Abstract
In Escherichia coli, the essential motor protein Rho promotes transcription termination in a tightly controlled manner that is not fully understood. Here, we show that the general post-transcriptional regulatory protein Hfq associates with Rho to regulate Rho function. The Hfq:Rho complex can be further stabilized by RNA bridging both factors in a configuration that inhibits the ATP hydrolysis and duplex unwinding activities of Rho and that mediates transcription antitermination at Rho-dependent terminators in vitro and in vivo. Antitermination at a prototypical terminator (λtR1) requires Hfq binding to an A/U-rich transcript region directly upstream from the terminator. Antitermination is modulated by trans-acting factors (NusG or nucleic acid competitors) that affect Hfq association with Rho or RNA. These data unveil a new Hfq function and a novel transcription regulatory mechanism with potentially important implications for bacterial RNA metabolism, gene silencing, and pathogenicity.
Keywords: hexameric helicase, RNA chaperone, transcription termination
Introduction
RNA biosynthesis is a fundamental process that is often regulated during the various phases of transcription as a function of other RNA metabolism transactions. In Escherichia coli, for instance, the synchronized translation of mRNAs’ open reading frames (ORFs) by ribosomes during their synthesis by RNA polymerase (RNAP) protects the transcription machinery from destabilization by Rho (Ciampi, 2006; Roberts et al, 2008; Rabhi et al, 2010), an essential (Das et al, 1976; Cardinale et al, 2008) and abundant (∼103 hexamers/cell; Geiselmann et al, 1992) ring-shaped, homo-hexameric protein motor (Figure 1A). Rho has generic ATP-dependent [5′ → 3′] RNA translocation, duplex unwinding, and protein displacement activities that it most likely uses to disrupt transcription elongation complexes at untranslated loci of the bacterial genome, termed Rho-dependent terminators (reviewed in Ciampi, 2006; Roberts et al, 2008; Rabhi et al, 2010). An estimated one-fifth of E. coli's transcription units (encoding genes and non-coding RNAs) are subjected to Rho-dependent termination (Peters et al, 2009). Rho also attenuates gene expression by promoting intracistronic transcription termination whenever mutations or environmental conditions perturb the coupling of translation with transcription (Roberts et al, 2008).
Figure 1.
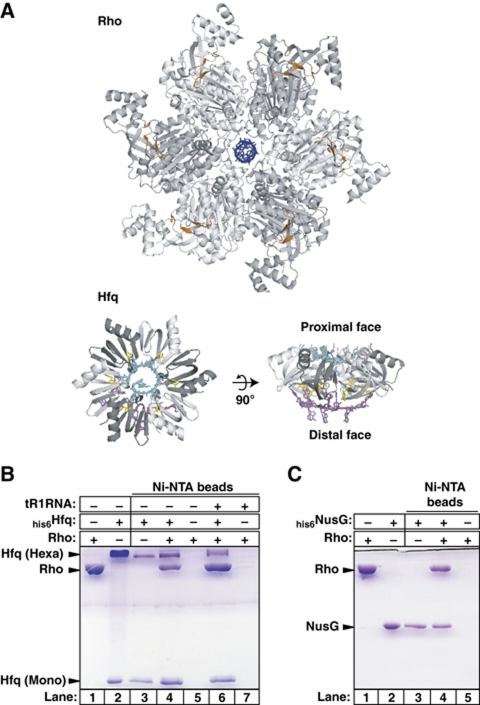
A direct Rho:Hfq interaction. (A) Same-scale structures of the Rho and Hfq hexamers. The r(UUUUUU) oligomer bound to the secondary binding site (SBS, which is strictly RNA specific) in the central channel of Rho (PDB #3ICE) is shown in blue. Residues composing the crown-like PBS of Rho are in orange. C-rich, 12–13 nt-long segments of RNA or DNA (PBS is not specific) are minimally required to span PBS clefts on adjacent Rho subunits (Skordalakes and Berger, 2003). The model of the Hfq hexamer has been built by structural alignment of two distinct crystal structures of RNA-bound Hfq (PDB #3GIB and #1KQ2) using the TopMatch server (http://topmatch.services.came.sbg.ac.at/). In the model, r(AUUUUUG) (in cyan) and r(A)9 (in magenta) oligomers are bound to the proximal and distal faces, respectively. The Tyr25 residues stacking on adenine bases are shown in yellow. Representative SDS–PAGE gels show Ni-NTA pull-down experiments with ‘bait’ his6Hfq (B) or his6NusG (C) and ‘prey’ Rho proteins. Note that Hfq hexamers are only partially denatured by SDS–PAGE (Carmichael et al, 1975; Arluison et al, 2002; Sukhodolets and Garges, 2003).
The classical model for Rho-dependent termination posits that Rho first binds to ∼70 nucleotide (nt)-long naked regions of nascent transcripts (i.e. RNA deprived of bound proteins or ribosomes), preferentially at unstructured C-rich/G-poor Rut (Rho utilization) sites (reviewed in Ciampi, 2006; Roberts et al, 2008; Rabhi et al, 2010). Then, Rho translocates towards the transcript's 3′-end in order to catch up with and dissociate RNAP with an efficiency that is thought to depend on the relative Rho and RNAP velocities (conditional ‘entry’ and kinetic competition model; Jin et al, 1992). Recent studies attempted to refine this model, notably by proposing that Rho is biased against expression of ‘foreign’ horizontally acquired genes in E. coli (Cardinale et al, 2008) or that Rho associates with RNAP throughout the transcription cycle (Epshtein et al, 2010). A tight Rho–RNAP association is supported indirectly by chromatin immunoprecipitation experiments (ChIP-chip assay), showing coincident RNAP and Rho distributions along transcription units (Mooney et al, 2009a). Such an association raises a question as to what regulates Rho outside ORFs (notably during transcription of long leader regions) when there is no ribosome trailing behind RNAP to increase its processivity (Proshkin et al, 2010) nor to prevent the mRNA from associating with Rho (Roberts et al, 2008; Rabhi et al, 2010). One possibility is that, as observed during transcription of ribosomal rrn operons or bacteriophage λ chromosome (reviewed in Condon et al, 1995; Roberts et al, 2008; Sen et al, 2008), accessory factors bind, in a conditional manner, to RNA and/or to the transcription machinery to form an antitermination complex resistant to Rho.
Antitermination complexes that resist to Rho-dependent termination signals within rrn and λ operons contain the NusA, NusB, NusE (ribosomal protein S10), and NusG factors as well as specific factors such as bacteriophage N protein for λN antitermination or ribosomal proteins (S2, S4, L1, L3, L4, and L13) and other unidentified cellular factor(s) for rrn antitermination (Condon et al, 1995; Torres et al, 2001; Roberts et al, 2008; Sen et al, 2008). Both rrn and λN antitermination complexes are stabilized by elaborate networks of interactions among their many components that include binding of a NusB–NusE heterodimer to a boxA RNA sequence found in the rrn and λ transcripts (Roberts et al, 2008; Sen et al, 2008) and binding of NusG to RNAP through its N-terminal domain (NusG-NTD) and to NusE through its C-terminal domain (NusG-CTD; Mooney et al, 2009b; Burmann et al, 2010). The bridge formed by NusG between RNAP and NusE (S10) could also connect physically the ribosome and RNAP during transcription of ORFs, thereby occluding the nascent RNA to Rho (Burmann et al, 2010). Thus, the interaction between NusE and NusG-CTD (Burmann et al, 2010) seems critical to protect the transcription machinery from Rho under various contexts. Importantly, NusG can also bind Rho through its CTD (Mooney et al, 2009b; Burmann et al, 2010) and stimulates Rho-dependent termination (Sullivan and Gottesman, 1992; Li et al, 1993; Burns et al, 1999). Regulatory plasticity is thus provided by NusG, which uses its NTD to bind RNAP and its CTD to bind Rho or NusE (S10) in a mutually exclusive manner (Mooney et al, 2009b; Burmann et al, 2010).
Although NusG is abundant in E. coli (∼104 molecules/cell; Li et al, 1993), ChIP-chip distributions suggest that it associates slowly with transcribing RNAPs (Mooney et al, 2009a). The NusG-binding site on RNAP-bound Rho (Epshtein et al, 2010) may thus be occupied by other factor(s), which could contribute to delay NusG association (Mooney et al, 2009a) and to repress Rho during transcription of 5′-leader mRNA regions. To test this hypothesis, we scrutinized putative Rho partners present in the E. coli ‘interactome’ that has been established by global tag affinity purification and mass spectrometry identification approaches (Butland et al, 2005; Hu et al, 2009) for features suggestive of physical or functional competition with NusG. One intriguing candidate is Hfq, a post-transcriptional regulator and virulence factor, which mediates gene silencing events by facilitating pairing of regulatory small non-coding RNAs (sRNAs) with their mRNA targets (reviewed in Gottesman et al, 2006; Brennan and Link, 2007; Chao and Vogel, 2010). Hfq has other roles in RNA metabolism and notably modulates the levels of several mRNAs, either by increasing their stability (Folichon et al, 2003) or through an unsolved transcriptional mechanism (Le Derout et al, 2010). In E. coli, Hfq is about as abundant as NusG (∼104 hexamers/cell) and is associated with components of the ribosome, RNase E, the nucleoid, and RNAP (reviewed in Sukhodolets and Garges, 2003; Butland et al, 2005; Brennan and Link, 2007; Hu et al, 2009). The Hfq protein forms stable, homo-hexameric rings having opposite polyA- and polyU-binding surfaces (Figure 1A; Brennan and Link, 2007; Link et al, 2009) and exhibits some topological similarities with NusG-CTD (see Supplementary data) and with YaeO (Gutierrez et al, 2007), the only cellular protein that, to our knowledge, is known to bind and inhibit Rho directly (Pichoff et al, 1998; Gutierrez et al, 2007).
