenia exposure to a variety of psychoactive substances is associated with dramatic brain structural abnormalities. Further analysis of the dataset, in particular investigating the relationship between these risk factors and longitudinal brain changes, is required. The current findings do however suggest that the developing brains of individuals at elevated genetic risk of schizophrenia are exquisitely vulnerable to the effects of a wide range of drugs of abuse. Given the findings of Job et al,46,47 it is most likely that this volume loss is occurring in gray matter, the very structure shown to be particularly susceptible to the effects of alcohol and cannabis in dual diagnosis studies of schizophrenia.20,42 The consequences of this brain tissue loss in adolescents at high risk of developing schizophrenia appear to be profound, this being reflected in the elevated risk of psychosis in those individuals with a history of either alcohol dependence or substantial exposure of cannabis.
Though the findings detailed above do seem robust, a number of points require further clarification. First, ascertainment of drug use relied on self-report of participants, which may not be reliable. While an objective method to determine history of drug use would have been desirable, unfortunately no appropriate tool exists. Given that we were interested in lifetime history of drug use, drug testing would have added little to the study. Furthermore, self-report of drug use is in fact a reliable tool in the research context.48
For some substances, the level of use that significantly correlated with structure volume in the regression model was not the highest level of exposure. For example, “excessive use” of alcohol rather than “dependence” positively correlated with right ventricular volume, despite mean ventricular volume in the latter group being greater than the former (4.79cm3 and 7.31cm3, respectively). In the case of alcohol exposure, this is explained by the number of subjects in the highest exposure group being relatively few compared with the excessive use category. This is not so for cannabis exposure however, there being more subjects in the “most days” than “frequent” category, yet the latter emerging as having a significant correlation with third ventricular volume. Given this, the latter analysis was repeated combining these 2 exposure groups to create a single dummy variable representing all subjects with a history of substantial exposure to cannabis. The combined exposure category correlated significantly with third ventricular volume, this being the case both with and without the inclusion of other illicit drugs in the regression model. This reinforces the veracity of the association between cannabis exposure and third ventricular volume.
The majority of findings from the regression analysis were robust to inclusion of past use of amphetamines, ecstasy, and LSD as additional regression blocks. That heavy use of alcohol was no longer a significant predictor of right lateral ventricular volume and frequent use of cannabis no longer a significant predictor of left ventricular volume are most likely due to the loss of power resulting from the inclusion of additional factors.
Data on control subjects have been included for completeness. Given their small numbers we have not focused on them in detail, but, as discussed above, the absence of structural associations comparable to those observed in high-risk subjects is in keeping with findings from other studies. The finding of decreased third ventricular volume with increasing history of alcohol exposure is notable however and requires further comment. This was unexpected and may not be reproducible in a larger sample of control subjects. It is notable however that nonconsumption of alcohol is very unusual in Scotland,49 and as such teetotal individuals may possess characteristics atypical of the general population. As such, it is conceivable that some unidentified confounding factor and/or factors are driving this unexpected relationship. Such factors may also be of relevance in understanding the comparable trend toward third ventricular volume reduction observed in this group in association with cannabis consumption.
It is the case that the current findings offer little insight into the processes occurring at a cellular or receptor level that result in the observed effects. The particular impact of cannabis on regions surrounding the third ventricle may offer some clues as to the circuits involved in mediating the propsychotic effects of this drug, but the structural consequences of the other substances investigated generally seem diffuse. Though speculative, it seems to us most likely that the genesis of these abnormalities involves an interaction between substance misuse and genetic risk to result in abnormalities of maturational processes. Clearly, however, considerable further work is required to clarify the nature of these processes and the mechanisms by which they are deranged by exposure to drugs of abuse.
While acknowledging the points above, it does therefore seem to be the case that a history of heavy use of alcohol in this high-risk population is indeed associated with lateral ventricular enlargement and frontal lobe volume loss; a history of frequent use of cannabis is associated with third ventricular enlargement; and repeated/frequent use of amphetamines and ecstasy is associated with increased lateral ventricular volume. These findings have major implications, particularly when viewed in combination with the elevated rate of development of schizophrenia associated with illicit drug and alcohol use that we also report. They not only add to our understanding of the pathogenesis of schizophrenia but also underline the potential hazards of even relatively modest recreational drug use by adolescent subjects at genetic risk of schizophrenia.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
UK Medical Research Council (G9226254, G9825423); Dr Mortimer and Theresa Sackler Foundation.
Supplementary Material
Acknowledgments
This study was carried out with the approval of the ethical committees of the relevant areas. We would like to thank all the subjects and family members for their participation in this study. Conflicts of interest: none declared.
