Abstract
EMBO J 30 14, 2762–2778 (2011); published online June 24 2011
EMBO Rep 12 6, 565–573 (2011); doi:; DOI: 10.1038/embor.2011.54
Mitochondria are remarkably dynamic organelles undergoing frequent fusion and fission events. Impairment thereof is linked to numerous neurodegenerative disorders and dysregulation of apoptosis. The principal players mediating mitochondrial fission are considered to be well known and largely conserved between yeast and mammals. However, how the essential fission factor Drp1 is recruited to mitochondria and how its activity is regulated are far more complex than previously assumed. According to a recent study (Otera et al, 2010), recruitment of Drp1 and mitochondrial fission can be exerted by Mff. Surprisingly, these processes do not appear to require Fis1, apparently contradicting several earlier reports on the role of Fis1. Two studies reported in EMBO Reports (Palmer et al, 2011) and in this issue of The EMBO Journal (Zhao et al, 2011) help to shed light on these unexpected findings. They identified two homologous vertebrate-specific negative regulators of Drp1-dependent fission termed: MIEF1/MiD51 and MiD49. They are able to recruit Drp1 to mitochondria but, importantly, rather than promoting fission, bind and inhibit Drp1. In a mutually exclusive manner, MIEF1/MiD51 can form a complex either with Drp1 or with Fis1. Thus, Fis1 may indirectly promote mitochondrial fission by its ability to sequester MIEF1/MiD51, preventing this novel factor from inhibiting mitochondrial fission. Future studies will have to decipher the complex interplay between these novel factors and how they regulate mitochondrial dynamics.
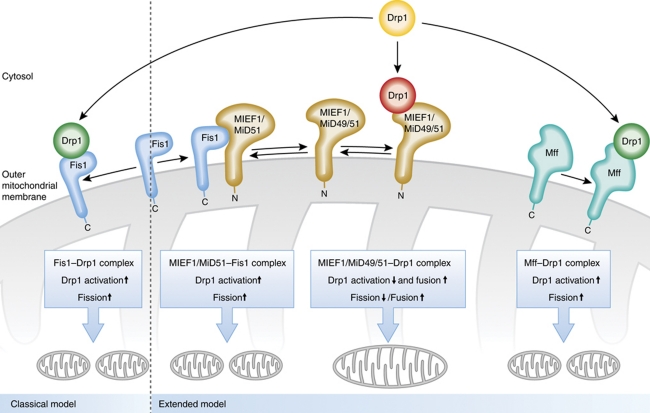
In yeast, three factors are known to be required for mitochondrial fission: Dnm1, Fis1 and Mdv1 (Chan, 2006; Westermann, 2010). Of those only Fis1 and Dnm1 have orthologues in vertebrates, namely Fis1 and Drp1, respectively. Drp1, a dynamin-like large GTPase shows a cytosolic localization that after recruitment to mitochondria and oligomerization mediates GTP-dependent fission. Downregulation of Fis1 leads to mitochondrial elongation and overexpression promoted fission consistent with data from yeast (Chan, 2006; Westermann, 2010). Fis1 was proposed to act as the mitochondrial receptor for Drp1-dependent fission (Figure 1). So, it appeared for quite some time in the field that the molecular functions of Drp1 and Fis1 are well conserved from yeast to humans. However, the general role of Fis1 in mitochondrial fission in mammals was questioned recently, for example, with the observation that a conditional knockout of this gene in a carcinoma cell culture model did not lead to a defect in mitochondrial fission, suggesting that Fis1 is dispensable for fission (Otera et al, 2010). The same study analysed in an impressive manner the molecular function of the mitochondrial fission factor, Mff, identified earlier (Gandre-Babbe and van der Bliek, 2008). Otera et al (2010) showed that this factor is able to recruit Drp1 to mitochondria, forms a complex with Drp1 and promotes mitochondrial fission (Figure 1). This was convincingly demonstrated as for example artificially linking Mff to the plasma membrane resulted in Drp1 recruitment to exactly that membrane; and downregulation of Mff led to a reduced number of Drp1-positive foci at the mitochondrial outer membrane accompanied by impairment of mitochondrial fission. In contrast, overexpression of Mff had the opposite effects. These findings strengthened the view that Fis1 is not an essential component of the fission machinery and that Mff may act in addition or alternatively as the mitochondrial receptor for Drp1. The apparent discrepancies to earlier studies in which downregulation of Fis1 impaired fission and upregulation of Fis1 promoted mitochondrial fragmentation were attributed to different types of cell lines and RNAi sequences used (Otera et al, 2010).
Figure 1.
Models for mitochondrial fission in vertebrates. Classical model, Fis1 acts as the mitochondrial receptor for Drp1 promoting fission. Extended model, Fis1, Mff and/or MIEF1/MiD51/Mid49 recruit Drp1 to the mitochondria. The Mff–Drp1 complex promotes mitochondrial fission. In contrast, the MIEF1/Mid49/51–Drp1 sequesters Drp1, inhibits Drp1 function and promotes fusion in an Mfn2-independent manner. Mitochondria become elongated. In an apparently mutually exclusive manner, MIEF1/MiD51 can also form a complex with Fis1 (MiD49 was omitted here as a MiD49–Fis1 complex was not demonstrated to exist yet). By that, the inhibitory effect of MIEF1/MiD51 on Drp1 function is reduced and hence mitochondrial fission is indirectly promoted by Fis1. Active Drp1, green. Inhibited Drp1, red.
