Abstract
Neuroligins are evolutionarily conserved postsynaptic cell-adhesion molecules that function, at least in part, by forming trans-synaptic complexes with presynaptic neurexins. Different neuroligin isoforms perform diverse functions and exhibit distinct intracellular localizations, but contain similar cytoplasmic sequences whose role remains largely unknown. Here, we analysed the effect of a single amino-acid substitution (R704C) that targets a conserved arginine residue in the cytoplasmic sequence of all neuroligins, and that was associated with autism in neuroligin-4. We introduced the R704C mutation into mouse neuroligin-3 by homologous recombination, and examined its effect on synapses in vitro and in vivo. Electrophysiological and morphological studies revealed that the neuroligin-3 R704C mutation did not significantly alter synapse formation, but dramatically impaired synapse function. Specifically, the R704C mutation caused a major and selective decrease in AMPA receptor-mediated synaptic transmission in pyramidal neurons of the hippocampus, without similarly changing NMDA or GABA receptor-mediated synaptic transmission, and without detectably altering presynaptic neurotransmitter release. Our results suggest that the cytoplasmic tail of neuroligin-3 has a central role in synaptic transmission by modulating the recruitment of AMPA receptors to postsynaptic sites at excitatory synapses.
Keywords: autism, neurexin, neuroligin, synaptogenesis, synaptic transmission
Introduction
Animal brains process information in vast parallel networks of neurons connected by synapses. Although synapses are primarily known for synaptic transmission, which consists of presynaptic neurotransmitter release and the postsynaptic reception of neurotransmitters, it should be noted that synapses fundamentally operate as intercellular junctions, wherein presynaptic and postsynaptic sides are connected by trans-synaptic cell-adhesion molecules. Neurexins and neuroligins are heterotypic cell-adhesion molecules that arguably constitute the best characterized trans-synaptic cell-adhesion pair (Ushkaryov et al, 1992; Ichtchenko et al, 1995). Presynaptic neurexins are differentially expressed in all neurons from three genes in two principal forms (α- and β-neurexins; Ullrich et al, 1995), whereas postsynaptic neuroligins are produced from four genes (NL1–NL4) that are largely co-expressed in most neurons (Ichtchenko et al, 1995). All neurexins and neuroligins bind to each other, although with distinct affinities dictated by isoforms and alternative splicing (Boucard et al, 2005; Chih et al, 2006; Comoletti et al, 2006; Graf et al, 2006; Arac et al, 2007). Neurexins and neuroligins are highly conserved evolutionarily except for NL4 which, at least in rodents, is poorly conserved and expressed at very low levels (Bolliger et al, 2008).
Neurexins and neuroligins are essential for synapse function. Triple knockout (KO) of all α-neurexins (Missler et al, 2003) or triple KO of NL1, NL2 and NL3 (Varoqueaux et al, 2006) is lethal, and produces nearly complete inactivation of synaptic transmission. Even single KO's of α-neurexins (Missler et al, 2003) or of neuroligins (Chubykin et al, 2007) induce major phenotypes. The general importance of neurexins and neuroligins is confirmed by the observation of multiple mutations in the genes encoding neurexins and neuroligins in neurological disorders. In particular, heterozygous deletion of neurexin-1α severely predisposes to autism and schizophrenia (Feng et al, 2006; Sebat et al, 2007; Szatmari et al, 2007; Kirov et al, 2008; Marshall et al, 2008; Walsh et al, 2008; Yan et al, 2008; Zahir et al, 2008; Kim et al, 2008a; Bucan et al, 2009; Glessner et al, 2009; Rujescu et al, 2009), whereas a single missense mutation in NL3 (R451C) and a large number of frameshift and missense mutations in NL4 lead to autism and/or mental retardation with complete penetrance (Jamain et al, 2003; Laumonnier et al, 2004; Yan et al, 2005; Talebizadeh et al, 2006; Lawson-Yuen et al, 2008; reviewed in Südhof, 2008).
Neuroligins are type I membrane proteins composed of a large extracellular domain homologous to cholinesterases, an extracellular O-glycosylated sequence, a single transmembrane region and a short cytoplasmic tail (Figure 1A). Neuroligin isoforms are differentially distributed among synapses in the same neuron (Song et al, 1999; Graf et al, 2004; Varoqueaux et al, 2004; Budreck and Scheiffele, 2007). The cytoplasmic tails of neuroligins are highly conserved, and include multiple sequence motifs that are present in all isoforms, raising the question of how the cytoplasmic sequences of neuroligins function. Among the conserved sequence motifs of cytoplasmic neuroligin tails, only the role of the C-terminal PDZ-binding sequence—which interacts with several PDZ domain proteins in vitro including PSD-95 (Irie et al, 1997)—is known, whereas the significance of the other sequence motifs remains unclear.
Figure 1.
R704C point mutation in the cytoplasmic tail of NL3 does not impair in vitro synaptogenic activity of NL3. (A) Diagram of the neuroligin domain structure (top; SP, signal peptide; EHD, esterase-homology domain; O-gly, O-glycosylation sequence; TMR, transmembrane region; PDZ, PDZ domain-binding motif) and alignment of neuroligin sequences surrounding the mutated residue R704C (bottom; arginine corresponding to R704 is shown in red typeface; intramembranous sequence, blue typeface; cytoplasmic sequence, black typeface; sequences show mouse NL1–NL4 and Drosophila neuroligin (NM_001170191.1)). (B) Co-immunoprecipitation experiment demonstrating that the R704C mutation does not block binding of NL3 to PSD-95. HA-tagged PSD-95 was transfected alone or together with Flag-tagged wild-type or R704C mutant NL3 into HEK293 cells, and co-immunoprecipitation of PSD-95 with NL3 was assayed. Data shown are representative immunoblot visualized by ECL. (C, D) Representative images (C) and quantitations (D) of artificial synapse formation on COS cells expressing mVenus only, or mVenus-fused wild-type NL3 (WT) or mutant NL3R704C (R704C). Synapses were quantified as the synapsin signal observed on the COS cells; transfection efficiency was measured as the mVenus signal. Scale bar=20 μm. (E, F) Representative images (E) and synapse quantitations (F) in neurons transfected with mVenus alone, or mVenus-fused wild-type (WT) or R704C-mutant NL3 (R704C). The synapse density per unit dendrite (E, left panels) or synapse size (E, right panels) was quantified based on the measurements of postsynaptic spines (as visualized with the mVenus signal) or presynaptic terminals (as measured by synapsin staining). Scale bar=5 μm. Data in (D, F) are mean values±s.e.m. (*P<0.05; ***P<0.001 by Student's t-test; (D) n=34 mVenus control-transfected, 27 WT NL3-transfected and 28 R704C NL3-transfected cells; (F) n=38 mVenus control-transfected, 41 WT NL3-transfected and 40 R704C NL3-transfected neurons; for both (D) and (F), three independent culture experiments were performed, with statistics based on the number of experiments and not the number of cells analysed).