Here, we show that Hfq associates stably with Rho and inhibits its ATP hydrolysis, duplex unwinding, and transcription termination activities. Consistent with these observations, transcription termination at the prototypical Rho-dependent λtR1 terminator is stronger in a hfq null mutant than in wild-type (WT) E. coli. In vitro, Hfq-mediated antitermination requires the interaction of Hfq with both Rho and RNA transcript and can be repressed by the addition of NusG, an A-rich oligomer, or an oligonucleotide that pairs with a specific region of the transcript upstream from the λtR1 Rut site. Our data unveil a new transcriptional antitermination mechanism that depends on trapping of the Rho–RNA complex into an inactive configuration by the bound Hfq. These data also reveal a complex interplay between key players of bacterial RNA metabolism and suggest that Rho-dependent transcription termination could contribute to sRNA-dependent gene silencing and/or control of virulence.
Results
Hfq forms a stable binary complex with Rho
Global analyses of E. coli's interaction network revealed that Rho and Hfq associate in the same protein complexe(s) in vivo (Butland et al, 2005; Hu et al, 2009). To determine if they can associate directly in the absence of other factors, we performed ‘pull-down’ trials on Ni-NTA agarose beads with purified Rho and hexahistidine-tagged Hfq (his6Hfq). We used a ‘low adsorption’ binding and washing procedure (see Materials and methods) whereby his6Hfq stably associates with the beads (Figure 1B, lane 3) whereas Rho alone does not (lane 5). When the beads, Rho, and his6Hfq are incubated together, a significant fraction of Rho is recovered with the beads (Figure 1B, lane 4). This fraction of Hfq-bound Rho was unaffected by the presence of RNase A or Benzonase nuclease in the mixture or if Rho and Hfq were incubated separately with the nucleases before their mixing (data not shown). This argues against a Rho:Hfq interaction being mediated by RNA contaminants (great care was also taken to avoid RNA contamination during preparation of Hfq and Rho; see Materials and methods). Moreover, poly-histidine did not promote Ni-NTA pull-down of Rho (not shown), excluding that Rho binds the his6-tag on Hfq. Thus, Rho and Hfq can form a genuine binary complex. Under the same experimental conditions, Rho also forms binary complexes with his6-tagged NusG (Figure 1C, lane 4) and YaeO (Supplementary Figure S1A), confirming previous observations (Pichoff et al, 1998; Kalarickal et al, 2010). Using fluorescence quenching titrations with Cy3-labelled Hfq (Supplementary Figure S2), we measured a Kd of 40 nM (25°C, pH 7, 100 mM NaCl) for the binary Rho:Hfq complex.
Hfq inhibits Rho-dependent transcription termination in vitro and in vivo
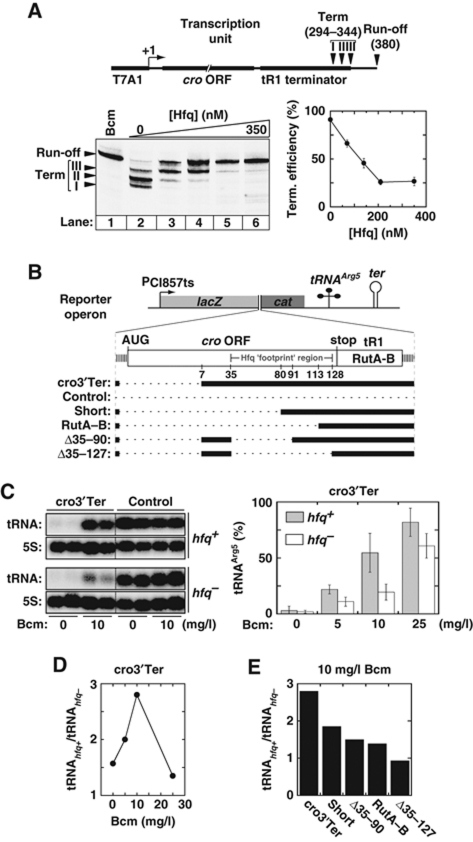
To determine if Hfq binding affects Rho activity, we performed in vitro transcription experiments with E. coli RNAP and DNA template pT7A1-λcro, which contains a region of the λ chromosome encompassing the cro ORF and Rho-dependent tR1 terminator (Figure 2A, schematic; Rabhi et al, 2011). With this template, Rho triggers efficient transcription termination at canonical release sites I, II, and III (Figure 2A, lane 2; Lau et al, 1982; Rabhi et al, 2011). The presence of bicyclomycin (Bcm), an antibiotic that specifically inhibits Rho (Rabhi et al, 2010), induces complete read-through of the tR1 terminator by RNAP (Figure 2A, lane 1). Similarly, Hfq represses Rho-dependent termination at λtR1 in a dose-dependent manner (Figure 2A, lanes 3–6 and graph). This effect is observed regardless of the order of addition of Hfq and other reaction components prior to transcription initiation with NTPs (data not shown).
Figure 2.
Hfq-mediated transcriptional antitermination. (A) Effect of Hfq on in vitro Rho-dependent transcription termination at the tR1 terminator. The composition of the DNA template (pT7A1-λcro) used in the in vitro transcription experiments is schematically depicted at the top of the panel. Gel bands corresponding to transcripts terminated at the canonical sites I, II, and III and to RNAP read-through of the terminator (Run-off transcripts) in the presence of 70 nM Rho are identified by arrowheads on the left side of the gel. The graph shows the global termination efficiency (sites I, II, and III) as a function of Hfq concentration. Bcm is a Rho-specific inhibitor. (B) A schematic of the reporter operons used in in vivo experiments. The thick black lines show which parts of cro-tR1 are present in the various operons. (C) Hfq reduces Rho-dependent tR1 termination in vivo in the presence of subinhibiting Bcm concentrations. Northern blots show tRNAarg5 and 5S RNA accumulation in hfq+ or hfq− cells carrying plasmid pOM10cI (Control operon) or pOM10cI-cro3′Tert (cro3′Ter operon). Total RNA was extracted from two different cultures in each case. Blots were successively hybridized with tRNAArg5 and 5S RNA probes. Blots separated by dotted lines are from the same membranes and were phosphor-imaged simultaneously. Histograms show normalized tRNAArg5 amounts (mean±error from 4–6 independent samples) in cells carrying the pOM10cI-cro3′Ter (cro3′Ter) plasmid. In each case, normalization assumes that 100% of tRNAArg5 is formed in the strain carrying the control pOM10cI plasmid. (D) Ratios of tRNAArg5 accumulated in hfq+ and hfq− cells from the cro3′Ter operon as a function of Bcm concentration. Bcm was added to culture media at the time of induction. (E) Ratios of tRNAArg5 accumulated in hfq+ and hfq− cells in the presence of 10 mg/l Bcm as a function of the reporter operon.
Similar observations were made with the distinct trpt’ terminator (Supplementary Figure S3), suggesting that Hfq-mediated read-through of Rho-dependent terminators has general relevance. Moreover, Hfq does not trigger efficient antitermination with phylogenetically distinct Rho from Mycobacterium tuberculosis (mtbRho; Supplementary Figure S4), a bacterium without Hfq homologue (Arnvig and Young, 2009). Note that mtbRho does not bind NusG efficiently (Kalarickal et al, 2010). Hfq also has no apparent effect on transcript release induced by the Rho-independent rrnB T1 terminator (data not shown) nor does it affect pausing of RNAP along the pT7A1-λcro template (Supplementary Figure S5). Thus, Hfq most likely promotes antitermination at Rho-dependent terminators by a mechanism involving a genuine Hfq:Rho interaction but without changing RNAP sensitivity to other signals regulating transcription elongation.
YaeO induces a comparable inhibition of Rho-dependent termination (Supplementary Figure S1B). However and in contrast to Hfq, the antitermination activity of purified YaeO declined rapidly upon time (data not shown), which probably explains why it was not detected previously (Pichoff et al, 1998).
To determine if Hfq also triggers antitermination in vivo, we constructed an artificial operon to measure cellular transcriptional read-through of the tR1 terminator. The operon contains the temperature-inducible λcI857ts promoter upstream from lacZ and cat genes and from sequences encoding tRNAArg5 and a Rho-independent terminator (cro3′Ter operon; Figure 2B) and is carried by a low-copy replicon (Espeli et al, 2001). The tR1 terminator and upstream cro sequences are inserted between the lacZ and cat genes in cro3′Ter (Figure 2B). This insert lacks the canonical Shine-Dalgarno sequence and the first 26 codons of the cro ORF (Figure 2B). These features ensure that transcriptional output from the λcI857ts promoter, tR1 termination, and potential Hfq-mediated antitermination are not affected by cro translation. Transient induction of the cro3′Ter operon is obtained following a temperature shift while synthesis and accumulation of the mature tRNAArg5 are used to measure read-through of the upstream tR1 terminator (see Materials and methods; Espeli et al, 2001 and references within).