However, an additional, attractive hypothesis explaining these findings is now provided by two parallel studies from Zhao et al (2011) and Palmer et al (2011). The latter group identified and characterized the role of MiD49 and MiD51 (mitochondrial dynamics proteins 49/51), which share about 45% amino-acid identity. In parallel, Zhao et al (2011) have identified and characterized the molecular function of MIEF1 (mitochondrial elongation factor 1), which notably is identical to MiD51. These studies demonstrate that MIEF1/MiD51 and MiD49 are able to recruit Drp1 to mitochondria (Figure 1). Importantly, instead of promoting mitochondrial fission, they rather block it by sequestering Drp1. MIEF1/MiD51 was further shown to have a pro-fusion activity independent of Mfn2, a known fusion factor located in the outer membrane. Interestingly, MIEF1/MiD51 is able to form two different protein complexes as it, in an apparently mutually exclusive manner, binds either to Drp1 or to Fis1. Consistently, overexpression of Fis1 partially reduces the inhibitory effect of MIEF1/MiD51 on Drp1. With these findings, these studies provide a rationale how Fis1 may, more indirectly, affect mitochondrial fission and explain part of the apparently contradicting results reported during the last years: Fis1 overexpression would sequester MIEF1/MiD51. That prevents the latter to act as a fission inhibitor as Drp1 cannot be sequestered efficiently anymore. Consequently, mitochondrial fission is promoted. Downregulation of Fis1 would increase the levels of available MIEF1/MiD51 molecules, resulting in a more efficient inactivation of Drp1. This certainly leaves the question why in the study by Otera et al (2010) Fis1 knockdown by various means did not appear to impact mitochondrial fission. We can only speculate, but based on the aforementioned model (Figure 1) lower levels of MIEF1/MiD51 or MiD49 in the cell types analysed could be a possible explanation. Future studies will have to test this.
The two studies by Zhao et al (2011) and Palmer et al (2011) are consistent regarding the role of MIEF1/MiD51/MiD49 in recruiting and inhibiting Drp1 function when overexpressed. However, there are some apparent discrepancies when MIEF1/MiD51/MiD49 are downregulated. Depletion of both MIEF1/MiD51 and MiD49 at the same time resulted in mitochondrial elongation and no effect on morphology was observed when only one of these proteins was depleted (Palmer et al, 2011). The authors propose that Drp1 recruitment is hampered when both proteins are depleted, which would explain these observations. They further suggest that MIEF1/MiD51 and MiD49 also can act as a bona fide fission factor as downregulation of MIEF1/MiD51 and MiD49 delayed CCCP-induced mitochondrial fragmentation in a similar way as downregulation of Drp1. In contrast, Zhao et al (2011) observed enhanced mitochondrial fragmentation upon depletion of MIEF1/Mid51. This is in line with the proposed role of MIEF1/Mid51 as a Drp1 inhibitor but requires that recruitment of Drp1 to mitochondria is maintained. Still, as MiD49 was not depleted in this study, and as Fis1 and Mff were still present, this may still occur sufficiently. These few discrepancies between the two studies can for example be attributed to differences in the way of downregulation and the cell lines used. However, we feel that they point to potential differences in the specific roles of MIEF1/MiD51 and MiD49, and it will be interesting to dissect them in the future.
Taken together, our view on how mitochondrial fission is regulated and which factors are involved has expanded quite a lot with these studies discussed here. How the different Drp1-containing complexes are formed and whether they can dynamically exchange subunits is an open question. Furthermore, it will be an exciting challenge to reveal the detailed interplay of these factors and how the different complexes are regulated.
Acknowledgments
We apologize for not being able to discuss all details of these interesting studies and to cite the work of many colleagues. We acknowledge financial support from the Cluster of Excellence ‘Macromolecular Complexes’ at the Goethe University Frankfurt DFG project EXC 115, the Deutsche Forschungsgemeinschaft DFG project RE-1575/1-1 and the BMBF project GerontoMitoSys.
Footnotes
The authors declare that they do not have any conflict of interest.
References
- Chan DC (2006) Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol 22: 79–99 [DOI] [PubMed] [Google Scholar]
- Gandre-Babbe S, van der Bliek AM (2008) The novel tail-anchored membrane protein Mff controls mitochondrial and peroxisomal fission in mammalian cells. Mol Biol Cell 19: 2402–2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otera H, Wang C, Cleland MM, Setoguchi K, Yokota S, Youle RJ, Mihara K (2010) Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J Cell Biol 191: 1141–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CS, Osellame LD, Laine D, Koutsopoulos OS, Frazier AE, Ryan MT (2011) MiD49 and MiD51, new components of the mitochondrial fission machinery. EMBO Rep 12: 565–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann B (2010) Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol 11: 872–884 [DOI] [PubMed] [Google Scholar]
- Zhao J, Liu T, Jin S, Wang X, Qu M, Uhlén P, Tomilin N, Shupliakov O, Lendahl U, Nistér M (2011) Human MIEF1 recruits Drp1 to mitochondrial outer membranes and promotes mitochondrial fusion rather than fission. EMBO J 30: 2762–2778 [DOI] [PMC free article] [PubMed] [Google Scholar]