Abstract
Background: It is well known that individuals with schizophrenia have impaired social cognition. The construct of social cognition involves several components, including perception, interpretation, and the ability to integrate context (Adolphs R. The neurobiology of social cognition. Curr Opin Neurobiol. 2001;11:231–239; Brothers L. The social brain: a project for integrating primate behavior and neurophysiology in a new domain. Concepts Neurosci. 1990;1:27–61). Importantly, a number of studies have suggested that deficits in context processing underlie cognitive dysfunction in schizophrenia (Penn DL, Corrigan PW, Bentall RP, Racenstein JM, Newman L. Social cognition in schizophrenia. Psychol Bull. 1997;121(1):114–132; Green MF, Nuechterlein KH. Should schizophrenia be treated as a neurocognitive disorder? Schizophr Bull. 1999;25:309–319). Thus, the purpose of the current study was to investigate the relationship between context processing and different aspects of social cognition in schizophrenia. Method: Individuals with schizophrenia (n = 41) and the healthy controls (n = 32) participated in this study. The participants completed 2 sections of The Awareness of Social Inference Test: (1) social inference minimal (SI-M) and (2) social inference enriched (SI-E). They also completed face and voice emotion discrimination tasks. In addition, we used the AX-Continuous Performance Test (AX-CPT) to measure context processing and the n-back task to measure working memory more generally. Results: AX-CPT performance in schizophrenia was positively correlated with both SI-M and SI-E performance but not with either the face or the voice discrimination. Furthermore, the correlation between AX-CPT performance and SI-M/SI-E performance was significantly stronger in individuals with schizophrenia than in controls. Conclusion: These results suggest that impairments in context processing are related to inferential components of social cognition in schizophrenia but not to the ability to recognition facial or vocal emotion. As such, deficits in context processing may contribute to deficits in both “hot” and “cold” aspects of cognition in schizophrenia.
Keywords: social cognition, context processing, schizophrenia
Introduction
Social cognition refers to the aspects of cognition required to perceive, interpret, and integrate social stimuli with given contextual cues for successful social interactions in our lives.1,2 It has long been known that individuals with schizophrenia have impaired social cognition.3 However, individuals with schizophrenia also have deficits in a range of cognitive domains,4,5 and it is not yet clear how such impairments may contribute to deficits in social cognition. For example, a large body of research suggests that context-processing abnormalities are a core cognitive disturbance in schizophrenia.6–8 As described below, context refers to the “information actively maintained in such a form that it can be used to mediate later task-appropriate behavior,”(p. 343)9 and is particularly critical in situations where there are different behavioral responses one can make to the same set of inputs in different contexts.7,10 At least some aspects of social cognition require the ability to integrate and apply such contextual information. As such, the goal of the current study is to examine whether deficits in context processing are associated with specific aspects of social cognition in schizophrenia.
One of the challenges of research on social cognition in schizophrenia is that the construct of social cognition is broad and includes a number of different processes.3,11 For example, a recent review suggested that there are at least 5 separable and hierarchical components to social-emotional processing.12 To date, the most frequently studied components of social cognition in schizophrenia have been emotion perception (ie, recognition of social-affective stimuli) and mental state inference, with a particular focus on theory of mind (TOM) paradigms. According to several reviews of emotion perception in schizophrenia,13–15 individuals with this illness display poor facial expression perception compared with healthy controls, both for identification and discrimination tasks. Furthermore, research has also shown that individuals with schizophrenia have impairments in emotion perception across other expressive channels besides faces, such as tone of voice and body gestures.13 More recently, researchers have examined the ability to use contextual information to modify emotion perception in schizophrenia. For example, Monkul et al.16 examined emotion intensity judgments of figures against a neutral background vs the same figures in a background that provided an emotional context. They found that individuals with schizophrenia showed less enhancement of emotion intensity judgments as a function of context than did controls. In another study, Huang et al.17 investigated information that set up a social context (eg, blame, praise, inquiry) on the categorization of happy and angry facial expressions. Individuals with schizophrenia showed less change in their categorization of faces as a function of context than did controls.
The ability to represent and infer the mental states of others has also been frequently studied in schizophrenia. In particular, many studies have examined TOM paradigms. Several systematic reviews18,19 and 2 recent meta-analyses20,21 showed that the magnitude of impairments for TOM performance in schizophrenia is large (Cohen’s d over 0.90) and that neurocognitive factors such as general intelligence at least partially contributed to TOM performance in schizophrenia. More recently, a series of studies have investigated context effects on some components of TOM performance (M.J. Green et al., in preparation).22,23 In these studies, individuals are presented with images of people either in neutral backgrounds or in backgrounds containing social contextual cues and are asked to judge what the person in the photo is thinking or feeling. Individuals with schizophrenia were less likely than controls to use the social context information to modify their judgments.22,23 In addition, Penn et al.24 investigated the role of contextual information in perceiving social stimuli using a battery of social perception tasks, providing some evidence for abnormal use of context cues during social cognition processing in schizophrenia.
Importantly, the research on nonsocial aspects of cognition in schizophrenia has also suggested that context processing may be a core deficit that underlies multiple domains of cognitive impairment in this illness.4–6,10 Context has been defined as to the ability to represent and maintain task-relevant information, which has to be integrated with current input to mediate task-appropriate behavior.7 Much of the prior work on context processing has focused on nonsocial cognitive tasks such as versions of the AX-Continuous Performance Test (CPT),9,25 the Stroop color-naming task,25,26 lexical disambiguation tasks,7 and measures of contextual influences in early visual processing.27,28 In nonsocial context-processing tasks, successful cognitive performance requires that individuals select and maintain task-relevant information (context) and use this information to inhibit task-irrelevant information. For example, in tasks such as the Stroop color-word task, the task instructions to attend to color and ignore word information can serve as context that allows the individual to successfully inhibit prepotent word information.