More than 20 mutations in NL4 were associated with autism (see references cited above). Most of these mutations predictably disrupt NL4 expression or folding, suggesting a loss-of-function mechanism during pathogenesis (Jamain et al, 2003; Laumonnier et al, 2004; Yan et al, 2005; Talebizadeh et al, 2006; Lawson-Yuen et al, 2008; reviewed in Südhof (2008) and Zhang et al (2009)). Some substitution mutations, however, do not indicate an obvious functional effect, raising the possibility that at least a subset of the described neuroligin mutations may be polymorphisms without functional consequence, or mediate gain-of-function effects. This is particularly true for the only mutation in a cytoplasmic residue of a neuroligin that has been described in autism, the R704C substitution (Yan et al, 2005). Although this substitution affects a highly conserved residue (Figure 1A), the arginine residue involved localizes close to the transmembrane region in a cluster of four positively charged residues, raising doubts about the significance of neutralizing a single charge in this cluster by the R704C mutation.
In the present study, we have tested the significance of R704 and its substitution to cysteine using in vitro and in vivo approaches. We performed these experiments in NL3 because this isoform is well conserved evolutionarily and highly expressed, and because we previously showed that a different autism-associated point mutation in NL3, R451C, produces a gain-of-function phenotype with distinct effects on synaptic transmission that differ from the NL3 KO phenotype (Tabuchi et al, 2007). Thus, we could relate the R704C-mutant phenotype to that of the NL3 KO and of the R451C mutation. Our results reveal that although the R704C substitution does not detectably alter the activity of NL3 in synapse formation, it produces a dramatic deficit in AMPA receptor-mediated synaptic transmission. This synaptic transmission deficit implies that the conserved juxtamembranous sequence motif of neuroligins performs a critical function in regulating synaptic transmission by a postsynaptic mechanism, and by extension, that the corresponding mutation in human NL4 is pathophysiologically significant.
Results
The R704C substitution does not detectably alter in vitro activities of NL3
To evaluate the effect of the R704C substitution on NL3 function in vitro, we examined binding of wild-type and R704C-mutant NL3 to PSD-95 (Irie et al, 1997). Co-immunoprecipitation experiments showed that the R704C mutation did not disrupt PSD-95 binding (Figure 1B), as would be expected given the distance of the R704 residue to the PDZ domain-binding motif of NL3 (Figure 1A). Next, we tested the ability of overexpressed wild-type or R704C-mutant NL3 to increase synapse densities on transfected COS cells in the artificial synapse formation assay (Figure 1C and D; Scheiffele et al, 2000), and in transfected neurons (Figure 1E and F; Boucard et al, 2005). In both assays, wild-type and R704C-mutant NL3 potently enhanced the number of detectable synapses, probably by stabilizing transient synapses formed on the transfected cells (Chubykin et al, 2007). Although there was a trend for a lower activity by R704C-mutant NL3, its effectiveness was not statistically different from that of wild-type NL3. Thus, the NL3R704C mutation does not significantly perturb the in vitro activity of NL3.
Generation of NL3 R704C (NL3R704C) KI mice
Next, we introduced the R704C mutation by homologous recombination into the mouse NL3 gene (Figure 2A). NL3R704C-mutant mice exhibited no obvious survival phenotype (adult male offspring from matings of heterozygous females with wild-type males: wild type, n=24 mice; R704C, n=28 mice).
Figure 2.
Generation and characterization of NL3R704C knock-in mice. (A) Homologous recombination strategy. The structures of the wild-type NL3 gene (E6–E8=exons 6–8), the targeting vector (DT, diphtheria toxin cassette and NEO, neomycin-resistance cassette), the recombined allele containing NEO (third line) and the recombined allele after flp excision of NEO are shown from top to bottom. Asterisk in exon 8 indicates R704C point mutation. (B, C) Representative immunoblots (B) and summary graphs of protein levels (C) in wild-type and NL3R704C-mutant brains analysed by quantitative immunoblotting using 125I-labelled secondary antibodies (n=4 pairs). (D, E) Further immunoblotting analysis of the protein levels of glutamate receptor subtypes in wild-type and NL3R704C-mutant brains analysed by quantitative immunoblotting using 125I-labelled secondary antibodies (n=5 WT and 6 R704C mice). Abbreviations used in (C, E): Syt1, synaptotagmin-1; Syb2, synaptobrevin-2; NR1, NR2a and NR2b, NMDA receptor subunit 1, 2a and 2b, respectively. Data in (C, E) are mean values±s.e.m. (*P<0.05 by Student's t-test).
Since previous data revealed that another autism-relevant neuroligin point mutation, the NL3R451C mutation, severely destabilized NL3 (Comoletti et al, 2004; Tabuchi et al, 2007; De Jaco et al, 2010), we quantified the levels of NL3 and of other synaptic proteins in NL3R704C-mutant mice. We detected only a modest decrease in NL3, but observed a small increase in the levels of the AMPA-type glutamate receptor subunit GluR1 (Figure 2B and C), suggesting a potential genetic interaction between GluR1 and NL3. To further explore the possibility that the R704C mutation alters glutamate receptor levels, we performed additional, more extensive quantitative immunoblotting analyses examining all major glutamate receptor subtypes (Figure 2D and E). These experiments confirmed the significant increase in GluR1 and additionally uncovered a significant increase in GluR3, suggesting that the R704C mutation increases AMPA-type glutamate receptor levels in the hippocampus.
We then examined the effect of the NL3R704C mutation on brain development and synapse formation. Immunocytochemical analyses of brain sections failed to uncover major abnormalities (Figure 3). Specifically, we quantified synapse densities in three different brain regions (the CA1 and CA3 regions of the hippocampus or in the somatosensory cortex) using three independent markers (synaptophysin as a marker for all synapses, and the vesicular glutamate (vGlut1) and GABA transporters (vGAT) as markers for excitatory and inhibitory synapses, respectively). The results showed that both the overall synapse density and size and the density and size of excitatory versus inhibitory synapses were not detectably altered by the NL3R704C mutation (Figure 3A and B).
Figure 3.
NL3R704C-mutant mice exhibit no changes in hippocampal or cortical synapse size and density. Immunohistochemistry for presynaptic markers was performed in the CA1 and the CA3 regions of the hippocampus and in the somatosensory cortex (SSC). (A) Representative low- (top of each series) and high-resolution images (bottom of each series) of sections from the hippocampal CA1and CA3 regions and the SSC as indicated on the left of wild-type (NL3 WT) and NL3R704C-mutant mice, immunostained for synaptophysin (left panels) or for the vesicular glutamate and GABA transporters vGlut1 and vGAT (right panels). (B), Summary graphs of the synapse densities (top) and size (bottom) as measured by synaptophysin staining (left) or by vGlut1 and vGAT staining (right). Data in (B) are mean values±s.e.m. (n=3 pairs). No statistically significant difference between wild-type and R704C-mutant mice was detected in the analysed brain regions.
Decreased excitatory synapse function in NL3R704C KI mice
To test whether the NL3R704C mutation altered synaptic transmission, we performed whole-cell voltage-clamp recordings in hippocampal CA1 pyramidal neurons in acute brain slices. Strikingly, the NL3R704C mutation decreased the frequency but not the amplitude of spontaneous mEPSCs, whereas it had no significant effect on mIPSCs (Figure 4A–F). This selective deficit in mEPSC frequency suggests that the NL3R704C mutation may alter presynaptic release probability, excitatory synapse number or postsynaptic AMPA receptor responses.
Figure 4.
