Abstract
Background
Glycemic variability has been proposed as a contributing factor in the development of diabetes complications. Multiple measures exist to calculate the magnitude of glycemic variability, but normative ranges for subjects without diabetes have not been described. For treatment targets and clinical research we present normative ranges for published measures of glycemic variability.
Methods
Seventy-eight subjects without diabetes having a fasting plasma glucose of <120 mg/dL (6.7 mmol/L) underwent up to 72 h of continuous glucose monitoring (CGM) with a Medtronic Minimed (Northridge, CA) CGMS® Gold device. Glycemic variability was calculated using EasyGV© software (available free for non-commercial use at www.easygv.co.uk), a custom program that calculates the SD, M-value, mean amplitude of glycemic excursions (MAGE), average daily risk ratio (ADRR), Lability Index (LI), J-Index, Low Blood Glucose Index (LBGI), High Blood Glucose Index (HBGI), continuous overlapping net glycemic action (CONGA), mean of daily differences (MODD), Glycemic Risk Assessment in Diabetes Equation (GRADE), and mean absolute glucose (MAG).
Results
Eight CGM traces were excluded because there were inadequate data. From the remaining 70 traces, normative reference ranges (mean±2 SD) for glycemic variability were calculated: SD, 0–3.0; CONGA, 3.6–5.5; LI, 0.0–4.7; J-Index, 4.7–23.6; LBGI, 0.0–6.9; HBGI, 0.0–7.7; GRADE, 0.0–4.7; MODD, 0.0–3.5; MAGE-CGM, 0.0–2.8; ADDR, 0.0–8.7; M-value, 0.0–12.5; and MAG, 0.5–2.2.
Conclusions
We present normative ranges for measures of glycemic variability in adult subjects without diabetes for use in clinical care and academic research.
Introduction
In clinical practice, the overall assessment of plasma glucose control is typically performed using hemoglobin A1c (HbA1c) in combination with fasting blood glucose and self-monitored capillary blood glucose profiles. HbA1c is the “gold standard” measure of glycemic exposure as it provides a biologically integrated indication of average glucose control during the 6–8 weeks prior to sampling.1 Both the Diabetes Control and Complications Trial (DCCT) and the United Kingdom Prospective Diabetes Study demonstrated that a lower HbA1c was associated with a reduction in the incidence of micro- and macrovascular complications.2–5 HbA1c targets are a key part of the American Diabetes Association's guidelines6 for optimizing diabetes control.
Despite HbA1c being the “gold standard” for the measure of glycemia, the DCCT showed that the progression of retinopathy associated with a given mean level of HbA1c was significantly lower in those patients treated intensively than in those treated conventionally over 5 to 9 years.7 Thus, for a given mean HbA1c, the incidence of retinopathy was increased in the conventional treatment group, suggesting an additional benefit from intensive insulin treatment above that accrued from HbA1c. It was suggested that the part of the additional microvascular risk seen in the conventional treatment group may be attributable to excess glycemic variability. This analysis was later retracted8 by the DCCT Study Group, which concluded that glucose variation may contribute to the risk of complications but can only explain a small part of the differences in risk between intensive and conventional therapy. In a separate analysis of the DCCT data assessing the relationship between variability and complication status, neither SD or the area under the curve of seven-point profile capillary glucose correlated with development or progression of neuropathy or retinopathy.9 However, this observation was based on using quarterly seven-point capillary blood glucose profiles and was limited to SD as a measure of variability.
Despite the reduction in microvascular complications in the intensive arm of the DCCT and the later retraction, an increased risk of hypoglycemia was observed that was not solely explained by the difference in HbA1c values. A re-analysis of the DCCT study has established that HbA1c, mean blood glucose, and glucose variability measurements each have an independent role in determining an individual's risk of hypoglycemia in type 1 diabetes.10
In clinical practice, glycemic variability may contribute in part to the HbA1c though its role remains uncertain. In vitro and in vivo data support glycemic variability as an independent risk factor for hypoglycemia, oxidative stress, endothelial dysfunction, and microvascular complications.11–15 However, these data are controversial. Mean amplitude of glycemic excursions (MAGE) was found to correlate significantly with oxidative stress as measured by urine isoprostane excretion in subjects with type 2 diabetes,13 but a similar study examining the relationship in subjects with type 1 diabetes and using a different assay found no association.16 So, although glycemic variations over time may play a role in the etiology of endothelial dysfunction and oxidative damage through a variety of pathways, the evidence is inconclusive.
With the introduction of continuous glucose monitoring (CGM) a more detailed glucose time series can be constructed that overcomes the problem of unrepresentative data inherent when irregular and infrequent glucose sampling is undertaken. CGM data sets are no different from other time series and can be analyzed using the same techniques, such as Fourier transformation and serial data array averaging.17 Simply defining the mean value of a data set plus its dispersion (a marker of variance) remains the most useful starting point in describing CGM data sets.