We measured tRNAArg5 accumulation in E. coli strain MC4100 (hfq+) and in an isogenic hfq null strain (hfq−; see Materials and methods). In the absence of Bcm, there is ∼95% less tRNAArg5 in cells carrying the cro3′Ter operon than in cells carrying the parent operon without cro-tR1 insert (Control; Figure 2C). Moreover, amounts of tRNAArg5 accumulated from cro3′Ter are very low in both hfq+ and hfq− strains (Figure 2C; 0 mg/l Bcm samples). This suggests that tR1 termination is very efficient with cro3′Ter and that endogenous Hfq hardly affects Rho under these optimal conditions. Consistent with tRNAArg5 accumulation being blocked by tR1 termination, the relative amounts of tRNAArg5 obtained with cro3′Ter increase significantly if Rho activity is mitigated by addition of Bcm to the culture media (Figure 2C). Interestingly, tRNAArg5 accumulates in greater amounts in hfq+ than in hfq− in the presence of Bcm (Figure 2C). This difference is highest at the subinhibitory concentration of 10 mg/l of Bcm (Figure 2D). Under this condition, ectopic expression of Hfq (from pBAD-Hfq plasmid; Sledjeski et al, 2001) in hfq− cells also boosts formation of tRNAArg5 from cro3′Ter (Supplementary Figure S6A). Altogether, these data show that, under appropriate conditions (i.e. suboptimal for Rho function), Hfq can also mediate transcription antitermination in vivo.
We also tested the effect of Hfq on tR1 termination with cro3′Ter variants deprived of additional segments of the cro ORF (Figure 2B, Short, RutA–B, Δ35–90, and Δ35–127 operons). Termination at tR1 remains very efficient and dependent on Bcm with these operon variants (Supplementary Figure S6B). However, the relative excess of tRNAArg5 formed in hfq+ versus hfq− cells in the presence of Bcm is significantly lower with the operon variants than with cro3′Ter (Figure 2E). Moreover, diminution of the tRNAhfq+/tRNAhfq− ratio is roughly correlated with the extent of the cro deletions, although downstream-most cro deletions appear to have the largest mitigating effects. For instance, there is almost no difference between the amounts of tRNAArg5 accumulated from the Δ35–127 construct in the hfq+ and hfq− strains or upon ectopic expression of Hfq in hfq− cells (Figure 2E; Supplementary Figure S6). These data strongly suggest that Hfq needs untranslated cro segments to trigger significant tR1 antitermination with our reporter system (see also below).
NusG represses Hfq-mediated antitermination
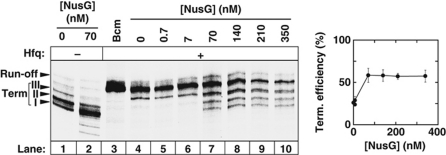
We tested the combined effect of Hfq and NusG on Rho in transcription termination experiments with the pT7A1-λcro template in vitro. In the absence of Hfq, NusG only weakly activates promoter-proximal termination sites that are not normally used by Rho (Figure 3, compare lanes 1 and 2; Li et al, 1993; Burns et al, 1999; Rabhi et al, 2011). In the presence of Hfq, however, NusG has a larger effect and restores tR1 termination in a dose-dependent manner (Figure 3, lanes 4–10). Similar observations were made with the trpt’ terminator (Supplementary Figure S3). Maximal recovery effects are reached with 70 nM NusG (Figure 3; Supplementary Figure S3), that is at a ratio of one NusG monomer per Rho hexamer in the transcription mixtures. This effective stoichiometry is the same as that of the Rho:NusG complex formed at equilibrium (Kd∼15 nM at 20°C, pH 7.9 in 100 mM KOAc; Pasman and von Hippel, 2000) and is not dependent on the order of addition of the transcription components (data not shown). Thus, counteraction of Hfq-mediated antitermination by NusG is likely due to the NusG canonical interaction with Rho. This counteraction is not total (Figure 3, compare lanes 7–10 with lanes 1 and 2; see also Supplementary Figure S3), suggesting that NusG does not totally displace Hfq from Rho, that the Hfq:Rho and NusG:Rho complexes are in dynamic equilibrium, or that Hfq and NusG bind Rho simultaneously during transcription. Further work is, however, required to rigorously assess and discriminate between these possibilities.
Figure 3.
NusG counteracts Hfq-mediated transcriptional antitermination at the tR1 terminator. A representative gel shows in vitro transcription termination experiments performed with the pT7A1-λcro template (Figure 2A) in the presence of 210 nM Hfq (+ lanes) and the various amounts of NusG indicated above gel lanes. The graph shows the global termination efficiency (sites I, II, and III) as a function of NusG concentration.
Hfq inhibits both ATPase and helicase activities of Rho
To define which step of Rho motor function is targeted by Hfq, we probed the effect of Hfq on Rho-directed RNA–DNA unwinding (Brennan et al, 1987). Standard strand-displacement (helicase) experiments (Walmacq et al, 2004) were performed with a synthetic RNA–DNA construct bearing the tR1 terminator upstream from a 21-bp duplex target (tR1RNA/D21; Figure 4A, schematic). Hfq inhibits Rho-directed unwinding of tR1RNA/D21 in a dose-dependent manner (Figure 4A). This experiment clearly shows that Hfq can affect Rho in the absence of RNAP and strongly suggests that Hfq-mediated antitermination is not due to a direct interference with the Rho–RNAP contacts triggering termination (Epshtein et al, 2010). Similar helicase experiments were performed with two shorter helicase substrates, one containing only the RutA–boxB–RutB region upstream from the RNA–DNA duplex (R106/D43; Supplementary Figure S7) and the other, unrelated one containing a synthetic Rut site upstream from a 23-bp RNA–DNA duplex (R132/D23; Walmacq et al, 2004). Although Hfq also reduces Rho-directed unwinding of these two substrates, the effects are much weaker than with tR1RNA/D21 (Supplementary Figure S7). These observations further support that Hfq requires specific RNA region(s) to inhibit Rho optimally.
Figure 4.
Effect of Hfq on Rho's RNA–DNA unwinding (A) and ATPase (B) activities. A schematic of the tR1RNA/D21 duplex used in helicase experiments is presented in (A) together with representative helicase gels and a graph showing the effect of Hfq on tR1RNA/D21 unwinding. The tR1RNA sequence is provided in Supplementary Figure S7.
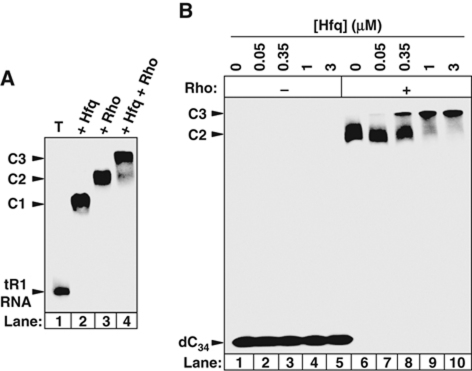
Next, we measured Rho's poly[rC]-dependent ATPase activity (Lowery-Goldhammer and Richardson, 1974; Rabhi et al, 2011) in the presence of Hfq. We observed that Hfq does not affect Rho's ATPase under these conditions (Figure 4B, dotted line). If tR1RNA replaces poly[rC] as the activating cofactor, however, Hfq inhibits Rho's ATPase in a dose-dependent manner (Figure 4B, black circles and lines) mimicking that observed for tR1RNA/D21 unwinding (Figure 4A, graph). Thus, Hfq can inhibit all the enzymatic activities of Rho (transcription termination, duplex unwinding, and ATP hydrolysis) but this inhibition depends on the nucleic acid cofactor. This dependence may stem from the strengthening of the Hfq:Rho complex by the cofactor. Indeed, a higher fraction of Rho is retained with his6Hfq on Ni-NTA beads in the presence of tR1RNA (Figure 1B, compare lanes 4 and 6), while both Rho and Hfq form stable complexes with tR1RNA (Figure 5A, lanes 2 and 3). By contrast, poly[rC] oligomers are very poor ligands for Hfq (Brennan and Link, 2007; Link et al, 2009) although they bind and activate Rho (Rabhi et al, 2010). Thus, Hfq probably needs to bind a specific RNA sequence/structure to inhibit Rho efficiently.
Figure 5.
Interactions within the ternary Hfq:Rho:RNA complex assessed by equilibrium band-shift assays. Representative gels show that (A) both Rho (20 nM) and Hfq (50 nM) interact with tR1RNA to form a ternary complex; (B) Hfq (concentrations indicated above gel lanes) interacts with the Rho–dC34 complex. Rho concentration was 20 nM (+ lanes).