Relatively little work has directly examined the relationship between nonsocial measures of context processing and social cognition in schizophrenia. As described above, there is evidence that both basic emotion perception and higher level mental state inferences can be influenced by context information. In addition, there is evidence that individuals with schizophrenia show impaired integration of context when performing both types of social cognition tasks. However, it may be that context is particularly important for higher level mental state judgments, as the inherent ambiguity and complexity of the stimuli used in such paradigms (often dynamic and temporally extended interactions) may allow for a greater influence of context than on more basic emotion perception or discrimination tests. In fact, some researchers have argued that the integration of contextual information is fundamentally necessary for high-level mental state influence but not necessarily for recognition of at least some components of social-affective stimuli.12 Interestingly, there are several studies that have examined the relationship between TOM deficits and visual context processing.27–29 For example, Uhlhaas et al.28 found significant correlations between impaired TOM performance on the Hinting task (a measure of mental state inference) and performance of visual size perception task (a measure of perceptual context processing). Schenkel et al.27 also found that deficits in TOM were associated with errors on the contour integration test (a measure of visual context processing). These types of context influences on perceptual organization occur at an early stage of visual processing, and these findings suggest that social cognition can be influenced by contextual impairments at early stages of visual processing. However, it is also possible that social cognition is influenced by contextual processing at later stages of processing, at the points at which current goals or prior experiences need to be integrated with ongoing processing in order to make appropriate social judgments and responses.
In the current study, we aimed to investigate the relationship between nonsocial measures of cognitive context processing and different aspects of social cognition in schizophrenia. Based on previous studies, we hypothesized that deficits in context processing would be more associated with deficits in high-level mental state inferences than with basic emotion perception. To address these questions, we examined performance on face and voice emotion discrimination tasks, as well as measures of social inference, Parts 2 and 3 of The Awareness of Social Inference Test30 (TASIT) in relationship to performance on the AX-CPT task as a measure of context processing.
Methods
Participants
Participants in the current study consisted of 41 individuals with schizophrenia (SCZ) and 32 healthy controls (CON). The groups were matched for gender, age, race, and parental education (see table 1). However, personal education was significantly higher in CON compared with SCZ. All subjects participated in a large-scale project investigating the relationship between emotion and cognitive deficits in schizophrenia and were the same participants reported on in Mathews and Barch’s study,31 which focused on the relationship between subjective emotional processing and social cognition. All SCZ were stable outpatients and medicated (see table 1). CON were community volunteers and had no current, past, or family history of psychotic or bipolar disorder and no current mood or anxiety disorder. All participants gave informed consent. Any participant was excluded for: (1) meeting Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for substance abuse or dependence at any time within the past 6 months; (2) the presence of any clinically unstable or severe medical disorder, or a medical disorder; (3) present or past head injury with documented neurological sequelae; (4) meeting DSM-IV criteria for mental retardation; or (5) pregnancy, history of claustrophobia, any metallic object in the body, history of heart rhythm abnormalities, or presence of a heart pacemaker.
Table 1.
Clinical and Demographic Characteristics
| Demographics | Schizophrenia (n = 41) |
Healthy Controls (n = 32) |
||
| Mean | SD | Mean | SD | |
| Age (years) | 36.9 | 8.89 | 36.3 | 10.85 |
| Sex (% male) | 63.4% | 65.60% | ||
| Race (% Caucasian) | 48.8% | 53.10% | ||
| Education (years) | 13.1* | 2.29 | 15.5 | 12.91 |
| Parental education (years) | 14.0 | 2.98 | 12.9 | 2.67 |
| Age of first hospitalization | 21.8 | 5.33 | ||
| Medication (%) | ||||
| Antipsychotics | 100% | |||
| Typical antipsychotics | 7.3% | |||
| Atypical antipsychotics | 90.2% | |||
| Both typical and atypicals | 2.4% | |||
| Antidepressants | 63.4% | |||
| Mood stabilizers | 4.9% | |||
| Anticholinergics | 19.5% | |||
| BDI | 15.32** | 9.65 | 5.68 | 7.32 |
| Positive symptoms | 3.61** | 2.71 | 0.03 | .18 |
| Disorganization symptoms | 2.12** | 1.95 | 0.19 | .47 |
| Negative symptoms | 7.27** | 3.67 | 1.03 | 1.24 |
*P < .05, **P < .001.
Diagnosis and Clinical Assessment
Participants’ diagnosis was by a trained Masters level clinician using the Structured Clinical Interview for DSM-IV. For the SCZ group, the same Masters level clinical used the Scale for the Assessment of Negative Symptoms32 and the Scale for the Assessment of Positive Symptoms33 to assess symptom severity. Interrater reliability was assessed at biweekly sessions calibration with the group of masters level clinicians involved in the Washington University Conte Center.
Measures
Social Cognition.