With the increased availability of CGM, clinicians face a considerable challenge in handling the large data sets generated. The situation is made the more daunting by the multiple modalities for estimation of glycemic variability, quality of glycemia, and glycemic risk18–26 that have been described and the lack or normative data sets with which to compare their data. Ambulatory glucose profiles have been described for subjects with normal glucose tolerance, demonstrating changes in glucose over time,27 and CGM has been used to demonstrate abnormal glucose profiles in insulinoma, cystic fibrosis, and bariatric surgery, among others.28–30 Here, we describe normative reference ranges for all of the described methods of variability assessment in subjects without diabetes from varying ethnic origins.
Subjects and Methods
Seventy-eight subjects were recruited by general advertisement. Prior to CGM they had a fasting laboratory plasma glucose measured and were included in the study if fasting glucose was less than 120 mg/dL (6.7 mmol/L). Subjects, with an overall equal male to female ratio (39:39), were drawn from the American white (n=44), Hispanic (n=13), Asian (n=7), and African American (n=6) populations. A CGM system (CGMS® Gold, Medtronic MiniMed, Northridge, CA) sensor measures subcutaneous tissue interstitial glucose levels continuously, recording values every 5 min, within a range of 40–400 mg/dL (2.2–22.2 mmol/L). The sensor was implanted in the anterior abdominal wall. Subjects were instructed in the use of the device, including calibration and management of sensor alarms. The monitoring period was up to 72 h (mean, 66 h) with calibration from capillary blood glucose samples at a minimum of 12 h.
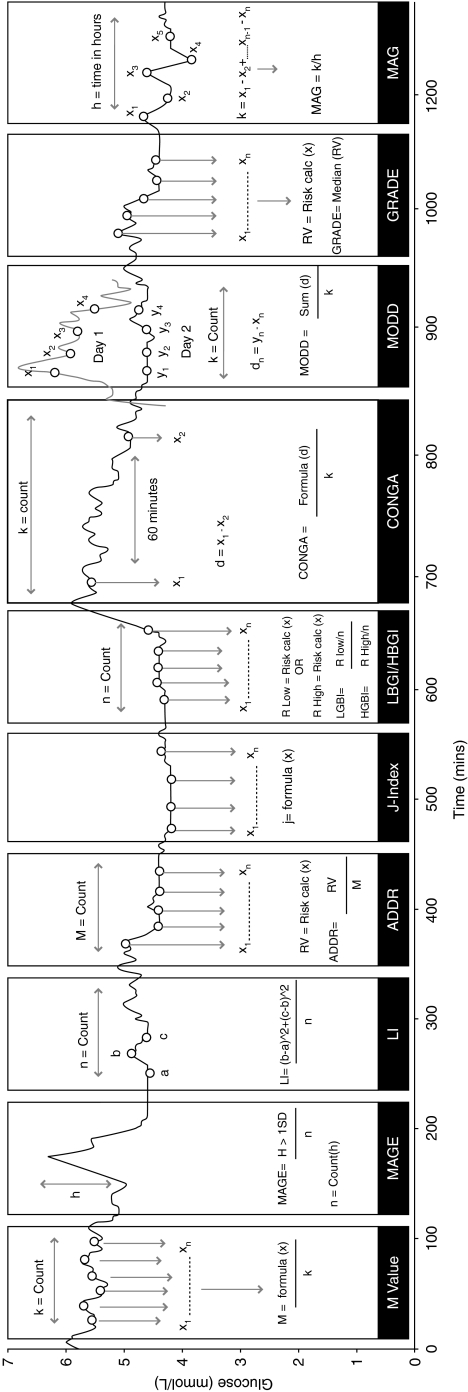
The mathematical formulae of the methods of assessment for glucose variability (Table 1) were taken from their original publications for inclusion in a computer program, EasyGV© (available free for non-commercial use at www.easygv.co.uk). The implementation and use of the methods of assessment have been exemplified in Figure 1. The EasyGV is used to calculate the following measures of glycemic variability:
Table 1.