Decreased frequency of spontaneous mEPSCs in NL3R704C-mutant mice. (A, D) Sample traces of mEPSCs (A) and mIPSCs (D). (B, E) Cumulative probability plots of mEPSC (B) and mIPSC amplitudes (E). Insets display box plots of the amplitudes (numbers list number of cells recorded; mEPSC: WT=11 cells/4 mice; R704C=11 cells/4 mice; mIPSC: WT=13 cells/4 mice and R704C=11 cells/4 mice). (C, F) Cumulative probability plots of mEPSC (C) and mIPSC frequency, as measured by the inter-event intervals (F). Insets display box plots of the actual frequencies (n=same as for (B, E)). Data represent mean values±s.e.m. Statistical significance (*P<0.05) was evaluated with a KS-test (cumulative probability plots) and Student's t-test (box plots). Box plots represent median and inter-quartile range; vertical lines represent 10th and 90th percentiles.
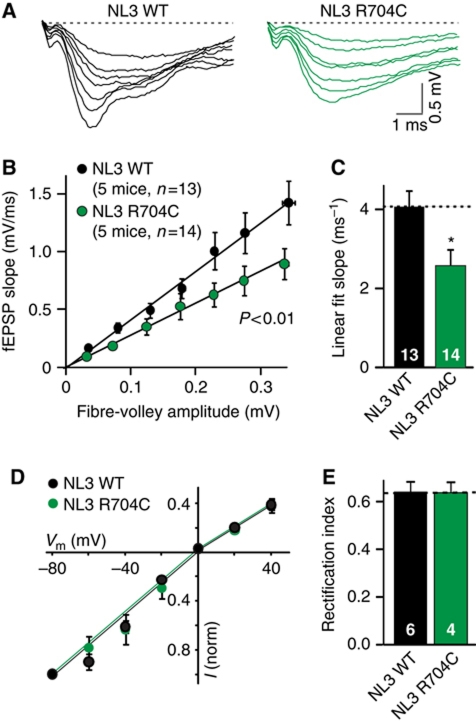
To further explore the possibility that the NL3R704C mutation causes a deficit in excitatory synaptic transmission, we measured excitatory synaptic strength by performing extracellular field recordings in the CA1 region of the hippocampus. In this assay, the slope of the postsynaptic fEPSP is measured as a function of the presynaptic fibre-volley amplitude (Figure 5A and B). Consistent with our mEPSC findings, the NL3R704C mutation produced a significant decrease in excitatory synaptic strength in the input–output experiments, as confirmed by measuring the slope of the linear fit for individual input–output experiments (Figure 5C). It is possible that the observed deficits are the result of changes in synaptic AMPA receptor subunit composition (the principal mediators of the synaptic response measured in Figure 5A–C). To exclude this possibility, we analysed the voltage dependence of synaptic AMPA receptor-mediated responses, but detected no change in the AMPA receptor rectification index (Figure 5D and E).
Figure 5.
NL3R704C mutation impairs AMPA receptor-mediated synaptic responses. (A–C) Sample traces (A), summary plots (B) and summary graph of the linear fit slopes (C) for input–output measurements obtained by extracellular field recordings in acute hippocampal slices from wild-type and NL3R704C-mutant mice (WT=13 slices/5 mice and R704C=14 slices/5 mice). (D) Current–voltage plot (I/V curve) for AMPA receptor-mediated synaptic responses. (E) AMPA receptor rectification index plotted as current at +40 mV relative to current at −40 mV (WT=6 cells/2 mice and R704C=4 cells/2 mice). Data are mean values±s.e.m. Statistical significance (*P<0.05) was evaluated by one-way ANOVA (B) or Student's t-test (C).
One potential explanation for the observed decreases in mEPSCs and input–output relations in NL3R704C-mutant mice is that the presynaptic release probability is altered in NL3R704C-mutant mice. Although this explanation was previously suggested for NL1 (Futai et al, 2007), it would be difficult to reconcile with the postsynaptic localization of NL3. Two different tests of release probability, measurements of paired-pulse facilitation and of the use-dependent block of NMDA receptor responses by MK-801, failed to detect a change in NL3R704C-mutant mice (Figure 6). Thus, the deficit in excitatory synaptic transmission in NL3R704C-mutant mice is not a consequence of changes in presynaptic release probability.
Figure 6.
Normal presynaptic release probability in NL3R704C-mutant mice. (A, B) Sample traces (A) and summary graphs (B) of paired-pulse facilitation measurements obtained with 50 and 80 ms inter-stimulus (numbers list number of cells recorded; WT=13 cells/3 mice and R704C=11 cells/3 mice). (C, D) Sample traces (C) and summary graph (D) obtained during measurements of the presynaptic release probability using the progressive block of NMDA receptor-mediated responses by MK-801. NMDA receptor EPSCs were monitored at +40 mV before and after addition of MK-801 (25 μM); traces in (C) depict the 1st, 10th and 50th NMDA receptor EPSC following MK-801 application. Experiments were performed in three parts: stable NMDA receptor-mediated EPSCs induced by 0.1 Hz stimulation were established; stimuli were stopped as MK-801 was bath applied for 8 min; stimuli were resumed. The weighted τ of the NMDA receptor response decay was calculated using a double exponential function: A1exp(−t/τ1)+A2exp(−t/τ2) (WT=9 cells/4 mice and R704C=7 cells/4 mice). Data represent mean values±s.e.m. Statistical significance was excluded using Student's t-test.
It is puzzling that the NL3R704C-mutant mice exhibit a decrease in excitatory mini frequency (Figure 4) and a decrease in evoked synaptic strength (Figure 5A–C), but no change in total excitatory synapse numbers as assessed by presynaptic staining for VGlut1 (Figure 3), because the first two observations would best be explained by a loss in synapse numbers, which is ruled out by the third observation. The limitation of staining for vGlut1 as a presynaptic marker for excitatory synapses, however, is that vGlut1 staining does not reveal whether excitatory synapses are functional. Thus, it is possible that the NL3R704C mutation decreases the number of mature, AMPA receptor containing excitatory synapses without dramatically affecting total excitatory synapse numbers.
To address this question, we measured the relative ratio of NMDA versus AMPA receptor-mediated synaptic responses using whole-cell recordings in acute hippocampal slices (Figure 7A). Consistent with the hypothesis that the NL3R704C mutation causes a selective reduction in AMPA but not in NMDA receptor-mediated synaptic transmission, the NMDA/AMPA ratio was increased in NL3R704C-mutant mice (Figure 7B). In agreement with a lack of an effect on NMDA receptor-mediated synaptic transmission by the NL3R704C mutation, NMDA receptor-dependent long-term potentiation was unchanged in NL3R704C-mutant mice (Figure 8).
Figure 7.
Increased ratio of NMDA to AMPA receptor-mediated EPSC in NL3R704C-mutant mice. (A, B) Sample traces (A) and summary graph (B) of measurements of the ratio of NMDA versus AMPA receptor-mediated synaptic responses monitored in slices. The NMDA/AMPA ratio was determined by sequentially evaluating EPSC amplitudes at −70 mV (AMPA) and at +40 mV (NMDA) holding potential; NMDA receptor-mediated responses were measured at 50 ms post-stimulus (WT=12 cells/3 mice and R704C=13 cells/3 mice). Data represent mean values±s.e.m. (*P<0.05 by Student's t-test).
Figure 8.