For emotion recognition, we employed the facial emotion and voice emotion discrimination tests developed by Kerr and Neale.34 As described below, Part 1 of TASIT was also an emotion recognition test. However, we used Kerr and Neale’s measures in the current study rather than part I of the TASIT because the Kerr and Neale measures have a longer history of use in studies with individuals with schizophrenia and have excellent psychometric properties.35 We chose not to have participants also complete part I of the TASIT to reduce subject burden. The facial emotion discrimination task consisted of 24 black and white photographs of facial emotion from Izard36; 4 each of happiness, sadness, anger, fear, surprise, and sham. Participants judged whether pairs of faces were both representing the same or a different emotion. The voice emotion discrimination test consisted of a 35-item discrimination task that used 18 neutral-content voiced sentences that expressed happiness, sadness, anger, fear, surprise, and shame in tone and prosody. Participants judged whether a pair of auditorily presented sentences represented the same or a different emotion. As the dependent measure for emotion recognition task, we used the overall accuracy scores for face and voice emotion discrimination.
To measure social inference, we used Parts 2 and 3 of TASIT.30 Section 2 of the TASIT was the test of social inference minimal (SI-M) that assessed conversational understanding using cues such as tone of voice and facial expression. There were 15-videotaped vignettes that involved sincere and sarcastic exchanges (if the sarcastic exchange was not recognized, the conversation was nonsensical). After each conversation, the participants were asked 4 types of questions about what the person in the conversation: (1) believes or knows, (2) means by what he/she said, (3) intends to do or act in the situation; and (4) feels. Part 3 of the TASIT was the test of social inference enriched (SI-E) which assessed the ability to use contextual knowledge (visual and verbal), in additional to voice and face cues, to derive meaning from the conversation. The contextual cues included edited video (such as zooming into a plate of food) and prologue with a third person to reveal the speakers “true” thoughts. There were 16-videotaped vignettes, and in each there was an untrue comment presented as either sarcasm (to amplify the truth) or as a lie (to minimize or conceal the truth). The same probe questions that were used in part 2 were used to assess understanding in part 3 as well. We collapsed across the 4 types questions and used the overall scores for each condition of the TASIT scales (SI-M and SI-E) as the dependent measures.
AX-Continuous Performance Test (AX-CPT).
We used the AX-CPT as a context-processing measures.7,37 Subjects were presented with cue-probe pairs and instructed to make a target response to an X (probe) only when it followed an A (cue) and to make a nontarget response otherwise. Target trials (AX) occur with high frequency (70%) and the remaining trials (30%) were split evenly among the other 3 nontarget trial types: BX (where B refers to any non-A cue), AY (where Y refers to any non-X cue), and BY (where B refers to any non-A cue and Y refers to any non-X probe). Performance on this task depends on the representation and maintenance of context provided by the cue (A or not A). On BX trials, the context provided by the B cue should be maintained and used to inhibit this bias to make a target response to X, which would lead to a false alarm. In contrast, context processing leads individuals to prepare to make a target response when an A is presented. This could lead to false alarms on AY trials, when this preparation to make a target response has to be overcome. As such, impaired context processing should lead individuals to make errors on BX trials but to perform relatively well on AY trials.
In addition, we manipulated the delay between the cue and probe. The cue-probe interval was 5 s on long-delay trials and 1 s on short-delay trials. The task was presented in 4 blocks of 50 trials, with 2 blocks of short-delay trials and 2 blocks of long-delayed trials. The order of short- and long-delayed trials was counterbalanced across subjects. Given the percentages of each trial type described above, this meant that there were 70 AX trials and 10 trials of each of the other trial types at both the short and long delays. As a dependent measure of the context processing, we used d′-context (short and long), which refers to sensitivity to context that was computed from the AX hits and BX false alarms.7,38
The N-Back Task.
We also administered the n-back as a more general measure of working memory (WM) to assess the specificity of any relationship between context processing (the AX-CPT) and social cognition.39,40 We used 3 conditions of the n-back task to examine performance as a function of memory load: (a) 0-back, (b) 1-back, and (c) 2-back. White letters against a black background were shown on a computer screen one at a time. In the 0-back condition, participants were asked to respond to a single prespecified target letter. In the 1-back condition, participants had to recognize whether the target letter was identical to the one right preceding it. In the 2-back condition, participants had to tell whether the target letter was identical to the one shown 2 trials back. Trials were blocked by condition, and 3 blocks of each condition (25 trials per block) were presented in counterbalanced order. Subjects were asked to press one button for targets (7 per block, 21 total per condition) and another button for nontargets (18 trials per block, 54 total per condition). As the dependent measures, we used accuracy for correct trials at each load level.
Results
The group differences on the social cognition and cognitive tasks have been presented in prior publications.31,41 Here, we present these group differences again for the subjects who completed both the cognitive and social cognition tasks for clarity prior to reporting on the relationships between the social cognition, context processing, and WM measure.
Social Cognition
We analyzed the TASIT (see table 2) using repeated measures analysis of variance (ANOVA) with group (CON, SCZ) as a between-subject factor and test section (SI-M, SI-E) as a within-subject factor. This ANOVA revealed a significant main effect of group (F1,71 = 23.56, P < .001), but no significant main effect of test section (F1,71 = 1.40, P = .24) and no significant group by test section interaction (F1,71 = 2.18, P = .14), with SCZ performing worse than CON on both test sections. The emotion perception measures were also analyzed using a repeated measures ANOVA with group as a between-subject factor and stimulus type (face, voice) as a within-subject factor. This ANOVA revealed a significant main effect of group (F1,71 = 7.29, P < .01), but no significant main effect of stimulus type (F1,71 = 2.47, P = .12) and no significant group by stimulus type interaction (F1,71 = 2.09, P = .15), with SCZ performing worse than CON on both stimulus types.
Table 2.