NusG does not affect Hfq-mediated inhibition of Rho's ATPase and helicase activities (data not shown), which contrasts with its antagonistic effect on Hfq-mediated antitermination (Figure 3; Supplementary Figure S3). This difference may be due to the local, effective NusG concentration which, in the absence of stabilizing RNAP contacts with NusG-NTD (Roberts et al, 2008; Mooney et al, 2009b), may not be sufficient to antagonize Hfq. In support for this proposal is the fact that NusG does not challenge efficiently the Rho:Hfq interaction in pull-down assays (Supplementary Figure S8). The Hfq versus NusG competition may thus be tuned by Hfq and NusG contacts with, respectively, the transcript sequence/structure and RNAP. In any case, our data support that NusG can only regulate Rho in the context of bacterial transcription (Nehrke et al, 1993; Pasman and von Hippel, 2000; Rabhi et al, 2010).
Hfq does not prevent Rho binding to RNA
Formation of a catalytically competent Rho–RNA complex is an elaborate process implicating two distinct binding sites on Rho. On the hexamer's N-terminal facet, the primary binding site (PBS; Figure 1A, orange residues), which preferentially binds single-stranded, YC-rich RNA or DNA (Y being a pyrimidine) anchors Rho to transcripts at Rut sites. Once bound to Rho's PBS, RNA must enter inside the hexamer ring (Figure 1A) to associate with secondary binding site components and activate Rho (reviewed in Ciampi, 2006; Roberts et al, 2008; Rabhi et al, 2010).
Using a gel retardation assay, we found that Hfq and Rho bind tR1RNA comparably well (Figure 5A, complexes C1 and C2, respectively). Incubation of tR1RNA with both Rho and Hfq yields a band super-shift consistent with formation of a ternary tR1RNA:Rho:Hfq complex (Figure 5A, complex C3). To ensure that Hfq does not perturb Rho anchoring to its substrate, we used a dC34 oligonucleotide that should not bind Hfq (Brennan and Link, 2007; Link et al, 2009). This was verified by the absence of gel shift of the band corresponding to 32P-labelled dC34 incubated in the presence of up to 3 μM Hfq (Figure 5B, lanes 1–5). Although dC34 cannot activate Rho, it binds its PBS efficiently (Rabhi et al, 2010) as evidenced by the shift of the 32P-labelled dC34 band in the presence of 50 nM Rho (Figure 5B, lane 6). A super-shift of this band in the presence of Rho and Hfq (Figure 5B, complex C3 in lanes 7–10) confirms that both proteins associate directly (see Figure 1B and above). However, a higher concentration of Hfq is required to form a ternary complex with dC34 than with tR1RNA (Figure 5A and B), confirming that both Hfq and Rho contacting RNA further stabilize the ternary complex (see above).
Formation of a ternary Hfq:Rho:dC34 complex (Figure 5B) contrasts with the effect of YaeO, which competitively displaces dC34 from Rho (Supplementary Figure S1C; Gutierrez et al, 2007). Thus, despite their topological similarity (Gutierrez et al, 2007) and affinities for Rho (Figure 1; Pichoff et al, 1998), YaeO and Hfq use distinct mechanisms to inhibit Rho.
Hfq binds to an upstream A/U-rich region of tR1RNA
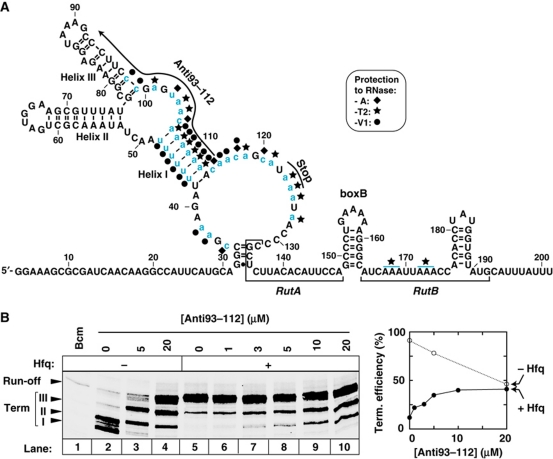
Rho binds two subsites (RutA and RutB) within tR1RNA (Figure 4A; Schwartz et al, 2007 and references therein). To identify the Hfq-binding site(s), we performed digestions of 32P-end-labelled tR1RNA with RNases A, T2, and V1. Hfq protects specific regions of tR1RNA from cleavage with the RNases (Supplementary Figure S9). Hfq ‘footprints’, which are summarized on the secondary structure of tR1RNA (Figure 6A), identify an A/U-rich cro region (positions 35–127) upstream from RutA as the major Hfq-binding site (blue nucleotides). Protections of RutB nucleotides from RNase T2 cleavage (Figure 6A, blue lines) also suggest that Hfq has some affinity for this region, which may contribute to inhibit Rho. However, Hfq hardly binds to (or displaces Rho from) R106/D43, a substrate containing the RutA–boxB–RutB region of λtR1RNA but lacking the upstream cro region (Supplementary Figure S10). These deficiencies are consistent with the lack of effect of Hfq on Rho-directed unwinding of R106/D43 (Supplementary Figure S7) and sustain that the upstream cro region contains the productive Hfq-binding site(s).
Figure 6.
Identification of the Hfq-binding region in tR1RNA. (A) Secondary structure of the relevant tR1RNA region based on RNase probing (Supplementary Figure S8) and MFOLD computation (Zuker, 2003). Several ambiguous RNase cleavages and structural constraints that could not be accounted for by the model suggest that regions 20–50 and 100–140 can adopt alternative conformations. Sites protected by Hfq from digestion with the RNases are identified by symbols (key is inset) and blue lowercase letters. Pairing of the Anti93–112 oligonucleotide, which was verified by oligonucleotide-induced cleavage of tR1RNA by RNase H (not shown), is depicted by an arrow. Nucleotides in region 165–175 that are protected by Hfq from digestion with RNase T2 (blue lines) could not be identified precisely. (B) The antitermination activity of Hfq is suppressed by the presence of oligonucleotide Anti93–112.
To confirm that the Hfq-binding site within cro is important for Hfq-mediated antitermination, we performed transcription termination experiments in the presence of U-rich oligonucleotide Anti93–112, which hybridizes with tR1RNA, thereby disrupting helices I and III and blocking access to the 93–112 region (Figure 6A, black arrow). The blocking Anti93–112 oligonucleotide has two distinct, opposite effects on transcription termination that could not be recapitulated with poly[rU] or an oligonucleotide that is not complementary to tR1RNA. First, Anti93–112 reduces Rho-dependent termination in the absence of Hfq (Figure 6B, lanes 3 and 4). Similar effects have been reported for sequence deletions or pairing of blocking oligonucleotides within cro, suggesting that Rho uses ancillary sequences within this region for optimal transcription termination in vitro (Lau and Roberts, 1985; Washburn et al, 1996; Gan and Richardson, 1999). Second, the blocking Anti93–112 oligonucleotide stimulates Rho-dependent termination in the presence of Hfq (Figure 6B, compare lanes 5 and 10), which no longer affects termination efficiency if Anti93–112 is present at a sufficiently high concentration (lanes 6–10 and graph). This shows that the antitermination activity of Hfq depends on the structural integrity and/or access to the 93–112 region and, by extension, validates the A/U-rich cro region (35–127; Figure 6A) as the one containing the effective Hfq-binding site(s). Note that deletions of sequences within this region also mitigate the effect of Hfq in vivo (see above and Figure 2E; Supplementary Figure S6). Regions preceding and encompassing the RutA–B sites of the trpt’ transcript are also strongly A/U-rich (Supplementary Figure S3) and may similarly befit Hfq, although we did not test this prediction explicitly.
Antitermination involves components on Hfq distal face
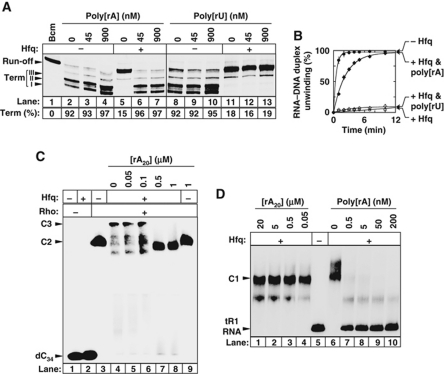
To determine if the RNA-binding faces of Hfq (Figure 1A) mediate its antitermination activity, we performed transcription termination experiments in the presence of poly[rA] or poly[rU] binding competitor. These polymers do not affect tR1 termination in the absence of Hfq (Figure 7A; lanes 2–4 and 8–10). However, poly[rA] is a strong suppressor of Hfq-mediated antitermination (Figure 7A, compare lanes 6 and 7 with lane 5) whereas poly[rU] is not (lanes 11–13). Poly[rA] also protects Rho-directed tR1RNA/D21 unwinding from inhibition by Hfq whereas poly[rU] does not (Figure 7B). A rA20 oligomer likewise suppresses Hfq inhibitory effects (data not shown). These data suggest that components on the distal, polyA-binding face of Hfq (Figure 1A) mediate Rho inhibition. To precise the origin of this mediation, we performed gel shift experiments with 32P-labelled dC34, Rho, Hfq, and competitor rA20. As shown in Figure 7C, rA20 challenges the formation of the dC34:Rho:Hfq complex (lanes 5–8), although it does not affect the binary dC34:Rho complex (lane 9). Because Hfq does not associate with dC34 (see above; Figure 7C, lane 2), these results strongly suggest that Hfq binds Rho through the Hfq distal face.
Figure 7.