Normal hippocampal LTP in NL3R704C-mutant mice. (A) Sample traces for extracellular field EPSP recordings performed before (1) and 60 min after (2) LTP induction with three 1 s stimulus trains at 100 Hz. (B) Summary graph for LTP experiments performed in wild-type and NL3R704C-mutant mice (WT=9 slices/5 mice and R704C=10 cells/5 mice). (C) Summary graph for the magnitude of LTP at 55–60 min post-induction. Data represent mean values±s.e.m.
Cultured neurons from NL3R704C KI mice
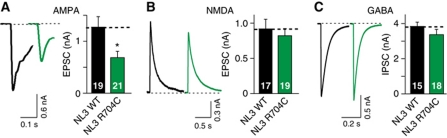
The slice physiology results provide evidence for a selective change in excitatory synapse function in NL3R704C-mutant mice, and suggest a specific impairment in AMPA but not in NMDA receptor-mediated responses. To quantitatively assess this phenotype in a reduced system, we measured synaptic responses in cultured hippocampal neurons (Maximov et al, 2007). Strikingly, cultured hippocampal neurons from NL3R704C-mutant mice displayed a major, selective decrease in AMPA receptor-mediated responses, without a change in NMDA receptor-mediated glutamatergic responses or GABA receptor-mediated inhibitory responses (Figure 9A–C). Thus, the R704C mutation specifically reduced postsynaptic AMPA receptor function in cultured neurons, consistent with the observations obtained in acute slices.
Figure 9.
Decreased AMPA receptor-mediated synaptic responses in cultured neurons from NL3R704C-mutant mice. (A–C) Sample traces (left) and summary graphs (right) of AMPA (A), NMDA (B) and GABA receptor- (C) mediated synaptic responses recorded in cultured hippocampal neurons (AMPA: WT=19 cells/3 cultures, R704C=21 cells/3 cultures; NMDA: WT=17 cells/3 cultures, R704C=19 cells/3 cultures; GABA: WT=15 cells/3 cultures and R704C=18 cells/3 cultures). Data shown are absolute postsynaptic current amplitudes as recorded using the approach of Maximov et al (2007). Data represent mean values±s.e.m.; statistical significance (*P<0.05) was evaluated by Student's t-test.
Discussion
Our data show that a single amino-acid substitution in a conserved cytoplasmic residue of NL3, R704C, has no significant effect on its synaptogenic activity in vitro, but dramatically alters excitatory synapse function in vivo. Specifically, we show that KI mice carrying the NL3R704C mutation exhibit a discrete and selective impairment of AMPA receptor-mediated synaptic transmission in hippocampal pyramidal neurons. As measured by multiple independent approaches (intracellular recordings of spontaneous synaptic responses in acute slices, extracellular recordings of input/output curves in acute slices and intracellular recordings of evoked responses in cultured neurons; Figures 4, 5 and 9), the decrease in synaptic strength in NL3R704C-mutant synapses is selective for AMPA receptor-mediated excitatory transmission since we observed no change in NMDA receptor-mediated responses or in GABA receptor-mediated synaptic transmission. As a result, the NMDA/AMPA receptor EPSC ratio is increased (Figure 7).
We observed no change in NMDA receptor protein levels (Figure 2), synapse numbers (Figure 3) or presynaptic release probability (Figure 6), but did observe a significant, possibly compensatory increase in the levels of GluR1 and GluR3 AMPA receptor subunits (Figure 2). The magnitude of the NL3R704C-mutant phenotype (40–50% decrease in AMPA receptor-mediated responses), and the fact that the R704C-mutant phenotype was consistently observed by multiple approaches and retained in cultured neurons, shows that it is robust. Thus, a single amino-acid substitution in the NL3 cytoplasmic tail caused a major effect on AMPA receptor-mediated synaptic transmission in the hippocampus. The changes we describe here are different from those previously observed in NL3R451C-mtuant and in NL3 KO mice (Tabuchi et al, 2007; Etherton et al, unpublished), suggesting a specific effect of different NL3 mutations on synaptic properties.
The most plausible hypothesis to account for the phenotype of the NL3R704C mutation is that in NL3R704C-mutant mice, a significant subset of synapses contain no, or only few, functional postsynaptic AMPA receptors, but maintain normal postsynaptic NMDA receptors. Although this hypothesis would explain our observations, alternative hypotheses cannot be ruled out. For example, it is possible that the NL3R704C mutation leads to selective elimination of a small number of synapses that are particularly active, such that no synapse loss would be apparent in our overall synapse density measurements, but could still have occurred and produced the phenotypic changes we observe. Our results do, however, exclude other alternative hypotheses. The NL3R704C mutation clearly does not decrease AMPA receptor-mediated responses uniformly in all synapses, since there was no significant change in mini amplitude, and the AMPA receptor subunit protein levels were increased, not decreased. Similarly, the NL3R704C mutation does not retrogradely suppress presynaptic release, since we observed no change in release probability, as assessed by two independent measures.
Although our experiments provide a detailed electrophysiological definition of the NL3R704C-mutant phenotype, we have not been able to define its molecular mechanism beyond the fact that it is due to a single point mutation. NL3 was not previously linked to the regulation of AMPA receptors, although NL1 and NL2 have been shown to profoundly modulate NMDA and GABA receptor-mediated synaptic transmission, respectively (Chubykin et al, 2007; Kim et al, 2008b; Gibson et al, 2009; Poulopoulos et al, 2009). What is potentially fascinating about the current findings are their implications regarding the function of the cytoplasmic tails of neuroligins. No binding partners for the mutated R704 sequence are known, and no trafficking deficit for NL3R704C-mutant protein was detected here (Figure 1). Given the mutant phenotype, a change in the interaction of NL3 with PSD-95 would have been interesting, but the R704 residue is too far away from the PDZ-binding motif of NL3 to alter PSD-95 binding, as confirmed in immunoprecipitation experiments (Figure 1B). It thus appears likely that the NL3R704C mutation alters the interaction of the conserved sequence motif containing the R704 residue with an as yet unidentified adaptor protein. Identifying this adaptor will be an important challenge that is made difficult by the juxtamembranous location and short length of the sequence motif.
A major motivation for the present analysis of the NL3R704C mutation was that the analogous mutation was observed in NL4 in an autism patient (Yan et al, 2005), and represents the only autism-associated mutation in a neuroligin cytoplasmic sequence. Although the R704C mutation involves a conserved residue present in all mammalian neuroligins, and is even retained in a Drosophila neuroligin (Figure 1A), its possible pathogenicity was questionable, since amino-acid sequence polymorphisms are not infrequently observed in proteins. We chose NL3 instead of NL4 to analyse the functional effect of the R704C mutation because NL4 is poorly conserved in mice (Bolliger et al, 2008), expressed at vanishingly low levels (Varoqueaux et al, 2006), and has no known synaptic function. NL3, on the other hand, is highly conserved and abundant in mice, and clearly associated with synaptic function (Tabuchi et al, 2007). Although our data do not directly show that the R704C mutation would have a functional effect in human NL4, the conservation of the residue involved and the dramatic alterations induced by the R704C mutation in NL3 function strongly suggest that the human NL4 R704C mutation also alters synaptic function in humans. Since different neuroligin isoforms perform distinct synaptic functions—at least in mice (Chubykin et al, 2007; Gibson et al, 2009)—the actual effect of the R704C mutation on NL4 is difficult to predict, but the probable existence of such an effect supports the overall notion that mutations in NL4 cause autism by altering synaptic transmission, a hypothesis that we have referred to as the synaptic hypothesis of autism (Südhof, 2008).