Performance on the Social Cognition Tasks
| Group |
Effect Size (Cohen’s D) | ||||
| Social Cognition | Schizophrenia (n = 41) |
Healthy Controls (n = 32) |
|||
| Mean | SD | Mean | SD | ||
| Face emotion discrimination | 25.07 | 2.42 | 26.19 | 2.73 | 0.43 |
| Voice emotion discrimination | 25.12 | 4.01 | 27.34 | 2.77 | 0.64 |
| TASIT | |||||
| Social inference-minimal, total | 45.93 | 8.67 | 54.16 | 4.24 | 1.21 |
| Social inference-enriched, total | 47.63 | 7.77 | 53.97 | 5.14 | 0.96 |
Note: TASIT, The Awareness of Social Inference Test.
AX-CPT
We analyzed error data using repeated measures of ANOVA, with group (CON, SCZ) as a between-subject factor and both delay (short, long) and trial-type (AX, AY, BX, BY) as within-subject factors. The ANOVA (table 3) revealed significant main effects of delay (F1,76 = 18.94, P < .001), group (F1,66 = 3.84, P = .05), and trial type (F3,198 = 7.35, P < .001). These main effects were modified by a significant group by trial-type interaction (F3,198 = 4.87, P = .01) as well as a significant trial-type by delay interaction (F3,198 = 6.14, P = .002). In addition, there was a significant 3-way interaction between group, trial-type, and delay (F3,198 = 4.09, P = .02). As shown in table 3, error rates were overall higher at the long compared with short delay, and SCZ made more errors than CON. Planned contrasts to determine the source of the group × trial-type interaction indicated that SCZ made AX errors (F1,66 = 8.9, P < .01) and BX errors (F1,66 = 5.64, P = .02) than CON but did not differ in AY (F1,66 = 0.75, P = .39) or BY errors (F1,66 = 0.15, P = .70). Planned contrasts to determine the source of the 3-way interaction between group, trial-type, and delay indicated that CON showed a significant increase in AY errors from the short to the long delay (F1,66 = 4.96, P = .03), while the SCZ showed a trend to decrease AY errors from the short to the long delay (F1,66 = 3.79, P = .06).
Table 3.
Performance on the AX-CPT and N-Back Tasks
| Group (n = 68) |
|||||||||
| Schizophrenia (n = 41) |
Healthy Controls (n = 27) |
Effect Size | |||||||
| AX-CPT | Errors | Reaction Times | Errors | Reaction Times | |||||
| Condition | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Short delay | |||||||||
| AX | 0.05 | 0.05 | 423.75 | 114.10 | 0.01 | 0.02 | 396.12 | 106.60 | |
| AY | 0.12 | 0.14 | 590.78 | 107.99 | 0.09 | 0.21 | 538.21 | 102.91 | |
| BX | 0.23 | 0.29 | 535.21 | 222.11 | 0.07 | 0.16 | 468.88 | 207.50 | |
| BY | 0.03 | 0.06 | 449.26 | 125.44 | 0.04 | 0.16 | 405.13 | 149.07 | |
| d′ | 2.56 | 1.15 | 3.50 | 0.62 | 1.02 | ||||
| Long delay | |||||||||
| AX | 0.20 | 0.25 | 496.58 | 112.83 | 0.08 | 0.14 | 426.11 | 92.38 | |
| AY | 0.07 | 0.10 | 596.51 | 97.88 | 0.16 | 0.20 | 550.46 | 71.14 | |
| BX | 0.24 | 0.34 | 543.65 | 208.86 | 0.09 | 0.14 | 471.71 | 181.74 | |
| BY | 0.03 | 0.05 | 488.11 | 109.14 | 0.04 | 0.19 | 442.79 | 127.79 | |
| d′ | 1.82 | 1.22 | 2.99 | 0.78 | 1.14 | ||||
| n-back | Accuracy | Reaction Times | Accuracy | Reaction Times | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| 0-back | 0.96 | 0.05 | 572.31 | 133.18 | 0.96 | 0.08 | 484.86 | 88.31 | 0.77 |
| 1-back | 0.89 | 0.08 | 693.95 | 195.47 | 0.93 | 0.09 | 541.96 | 119.10 | 0.94 |
| 2-back | 0.80 | 0.10 | 793.51 | 244.83 | 0.88 | 0.11 | 680.38 | 184.82 | 0.52 |
Note: AX-CPT, AX-Continuous Performance Test.
N-Back Task
Accuracy and reaction times for 0-, 1-, 2- back tasks were presented in table 3. We analyzed the data with a repeated measures ANOVA with group as a between-subject factor and condition (0-, 1-, 2-back tasks) as a within-subject factor. The accuracy ANOVA revealed a main effect of group (F1,68 = 7.02, P = .01) and condition (F2,136 = 55.33, P < .001) as well as a significant group by condition interaction (F2,136 = 5.74, P < .01). Simple effects tests indicated that SCZ group performed significantly worse than CON on 1-back (F1,68 = 4.54, P < .05) and 2-back tasks (F1,68 = 10.49, P < .01). However, there was no significant difference between 2 groups on 0-back task (F1,68 = 0.00, P = .99).
The Relationship between Context Processing, WM, and Social Cognition
SCZ.
Consistent with our predictions (see table 4), performance on TASIT, SI-E for SCZ was positively correlated with d′-context short- and long-delay scores, and TASIT, SI-M was correlated with d′-context short delay. In contrast, neither the face nor the voice discrimination tasks were correlated with AX-CPT performance at either the short or long delay. There were no significant correlations between performance on the social cognition tasks and 2-back performance.
Table 4.