References
- 1.Wright IC, Rabe-Hesketh S, Woodruff PWR, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 2.Lawrie SM, McIntosh AM, Hall J, Owens DGC, Johnstone EC. Brain structure and function changes during the development of schizophrenia: the evidence from studies of subjects at increased genetic risk. Schizophr Bull. 2008;34:330–340. doi: 10.1093/schbul/sbm158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boos H, Aleman A, Cahn W, Pol HH, Kahn RS. Brain volumes in relatives of patients with schizophrenia: a meta-analysis. Arch Gen Psychiatry. 2007;64:297. doi: 10.1001/archpsyc.64.3.297. [DOI] [PubMed] [Google Scholar]
- 4.Margolese HC, Malchy L, Negrete JC, Tempier R, Gill K. Drug and alcohol use among patients with schizophrenia and related psychoses: levels and consequences. Schizophr Res. 2004;67:157–166. doi: 10.1016/S0920-9964(02)00523-6. [DOI] [PubMed] [Google Scholar]
- 5.McCreadie RG. Use of drugs, alcohol and tobacco by people with schizophrenia: case-control study. Br J Psychiatry. 2002;181:321–325. doi: 10.1192/bjp.181.4.321. [DOI] [PubMed] [Google Scholar]
- 6.Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. J Psychopharmacol. 2005;19:187. doi: 10.1177/0269881105049040. [DOI] [PubMed] [Google Scholar]
- 7.Zammit S, Allebeck P, Andreasson S, Lundberg I, Lewis G. Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. Br Med J. 2002;325:1199. doi: 10.1136/bmj.325.7374.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore THM, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- 9.Mueser KT, Drake RE, Wallach MA. Dual diagnosis: a review of etiological theories. Addict Behav. 1998;23:717–734. [PubMed] [Google Scholar]
- 10.Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–998. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- 11.Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res. 1997;21:521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- 12.Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ. Cortical gray matter loss in treatment-naive alcohol dependent individuals. Alcohol Clin Exp Res. 2002;26:558. [PMC free article] [PubMed] [Google Scholar]
- 13.Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW. Hippocampal volume in patients with alcohol dependence. Arch Gen Psychiatry. 1999;56:356–363. doi: 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcohol Clin Exp Res. 1995;19:110–122. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- 16.Quickfall J, Crockford D. Brain neuroimaging in cannabis use: a review. J Neuropsychiatry Clin Neurosci. 2006;18:318. doi: 10.1176/jnp.2006.18.3.318. [DOI] [PubMed] [Google Scholar]
- 17.Martín-Santos R, Fagundo AB, Crippa JA, et al. Neuroimaging in cannabis use: a systematic review of the literature. Psychol Med. 2009:1–17. doi: 10.1017/S0033291709990729. [DOI] [PubMed] [Google Scholar]
- 18.Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon DH, Lim KO. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Arch Gen Psychiatry. 1998;55:905–912. doi: 10.1001/archpsyc.55.10.905. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan EV, Mathalon DH, Lim KO, Marsh L, Pfefferbaum A. Patterns of regional cortical dysmorphology distinguishing schizophrenia and chronic alcoholism. Biol Psychiatry. 1998;43:118–131. doi: 10.1016/S0006-3223(97)00264-3. [DOI] [PubMed] [Google Scholar]
- 20.Mathalon DH, Pfefferbaum A, Lim KO, Rosenbloom MJ, Sullivan EV. Compounded brain volume deficits in schizophrenia-alcoholism comorbidity. Arch Gen Psychiatry. 2003;60:245–252. doi: 10.1001/archpsyc.60.3.245. [DOI] [PubMed] [Google Scholar]
- 21.Szeszko PR, Robinson DG, Sevy S, et al. Anterior cingulate grey-matter deficits and cannabis use in first-episode schizophrenia. Br J Psychiatry. 2007;190:230–236. doi: 10.1192/bjp.bp.106.024521. [DOI] [PubMed] [Google Scholar]
- 22.Bangalore SS, Prasad KMR, Montrose DM, Goradia DD, Diwadkar VA, Keshavan MS. Cannabis use and brain structural alterations in first episode schizophrenia—a region of interest, voxel based morphometric study. Schizophr Res. 2008;99:1–6. doi: 10.1016/j.schres.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 23.Hodges A, Byrne M, Grant E, Johnstone EC. People at risk of schizophrenia. sample characteristics of the first 100 cases in the Edinburgh high-risk study. Br J Psychiatry. 1999;174:547–553. doi: 10.1192/bjp.174.6.547. [DOI] [PubMed] [Google Scholar]
- 24.Johnstone EC, Abukmeil SS, Byrne M, et al. Edinburgh high risk study—findings after four years: demographic, attainment and psychopathological issues. Schizophr Res. 2000;46:1–15. doi: 10.1016/s0920-9964(99)00225-x. [DOI] [PubMed] [Google Scholar]
- 25.Johnstone EC, Ebmeier KP, Miller P, Owens DGC, Lawrie SM. Predicting schizophrenia: findings from the Edinburgh high-risk study. Br J Psychiatry. 2005;186:18–25. doi: 10.1192/bjp.186.1.18. [DOI] [PubMed] [Google Scholar]