Measures of Glycemic Variability with the Equations for Glucose Measurements
| Measure | Formulae | Variables |
|---|---|---|
| M-value*15 | 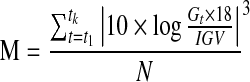 |
G=glucose measured |
| IGV=ideal glucose value | ||
| k=total number of observations | ||
| N=total number of readings | ||
| MAGE16 | 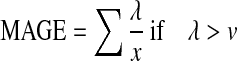 |
λ=blood glucose changes from peak to nadir |
| x=number of valid observations | ||
| v=1 SD of mean glucose for a 24-h period | ||
| Lability Index17 | 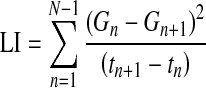 |
G=glucose measured |
| N=total number of readings in a week | ||
| t=time | ||
| ADRR*18 | 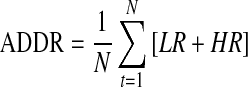 |
N=total number of readings |
| LR=risk value attributed to low glucose | ||
| HR=risk value attributed to high glucose | ||
| J-Index19 | J=0.324×(MBG+SD)2 | MBG=mean glucose levels |
| SD=SD of glucose levels | ||
| LBGI/HBGI*20 | 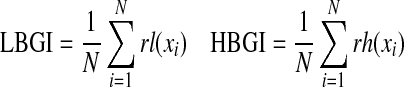 |
N=number of readings |
| rl=risk value associated with a low glucose (if x<0) | ||
| rh=risk value associated with a high glucose (if x>0) | ||
| x=nonlinear transformation of glucose measured | ||
| CONGA21 |
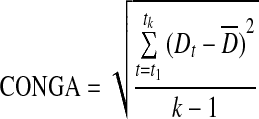 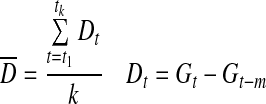
|
k=number of observations with an observation n×60 min ago |
| m=n×60 | ||
| G=glucose measured | ||
| MODD22 | 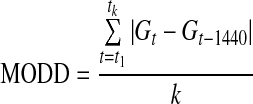 |
k=number of observations with an observation 24 h ago |
| G=glucose measured | ||
| t=time (in min) | ||
| GRADE*23 | GRADE=median(425×{log[log(Gn)]+0.16}2) | G=glucose measured |
| MAG24 | 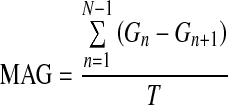 |
G=glucose measured |
| N=number of glucose measurements | ||
| T=total time (in h) |
Glucose was measured in mmol/L.
Indicates methods that assess the quality of glycemia. Three of the measures defined good control in people with type 2 diabetes: M-value, 0≤M≤18 is good control, 19≤M≤31 is fair control, and 32≤M is poor control; J-Index, 10≤J≤20 is ideal control, 20<J≤30 is good control, 30<J≤40 is poor control, and J>40 is lack of control; and Glycemic Risk Assessment in Diabetes Equation (GRADE), median GRADE <5 is good control.
ADRR, average daily risk ratio; CONGA, continuous overlapping net glycemic action; HBGI, High Blood Glucose Index; LBGI, Low Blood Glucose Index; LI, Lability Index; MAG, mean absolute glucose; MAGE, mean amplitude of glucose excursions; MODD, mean of daily differences.
FIG. 1.
Graphical illustration of how each of the 10 methods of glycemic variability assessment are calculated from a continuous glucose monitoring trace: average daily risk ratio (ADRR), continuous overlapping net glycemic action (CONGA), Glycemic Risk Assessment in Diabetes Equation (GRADE), High Blood Glucose Index (HBGI), Low Blood Glucose Index (LBGI), J-Index, Lability Index (LI), mean absolute glucose (MAG), mean amplitude of glucose excursions (MAGE), and mean of daily differences (MODD). In practice each method would independently assess the entire trace.
SD
The SD is a widely used measurement of variability used in the assessment of glycemic profiles. It shows how much variation or dispersion there is from the average.
M-value18
The M-value is calculated on each glucose value using a formula and then is divided by the total number of values to produce a mean. The M-value has several different versions, and the initial glucose value can be set in EasyGV. The default of 120 or M120 was used for analysis.
MAGE19
The MAGE is calculated using the formula as the mean height of excursions (greater than 1 SD).
Average daily risk ratio20
The average daily risk ratio (ADRR) is calculated by transforming each glucose value using a formula and then attributing a risk value to the transformed point.
Lability Index21
The Lability Index (LI) formula processes three glucose values to calculate a lability value and then moves to the next three glucose values, and so on. The LI is the mean of these values. The LI time frame can be changed in EasyGV; the default is 60 min, and this was used for analysis.
J-Index22
The J-Index is calculated using a simple formula on each of the data points.
Low Blood Glucose Index and High Blood Glucose Index23
The Low Blood Glucose Index (LBGI) and High Blood Glucose Index (HBGI) formulae are implemented by converting glucose values into risk scores. If the glucose risk score is below 0, then the risk is labeled as LBGI, and if it is above 0, then it is labeled as HBGI.
Continuous overlapping net glycemic action24
Continuous overlapping net glycemic action (CONGA) is calculated by determining the difference between values at different set intervals, and the difference is then applied to the CONGA formula. In EasyGV an operator can change the interval used. The default is 60 min or CONGA1; this was used for the analysis.
Mean of daily differences25
The mean of daily differences (MODD) formula is calculated as the average of the difference between values on different days but at the same time.
Glycemic Risk Assessment in Diabetes Equation26
The Glycemic Risk Assessment in Diabetes Equation (GRADE) formula converts glucose values to a risk score, calculates the median, and provides the risk attributable to hypoglycemia and hyperglycemia.
Mean absolute glucose31
Mean absolute glucose (MAG) calculates the sum of the differences between successive glucose values divided by the total time measured in hours.