Relationship between Social and Nonsocial Cognition Measures
| TASIT SI-M | TASIT SI-E | Face Discrimination | Voice Discrimination | |
| Schizophrenia | ||||
| d′ short | 0.34* | 0.45** | −0.19 | 0.19 |
| d′ long | 0.23 | 0.38* | −0.15 | 0.12 |
| 2-back accuracy | 0.14 | 0.19 | 0.12 | 0.15 |
| Healthy controls | ||||
| d′ short | −0.13 | 0.07 | −0.10 | 0.09 |
| d′ long | −0.09 | 0.09 | −0.10 | 0.09 |
| 2-back accuracy | 0.12 | 0.40* | 0.21 | 0.45* |
Note: TASIT, The Awareness of Social Inference Test; SI-M, social inference minimal; SI-E, social inference enriched. *P < .05, **P < .001.
To determine whether the TASIT was significantly more correlated with AX-CPT performance than was face or voice discrimination performance, we compared these correlations using procedures for comparing correlated correlation coefficients.42 These analyses indicated that the TASIT, SI-E was significantly more correlated with both d′-context at the short delay (Z = −3.09, P < .01) and at the long delay (Z = −2.56, P < .01) than was face discrimination. We found similar trends for the TASIT, SI-E to be more correlated with d′ context than voice discrimination for both the short (Z = −1.36, P = .08) and long (Z = −1.28, P = .09) delays on the AX-CPT. In addition, the TASIT, SI-M was significantly more correlated with d′ context at the long delay (Z = −1.80, P < .05) than was face discrimination. Although there were no significant correlations between TASIT and 2-back performance, the magnitude of the correlations did not differ significantly between the TASIT and the AX-CPT vs the 2-back.
CON.
As presented in table 4, TASIT (SI-M and SI-E) was not correlated with either d′ context short or long among CON. However, TASIT, SI-E was correlated with 2-back accuracy (r = .40, P = .03). Neither the face nor voice discrimination task was correlated with d′ context short and long. However, none of these correlations differed significantly from each other (all Ps >.05).
CON Vs SCZ.
We also examined whether the magnitude of the correlations between the TASIT and the AX-CPT differed between CON and SCZ. Fisher’s R to Z transformations indicated that there was a significantly stronger correlation between d′-context at the short delay and both TASIT, SI-M (Z = −1.99, P < .05) and TASIT, SI-E (Z = −1.67, P < .05) among SCZ compared with CON. None of the other correlations differed significantly between the 2 groups.
Mediation Analyses
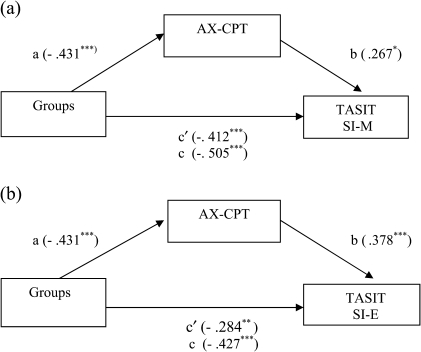
As described above, SCZ performed more poorly than CON on both sections of the TASIT and on the AX-CPT. Furthermore, there was a strong correlation between AX-CPT (d′ context short) and TASIT (SI-M and SI-E) for SCZ. Thus, to test whether performance on the AX-CPT mediated the group differences on TASIT (SI-M and SI-E), we conducted mediation analyses. We focused on d′ context at the short delay rather than the long delay as it showed a numerically stronger correlation with the social cognition measures, and we felt it would be redundant to include both the short and long delay measures. The Sobel test43 indicated that d′ context short significantly mediated the group differences on the TASIT, SI-M (Z = −2.03, P < .05) and SI-E (Z = −2.52, P < .01). However, the group effects for both SI-M and SI-E were still significant even with AX-CPT in the model, suggesting that this mediation was partial rather than full (see figure 1). We also calculated the proportion of the total effect of the group differences in the TASIT measures that was carried through the mediator (d′ context) as a measure of the effect size. These analyses indicated that AX-CPT accounted for approximately 23% and 38% of the shared variance in performance on TASIT, SI-M and SI-E, respectively. These findings suggest that context processing assessed by AX-CPT partially mediated the deficits of social inference performance (TASIT) in schizophrenia, as indicated by a significant indirect path in figure 1.
Fig. 1.
The Mediation Effect of the AX-CPT on the TASIT. AX-CPT, AX-Continuous Performance Test; TASIT, The Awareness of Social Inference Test; SI-M, social inference minimal; SI-E, social inference enriched.
Discussion
The goal of this study was to investigate the relevance of context processing to different aspects of social cognition (emotion perception, social inference) in schizophrenia. We predicted that mental state inference would be more associated with context processing measured by AX-CPT than simple recognition of basic emotions assessed by face and voice discrimination tasks. Consistent with our hypothesis, social inference performance on the TASIT showed a significant association with AX-CPT performance among individuals with schizophrenia, while emotion recognition was not associated with AX-CPT performance. Our findings are consistent with previous work regarding the relevance of context processing to mental state inference. For example, prior research on perceptual context processing suggested that the ability to integrate the surrounding visual context was associated with impaired performance on several TOM tasks in schizophrenia.27,28 More recently, a series of studies by Green et al.22,23 found that individuals with schizophrenia showed reduced visual attention to context information, which was associated with impaired mental state inference. Taken together, this body of work begins to suggest that a key component of impaired social inference in schizophrenia may be difficulties attending to or integrating context information.