Interaction with the distal, polyA-binding face of Hfq is critical for Rho inhibition. (A) Effect of polynucleotides (concentrations in nucleotides are indicated above gel lanes) on Hfq-mediated transcription antitermination at λtR1 in the presence of 70 nM Rho. In vitro transcriptions were performed with the pT7A1-λcro template (Figure 2A) and Hfq (+ lanes) present at a concentration of 210 nM. Global termination efficiencies (Term) are indicated below gel lanes. (B) Effect of the polynucleotides (900 nM, in nucleotides) on Rho-directed unwinding of the tR1RNA/D21 duplex. The concentrations of Rho and Hfq (in selected samples) were 20 nM and 210 nM, respectively. (C) Oligomer rA20 prevents the association of Hfq with the binary Rho–dC34 complex. Assay conditions are as in Figure 5B. Concentration of Hfq was 375 nM. (D) Oligomer rA20 does not displace Hfq from tR1RNA whereas poly[rA] does.
We performed similar experiments with a Hfq mutant, Y25A, which binds polyA oligomers ∼100 times less efficiently than WT Hfq (Sun and Wartell, 2006). Y25A and WT Hfq form equally stable complexes with tR1RNA (Supplementary Figure S11A), confirming that nucleotides other than adenines contribute to the tR1RNA–Hfq interaction (see above and Figure 6A). However, Y25A does not associate with the dC34:Rho complex at testable protein concentrations (Supplementary Figure S11B, lanes 6–10). Thus, the Hfq distal-face Y25A mutation dramatically weakens Hfq interaction with Rho. The decrease in affinity of Y25A for poly[rA] is ascribed to the loss of stacking of Tyr25 residues onto adenines (Figure 1A; Link et al, 2009). Similar stacking contact(s) between Tyr25 residue(s) on Hfq and aromatic side-chain(s) on Rho may stabilize the interaction between both proteins. Consistent with a defective interaction with Rho, Y25A has weaker antitermination activity than WT Hfq and is easily outcompeted by NusG (Supplementary Figure S11C). Yet, the antitermination activity of Y25A is suppressed by rA20 or poly[rA] (Supplementary Figure S11D and data not shown), suggesting that, although weakened, inhibitory contacts between Rho and Y25A still form in the antitermination complex.
Poly[rA] but not rA20 precludes tR1RNA binding to Y25A and WT Hfq in equilibrium binding assays (Figure 7D and data not shown). This difference may be related to the recent discovery of an additional contact surface on the periphery of the Hfq torus with specificity for long RNAs (Beich-Frandsen et al, 2011). Thus, Poly[rA] may suppress the antitermination activity of Y25A and WT Hfq by preventing both Rho and tR1RNA interactions with Hfq (but note that the effects of rA20 and the Anti93–112 oligonucleotide presented above suggest that disrupting either one of the two interactions is sufficient). Furthermore, Hfq-mediated antitermination at the trpt’ terminator is suppressed by rA20 and poly[rA] but is also moderately affected by poly[rU] (Supplementary Figure S12). Thus, Hfq-dependent antitermination can vary with both cis- (e.g. template sequence) and trans- (e.g. nucleic acid ligand) acting components and upon their matching combination.
Discussion
In E. coli, the elongation of transcripts by RNAP is often terminated by Rho (Cardinale et al, 2008; Peters et al, 2009) an essential ring-shaped protein motor (Rabhi et al, 2010). Rho-dependent termination relies on multiple interactions among Rho, RNA, RNAP, and cofactor NusG, which are regulatory targets for transcription factors, some of which remain unidentified (Roberts et al, 2008; Sen et al, 2008). In a search for new host inhibitors of Rho, we found that Hfq, a protein displaying some topological similarities with NusG-CTD (Supplementary data), can associate with Rho (Figure 1B; Supplementary Figure S2) and can inhibit all of its enzymatic activities (Figures 2 and 4). Yet, Hfq does not simply perturb Rho allosterically since it cannot inactivate Rho if the RNA cofactor is a poor Hfq ligand (poly[rC]; Figure 4B). Conversely, Hfq inhibits Rho if the cofactor contains Hfq-preferred A/U-rich regions (Brennan and Link, 2007), as in tr1RNA or trpt’ transcripts (Figures 2 and 4; Supplementary Figures S3 and S7). Inhibition requires Rho interaction with Hfq's distal face (Figure 7C) and Hfq binding RNA near Rut sites (Figure 6). Yet, Hfq does not displace Rho from the Rut site, at least in the case of λtR1 (Supplementary Figures S7 and S10). Primary Rho and Hfq interactions with RNA appear to co-stabilize the ternary Rho:Hfq:RNA complex (Figures 1B and 5A). Altogether, these features suggest that bound Hfq precludes productive Rho–RNA configuration(s) (Rabhi et al, 2010). Topological constraints in the ternary Rho:Hfq:RNA complex may, for instance, impede conformational rearrangements driving RNA inside the central Rho channel (Skordalakes and Berger, 2003; Canals et al, 2010). Additionally, RNA-bound Hfq may also preclude sterically Rho from using ancillary transcript sequences located upstream from the canonical Rut site (see above and Washburn et al, 1996).
Protein factors known to bind Rho to repress its function are rare. On one hand, the coat protein Psu from bacteriophage P4 binds Rho and alters its capacity to utilize ATP (Pani et al, 2006). On the other hand, YaeO is, to our knowledge, the only other host protein known to bind Rho and to trigger antitermination at Rho-dependent terminators (Supplementary Figure S1; Pichoff et al, 1998). Although YaeO and Hfq display topological similarities (Gutierrez et al, 2007), they use distinct mechanisms to inhibit Rho: YaeO displaces C-rich oligomers from Rho's PBS (Gutierrez et al, 2007) whereas Hfq does not (Figure 5B).
The YaeO protein is dispensable in E. coli (Gerdes et al, 2003), suggesting that its antitermination activity (Pichoff et al, 1998) is accessory. Although YaeO is expressed in E. coli (Taoka et al, 2004), its association with Rho was undetected in global analyses of the cellular interactome (Butland et al, 2005; Hu et al, 2009). This could be related to the fast inactivation of YaeO that we observed in vitro (see Results) or may indicate that the YaeO–Rho complex forms only under specific conditions in vivo. By contrast, Hfq, a very abundant and stable protein in E. coli (Brennan and Link, 2007), was consistently associated to Rho in the cellular interactome (Butland et al, 2005; Hu et al, 2009). This suggests that a functional Rho:Hfq interaction is frequent in E. coli, although the specifically targeted transcription units remain currently unknown. A functional Rho:Hfq interaction is supported by the recent discovery that deleting hfq yields cells that are more resistant to Bcm (Tran et al, 2011), which is consistent with our data showing better Rho-dependent tR1 termination in hfq− cells in the presence of Bcm (Figure 2C–E). Hereafter, we speculate on the possible biological role(s) of the Rho:Hfq interaction.
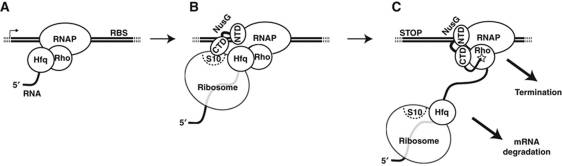
Our data with the cro3′Ter operon (Figure 2B) show that Hfq can increase read-through of Rho-dependent terminators in vivo (Figure 2C–E). This is highly significant given that conditions for terminator read-through may not have been optimal with our in vivo reporter system. For instance, we did not probe the effect of Hfq on cro3′Ter transcription independently from that of NusG, yet NusG reduces Hfq-mediated antitermination in vitro (Figure 3; Supplementary Figure S3). We note that Rho-dependent polarity in several terminators (including λtR1) is actually suppressed in NusG-deficient cells (Sullivan and Gottesman, 1992). Thus, it is possible that Hfq-mediated antitermination is maximized in vivo when NusG cannot counter Hfq. This could be the case during synthesis of mRNA leader regions (Figure 8A) when NusG has not yet associated with RNAP (Mooney et al, 2009a) or during synthesis of ORFs when NusG-CTD binds S10 rather than Rho (Figure 8B; Burmann et al, 2010). Hfq association with RNAP and ribosomal proteins (Sukhodolets and Garges, 2003; Butland et al, 2005; Brennan and Link, 2007; Hu et al, 2009) suggests that Hfq may travel with the tandem translation/transcription complex and contribute to read-through of ORFs by binding and inactivating Rho within it (Figure 8B). The antitermination activity of the tandem complex may therefore be multi-factorial (Figure 8B), as certainly is the case for the composite rrn and λN antitermination complexes (Roberts et al, 2008; Sen et al, 2008). The recurrence of boxA-like sequences in Rho-dependent terminators (Ciampi, 2006) and binding of Hfq-RNAP mediator S1 protein (Sukhodolets and Garges, 2003) to boxA RNA (Mogridge and Greenblatt, 1998) further suggests a commonality of mechanisms. Interestingly, disruption of the tandem translation/transcription complex at the end of ORFs (or upon conditional perturbation; Roberts et al, 2008; Rabhi et al, 2010) may both activate transcription termination and recruitment of RNA degradation factors that frequently associate with Hfq (Butland et al, 2005; Brennan and Link, 2007; Hu et al, 2009), thereby linking the three major steps of RNA metabolism in the same regulatory mechanism (Figure 8C).