The measures of variability may be statistical measures of variability or derivations that are adjusted to provide an estimate of risk. It is appropriate to include these together as clinically they are used to grade the quality of glucose control. The results from each of the methods have been based on the total data available. One of the methods, the MAGE formula, is a mathematical score of glycemic variability based on an operator's definition of where a peak or trough begins and ends. This method was modified (MAGE-CGM) to select a peak or trough based on direction of change (rising or falling) of the preceding and succeeding data points. The MAGE-CGM formula also contains a 15-min lag window for the direction of change based on the known delay between interstitial fluid glucose measurement and plasma glucose concentrations.32
The CGM profiles were analyzed using the methods in the EasyGV program as well as the percentage of time spent less than 63 mg/dL (3.5 mmol/L) and percentage of time spent greater than 126 mg/dL (7.0 mmol/L). Results from the methods of glycemic assessment were assessed for normality using the z-test for skewness33 before normative ranges were defined. The normative ranges were assessed using five representative CGM data sets (mean number of glucose data points, 887) from people with type 1 diabetes having a mean±SD age of 47.8±11.1 years, body mass index of 22.2±3.0 kg/m2, duration of diabetes of 29.6±6.3 years, and HbA1c of 7.5±0.8%. The degree of correlation between the methods of glycemic variability was identified using Spearman's correlation coefficient.
Results
Seventy-eight CGM traces were initially collected. Of these traces, two failed to collect any data, and six were excluded, as their duration was less than 24 h. The remaining 70 traces had a mean (±SD) of 790±78.9 measurements (mean, 66 h), with no difference in mean or SD for glycemia between the ethnic groups. Fasting blood glucose concentrations were all less than 120 mg/dL (6.7 mmol/L). Four ethnic groups with a mean age of 27.9±5.2 years were studied with an equal representation of the sexes.
Time spent less than 63 mg/dL (3.5 mmol/L) was on average 1.2% for all subjects, and the percentage of time spent greater than 126 mg/dL (7.0 mmol/L) was on average 2.1% for all subjects, with no differences between ethnic groups. Therefore 96.7% of the time was spent in the euglycemic state.
The z-test for skewness indicated that the results for LI, HGBI, GRADE, MODD, and ADRR were positively skewed. Log transformation removed the asymmetry for these methods. The geometric mean and SD for these results and the mean and SD for the remaining measures of glycemic variability are shown in Table 2.
Table 2.
Mean and SD for Measures of Glucose and Glycemic Variability in Populations Without Diabetes
| |
|
|
|
Mean (SD) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethnicity | n | Mean (SD) age | Mean CGM count | Glucose | SD | CONGA | LI* | J-Index† | LBGI† | HBGI*† | GRADE*† | MODD*† | MAGE-CGM | ADRR*† | M-value† | MAG |
| Asian | 7 | 29.9 (5.2) | 766.4 | 5.3 (0.4) | 1.7 (0.9) | 4.8 (0.4) | 0.2 (3.5) | 16.0 (3.5) | 2.0 (1.1) | 0.3 (3.8) | 0.2 (2.0) | 0.7 (1.4) | 1.3 (0.7) | 0.6 (2.8) | 2.6 (1.8) | 1.1 (0.6) |
| African American | 6 | 26.7 (5.0) | 712.0 | 5.2 (0.3) | 1.9 (1.1) | 4.7 (0.4) | 0.5 (3.7) | 16.7 (5.5) | 3.7 (2.9) | 0.5 (2.0) | 0.5 (2.2) | 0.9 (1.7) | 1.3 (1.1) | 1.3 (2.3) | 5.5 (5.0) | 1.6 (0.8) |
| Caucasian | 44 | 27.3 (5.8) | 780.9 | 5.0 (0.5) | 1.5 (0.7) | 4.4 (0.6) | 0.4 (1.9) | 13.7 (4.9) | 3.5 (1.9) | 0.4 (4.2) | 0.4 (2.0) | 0.8 (1.3) | 1.4 (0.5) | 0.4 (4.5) | 5.5 (4.1) | 1.4 (0.3) |
| Hispanic | 13 | 27.7 (1.8) | 803.5 | 5.1 (0.4) | 1.3 (0.7) | 4.6 (0.4) | 0.3 (1.7) | 13.6 (4.1) | 2.5 (1.2) | 0.2 (3.1) | 0.2 (2.0) | 0.7 (1.4) | 1.2 (0.7) | 0.4 (3.8) | 3.5 (1.9) | 1.1 (0.3) |
| All | 70 | 27.9 (5.2) | 790.8 | 5.1 (0.5) | 1.5 (0.7) | 4.6 (0.5) | 0.4 (2.2) | 14.3 (4.7) | 3.1 (1.9) | 0.2 (3.8) | 0.4 (2.1) | 0.8 (1.4) | 1.4 (0.7) | 0.5 (4.1) | 4.7 (3.8) | 1.3 (0.4) |
Glucose was measured in mmol/L.
Geometric mean and SD used for non-normally distributed results.
Indicates methods that assess the quality of glycemia.
ADRR, average daily risk ratio; CGM, continuous glucose monitoring; CONGA, continuous overlapping net glycemic action; GRADE, Glycemic Risk Assessment of Diabetes Equation; HBGI, High Blood Glucose Index; LBGI, Low Blood Glucose Index; LI, Lability Index; MAG, mean absolute glucose; MAGE, mean amplitude of glucose excursions; MODD, mean of daily differences.