In addition to finding an association between AX-CPT and TASIT performance in schizophrenia, we also found that AX-CPT performance mediated the influence of diagnostic status on TASIT performance. The finding that some of the group differences in social cognition were accounted for by context processing is consistent with previously reported associations between neurocognitive impairments, social cognition, and functioning.44–46 However, this mediation was only partial, and group differences in TASIT performance still remained after accounting for variance associated with context processing. This suggests that there are impairments in mental state inference among individuals with schizophrenia that are not accounted for by deficits in the type of context processing measured by the AX-CPT. The AX-CPT uses nonsocial stimuli and thus may fail to capture components of context processing that are unique or specific to social situations or factors other than context processing that are necessary for accurate social inference. For example, a perceiver’s prior experiences with specific social stimuli or cues (faces, body gestures, etc.) could also serve as relevant contextual information that modulates the perceiver’s responses to a current social situation.3 If individuals with schizophrenia have deficits in either basic components of social stimulus perception,47,48 or deficits in the ability to retrieve prior experiences with similar stimuli or cues (given their known impairments in episodic memory function),49 then these factors may contribute to impaired social inference independent of deficits in context processing per se.
Although we found that mental state inference in schizophrenia was significantly associated with context processing measured by AX-CPT, it was not associated with more general WM function as assessed by n-back performance among individuals with schizophrenia. It is possible that this reflects a psychometric confound, as examination of the coefficients of variation (standardized measures of variance that account for scaling differences) indicated that performance on the AX-CPT was more variable than performance on the 2-back among individuals with schizophrenia. However, it is also possible that this finding suggests that mental state inference may be more dependent on integrating context information than on general WM demands. As discussed by Ochsner,12 to represent and understand complex mental states, individuals may need to integrate context information into their existing mental representations derived from past experiences and modify such mental representations by taking into account others’ past experiences and goals. These processes may then help an individual reappraise the meaning of social stimuli in a context appropriate way. According to Ochsner’s model,50,51 this reappraisal is associated with activity of dorsal-lateral prefrontal regions, which are known to be important for context processing, though they are also engaged by WM tasks more generally.52
We also examined the association between context processing and emotion perception. Somewhat surprisingly, we did not find any relationship between context processing and voice or face emotion perception among individuals with schizophrenia. In one sense, this result could be seen as inconsistent with prior work showing that: (a) context can influence emotion perception in healthy individuals53 (M.J. Green et al., in preparation) and (b) individuals with schizophrenia show a deficit in their ability to use context to modulate emotion perception.16,17 However, unlike these prior studies, our emotion perception task did not contain any contextual information that either controls or patients could use to modify their emotion recognition judgments. Thus, we may not have found a relationship between emotion perception and context processing in our participants with schizophrenia because our emotion perception tasks did not tap contextual integration. As such, it would be important in future work to examine whether context processing—as measured by tasks such as the AX-CPT—is associated with emotion perception in paradigms or situations where it is possible to use contextual information to modify performance (M.J. Green et al., in preparation).22,23
Although the results of this study have interesting implications for understanding the relevance of context processing for mental state inference in schizophrenia, there were several limitations to the study. First, all participants with schizophrenia were chronic outpatients. This may restrict generalization of the current findings to other stage of illness (eg, acute patients, those early in the course of the illness, or unmedicated patients). In addition, all of our patients were medicated on stable doses of antipsychotics, and we cannot rule out the possibility that medications influenced the relationship between context processing and our social cognition measures. However, many medicated individuals with schizophrenia continue to experience social cognition impairments, and thus it is important to understand what factors influence social cognition even among medicated individuals with schizophrenia. In addition, as noted above, it will be important in future work to use a wider range of tasks (eg, AX-CPT type tasks with social stimuli, emotion perception tasks that allow contextual modulation) that may allow for more sensitive tests of the influence of context processing on emotion perception as well as higher level mental state inference components of social cognition. Lastly, we found that many of the relationships between context processing and social cognition were present among individuals with schizophrenia and not among controls. However, it is difficult to tell whether this reflects unique relationships present only in individuals with schizophrenia and not controls, or the fact that the controls performed overall better and thus a restriction of range prevented us from seeing similar associations in controls. In future research, it would be important to examine performance on tasks that are challenging even for controls to determine whether such relationships between nonsocial measures of context processing and social cognition are present regardless of disease state.
Funding
National Institute of Mental Health R01 (MH066031 to D.M.B.).
Acknowledgments
The authors would like to thank the participants in this study, who gave generously of their time. The authors would also like to thank Carol Cox for her help in preparing this manuscript.