Materials and methods
Antibodies
Monoclonal GFP antibody (clone 3E6) was purchased from Invitrogen, Eugene, OR, USA; all other antibodies were described previously (Chubykin et al, 2005; Tabuchi et al, 2007).
In vitro function assays
Artificial synapse formation assays. Artificial synapse formation assays were performed with COS-7 cells as described (Ko et al, 2009). Briefly, COS-7 cells were transfected with FuGene-6 (Roche) with expression vectors encoding mVenus fusion proteins of wild-type or R704C-mutant NL3, or with mVenus alone (as a negative control). After 24 h, transfected COS-7 cells were trypsinized, added onto hippocampal neurons at DIV9, further co-cultured for 48 h, and immunostained with GFP and synapsin antibodies at DIV11. All images were acquired by confocal microscopy. For quantitative analyses, the contours of the transfected COS-7 cells were selected as the region of interest. Fluorescence intensity of synapsin puncta normalized to each COS-7 cell area was quantified for both red and green channels with MetaMorph (Molecular Devices). Statistical significance was determined by Student's t-test and all the data were expressed as the mean values±s.e.m.
Neuronal transfection assays. Primary rat hippocampal neuron cultures were prepared from E18 (embryonic 18 days) embryos as described (Ko et al, 2009). Neurons were transfected with expression vectors encoding mVenus fusion proteins of wild-type or R704C-mutant NL3, or with mVenus alone (as a negative control) at DIV10, and immunostained at DIV14 with synapsin (E028; 1:500) and EGFP antibodies (1:300). The neurons were fixed with 4% paraformaldehyde/4% sucrose for 10 min at room temperature, permeabilized with 0.2% Triton X-100 in phosphate-buffered saline (PBS) for 5 min at 4°C, blocked with 3% horse serum/0.1% crystalline grade BSA in PBS for 30 min at room temperature, and incubated with the respective primary and secondary antibodies in blocking solution for 1 h at room temperature, respectively. Transfected neurons were randomly chosen and imaged using a confocal microscope (LSM510, Zeiss); all the image settings were kept constant. Z-stacked images were maximally projected and analysed using MetaMorph (Molecular Devices) with area size and density of spines and presynaptic terminals per 50 μm of dendrite. To quantify the synaptic puncta size, we thresholded all images equally and measured the average pixel intensities along the dendritic segments in the transfected neurons by manually tracing each punctum.
Generation of NL3R704C KI mice
A 11.3-kb mouse genomic clone encompassing exons 6–8 of the NL3 gene was isolated from a 129/SvJ mouse genomic library and subcloned into NotI site of pBluescriptII KS (−) for targeting vector construction. The nucleotide sequence encoding R704 in exon 8 was mutated to encode C704. A neomycin-resistance cassette (for positive selection) surrounded by FRT sites (flp-recombinase recognition sequences for removal of the neo cassette) was inserted into the HindIII site in the intron 3′ of exon 7. Since the neomycin-resistance cassette was lifted from the vector for the R451C-knock-in mice (Tabuchi et al, 2007), it contained a loxP site that had no use in the present mouse. A diphtheria toxin gene cassette was attached to the 5′ end of the vector for negative selection. Embryonic R1 stem cells were electroporated with the targeting vector, and cell clones resistant to positive and negative selection were screened by Southern analysis using a 5′ outside probe to detect a size shift by BglII digestion. Homologously recombined clones were injected into blastocysts of C57/BL6 mice to generate chimeric mice. Germline transmission was monitored by PCR using oligonucleotide primers KT06470 (5′-GGCGATTTCACCTGCCTACAG-3′) and KT06471 (5′-TTGGGAGTCATGAGTGGGATG-3′) to detect the point mutation. The neomycin-resistance gene cassette was removed by flip recombination by crossing the initial mutant mice with flip transgenic mice. Genotyping was performed by PCR using oligonucleotide primers: KT06470 (5′-GGCGATTTCACCTGCCTACAG-3′) and KT06471 (5′-TTGGGAGTCATGAGTGGGATG-3′) to detect the point mutation (conditions: 1 × 93°C 10 min, 40 × 93°C 30 s, 75°C 45 s, 65°C 3 min and 1 × 65°C 10 min).
Biochemical measurements
Protein levels were quantified in total brain homogenates from four pairs of adult male mice using quantitative immunoblotting as described previously (Tabuchi et al, 2007). Signals were detected with iodinated secondary antibodies, and monitored with a phosphoimager. Levels were normalized for the signals of control proteins (GDI and β-actin).
Morphometric analyses
Morphometric analyses were performed as described previously (Tabuchi et al, 2007). Male KI and wild-type littermate control mice were anesthetized and perfusion fixed with 4% fresh paraformaldehyde in 100 mM phosphate buffer (pH 7.4). Brains were removed and immersion fixed for 4 h in the same fixative and cryoprotected with 30% sucrose in PBS for 2 days at 4°C. In all, 30 μm serial parasagittal sections were cut on a cryomicrotome, and floated and washed in PBS. For immunostaining, sections were blocked with 3% goat serum/0.3% Triton X-100 in PBS for 1 h at room temperature, and incubated with anti-synaptophysin monoclonal antibody (Millipore, Billerica, MA, USA) at 1:500 dilution, anti-vesicular glutamate transporter 1 (vGlut1) monoclonal antibody (Synaptic Systems, Göttingen, Germany) at 1:1000 dilution, and/or anti-vesicular GABA transporter (VGAT) polyclonal antibody (Millipore) at 1:500 dilution overnight at 4°C, followed by incubation with Alexa Fluor 488 or 633 goat anti-mouse IgG (Invitrogen) at 1:1000 dilution. Sections were transferred onto SuperFrost slides and mounted under glass coverslips with Vectashield with 4′, 6′-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA, USA). Sections of the CA1 and CA3 subfields of the hippocampus were imaged with a Leica TCS2 laser-scanning confocal microscope (Leica Microsystems, Wetzlar, Germany) at × 63, and the stratum radiatum layers were magnified five-fold. For each experimental series, all images were acquired with identical settings for laser power, photomultiplier gain and offset with a pinhole diameter. Images were imported into ImageJ 1.41 software and converted into binary data for morphometric analysis. Synaptic densities and sizes were analysed under fixed thresholds across all slides. Thresholds were chosen within the range that allowed outlining as many immunopositive puncta as possible throughout all images. The number and size of puncta were detected using the ‘analyse particle’ module of the program. The average number and size of puncta were normalized with data from wild-type to determine synaptic density and size, respectively. Statistical significance was determined by Student's t-test.
Neuronal culture
Primary hippocampal neurons were isolated from newborn litters of wild-type and NL3R704C mice and cultured as described (Maximov et al, 2007, 2009; Xu et al, 2009). Briefly, hippocampi were isolated and dissociated by papain digestion (10 U/ml, with 1 mM CaCl2 and 0.5 mM EDTA). Dissociated neurons were plated on Matrigel-coated glass coverslips. Culture media contained MEM (Invitrogen) fortified with B27 (Invitrogen), transferrin, fetal bovine serum, AraC (Sigma) and glucose. Neurons were cultured for 14–16 days before experiments. Following neuronal culture, pups were genotyped and only wild-type and NL3R704C cultures were used for experiments.