The normative ranges for each of the methods of glycemic assessment were then defined as the geometric mean±2 SD or the mean±2 SD as appropriate (Table 3). The mean values for the five representative data sets in people with type 1 diabetes were all outside of the normative ranges defined: mean SD, 3.9; CONGA, 8.5; LI, 1.9; J-Index, 50.6; LBGI, 8.7; HBGI, 11.5; GRADE, 7.1; MODD, 4.6; MAGE-CGM, 3.2; ADDR, 24.1; M-value, 20.0; and MAG, 2.5.
Table 3.
Normal Range for the Population Without Diabetes (n=70)
| Method of assessment | Low (mean−2 SD) | High (mean+2 SD) |
|---|---|---|
| SD | 0.0 | 3.0 |
| CONGA | 3.6 | 5.5 |
| LI* | 0.0 | 4.7 |
| J-Index | 4.7 | 23.6 |
| LBGI† | 0.0 | 6.9 |
| HBGI*† | 0.0 | 7.7 |
| GRADE*† | 0.0 | 4.6 |
| MODD* | 0.0 | 3.5 |
| MAGE-CGM | 0.0 | 2.8 |
| ADDR*† | 0.0 | 8.7 |
| M-Value† | 0.0 | 12.5 |
| MAG | 0.5 | 2.2 |
Low and high are defined as mean±2 SD.
Geometric mean±2 SD.
Indicates methods that assess the quality of glycemia.
ADRR, average daily risk ratio; CGM, continuous glucose monitoring; CONGA, continuous overlapping net glycemic action; GRADE, Glycemic Risk Assessment of Diabetes Equation; HBGI, High Blood Glucose Index; LBGI, Low Blood Glucose Index; LI, Lability Index; MAG, mean absolute glucose; MAGE, mean amplitude of glucose excursions; MODD, mean of daily differences.
The Spearman's correlation coefficient between the methods of glycemic variability (Table 4) elucidates the degree to which the methods are related in the normoglycemic range.
Table 4.
Correlation Coefficients Among the Measures of Glycemic Variability on 70 Subjects with Normoglycemia Using a Two-Tailed Spearman Correlation
| CONGA | LI | J-Index | LBGI† | HBGI† | GRADE† | MODD | MAGE-CGM | ADRR† | M-value† | MAG | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SD | Correlation | 0.30* | 0.17 | 0.84** | −0.15 | 0.20 | 0.28* | 0.21 | −0.08 | 0.25* | −0.17 | 0.21 |
| Significance | 0.01 | 0.16 | <0.001 | 0.22 | 0.09 | 0.02 | 0.08 | 0.50 | 0.04 | 0.16 | 0.08 | |
| CONGA | Correlation | −0.18 | 0.70** | −0.88** | 0.10 | 0.08 | −0.06 | −0.03 | 0.35** | −0.87** | −0.13 | |
| Significance | 0.14 | <0.001 | <0.001 | 0.39 | 0.52 | 0.63 | 0.78 | 0.00 | <0.001 | 0.27 | ||
| LI | Correlation | 0.07 | 0.27* | 0.63** | 0.33** | 0.74** | 0.67** | 0.59** | 0.28* | 0.82* | ||
| Significance | 0.55 | 0.02 | <0.001 | 0.00 | <0.001 | <0.001 | <0.001 | 0.02 | 0.01 | |||
| J-Index | Correlation | −0.55** | 0.26* | 0.29* | 0.11 | −0.05 | 0.42** | −0.56** | 0.12 | |||
| Significance | <0.001 | 0.03 | 0.01 | 0.38 | 0.67 | <0.001 | <0.001 | 0.30 | ||||
| LBGI† | Correlation | 0.03 | 0.09 | 0.31** | 0.12 | −0.19 | 0.99** | 0.21 | ||||
| Significance | 0.83 | 0.45 | 0.01 | 0.32 | 0.11 | <0.001 | 0.08 | |||||
| HBGI† | Correlation | 0.38** | 0.51** | 0.40** | 0.93** | −0.01 | 0.45* | |||||
| Significance | 0.00 | <0.001 | <0.001 | <0.001 | 0.91 | 0.01 | ||||||
| GRADE† | Correlation | 0.40** | 0.16 | 0.48** | 0.06 | 0.31* | ||||||
| Significance | <0.001 | 0.20 | <0.001 | 0.60 | 0.01 | |||||||
| MODD | Correlation | 0.57** | 0.51** | 0.30* | 0.60* | |||||||
| Significance | <0.001 | <0.001 | 0.01 | <0.001 | ||||||||
| MAGE-CGM | Correlation | 0.41** | 0.15 | 0.49** | ||||||||
| Significance | <0.001 | 0.22 | <0.001 | |||||||||
| ADRR† | Correlation | −0.22 | 0.44* | |||||||||
| Significance | 0.06 | <0.001 | ||||||||||
| M-value | Correlation | 0.23 | ||||||||||
| Significance | 0.06 |
Significance of correlations: *at the 0.05 level, **at the 0.01 level.
Indicates methods that assess the quality of glycemia.