References
- 1.Adolphs R. The neurobiology of social cognition. Curr Opin Neurobiol. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- 2.Brothers L. The social brain: a project for integrating primate behavior and neurophysiology in a new domain. Concepts Neurosci. 1990;1:27–61. [Google Scholar]
- 3.Penn DL, Corrigan PW, Bentall RP, Racenstein JM, Newman L. Social cognition in schizophrenia. Psychol Bull. 1997;121(1):114–132. doi: 10.1037/0033-2909.121.1.114. [DOI] [PubMed] [Google Scholar]
- 4.Green MF, Nuechterlein KH. Should schizophrenia be treated as a neurocognitive disorder? Schizophr Bull. 1999;25:309–319. doi: 10.1093/oxfordjournals.schbul.a033380. [DOI] [PubMed] [Google Scholar]
- 5.Hemsley DR. The development of a cognitive model of schizophrenia: placing it in context. Neurosci Biobehav Rev. 2005;29:977–988. doi: 10.1016/j.neubiorev.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Shakow D. Segmental set: a theory of the formal psychological deficit in schizophrenia. Arch Gen Psychiatry. 1962;6:1–17. doi: 10.1001/archpsyc.1962.01710190003001. [DOI] [PubMed] [Google Scholar]
- 7.Cohen JD, Barch DM, Carter C, Servan-Schreiber D. Context processing deficits in schizophrenia: converging evidence from three theoretically motivated cognitive tasks. J Abnorm Psychol. 1999;108(1):120–133. doi: 10.1037//0021-843x.108.1.120. [DOI] [PubMed] [Google Scholar]
- 8.Phillips WA, Silverstein SM. Convergence of biological and psychological perspectives on cognitive coordination in schizophrenia. Behav Brain Sci. 2003;26(1):65–82. doi: 10.1017/s0140525x03000025. [DOI] [PubMed] [Google Scholar]
- 9.McClure MM, Barch DM, Flory JD, Harvey PD, Siever LJ. Context processing in schizotypal personality disorder: evidence of specificity of impairment to the schizophrenia spectrum. J Abnorm Psychol. 2008;117:342–354. doi: 10.1037/0021-843X.117.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen JD, Servan-Schreiber D. Context, cortex, and dopamine: a connectionist approach to behavior and biology in schizophrenia. Psychol Rev. 1992;99(1):45–77. doi: 10.1037/0033-295x.99.1.45. [DOI] [PubMed] [Google Scholar]
- 11.Green MF, Olivier B, Crawley JN, Penn DL, Silverstein S. Social cognition in schizophrenia: recommendations from the measurement and treatment research to improve cognition in schizophrenia new approaches conference. Schizophr Bull. 2005;31:882–887. doi: 10.1093/schbul/sbi049. [DOI] [PubMed] [Google Scholar]
- 12.Ochsner KN. The social-emotional processing stream: five core constructs and their translational potential for schizophrenia and beyond. Biol Psychiatry. 2008;64(1):48–61. doi: 10.1016/j.biopsych.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trémeau F. A review of emotion deficits in schizophrenia. Dialogues Clin Neurosci. 2006;8(1):59–70. doi: 10.31887/DCNS.2006.8.1/ftremeau. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards JJH, Pattison PE. Emotion recognition via facial expression and affective prosody in schizophrenia: a methodological review. Clin Psychol Rev. 2002;22:789–832. doi: 10.1016/s0272-7358(02)00130-7. [DOI] [PubMed] [Google Scholar]
- 15.Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr Bull. doi: 10.1093/schbul/sbn192. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monkul ES, Green MJ, Barrett JA, Robinson JL, Velligan DI, Glahn DC. A social cognitive approach to emotional intensity judgment deficits in schizophrenia. Schizophr Res. 2007;94:245–252. doi: 10.1016/j.schres.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 17.Huang J, Chan RC, Lu X, Tong Z. Emotion categorization perception in schizophrenia in conversations with different social contexts. Aust N Z J Psychiatry. 2009;43:438–445. doi: 10.1080/00048670902817646. [DOI] [PubMed] [Google Scholar]
- 18.Brüne M. “Theory of mind” in schizophrenia: a review of the literature. Schizophr Bull. 2005;31(1):21–42. doi: 10.1093/schbul/sbi002. [DOI] [PubMed] [Google Scholar]
- 19.Harrington L, Siegert RJ, McClure J. Theory of mind in schizophrenia: a critical review. Cogn Neuropsychiatry. 2005;10(4):249–286. doi: 10.1080/13546800444000056. [DOI] [PubMed] [Google Scholar]
- 20.Sprong M, Schothorst P, Vos E, Hox J, Engeland HV. Theory of mind in schizophrenia: meta-analysis. Br J Psychiatry. 2007;191:5–13. doi: 10.1192/bjp.bp.107.035899. [DOI] [PubMed] [Google Scholar]
- 21.Bora E, Yucel M, Pantelis C. Theory of mind impairment in schizophrenia: meta-analysis. Schizophr Res. 2009;109(1–3):1–9. doi: 10.1016/j.schres.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 22.Green MJ, Waldron JH, Coltheart M. Emotional context processing is impaired in schizophrenia. Cogn Neuropsychiatry. 2007;12(3):259–280. doi: 10.1080/13546800601051847. [DOI] [PubMed] [Google Scholar]
- 23.Green MJ, Waldron JH, Simpson I, Coltheart M. Visual processing of social context during mental state perception in schizophrenia. J Psychiatry Neurosci. 2008;33(1):34–42. [PMC free article] [PubMed] [Google Scholar]
- 24.Penn DL, Ritchie M, Francis J, Combs D, Martin J. Social perception in schizophrenia: the role of context. Psychiatry Res. 2002;109(2):149–159. doi: 10.1016/s0165-1781(02)00004-5. [DOI] [PubMed] [Google Scholar]
- 25.Abramczyk RR, Jordan DE, Hegel M. “Reverse” Stroop effect in the performance of schizophrenics. Percept Mot Skills. 1983;56(1):99–106. doi: 10.2466/pms.1983.56.1.99. [DOI] [PubMed] [Google Scholar]