Figure 8.
Possible contexts for Hfq-mediated antitermination. (A) Hfq may be required to repress Rho-dependent termination optimally during transcription of specific, long (>70 nt) leader regions. (B) Hfq may contribute to Rho inactivation in the tandem transcription/translation complex. Scavenging of NusG-CTD by S10 (NusE) and occlusion of RNA by the ribosome are also likely important factors (Burmann et al, 2010). (C) Uncoupling of translation from transcription may activate Rho-dependent termination by allowing NusG-CTD to bind Rho and by helping Rho to form a productive termination complex with RNA upon disruption of the Hfq–Rho interaction. These events may also create a signal for degradation of the mRNA.
Despite evidence for a molecular partnership between Rho and Hfq (Butland et al, 2005) and for Hfq-dependent regulation at the transcriptional level (Le Derout et al, 2010), the Rho–Hfq connection has been heretofore overlooked. The expressions of the proU and bgl operons are under the antagonistic influence of Rho and Hfq but effects are complex and may be indirect (Rajkumari and Gowrishankar, 2001; Dole et al, 2004). Nevertheless, important biological functions may be controlled by Hfq-mediated Rho inhibition. A potential target of such control is the expression of virulence genes, notably in enterobacteria where Rho and Hfq seem equally important (Chao and Vogel, 2010; Rabhi et al, 2010). Horizontal DNA transfer fosters bacterial virulence (Chao and Vogel, 2010), yet Rho preferentially represses expression of foreign DNA (Cardinale et al, 2008). Hfq relieving Rho-dependent silencing of horizontally acquired genes may thus contribute to explain the tight dependence of virulence factors on Hfq (Chao and Vogel, 2010). Circumstantial evidence comes from rho defects conferring hyperinvasive phenotypes to Salmonella typhimurium (Lee et al, 1992). Conversely, Rho-dependent termination may strengthen Hfq-mediated gene silencing (Gottesman et al, 2006; Brennan and Link, 2007; Vogel, 2009) when conditions altering the Rho–Hfq relationship arise. Our findings that nucleic acids provided in trans can contextually suppress Hfq inhibition of Rho (Figures 6B and 7; Supplementary Figure S12) suggest that such regulatory conditions could develop with some sRNA/mRNA pairs involved in Hfq-mediated silencing. Our discovery of a direct functional relationship between Rho and Hfq therefore opens new avenues of investigation for a better understanding of the roles of these two key factors in bacterial metabolism and pathogenicity.
Materials and methods
Materials
We purchased chemicals, oligonucleotides, homopolymers, and Sigma-saturated E. coli RNAP from Sigma-Aldrich, Eurogentec, GE-Healthcare and Midland CRC (USA), and Epicentre Biotechnologies, respectively. The pBAD-Hfq plasmid (Sledjeski et al, 2001) was kindly provided by N Majdalani and S Gottesman (NCI, NIH, MD). The hfq− strain was obtained by P1 transduction of the ΔHfq mutant from the Keio collection (Baba et al, 2006) into E. coli strain MC4100.
We prepared the DNA templates for transcriptions with T7 or E. coli RNAP by PCR amplification of custom-made DNA fragments described previously (Rabhi et al, 2011) or purchased from GenScript (USA). Plasmid pOMcI-cro3′Ter was constructed by subcloning the PCR-amplified region of λDNA (Invitrogen) encoding the cro ORF (minus first 26 codons) and tR1 terminator into the filled-in BamHI site of previously described plasmid pOM10cI (Espeli et al, 2001). Plasmids pOMcI-Short, pOMcI-RutA–B, pOMcI-Δ35–90, and pOMcI-Δ35–127, which bear variants of the cro3′Ter operon (Figure 2B), were prepared by similar PCR and subcloning procedures. We followed published procedures (Boudvillain et al, 2010) to prepare the tR1RNA transcript and the RNA–DNA constructs used in helicase experiments.
We prepared Rho from E. coli or M. tuberculosis, NusG, and YaeO as described previously (Artsimovitch and Landick, 2000; Gutierrez et al, 2007; Boudvillain et al, 2010; Kalarickal et al, 2010) from cultures of BL21(DE3) cells harbouring, respectively, the pET28b-Rho (kindly provided by J Berger, UC Berkeley), pET28b-mtbRho (kindly provided by R Sen, CDFD, Hyderabad, India), pIA247 (kindly provided by I Artsimovitch, Ohio State University), or pET15b-YaeO (kindly provided by K Gehring, McGill University) plasmid. We stored Rho and NusG at −20°C in storage buffer (100 mM KCl, 10 mM Tris–HCl, pH 7.9, 0.1 mM EDTA, 0.1 mM DTT, and 50% glycerol). Because the inhibitory activity of YaeO declined rapidly upon storage regardless of the buffer and temperature conditions that were tried, we performed experiments with YaeO preparations that were not older than 2–3 days. We prepared Hfq using a procedure specifically designed to avoid RNA contamination, as described previously (Hwang et al, 2011). We stored Hfq at 4°C in 50 mM Tris–HCl pH 7.5, 1 mM EDTA, 10% glycerol, and 50 mM NH4Cl. Absence of significant RNA contamination of the protein preparations was verified by high A280/A260 ratios of optical absorbances, by the absence of detectable bands upon Sybr green staining of sample-resolved PAGE (polyacrylamide gel electrophoresis) gels, indicating that <1 ng of RNA/gel lane were present, and, for Rho, by the lack of detectable ATP hydrolysis activity (which is strictly RNA dependent; Rabhi et al, 2010) after incubation for 30 min at 37°C. The Rho and Hfq concentrations are expressed in hexamers throughout the manuscript.
Pull-down experiments with his-tagged proteins
We washed aliquots (100 μl) of Ni-NTA agarose beads (Qiagen) thrice with 200 μl of interaction buffer (IB; 20 mM Tris–HCl, pH 7.9, 100 mM KCl, 1 mM DTT, 0.1% Triton-X100, and 20 mM PMSF) and then mixed beads with 50 pmol of His-tagged protein (his6NusG, his6Hfq, or his6YaeO) in 500 μl of IB before incubation for 2 h at 4°C. We washed beads thrice again with 500 μl of IB supplemented with 0.1% BSA and 20 mM imidazole (‘low adsorption’ buffer) before addition of 150 pmol of Rho (we also added an equimolar amount of tR1RNA in selected samples). We incubated beads overnight at 4°C in 500 μl of ‘low adsorption’ buffer. After centrifugation, we discarded the supernatant and washed beads thrice with 800 μl of ‘low adsorption’ buffer. We re-suspended beads in SDS loading buffer (2% SDS, 12% glycerol, 0.1 mM Tris–HCl pH 6.8, 0.35 M 2-mercapto-ethanol, and 100 mM imidazole), heated them for 5 min at 90°C, and loaded them on 12.5% SDS–PAGE gels.
Transcription termination experiments
Standard transcription termination experiments were performed as described previously (Zou and Richardson, 1991; Wei and Richardson, 2001) with minor modifications. Briefly, we incubated mixtures of DNA template (0.1 pmol), E. coli RNAP (0.45 pmol), Rho (1.4 pmol), SUPERase-In (0.5 U/μl; Ambion), with or without NusG in 18 μl of transcription buffer (40 mM Tris–HCl, pH 8.0, 50 mM KCl, 5 mM MgCl2, 1.5 mM DTT) for 10 min at 37°C (control reactions contained 3 nmol of Bcm, kindly provided by K Schnetz, University of Cologne, Germany). We started transcriptions by adding 2 μl of initiation mix (2 mM ATP, GTP, and CTP and 0.2 mM UTP, 2.5 μCi/μl of 32P-αUTP, and 250 μg/ml rifampicin in transcription buffer) before incubation for 20 min at 37°C. In competition experiments, we added poly[rA] or poly[rU] with the initiation mix to limit their interference with transcription initiation. We stopped transcription reactions by adding 4 μl of EDTA (0.5 M), 6 μl of tRNA (0.25 mg/ml), and 80 μl of sodium acetate (0.42 M) before precipitation at −20°C with 330 μl of ethanol. We dissolved reaction pellets in denaturing loading buffer (95% formamide, 5 mM EDTA), and analysed them by denaturing 5% PAGE. Normalization of band intensities for the U contents of the corresponding transcripts and calculation of termination efficiencies were performed as described previously (Washburn et al, 1996). See supplementary data for additional transcription procedures.
In vivo experiments with synthetic tR1-containing operons
We incubated E. coli cells carrying the control pOM10cI (Espeli et al, 2001) or reporter operon plasmid at 30°C in LB supplemented with spectinomycin (and kanamycin for hfq− cells) until A600∼0.3. We then incubated cultures at 42°C for 40 min to induce operon expression from the λCI857ts promoter. We extracted total RNA from independent cultures using a hot phenol procedure, as described previously (Espeli et al, 2001). We resolved RNA samples on formaldehyde/1.5% agarose gels and electro-blotted them on positive TM membranes (MPbiochemicals) with a TransBlot SD transfer cell (Bio-Rad). We performed successive hybridizations with 32P-end-labelled tRNA and 5S RNA probes as well as quantification and normalization of hybridization signals as described previously (Espeli et al, 2001). Briefly, band intensities were measured using the rectangle quantitation and local average background functions of ImageQuantTL. Normalized tRNAArg5 levels were calculated with the following equation:
where ItRNA and I5S are the intensities of the blots corresponding to tRNAArg5 and 5S RNA, respectively.