ADRR, average daily risk ratio; CGM, continuous glucose monitoring; CONGA, continuous overlapping net glycemic action; GRADE, Glycemic Risk Assessment of Diabetes Equation; HBGI, High Blood Glucose Index; LBGI, Low Blood Glucose Index; LI, Lability Index; MAG, mean absolute glucose; MAGE, mean amplitude of glucose excursions; MODD, mean of daily differences.
Conclusions
In this article we describe normative ranges for measures of glycemic variability obtained from using a single-source analytical program, EasyGV. The program makes no attempt to define the best algorithm as each formula makes different assumptions about what constitutes variability. The population chosen is North American and occupies a narrow age range. Values obtained for the M-value, J-Index, and GRADE are in agreement with the originally published descriptions for the methodologies, with mean GRADE less than 5, mean J-Index less than 20, and a mean M-value under 18. The values were obtained from a young population with mean tissue glucose of 91.8 mg/dL (5.1 mmol/L), suggesting that glucose metabolism was within normal limits over the course of the monitoring period. This is confirmed with a mean of 96.7% of time spent in the euglycemic range for all subjects. The difference in the percentage of time spent less than 63 mg/dL (3.5 mmol/L) between the ethnic groups was significant. However, this may be an artifact of the CGM sensor as the accuracy of sensing becomes degraded at low glucose levels. In addition, the average time spent less than 63 mg/dL (3.5 mmol/L) was 1.2%, so it can be heavily affected by a single outlier. The high degree of correlation between the methods of glycemic variability assessment may similarly be related to the fact that these subjects have tight glucose control with a low level of glycemic variability.
The normative ranges provide a guide for both clinical care and academic assessment of glucose variability and may offer an alternative treatment target in people with type 1 and type 2 diabetes. The five representative CGM data sets from people with type 1 diabetes provide some assurance that the normative ranges defined have clinical value, and it should also be noted that the values for glycemic variability reported in other series also fall outside of the normative ranges reported here.15,16 They may be especially useful for guiding treatment in labile type 1 diabetes where assessment of CGM data is used alongside self-monitored capillary blood glucose and HbA1c. They may also provide additional data when HbA1c values are perturbed or uninterpretable as in hemoglobinopathies.
A recent study has reported reference ranges for glycemic variability in Chinese subjects,34 and the data presented here provide ranges for other ethnicities. The data analyzed in this article add to the body of knowledge looking at normal values, allowing for better identification of abnormalities. These are important when looking at glucose excursions in situations such as cystic fibrosis, insulinomas, or post-bariatric surgery. However, larger studies are required to robustly validate the use of variability measures as treatment targets for diabetes.
The inter-relationships between measures of variability were assessed. It should be noted that GRADE, J-Index, ADRR, HBGI, LBGI, and M-value are measures of quality of glycemic control and not specifically variability. Despite this difference, we believe it is appropriate to examine the metrics together as clinically they are all validated to assess glycemia. There is a large level of agreement among the measures, with 38 out of 66 correlations being significant at the P<0.05 level. It is expected that measures dependent on mean tissue glucose and SD, such as the J-Index, will show close correlations with the SD, but we may expect that measures of quality of glucose control will have a poor correlation with other measures of variability. However, this does not seem to be the case with ADRR showing significant correlation with nine out of 11 measures and J-Index, LBGI, and HBGI correlated with seven. Indeed, SD correlated with only four other measures. It remains unclear which of the measures of glycemia calculated represents a “gold standard,” and each is measuring a different facet of glucose change over time, but the general agreement among the values suggests utility in all of them. However, before measures of variability from CGM data can be used for clinical targets it is important to identify what is being measured and the best way to measure it.
We examined a total of 55,000 data points for this analysis, but we acknowledge that the sample could have been greater and could embrace other categories of race and age. These data may, however, allow a baseline on which other data can be used as comparisons. Normative data are essential if hypothesis testing of, for example, glycemic variability as a treatment target in diabetes is to be undertaken.
CGM accuracy remains an ongoing issue in people with diabetes, and peaks and nadirs can be rounded and underestimated by the technology. However, in the data set without diabetes the magnitude and slope of these peaks and troughs are notably less, and accuracy becomes a smaller issue with lower values for the mean absolute difference between correlation points and tissue glucose.
Glucose metabolic status was defined by a fasting laboratory plasma glucose measurement, and, apart from this, no other investigations were carried out to define normoglycemia. We therefore prefer to use the term normative range rather than claim that these profiles represent a standard. The CGM data used for the study were obtained from subjects with a fasting plasma glucose of <120 mg/dL, and although this ensures that the study excludes subjects with diabetes, it is possible that subjects had impaired fasting glucose. However, the mean non-fasting tissue glucose for the study cohort was 5.1 mmol/L, suggesting a normal glucose profile over the study period.
These normative ranges should be applicable to other age groups but need to be explored further in pregnancy and perhaps in children and adolescents. The ranges could be used to guide treatment targets for therapies in type 1 and 2 diabetes as several of the measures are sensitive to episodes of hypoglycemia, thereby providing a more comprehensive estimate of glycemic control than that currently available from finger prick blood glucose testing.