Slice electrophysiology
Electrophysiological recordings were performed essentially as described (Tabuchi et al, 2007; Etherton et al, 2009). P28-P40 mouse brains were removed and immediately immersed in ice-cold dissection solution (in mM: 222 sucrose; 11 glucose; 26 NaHCO3; 1 NaH2PO4; 3 KCl; 7 MgCl2; 0.5 CaCl2). The chilled brain was then bisected and each hemisphere blocked to appropriately arrange the hippocampus for transverse hippocampal slice collection. Acute hippocampal slices were collected at specific thicknesses for extracellular (0.4 mm) and whole-cell (0.3 mm) recordings. Hippocampal slices were made using a Leica VT 1200S, transferred to a recovery chamber containing modified artificial cerebrospinal fluid (ACSF) (in mM: 126 NaCl; 3 KCl; 1.25 NaH2PO4; 26 NaHCO3; 10 glucose; 2.5 CaCl2; and 1.3 MgCl2 saturated with 95% O2/5% CO2, pH 7.4), and were allowed to recover first at 32.5°C for 30 min and then at room temperature for 1 h. All recordings were done with ACSF at 28–30°C except where mentioned otherwise.
For extracellular field recordings, patch pipettes (2–4 MΩ) were filled with different internal solutions for input–output analysis (ACSF) and LTP (1 M NaCl, 10 mM HEPES, pH 7.4). Slices were placed in the recording chamber and allowed to recover for 10 min before recordings. A stimulus electrode was placed in the stratum radiatum, and the recording electrode was placed nearby. Input–output measurements were performed as described previously (Etherton et al, 2009). Five to ten traces were averaged at each stimulation intensity and the amplitude of the presynaptic fibre-volley was measured relative to the slope of the fEPSP. The stimulation rate was 0.1 Hz. The average linear fit slope was calculated as the slope of the linear input–output relationship for each slice. LTP experiments were performed as described previously, with modifications (Kaeser et al, 2008). For LTP experiments, recordings were performed at room temperature. A stable baseline response was established at 30–40% of the maximal response. Baseline responses were collected for at least 15 min. If the fEPSP slope changed >10% during the baseline period, recordings were discontinued. LTP was induced with three 1 s 100 Hz stimulus trains separated by 20 s intervals. fEPSPs were monitored at 0.05 Hz. LTP was then monitored for 60 min post-induction. The magnitude of LTP was normalized to the average slope of the fEPSP during the baseline period. In all, 50 μM picrotoxin (Sigma) was included in ACSF for all recordings.
For whole-cell voltage-clamp recordings in acute hippocampal slices, patch pipettes (2–4 MΩ) were filled with excitatory specific (in mM: 117.5 CsMeSO4, 10 HEPES, 10 TEA-Cl, 15.5 CsCl, 1 MgCl2, 10 Na-phosphocreatine, 8 NaCl, 0.3 NaGTP, 4 MgATP, 5 EGTA and 1 QX-314) or inhibitory-specific internal solutions (in mM: 120 CsCl, 10 HEPES, 5 NaCl, 1 MgCl2, 0.3 NaGTP, 3 MgATP, 10 EGTA and 5 QX-314). For all excitatory and inhibitory recordings, 50 μM picrotoxin or 10 μM NBQX (Tocris) and 50 μM AP5 (Tocris) were included in the ACSF, respectively.
mEPSC and mIPSC recordings were performed with CA1 pyramidal neurons voltage clamped at −70 mV. In all, 0.5 μM TTX (Tocris) was included in ACSF to block action potential evoked responses. For each experiment, 10 traces of 30 s duration were collected with a −5 mV voltage step between each trace to monitor membrane statistics. Cumulative probability plots were constructed using 200 events. Miniature amplitude threshold was set at 6 pA. The Kolmogorov–Smirnov test was used for measuring statistical significance for the cumulative probability plot. For box plots, statistical significance was assessed with an unpaired Student's t-test.
NMDA/AMPA receptor EPSC analysis was performed in the presence of 50 μM picrotoxin. Evoked EPSCs were collected at two holding potentials. At −70 mV, responses were collected and the peak amplitude identified as the AMPA receptor-mediated response. Cells were then voltage clamped at +40 mV, and the amplitude of the evoked EPSC 50 ms post-stimulus was identified as the NMDA-R-mediated response. Twenty traces were collected at 0.1 Hz for each membrane potential.
Paired-pulse facilitation experiments were performed at −70 mV holding potential. The amplitude of the second EPSC was measured relative to the amplitude of the first EPSC. PPF was measured at two inter-stimulus intervals, 50 and 80 ms. Twenty traces were collected for each inter-stimulus interval. MK-801 experiments were performed at +40 mV holding potential as described previously (Schoch et al, 2002); in the presence of 50 μM picrotoxin and 10 μM NBQX to isolate NMDA-R-mediated responses. Baseline responses were collected for 8 min before MK-801 wash-in. If baseline responses changed >20%, experiments were terminated. Once a stable baseline was achieved, MK-801 was washed into the recording chamber. MK-801 was perfused into the chamber for at least 6 min without stimulation. After 6 min, stimulation was resumed and the rate of the NMDA-R EPSC block was monitored. The time constant of decay was calculated using a double exponential function: A1exp(−t/τ1)+A2exp(−t/τ2) (Wasling et al, 2004).
AMPA-R-mediated current–voltage experiments were conducted with 50 μM picrotoxin and 50 μM AP5 in the ACSF and 0.1 mM spermine added to the internal solution. Six to eight traces were collected and averaged at each holding potential. Summary graph was constructed by normalizing all values to the AMPA-R EPSC at −80 mV. Rectification index was calculated as the AMPA-R-mediated response at +40/−40 mV.
For all whole-cell recordings, membrane statistics were monitored after each trace. All evoked whole-cell recordings were collected at 0.1 Hz. Whole-cell recording criteria were as follows: Ra was <25 MΩ and cells were rejected if Ra or Rm changed >20% over the course of the experiment. All recordings were digitized at 10 kHz and filtered at 2 kHz. Recordings were monitored with a Multiclamp 700B (Molecular Devices) and analysed offline using pClamp (Molecular Devices). Statistical significance of data was evaluated using a Student's t-test. For cumulative probability plots, a Kolmogorov–Smirnov test with a significance of P<0.05 was used. All experiments were performed on male wild-type and NL3R704C littermate pairs. All recordings and analysis were done with experimenter blind to genotype.
Cell culture electrophysiology
Cell culture electrophysiology was done as described previously (Maximov et al, 2007, 2009; Xu et al, 2009; Zhang et al, 2009) and with similar criteria to the aforementioned acute slice electrophysiology. Briefly, recordings were performed on DIV14-16 hippocampal cultures. Evoked synaptic responses were triggered with a homemade bipolar electrode. The stimulus electrode was placed 150 μm from soma of patched neuron. Stimulus intensity was adjusted to achieve maximal synaptic responses for each cell. Cells were patched with a modified internal solution (in mM: 117.5 CsMeSO4, 10 HEPES, 10 TEA-Cl, 15.5 CsCl, 1 MgCl2, 10 Na-phosphocreatine, 8 NaCl, 0.3 NaGTP, 4 MgATP, 5 EGTA and 5 QX-314). Recordings were performed in modified ACSF (in mM: 126 NaCl; 3 KCl; 1.25 NaH2PO4; 26 NaHCO3; 10 glucose; 2 CaCl2; and 2 MgCl2 saturated with 95% O2/5% CO2, pH 7.4). After break-in, series resistance was compensated to 5–7 MΩ. AMPA-R-mediated EPSCs were isolated with 50 μM picrotoxin and 50 μM AP5. NMDA-R-mediated EPSCs were isolated with 50 μM picrotoxin and 10 μM NBQX. GABA-R-mediated IPSCs were isolated with 10 μM NBQX and 50 μM AP5. AMPA-R- and GABA-R-mediated evoked responses were recorded while holding the neuron at −70 mV. NMDA-R-mediated evoked responses were performed while holding the neuron at +40 mV. Statistical significance was calculated by an unpaired Student's t-test. Data represent mean values±s.e.m. Experimenter was blind to genotype throughout data collection and analysis.