Helicase assays
We performed unwinding experiments as described previously (Schwartz et al, 2007). Briefly, we mixed RNA–DNA substrates (5 nM, final concentration) with Rho (20 nM, final concentration) in helicase buffer (20 mM HEPES, pH 7.5, 0.1 mM EDTA, 0.5 mM DTT, 150 mM potassium acetate) before incubation for 3 min at 30°C. We then added ATP, MgCl2 (1 mM, final concentrations), and DNA trap (400 nM, final concentration; the DNA trap oligonucleotide is complementary to the released DNA strand) before further incubation at 30°C. We withdrew reaction aliquots at various times, mixed them with four volumes of quench buffer (100 mM EDTA, 2.5% SDS, 15% Ficoll-400) before loading them on 7.5% PAGE gels that contained 0.5% SDS.
ATPase assays
We measured the steady-state ATPase activity of Rho at 25°C with the EnzCheck Phosphate Assay kit (Molecular Probes) as described previously (Rabhi et al, 2011). Reactions mixtures contained 20 nM Rho, 20 nM of tR1RNA or poly[rC] fragments, and Hfq at the concentrations indicated in Figure 4B.
Electrophoretic mobility shift assays
We mixed the 32P-end-labelled tR1RNA or dC34 oligonucleotide (0.1 nM, final concentration), with various concentrations of Rho and/or Hfq in binding buffer (150 mM potassium acetate, 20 mM HEPES, pH 7.5, 0.1 mM EDTA, 0.5 mM DTT; 0.5 U/μl SUPERase-In, 0.1 mg/ml tRNA, and 0.1% BSA). We incubated the mixtures for 30 min at 20°C before adding 2.5 μl Ficoll-400 and loading them on a 4% (for tR1RNA) or stacked 4/12% (for dC34 oligonucleotide) PAGE gel that contained 0.05% Triton-X100.
We analysed PAGE gels containing 32P-labelled materials with a Typhoon-Trio imager (GE-Healthcare).
Supplementary Material
Acknowledgments
We gratefully acknowledge N Hervouet-Coste and C Mosrin-Huaman for helpful technical advice, Alexey Nikulin for help with structural comparison, and Richard A Lease for critical reading of the manuscript. This work was supported by a grant from the Conseil Régional du Centre.
Author contributions: MR, OE, AS, VA, and MB conceived and designed the experiments. MR, OE, AS, and BC performed the experiments. MR, OE, AS, RR, VA, and MB analysed the data. OE, RR, VA, and MB contributed reagents/materials/logistical support. VA and MB coordinated the study and wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Arluison V, Derreumaux P, Allemand F, Folichon M, Hajnsdorf E, Regnier P (2002) Structural modelling of the Sm-like protein Hfq from Escherichia coli. J Mol Biol 320: 705–712 [DOI] [PubMed] [Google Scholar]
- Arnvig KB, Young DB (2009) Identification of small RNAs in Mycobacterium tuberculosis. Mol Microbiol 73: 397–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artsimovitch I, Landick R (2000) Pausing by bacterial RNA polymerase is mediated by mechanistically distinct classes of signals. Proc Natl Acad Sci USA 97: 7090–7095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2: 2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beich-Frandsen M, Vecerek B, Konarev PV, Sjoblom B, Kloiber K, Hammerle H, Rajkowitsch L, Miles AJ, Kontaxis G, Wallace BA, Svergun DI, Konrat R, Blasi U, Djinovic-Carugo K (2011) Structural insights into the dynamics and function of the C-terminus of the E. coli RNA chaperone Hfq. Nucleic Acids Res (doi:10.1093/nar/gkq1346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudvillain M, Walmacq C, Schwartz A, Jacquinot F (2010) Simple enzymatic assays for the in vitro motor activity of transcription termination factor Rho from Escherichia coli. In Helicases: Methods and Protocols, Abdelhaleem M (ed), Vol. 587, pp 137–154. Totowa: Humana Press Inc. [DOI] [PubMed] [Google Scholar]
- Brennan CA, Dombroski AJ, Platt T (1987) Transcription termination factor rho is an RNA-DNA helicase. Cell 48: 945–952 [DOI] [PubMed] [Google Scholar]
- Brennan RG, Link TM (2007) Hfq structure, function and ligand binding. Curr Opin Microbiol 10: 125–133 [DOI] [PubMed] [Google Scholar]
- Burmann BM, Schweimer K, Luo X, Wahl MC, Stitt BL, Gottesman ME, Rosch P (2010) A NusE:NusG complex links transcription and translation. Science 328: 501–504 [DOI] [PubMed] [Google Scholar]
- Burns CM, Nowatzke WL, Richardson JP (1999) Activation of Rho-dependent transcription termination by NusG. Dependence on terminator location and acceleration of RNA release. J Biol Chem 274: 5245–5251 [DOI] [PubMed] [Google Scholar]
- Butland G, Peregrin-Alvarez JM, Li J, Yang W, Yang X, Canadien V, Starostine A, Richards D, Beattie B, Krogan N, Davey M, Parkinson J, Greenblatt J, Emili A (2005) Interaction network containing conserved and essential protein complexes in Escherichia coli. Nature 433: 531–537 [DOI] [PubMed] [Google Scholar]
- Canals A, Uson I, Coll M (2010) The structure of RNA-free Rho termination factor indicates a dynamic mechanism of transcript capture. J Mol Biol 400: 16–23 [DOI] [PubMed] [Google Scholar]
- Cardinale CJ, Washburn RS, Tadigotla VR, Brown LM, Gottesman ME, Nudler E (2008) Termination factor Rho and its cofactors NusA and NusG silence foreign DNA in E. coli. Science 320: 935–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael GG, Weber K, Niveleau A, Wahba AJ (1975) The host factor required for RNA phage Qbeta RNA replication in vitro. Intracellular location, quantitation, and purification by polyadenylate-cellulose chromatography. J Biol Chem 250: 3607–3612 [PubMed] [Google Scholar]
- Chao Y, Vogel J (2010) The role of Hfq in bacterial pathogens. Curr Opin Microbiol 13: 24–33 [DOI] [PubMed] [Google Scholar]
- Ciampi MS (2006) Rho-dependent terminators and transcription termination. Microbiology 152: 2515–2528 [DOI] [PubMed] [Google Scholar]
- Condon C, Squires C, Squires CL (1995) Control of rRNA transcription in Escherichia coli. Microbiol Rev 59: 623–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Court D, Adhya S (1976) Isolation and characterization of conditional lethal mutants of Escherichia coli defective in transcription termination factor rho. Proc Natl Acad Sci USA 73: 1959–1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dole S, Klingen Y, Nagarajavel V, Schnetz K (2004) The protease Lon and the RNA-binding protein Hfq reduce silencing of the Escherichia coli bgl operon by H-NS. J Bacteriol 186: 2708–2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epshtein V, Dutta D, Wade J, Nudler E (2010) An allosteric mechanism of Rho-dependent transcription termination. Nature 463: 245–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeli O, Moulin L, Boccard F (2001) Transcription attenuation associated with bacterial repetitive extragenic BIME elements. J Mol Biol 314: 375–386 [DOI] [PubMed] [Google Scholar]
- Folichon M, Arluison V, Pellegrini O, Huntzinger E, Regnier P, Hajnsdorf E (2003) The poly(A) binding protein Hfq protects RNA from RNase E and exoribonucleolytic degradation. Nucleic Acids Res 31: 7302–7310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan E, Richardson JP (1999) ATP and other nucleotides stabilize the Rho-mRNA complex. Biochemistry 38: 16882–16888 [DOI] [PubMed] [Google Scholar]
- Geiselmann J, Yager T, Gill S, Calmettes P, von Hippel P (1992) Physical properties of the Escherichia coli transcription termination factor rho. 1. Association states and geometry of the rho hexamer. Biochemistry 31: 111–121 [DOI] [PubMed] [Google Scholar]
- Gerdes SY, Scholle MD, Campbell JW, Balazsi G, Ravasz E, Daugherty MD, Somera AL, Kyrpides NC, Anderson I, Gelfand MS, Bhattacharya A, Kapatral V, D’Souza M, Baev MV, Grechkin Y, Mseeh F, Fonstein MY, Overbeek R, Barabasi AL, Oltvai ZN et al. (2003) Experimental determination and system level analysis of essential genes in Escherichia coli MG1655. J Bacteriol 185: 5673–5684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S, McCullen CA, Guillier M, Vanderpool CK, Majdalani N, Benhammou J, Thompson KM, FitzGerald PC, Sowa NA, FitzGerald DJ (2006) Small RNA regulators and the bacterial response to stress. Cold Spring Harb Symp Quant Biol 71: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez P, Kozlov G, Gabrielli L, Elias D, Osborne MJ, Gallouzi IE, Gehring K (2007) Solution structure of YaeO, a Rho-specific inhibitor of transcription termination. J Biol Chem 282: 23348–23353 [DOI] [PubMed] [Google Scholar]