In conclusion, we present normative ranges for measures of glycemic variability in adult subjects without diabetes for use in clinical care and academic research.
Acknowledgments
N.R.H. would like to acknowledge Takeda Pharmaceuticals PLC for the support of his salary. D.R.M and N.R.H. would also like to acknowledge the National Institute for Health Research for their support of this work.
Author Disclosure Statement
No competing financial interest exists for any of the authors.
References
- 1.Goldstein DE. Little RR. Wiedmeyer HM. England JD. McKenzie EM. Glycated hemoglobin: methodologies and clinical applications. Clin Chem. 1986;32:B64–B70. [PubMed] [Google Scholar]
- 2.The effect of intensive treatment of diabetes on development and progression of long-term complications in insulin-dependent diabetes melitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–854. Erratum in: Lancet 1998;352:1558. [PubMed] [Google Scholar]
- 4.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) UK Prospective Diabetes Study (UPKPS) Group. Lancet. 1998;352:854–865. Erratum in: Lancet 1999;354:602. [PubMed] [Google Scholar]
- 5.Nathan DM. Cleary PA. Backlund JY. Genuth SM. Lachin JM. Orchard TJ. Raskin P. Zinman B. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group: Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Diabetes Association: Standards of medical care in diabetes. Diabetes Care. 2005;28(Suppl 1):S4–S36. [PubMed] [Google Scholar]
- 7.The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the Diabetes Control and Complications Trial. Diabetes. 1995;44:968–983. [PubMed] [Google Scholar]
- 8.Lachin JM. Genuth S. Nathan DM. Zinman B. Rutledge BN. Effect of glycemic exposure on the risk of microvascular complications in the Diabetes Control and Complications Trial—revisited. Diabetes. 2008;57:995–1001. doi: 10.2337/db07-1618. [DOI] [PubMed] [Google Scholar]
- 9.Kilpatrick ES. Rigby AS. Atkin SL. The effect of glucose variability on the risk of microvascular complications in type 1 diabetes. Diabetes Care. 2006;29:1486–1490. doi: 10.2337/dc06-0293. [DOI] [PubMed] [Google Scholar]
- 10.Kilpatrick ES. Rigby AS. Goode K. Atkin SL. Relating mean blood glucose and glucose variability to the risk of multiple episodes of hypoglycaemia in type 1 diabetes. Diabetologia. 2007;50:2553–2561. doi: 10.1007/s00125-007-0820-z. [DOI] [PubMed] [Google Scholar]
- 11.Risso A. Mercuri F. Quagliaro L. Damante G. Ceriello A. Intermittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. Am J Physiol Endocrinol Metab. 2001;281:E924–E930. doi: 10.1152/ajpendo.2001.281.5.E924. [DOI] [PubMed] [Google Scholar]
- 12.Quagliaro L. Piconi L. Assaloni R. Martinelli L. Motz E. Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003;52:2795–2804. doi: 10.2337/diabetes.52.11.2795. [DOI] [PubMed] [Google Scholar]
- 13.Piconi L. Quagliaro L. Da Ros R. Assaloni R. Giugliano D. Esposito K. Szabó C. Ceriello A. Intermittent high glucose enhances ICAM-1, VCAM-1, E-selectin and interleukin-6 expression in human umbilical endothelial cells in culture: the role of poly(ADP-ribose) polymerase. J Thromb Haemost. 2004;2:1453–1459. doi: 10.1111/j.1538-7836.2004.00835.x. [DOI] [PubMed] [Google Scholar]
- 14.Schiekofer S. Andrassy M. Chen J. Rudofsky G. Schneider J. Wendt T. Stefan N. Humpert P. Fritsche A. Stumvoll M. Schleicher E. Häring HU. Nawroth PP. Bierhaus A. Acute hyperglycemia causes intracellular formation of CML and activation of ras, p42/44 MAPK, and nuclear factor kappaB in PBMCs. Diabetes. 2003;52:621–633. doi: 10.2337/diabetes.52.3.621. [DOI] [PubMed] [Google Scholar]
- 15.Monnier L. Mas E. Ginet C. Michel F. Villon L. Cristol JP. Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 16.Wentholt IM. Kulik W. Michels RP. Hoekstra JB. DeVries JH. Glucose fluctuations and activation of oxidative stress in patients with type 1 diabetes. Diabetologia. 2008;51:183–190. doi: 10.1007/s00125-007-0842-6. [DOI] [PubMed] [Google Scholar]
- 17.Matthews DR. Time series analysis in endocrinology. Acta Paediatr Scand Suppl. 1988;347:55–62. [PubMed] [Google Scholar]
- 18.Schlichtkrull J. Munck O. Jersild M. The M-valve, an index of blood-sugar control in diabetics. Acta Med Scand. 1965;177:95–102. doi: 10.1111/j.0954-6820.1965.tb01810.x. [DOI] [PubMed] [Google Scholar]