Supplementary Material
Acknowledgments
This work was supported by grants from NIMH (R37 MH052804 to TCS) and the Simons Foundation (SF177850 to TCS), and by a long-term fellowship from International Human Frontier Science Program Organization (LT00021/2008-L to JK).
Author contribution: MRE, KT, MS and JK designed and performed the experiments, MRE and TCS analysed the data and wrote the paper; TCS conceived the project and organized its execution.
Footnotes
The authors declare that they have no conflict of interest.
References
- Arac D, Boucard AA, Ozkan E, Strop P, Newell E, Südhof TC, Brunger AT (2007) Structures of neuroligin-1 and the neuroligin-1/neurexin-1β complex reveal specific protein-protein and protein-Ca2+ interactions. Neuron 56: 992–1003 [DOI] [PubMed] [Google Scholar]
- Bolliger MF, Pei J, Maxeiner S, Boucard AA, Grishin NV, Südhof TC (2008) Unusually rapid evolution of neuroligin-4 in mice. Proc Natl Acad Sci USA 105: 6421–6426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucard AA, Chubykin AA, Comoletti D, Taylor P, Südhof TC (2005) A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to α- and β-neurexins. Neuron 48: 229–236 [DOI] [PubMed] [Google Scholar]
- Bucan M, Abrahams BS, Wang K, Glessner JT, Herman EI, Sonnenblick LI, Alvarez Retuerto AI, Imielinski M, Hadley D, Bradfield JP, Kim C, Gidaya NB, Lindquist I, Hutman T, Sigman M, Kustanovich V, Lajonchere CM, Singleton A, Kim J, Wassink TH et al. (2009) Genome-wide analyses of exonic copy number variants in a family-based study point to novel autism susceptibility genes. PLoS Genet 5: e1000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budreck EC, Scheiffele P (2007) Neuroligin-3 is a neuronal adhesion protein at GABAergic and glutamatergic synapses. Eur J Neurosci 26: 1738–1748 [DOI] [PubMed] [Google Scholar]
- Chih B, Gollan L, Scheiffele P (2006) Alternative splicing controls selective trans-synaptic interactions of the neuroligin-neurexin complex. Neuron 51: 171–178 [DOI] [PubMed] [Google Scholar]
- Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, Südhof TC (2007) Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron 54: 919–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubykin AA, Liu X, Comoletti D, Tsigelny I, Taylor P, Südhof TC (2005) Dissection of synapse induction by neuroligins: effect of a neuroligin mutation associated with autism. J Biol Chem 280: 22365–22374 [DOI] [PubMed] [Google Scholar]
- Comoletti D, De Jaco A, Jennings LL, Flynn RE, Gaietta G, Tsigelny I, Ellisman MH, Taylor P (2004) The Arg451Cys-neuroligin-3 mutation associated with autism reveals a defect in protein processing. J Neurosci 24: 4889–4893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoletti D, Flynn RE, Boucard AA, Demeler B, Schirf V, Shi J, Jennings LL, Newlin HR, Südhof TC, Taylor P (2006) Gene selection, alternative splicing, and post-translational processing regulate neuroligin selectivity for beta-neurexins. Biochemistry 45: 12816–12827 [DOI] [PubMed] [Google Scholar]
- De Jaco A, Lin MZ, Dubi N, Comoletti D, Miller M, Camp S, Ellisman M, Butko MT, Tsien RY, Taylor P (2010) Neuroligin trafficking deficiencies arising from mutations in the {alpha}/{beta}-hydrolase fold protein family. J Biol Chem 285: 28674–28682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton MR, Blaiss CA, Powell CM, Südhof TC (2009) Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proc Natl Acad Sci USA 106: 17998–18003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Schroer R, Yan J, Song W, Yang C, Bockholt A, Cook EH Jr, Skinner C, Schwartz CE, Sommer SS (2006) High frequency of neurexin 1β signal peptide structural variants in patients with autism. Neurosci Lett 409: 10–13 [DOI] [PubMed] [Google Scholar]
- Futai K, Kim MJ, Hashikawa T, Scheiffele P, Sheng M, Hayashi Y (2007) Retrograde modulation of presynaptic release probability through signaling mediated by PSD-95-neuroligin. Nat Neurosci 10: 186–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JR, Huber KM, Südhof TC (2009) Neuroligin-2 deletion selectively decreases inhibitory synaptic transmission originating from fast-spiking, but not from somatostatin-positive interneurons. J Neurosci 29: 13883–138897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glessner JT, Wang K, Cai G, Korvatska O, Kim CE, Wood S, Zhang H, Estes A, Brune CW, Bradfield JP, Imielinski M, Frackelton EC, Reichert J, Crawford EL, Munson J, Sleiman PM, Chiavacci R, Annaiah K, Thomas K, Hou C et al. (2009) Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature 459: 569–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf ER, Kang Y, Hauner AM, Craig AM (2006) Structure function and splice site analysis of the synaptogenic activity of the neurexin-1β LNS domain. J Neurosci 26: 4256–4265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM (2004) Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell 119: 1013–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Missler M, Moomaw C, Südhof TC (1995) Neuroligin 1: a splice site-specific ligand for beta-neurexins. Cell 81: 435–443 [DOI] [PubMed] [Google Scholar]
- Irie M, Hata Y, Takeuchi M, Ichtchenko K, Toyoda A, Hirao K, Takai Y, Rosahl TW, Südhof TC (1997) Binding of neuroligins to PSD-95. Science 277: 1511–1515 [DOI] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T (2003) Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet 34: 27–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser PS, Kwon HB, Blundell J, Chevaleyre V, Morishita W, Malenka RC, Powell CM, Castillo PE, Südhof TC (2008) RIM1alpha phosphorylation at serine-413 by protein kinase A is not required for presynaptic long-term plasticity or learning. Proc Natl Acad Sci USA 105: 14680–14685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HG, Kishikawa S, Higgins AW, Seong IS, Donovan DJ, Shen Y, Lally E, Weiss LA, Najm J, Kutsche K, Descartes M, Holt L, Braddock S, Troxell R, Kaplan L, Volkmar F, Klin A, Tsatsanis K, Harris DJ, Noens I et al. (2008a) Disruption of neurexin 1 associated with autism spectrum disorder. Am J Hum Genet 82: 199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jung SY, Lee YK, Park S, Choi JS, Lee CJ, Kim HS, Choi YB, Scheiffele P, Bailey CH, Kandel ER, Kim JH (2008b) Neuroligin-1 is required for normal expression of LTP and associative fear memory in the amygdala of adult animals. Proc Natl Acad Sci USA 105: 9087–9092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov G, Gumus D, Chen W, Norton N, Georgieva L, Sari M, O’Donovan MC, Erdogan F, Owen MJ, Ropers HH, Ullmann R (2008) Comparative genome hybridization suggests a role for NRXN1 and APBA2 in schizophrenia. Hum Mol Genet 17: 458–465 [DOI] [PubMed] [Google Scholar]