- Hu P, Janga SC, Babu M, Diaz-Mejia JJ, Butland G, Yang W, Pogoutse O, Guo X, Phanse S, Wong P, Chandran S, Christopoulos C, Nazarians-Armavil A, Nasseri NK, Musso G, Ali M, Nazemof N, Eroukova V, Golshani A, Paccanaro A et al. (2009) Global functional atlas of Escherichia coli encompassing previously uncharacterized proteins. PLoS Biol 7: e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang W, Arluison V, Hohng S (2011) Dynamic competition of DsrA and rpoS fragments for the proximal binding site of Hfq as a means for efficient annealing. Nucleic Acids Res (doi:10.1093/nar/gkr075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin DJ, Burgess RR, Richardson JP, Gross CA (1992) Termination efficiency at rho-dependent terminators depends on kinetic coupling between RNA polymerase and rho. Proc Natl Acad Sci USA 89: 1453–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalarickal NC, Ranjan A, Kalyani BS, Wal M, Sen R (2010) A bacterial transcription terminator with inefficient molecular motor action but with a robust transcription termination function. J Mol Biol 395: 966–982 [DOI] [PubMed] [Google Scholar]
- Lau LF, Roberts JW (1985) Rho-dependent transcription termination at lambda R1 requires upstream sequences. J Biol Chem 260: 574–584 [PubMed] [Google Scholar]
- Lau LF, Roberts JW, Wu R (1982) Transcription terminates at lambda tR1 in three clusters. Proc Natl Acad Sci USA 79: 6171–6175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Derout J, Boni IV, Regnier P, Hajnsdorf E (2010) Hfq affects mRNA levels independently of degradation. BMC Mol Biol 11: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CA, Jones BD, Falkow S (1992) Identification of a Salmonella typhimurium invasion locus by selection for hyperinvasive mutants. Proc Natl Acad Sci USA 89: 1847–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Mason SW, Greenblatt J (1993) Elongation factor NusG interacts with termination factor rho to regulate termination and antitermination of transcription. Genes Dev 7: 161–172 [DOI] [PubMed] [Google Scholar]
- Link TM, Valentin-Hansen P, Brennan RG (2009) Structure of Escherichia coli Hfq bound to polyriboadenylate RNA. Proc Natl Acad Sci USA 106: 19292–19297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery-Goldhammer C, Richardson JP (1974) An RNA-dependent nucleoside triphosphate phosphohydrolase (ATPase) associated with rho termination factor. Proc Natl Acad Sci USA 71: 2003–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogridge J, Greenblatt J (1998) Specific binding of Escherichia coli ribosomal protein S1 to boxA transcriptional antiterminator RNA. J Bacteriol 180: 2248–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney RA, Davis SE, Peters JM, Rowland JL, Ansari AZ, Landick R (2009a) Regulator trafficking on bacterial transcription units in vivo. Mol Cell 33: 97–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney RA, Schweimer K, Rosch P, Gottesman M, Landick R (2009b) Two structurally independent domains of E. coli NusG create regulatory plasticity via distinct interactions with RNA polymerase and regulators. J Mol Biol 391: 341–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehrke KW, Zalatan F, Platt T (1993) NusG alters rho-dependent termination of transcription in vitro independent of kinetic coupling. Gene Expr 3: 119–133 [PMC free article] [PubMed] [Google Scholar]
- Pani B, Banerjee S, Chalissery J, Abishek M, Loganathan RM, Suganthan RB, Sen R (2006) Mechanism of inhibition of Rho-dependent transcription termination by bacteriophage P4 protein Psu. J Biol Chem 281: 26491–26500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasman Z, von Hippel PH (2000) Regulation of rho-dependent transcription termination by NusG is specific to the Escherichia coli elongation complex. Biochemistry 39: 5573–5585 [DOI] [PubMed] [Google Scholar]
- Peters JM, Mooney RA, Kuan PF, Rowland JL, Keles S, Landick R (2009) Rho directs widespread termination of intragenic and stable RNA transcription. Proc Natl Acad Sci USA 106: 15406–15411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichoff S, Alibaud L, Guedant A, Castanie MP, Bouche JP (1998) An Escherichia coli gene (yaeO) suppresses temperature-sensitive mutations in essential genes by modulating Rho-dependent transcription termination. Mol Microbiol 29: 859–869 [DOI] [PubMed] [Google Scholar]
- Proshkin S, Rahmouni AR, Mironov A, Nudler E (2010) Cooperation between translating ribosomes and RNA polymerase in transcription elongation. Science 328: 504–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabhi M, Gocheva V, Jacquinot F, Lee A, Margeat E, Boudvillain M (2011) Mutagenesis-based evidence for an asymmetric configuration of the ring-shaped transcription termination factor Rho. J Mol Biol 405: 497–518 [DOI] [PubMed] [Google Scholar]
- Rabhi M, Rahmouni AR, Boudvillain M (2010) Transcription termination factor Rho: a ring-shaped RNA helicase from bacteria. In RNA Helicases, Jankowsky E (ed), Vol. 19, pp 243–271. Cambridge (UK): RSC Publishing [Google Scholar]
- Rajkumari K, Gowrishankar J (2001) In vivo expression from the RpoS-dependent P1 promoter of the osmotically regulated proU operon in Escherichia coli and Salmonella enterica serovar Typhimurium: activation by rho and hns mutations and by cold stress. J Bacteriol 183: 6543–6550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JW, Shankar S, Filter JJ (2008) RNA polymerase elongation factors. Annu Rev Microbiol 62: 211–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A, Walmacq C, Rahmouni AR, Boudvillain M (2007) Noncanonical interactions in the management of RNA structural blocks by the transcription termination rho helicase. Biochemistry 46: 9366–9379 [DOI] [PubMed] [Google Scholar]
- Sen R, Chalissery J, Muteeb G (2008) Nus factors of Escherichia coli. In EcoSal-Escherichia coli and Salmonella: Cellular and Molecular Biology, Böck A, Curtiss III R, Kaper JB, Karp PD, Neidhardt FC, Nyström T, Slauch JM, Squires CL, Ussery D (eds), http://www.ecosal.org##Washington, DC: ASM Press Vol. 4.5.3.1 [Google Scholar]
- Skordalakes E, Berger JM (2003) Structure of the rho transcription terminator. Mechanism of mRNA recognition and helicase loading. Cell 114: 135–146 [DOI] [PubMed] [Google Scholar]
- Sledjeski DD, Whitman C, Zhang A (2001) Hfq is necessary for regulation by the untranslated RNA DsrA. J Bacteriol 183: 1997–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhodolets MV, Garges S (2003) Interaction of Escherichia coli RNA polymerase with the ribosomal protein S1 and the Sm-like ATPase Hfq. Biochemistry 42: 8022–8034 [DOI] [PubMed] [Google Scholar]
- Sullivan SL, Gottesman ME (1992) Requirement for E. coli NusG protein in factor-dependent transcription termination. Cell 68: 989–994 [DOI] [PubMed] [Google Scholar]
- Sun X, Wartell RM (2006) Escherichia coli Hfq binds A18 and DsrA domain II with similar 2:1 Hfq6/RNA stoichiometry using different surface sites. Biochemistry 45: 4875–4887 [DOI] [PubMed] [Google Scholar]
- Taoka M, Yamauchi Y, Shinkawa T, Kaji H, Motohashi W, Nakayama H, Takahashi N, Isobe T (2004) Only a small subset of the horizontally transferred chromosomal genes in Escherichia coli are translated into proteins. Mol Cell Proteomics 3: 780–787 [DOI] [PubMed] [Google Scholar]
- Torres M, Condon C, Balada JM, Squires C, Squires CL (2001) Ribosomal protein S4 is a transcription factor with properties remarkably similar to NusA, a protein involved in both non-ribosomal and ribosomal RNA antitermination. EMBO J 20: 3811–3820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran L, van Baarsel JA, Washburn RS, Gottesman ME, Miller JH (2011) Single gene deletion mutants of Escherichia coli with altered sensitivity to bicyclomycin, an inhibitor of transcription termination factor Rho. J Bacteriol 193: 2229–2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J (2009) A rough guide to the non-coding RNA world of Salmonella. Mol Microbiol 71: 1–11 [DOI] [PubMed] [Google Scholar]
- Walmacq C, Rahmouni AR, Boudvillain M (2004) Influence of substrate composition on the helicase activity of transcription termination factor Rho: reduced processivity of Rho hexamers during unwinding of RNA-DNA hybrid regions. J Mol Biol 342: 403–420 [DOI] [PubMed] [Google Scholar]
- Washburn RS, Jin DJ, Stitt BL (1996) The mechanism of early transcription termination by Rho026. J Mol Biol 260: 347–358 [DOI] [PubMed] [Google Scholar]
- Wei RR, Richardson JP (2001) Mutational changes of conserved residues in the Q-loop region of transcription factor Rho greatly reduce secondary site RNA-binding. J Mol Biol 314: 1007–1015 [DOI] [PubMed] [Google Scholar]
- Zou LL, Richardson JP (1991) Enhancement of transcription termination factor rho activity with potassium glutamate. J Biol Chem 266: 10201–10209 [PubMed] [Google Scholar]
- Zuker M (2003) Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 31: 3406–3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.