- 26.Whsocki JJ, Sweet JJ. Identification of brain-damaged, schizophrenic, and normal medical patients using a brief neuropsychological screening battery. Int J Clin Neuropsyc. 1985;7(1):40–44. [Google Scholar]
- 27.Schenkel LS, Spaulding WD, Silverstein SM. Poor premorbid social functioning and theory of mind deficit in schizophrenia: evidence of reduced context processing? J Psychiatr Res. 2005;39:499–508. doi: 10.1016/j.jpsychires.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Uhlhaas PJ, Phillips WA, Schenkel LS, Silverstein SM. Theory of mind and perceptual context-processing in schizophrenia. Cogn Neuropsychiatry. 2006;11:416–436. doi: 10.1080/13546800444000272. [DOI] [PubMed] [Google Scholar]
- 29.Schenkel LS, Spaulding WD. Presented at: 17th Annual Meeting of the Society for Research in Psychopathology; San Francisco, CA. Social inference in schizophrenia: relationships with childhood functioning and later cognitive impairment. September, 2002. [Google Scholar]
- 30.McDonald S, Flanagan S, Rollins J. The Awareness of Social Inference Test. Suffolk, UK: Thames Valley Test Company Limited; 2002. [Google Scholar]
- 31.Mathews JR, Barch DM. Emotion responsivity, social cognition, and functional outcome in schizophrenia. J Abnorm Psychol. 2010;119(1):50–59. doi: 10.1037/a0017861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andreason NC. Scale for the Assessment of Negative Symptoms. Iowa City: University of Iowa; 1983a. [Google Scholar]
- 33.Andreason NC. The Scale for the Assessment of Positive Symptoms. Iowa City: University of Iowa; 1983b. [Google Scholar]
- 34.Kerr SL, Neale JM. Emotion perception in schizophrenia: specific deficit or further evidence of generalized poor performance? J Abnorm Psychol. 1993;102:312–318. doi: 10.1037//0021-843x.102.2.312. [DOI] [PubMed] [Google Scholar]
- 35.Kee KS, Green MF, Mintz J, Brekke JS. Is emotion processing a predictor of functional outcome in schizophrenia? Schizophr Bull. 2003;29:487–497. doi: 10.1093/oxfordjournals.schbul.a007021. [DOI] [PubMed] [Google Scholar]
- 36.Izard CE. The Face of Emotion. New York: Appleton-Century-Crofts; 1971. [Google Scholar]
- 37.Servan-Schreiber D, Cohen JD, Steingard S. Schizophrenic deficits in the processing of context. A test of a theoretical model. Arch Gen Psychiatry. 1996;53:1105–1112. doi: 10.1001/archpsyc.1996.01830120037008. [DOI] [PubMed] [Google Scholar]
- 38.Swets JA, Sewall ST. Invariance of signal detectability over stages of practice and levels of motivation. J Exp Psychol. 1963;66(2):120–126. [Google Scholar]
- 39.Casey BJ, Cohen JD, Jezzard P, et al. Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. Neuroimage. 1995;2:221–229. doi: 10.1006/nimg.1995.1029. [DOI] [PubMed] [Google Scholar]
- 40.Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- 41.Barch DM. Neuropsychological abnormalities in schizophrenia and major mood disorders: similarities and differences. Curr Psychiatry Rep. 2009;11:313–319. doi: 10.1007/s11920-009-0045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meng X-L, Rosenthal R, Rubin DB. Comparing correlated correlation coefficients. Psychol Bull. 1992;111(1):172–175. [Google Scholar]
- 43.Morgan-Lopez AA, MacKinnon DP. Demonstration and evaluation of a method for assessing mediated moderation. Behav Res Methods. 2006;38(1):77–87. doi: 10.3758/bf03192752. [DOI] [PubMed] [Google Scholar]
- 44.Addington J, Addington D. Neurocognitive and social functioning in schizophrenia. Schizophr Bull. 1999;25(1):173–182. doi: 10.1093/oxfordjournals.schbul.a033363. [DOI] [PubMed] [Google Scholar]
- 45.Addington J, Saeedi H, Addington D. Facial affect recognition: a mediator between cognitive and social functioning in psychosis? Schizophr Res. 2006;85(1–3):142–150. doi: 10.1016/j.schres.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 46.Pinkham AE, Penn DL. Neurocognitive and social cognitive predictors of interpersonal skill in schizophrenia. Psychiatry Res. 2006;143(2–3):167–178. doi: 10.1016/j.psychres.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 47.Addington J, Addington D. Facial affect recognition and information processing in schizophrenia and bipolar disorder. Schizophr Res. 1998;32:171–181. doi: 10.1016/s0920-9964(98)00042-5. [DOI] [PubMed] [Google Scholar]
- 48.Sergi MJ, Green MF. Social perception and early visual processing in schizophrenia. Schizophr Res. 2003;59:233–241. doi: 10.1016/s0920-9964(01)00405-4. [DOI] [PubMed] [Google Scholar]
- 49.Ranganath C, Minzenberg MJ, Ragland JD. The cognitive neuroscience of memory function and dysfunction in schizophrenia. Biol Psychiatry. 2008;64(1):18–25. doi: 10.1016/j.biopsych.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 51.Ochsner KN, Gross JJ. Cognitive emotion regulation: insights from social cognitive and affective neuroscience. Currr Dir Psychol Sci. 2008;17:153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barch DM, Braver TS, Nystom LE, Forman SD, Noll DC, Cohen JD. Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia. 1997;35:1373–1380. doi: 10.1016/s0028-3932(97)00072-9. [DOI] [PubMed] [Google Scholar]
- 53.de Gelder B, Vroomen J, de Jong SJ, Masthoff ED, Trompenaars FJ, Hodiamont P. Multisensory integration of emotional faces and voices in schizophrenics. Schizophr Res. 2005;72:195–203. doi: 10.1016/j.schres.2004.02.013. [DOI] [PubMed] [Google Scholar]