- Ko J, Zhang C, Arac D, Boucard AA, Brunger AT, Sudhof TC (2009) Neuroligin-1 performs neurexin-dependent and neurexin-independent functions in synapse validation. EMBO J 28: 3244–3255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumonnier F, Bonnet-Brilhault F, Gomot M, Blanc R, David A, Moizard MP, Raynaud M, Ronce N, Lemonnier E, Calvas P, Laudier B, Chelly J, Fryns JP, Ropers HH, Hamel BC, Andres C, Barthelemy C, Moraine C, Briault S (2004) X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am J Hum Genet 74: 552–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson-Yuen A, Saldivar JS, Sommer S, Picker J (2008) Familial deletion within NLGN4 associated with autism and Tourette syndrome. Eur J Hum Genet 16: 614–618 [DOI] [PubMed] [Google Scholar]
- Marshall CR, Noor A, Vincent JB, Lionel AC, Feuk L, Skaug J, Shago M, Moessner R, Pinto D, Ren Y, Thiruvahindrapduram B, Fiebig A, Schreiber S, Friedman J, Ketelaars CE, Vos YJ, Ficicioglu C, Kirkpatrick S, Nicolson R, Sloman L et al. (2008) Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet 82: 477–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximov A, Pang ZP, Tervo DG, Südhof TC (2007) Monitoring synaptic transmission in primary neuronal cultures using local extracellular stimulation. J Neurosci Methods 161: 75–87 [DOI] [PubMed] [Google Scholar]
- Maximov A, Tang J, Yang X, Pang ZP, Südhof TC (2009) Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science 323: 516–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K, Südhof TC (2003) α-Neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature 423: 939–948 [DOI] [PubMed] [Google Scholar]
- Poulopoulos A, Aramuni G, Meyer G, Soykan T, Hoon M, Papadopoulos T, Zhang M, Paarmann I, Fuchs C, Harvey K, Jedlicka P, Schwarzacher SW, Betz H, Harvey RJ, Brose N, Zhang W, Varoqueaux F (2009) Neuroligin 2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin. Neuron 63: 628–642 [DOI] [PubMed] [Google Scholar]
- Rujescu D, Ingason A, Cichon S, Pietiläinen OP, Barnes MR, Toulopoulou T, Picchioni M, Vassos E, Ettinger U, Bramon E, Murray R, Ruggeri M, Tosato S, Bonetto C, Steinberg S, Sigurdsson E, Sigmundsson T, Petursson H, Gylfason A, Olason PI et al. (2009) Disruption of the neurexin 1 gene is associated with schizophrenia. Hum Mol Genet 18: 988–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T (2000) Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell 101: 657–669 [DOI] [PubMed] [Google Scholar]
- Schoch S, Castillo PE, Jo T, Mukherjee K, Geppert M, Wang Y, Schmitz F, Malenka RC, Südhof TC (2002) RIM1alpha forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature 415: 321–326 [DOI] [PubMed] [Google Scholar]
- Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, Yamrom B, Yoon S, Krasnitz A, Kendall J, Leotta A, Pai D, Zhang R, Lee YH, Hicks J, Spence SJ, Lee AT, Puura K, Lehtimäki T, Ledbetter D et al. (2007) Strong association of de novo copy number mutations with autism. Science 316: 445–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JY, Ichtchenko K, Südhof TC, Brose N (1999) Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci USA 96: 1100–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC (2008) Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455: 903–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari P, Paterson AD, Zwaigenbaum L, Roberts W, Brian J, Liu XQ, Vincent JB, Skaug JL, Thompson AP, Senman L, Feuk L, Qian C, Bryson SE, Jones MB, Marshall CR, Scherer SW, Vieland VJ, Bartlett C, Mangin LV, Goedken R et al. (2007) Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet 39: 319–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, Südhof TC (2007) A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science 318: 71–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talebizadeh Z, Lam DY, Theodoro MF, Bittel DC, Lushington GH, Butler MG (2006) Novel splice isoforms for NLGN3 and NLGN4 with possible implications in autism. J Med Genet 43: e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich B, Ushkaryov YA, Südhof TC (1995) Cartography of neurexins: more than 1000 isoforms generated by alternative splicing and expressed in distinct subsets of neurons. Neuron 14: 497–507 [DOI] [PubMed] [Google Scholar]
- Ushkaryov YA, Petrenko AG, Geppert M, Südhof TC (1992) Neurexins: synaptic cell surface proteins related to the alpha-latrotoxin receptor and laminin. Science 257: 50–56 [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Südhof TC, Brose N (2006) Neuroligins determine synapse maturation and function. Neuron 51: 741–754 [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Jamain S, Brose N (2004) Neuroligin 2 is exclusively localized to inhibitory synapses. Eur J Cell Biol 83: 449–456 [DOI] [PubMed] [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, Nord AS, Kusenda M, Malhotra D, Bhandari A, Stray SM, Rippey CF, Roccanova P, Makarov V, Lakshmi B, Findling RL, Sikich L, Stromberg T, Merriman B, Gogtay N et al. (2008) Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science 320: 539–543 [DOI] [PubMed] [Google Scholar]
- Wasling P, Hanse E, Gustafsson B (2004) Developmental changes in release properties of the CA3-CA1 glutamate synapse in rat hippocampus. J Neurophysiol 92: 2714–2724 [DOI] [PubMed] [Google Scholar]
- Xu J, Pang ZP, Shin OH, Südhof TC (2009) Synaptotagmin-1 functions as a Ca2+ sensor for spontaneous release. Nat Neurosci 12: 759–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Noltner K, Feng J, Li W, Schroer R, Skinner C, Zeng W, Schwartz CE, Sommer SS (2008) Neurexin 1α structural variants associated with autism. Neurosci Lett 438: 368–370 [DOI] [PubMed] [Google Scholar]
- Yan J, Oliveira G, Coutinho A, Yang C, Feng J, Katz C, Sram J, Bockholt A, Jones IR, Craddock N, Cook EH Jr, Vicente A, Sommer SS (2005) Analysis of the neuroligin 3 and 4 genes in autism and other neuropsychiatric patients. Mol Psychiatry 10: 329–332 [DOI] [PubMed] [Google Scholar]
- Zahir FR, Baross A, Delaney AD, Eydoux P, Fernandes ND, Pugh T, Marra MA, Friedman JM (2008) A patient with vertebral, cognitive and behavioural abnormalities and a de novo deletion of NRXN1α. J Med Genet 45: 239–243 [DOI] [PubMed] [Google Scholar]
- Zhang C, Milunsky JM, Newton S, Ko J, Zhao G, Maher TA, Tager-Flusberg H, Bolliger MF, Carter AS, Boucard AA, Powell CM, Südhof TC (2009) A neuroligin-4 missense mutation associated with autism impairs neuroligin-4 folding and endoplasmic reticulum export. J Neurosci 29: 10843–10854 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.