- 19.Service FJ. Molnar GD. Rosevear JW. Ackerman E. Gatewood LC. Taylor WF. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19:644–655. doi: 10.2337/diab.19.9.644. [DOI] [PubMed] [Google Scholar]
- 20.Kovatchev BP. Otto E. Cox D. Gonder-Frederick L. Clarke W. Evaluation of a new measure of blood glucose variability in diabetes. Diabetes Care. 2006;29:2433–2438. doi: 10.2337/dc06-1085. [DOI] [PubMed] [Google Scholar]
- 21.Ryan EA. Shandro T. Green K. Paty BW. Senior PA. Bigam D. Shapiro AM. Vantyghem MC. Assessment of the severity of hypoglycemia and glycemic lability in type 1 diabetic subjects undergoing islet transplantation. Diabetes. 2004;53:955–962. doi: 10.2337/diabetes.53.4.955. [DOI] [PubMed] [Google Scholar]
- 22.Wojcicki JM. “J”-index. A new proposition of the assessment of current glucose control in diabetic patients. Horm Metab Res. 1995;27:41–42. doi: 10.1055/s-2007-979906. [DOI] [PubMed] [Google Scholar]
- 23.Kovatchev BP. Cox DJ. Kumar A. Gonder-Frederick L. Clarke WL. Algorithmic evaluation of metabolic control and risk of severe hypoglycemia in type 1 and type 2 diabetes using self-monitoring blood glucose data. Diabetes Technol Ther. 2003;5:817–828. doi: 10.1089/152091503322527021. [DOI] [PubMed] [Google Scholar]
- 24.McDonnell CM. Donath SM. Vidmar SI. Werther GA. Cameron FJ. A novel approach to continuous glucose analysis utilizing glycemic variation. Diabetes Technol Ther. 2005;7:253–263. doi: 10.1089/dia.2005.7.253. [DOI] [PubMed] [Google Scholar]
- 25.Molnar GD. Taylor WF. Ho MM. Day-to-day variation of continuously monitored glycaemia: a further measure of diabetic instability. Diabetologia. 1972;8:342–348. doi: 10.1007/BF01218495. [DOI] [PubMed] [Google Scholar]
- 26.Hill NR. Hindmarsh PC. Stevens RJ. Stratton IM. Levy JC. Matthews DR. A method for assessing quality of control from glucose profiles. Diabet Med. 2007;24:753–758. doi: 10.1111/j.1464-5491.2007.02119.x. [DOI] [PubMed] [Google Scholar]
- 27.Mazze RS. Strock E. Wesley D. Borgman S. Morgan B. Bergenstal R. Cuddihy R. Characterizing glucose exposure for individuals with normal glucose tolerance using continuous glucose monitoring and ambulatory glucose profile analysis. Diabetes Technol Ther. 2008;10:149–159. doi: 10.1089/dia.2007.0293. [DOI] [PubMed] [Google Scholar]
- 28.Munir A. Choudhary P. Harrison B. Heller S. Newell-Price J. Continuous glucose monitoring in patients with insulinoma. Clin Endocrinol (Oxf) 2008;68:912–918. doi: 10.1111/j.1365-2265.2007.03161.x. [DOI] [PubMed] [Google Scholar]
- 29.O'Riordan SM. Hindmarsh P. Hill NR. Matthews DR. George S. Greally P. Canny G. Slattery D. Murphy N. Roche E. Costigan C. Hoey H. Validation of continuous glucose monitoring in children and adolescents with cystic fibrosis: a prospective cohort study. Diabetes Care. 2009;32:1020–1022. doi: 10.2337/dc08-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marfella R. Barbieri M. Ruggiero R. Rizzo MR. Grella R. Mozzillo AL. Docimo L. Paolisso G. Bariatric surgery reduces oxidative stress by blunting 24-h acute glucose fluctuations in type 2 diabetic obese patients. Diabetes Care. 2010;33:287–289. doi: 10.2337/dc09-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hermanides J. Vriesendorp TM. Bosman RJ. Zandstra DF. Hoekstra JB. Devries JH. Glucose variability is associated with intensive care unit mortality. Crit Care Med. 2010;38:838–842. doi: 10.1097/CCM.0b013e3181cc4be9. [DOI] [PubMed] [Google Scholar]
- 32.Wentholt IM. Hart AA. Hoekstra JB. Devries JH. Relationship between interstitial and blood glucose in type 1 diabetes patients: delay and the push–pull phenomenon revisited. Diabetes Technol Ther. 2007;9:169–175. doi: 10.1089/dia.2006.0007. [DOI] [PubMed] [Google Scholar]
- 33.Altman DG. Pratical Statistics for Medical Research. London: Chapman & Hall; 1991. [Google Scholar]
- 34.Zhou J. Li H. Ran X. Yang W. Li Q. Peng Y. Li Y. Gao X. Luan X. Wang W. Jia W. Establishment of normal reference ranges for glycemic variability in Chinese subjects using continuous glucose monitoring. Med Sci Monit. 2011;17:CR9–CR13. doi: 10.12659/MSM.881318. [DOI] [PMC free article] [PubMed] [Google Scholar]