- 26.Whalley HC, Kestelman JN, Rimmington JE, et al. Methodological issues in volumetric magnetic resonance imaging of the brain in the Edinburgh high risk project. Psychiatry Res. 1999;91:31–44. doi: 10.1016/s0925-4927(99)00012-8. [DOI] [PubMed] [Google Scholar]
- 27.Suddath RL, Christison GW, Torrey EF, Casanova MF, Weinberger DR. Anatomical abnormalities in the brains of monozygotic twins discordant for schizophrenia. N Engl J Med. 1990;322:789–794. doi: 10.1056/NEJM199003223221201. [DOI] [PubMed] [Google Scholar]
- 28.Shenton ME, Kikinis R, Jolesz FA, et al. Abnormalities of the left temporal lobe and thought disorder in schizophrenia. A quantitative magnetic resonance imaging study. N Engl J Med. 1992;327:604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- 29.Lawrie SM, Whalley HC, Abukmeil SS, et al. Brain structure, genetic liability, and psychotic symptoms in subjects at high risk of developing schizophrenia. Biol Psychiatry. 2001;49:811–823. doi: 10.1016/s0006-3223(00)01117-3. [DOI] [PubMed] [Google Scholar]
- 30.Wechsler D. Wechsler Adult Intelligence Scale Revised. New York, NY: Psychological Corporation; 1981. [Google Scholar]
- 31.Dickey CC, Shenton ME, Hirayasu Y, et al. Large CSF volume not attributable to ventricular volume in schizotypal personality disorder. Am J Psychiatry. 2000;157:48–54. doi: 10.1176/ajp.157.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smit F, Bolier L, Cuijpers P. Cannabis use as a probable causative factor in the later development of schizophrenia. Ned Tijdschr Geneeskd. 2003;147:2178–2183. [PubMed] [Google Scholar]
- 33.Drake RE, Mueser KT. Co-occurring alcohol use disorder and schizophrenia. Alcohol Res Health. 2002;26:99–103. [Google Scholar]
- 34.Potvin S, Stip E, Roy JY. Schizophrenia and addiction: an evaluation of the self-medication hypothesis. Encephale. 2003;29(3 pt 1):193–203. [PubMed] [Google Scholar]
- 35.Chambers RA, Krystal JH, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biol Psychiatry. 2001;50:71–83. doi: 10.1016/s0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Bellis MD, Clark DB, Beers SR, et al. Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- 37.De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol Clin Exp Res. 2005;29:1590. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- 38.Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol Clin Exp Res. 2008;32:386–394. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gouzoulis-Mayfrank E, Daumann J. Neurotoxicity of methylenedioxyamphetamines (MDMA; ecstasy) in humans: how strong is the evidence for persistent brain damage? Addiction. 2006;101:348. doi: 10.1111/j.1360-0443.2006.01314.x. [DOI] [PubMed] [Google Scholar]
- 40.Thompson PM, Hayashi KM, Simon SL, et al. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berman S, O'Neill J, Fears S, Bartzokis G, London ED. Abuse of amphetamines and structural abnormalities in the brain. Ann N Y Acad Sci. 2008;1141(1 Addiction Reviews 2008):195–220. doi: 10.1196/annals.1441.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rais M, Cahn W, Van Haren N, et al. Excessive brain volume loss over time in cannabis-using first-episode schizophrenia patients. Am J Psychiatry. 2008;165:490. doi: 10.1176/appi.ajp.2007.07071110. [DOI] [PubMed] [Google Scholar]
- 43.Soyka M. Alcoholism and schizophrenia. Addiction. 2000;95:1613–1618. doi: 10.1080/09652140020000849. [DOI] [PubMed] [Google Scholar]
- 44.Howes OD, McDonald C, Cannon M, Arseneault L, Boydell J, Murray RM. Pathways to schizophrenia: the impact of environmental factors. Int J Neuropsychopharmacol. 2004;7:7–13. doi: 10.1017/S1461145704004122. [DOI] [PubMed] [Google Scholar]
- 45.Picchioni MM, Murray RM. Overvalued ideas about alcoholism and schizophrenia. Addiction. 2000;95:1860–1863. [PubMed] [Google Scholar]
- 46.Job DE, Whalley HC, Johnstone EC, Lawrie SM. Grey matter changes over time in high risk subjects developing schizophrenia. Neuroimage. 2005;25:1023–1030. doi: 10.1016/j.neuroimage.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Job DE, Whalley HC, McIntosh AM, Owens DG, Johnstone EC, Lawrie SM. Grey matter changes can improve the prediction of schizophrenia in subjects at high risk. BMC Med. 2006;4:29. doi: 10.1186/1741-7015-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cantwell R. Substance use and schizophrenia: effects on symptoms, social functioning and service use. Br J Psychiatry. 2003;182:324–329. doi: 10.1192/bjp.182.4.324. [DOI] [PubMed] [Google Scholar]
- 49.Scottish Government. Revised Alcohol Consumption Estimates From the 2003 Scottish Health Survey. Edinburgh, UK: Scottish Government; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.