Abstract
Basal insulin analogs are recognized as an effective method of achieving and maintaining glycemic control for patients with type 2 diabetes. However, the progressive nature of the disease means that some individuals may require additional ways to maintain their glycemic goals. Intensification in these circumstances has traditionally been achieved by the addition of short-acting insulin to cover postprandial glucose excursions that are not targeted by basal insulin. However, intensive insulin regimens are associated with a higher risk of hypoglycemia and weight gain, which can contribute to a greater burden on patients. The combination of basal insulin with a glucagon-like peptide-1 (GLP-1) mimetic is a potentially attractive solution to this problem for some patients with type 2 diabetes. GLP-1 mimetics target postprandial glucose and should complement the activity of basal insulins; they are also associated with a relatively low risk of associated hypoglycemia and moderate, but significant, weight loss. Although the combination has not been approved by regulatory authorities, preliminary evidence from mostly small-scale studies suggests that basal insulins in combination with GLP-1 mimetics do provide improvements in A1c and postprandial glucose with concomitant weight loss and no marked increase in the risk of hypoglycemia. These results are promising, but further studies are required, including comparisons with basal–bolus therapy, before the complex value of this association can be fully appreciated.
Introduction
The last decade has seen a dramatic increase in the number of therapeutic options available for the treatment of type 2 diabetes. Although this upsurge in innovation is to be welcomed, it has created its own challenges that include how best to incorporate new agents into clinical practice in order to maximize the benefits to patients. Treatment algorithms have been devised in order to provide guidance to healthcare professionals and are updated on a regular basis to reflect advances in care. The current recommendations for type 2 diabetes developed by the American Diabetes Association and the European Association for the Study of Diabetes suggest that initial intervention should focus on lifestyle changes and the use of metformin but that basal insulin or a sulfonylurea should be added if A1c levels remain ≥7% for 2–3 months; moreover, basal insulin is recommended for patients with A1c levels >8.5% or who have symptoms associated with hyperglycemia.1 This approach is effective, and numerous studies have shown that basal insulin, in combination with metformin, improves A1c to ≤7% in many patients.2–12 Thiazolidinediones or glucagon-like peptide-1 (GLP-1) mimetics are also alternative options for those who have failed metformin monotherapy, although when the most recent guidleines were written these options were considered as “less well validated” than the core therapies of metformin plus basal insulin or a sulfonylurea.1
However, with disease progression, individuals may require additional means by which to maintain their blood glucose at target levels. Treatment intensification is often achieved by the addition of a short-acting insulin to cover postprandial glucose excursions.13,14 The American Diabetes Association/European Association for the Study of Diabetes consensus statement proposes the add-on of short-acting insulin at mealtimes to correct postprandial hyperglycemia,1 and studies have demonstrated the efficacy of this approach.9,15 This strategy recommends that in patients on basal insulin who are no longer achieving target A1c, one injection of short-acting insulin should be added to a single meal according to blood glucose levels, followed by the addition of further prandial injections if the A1c levels continue to be out of range.1 It should be noted, however, that the more intensively diabetes is treated, the greater the risk of hypoglycemia and weight gain. The very aggressive glycemic targets in the intensive arm of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial were associated with a threefold increase in hypoglycemia episodes compared with a standard regimen (annual incidence of hypoglycemia, 3.1% vs. 1.0%)16 and a twofold increase in the number of patients gaining more than 10 kg in weight (overall incidence, 27.8% vs. 14.1%).17 Therefore, one of the key challenges to implementing intensive therapy is to use strategies to mitigate against the risk of hypoglycemia and weight gain.
The identification of the role of endogenous GLP-1 in postprandial glucose metabolism and the introduction of GLP-1 mimetics into clinical practice have opened another avenue that warrants attention—the combination of basal insulin plus GLP-1 mimetics. Endogenous GLP-1 is secreted in anticipation of a meal and in response to ingested glucose in order to potentiate endogenous glucose-stimulated insulin secretion. Accordingly, GLP-1 mimetics, which target postprandial glucose, should complement the activity of basal insulins, which are typically titrated based on fasting glucose levels. The GLP-1 mimetics that are either approved or in development have varying abilities to replicate the strong prandial effect of endogenous GLP-1. Therefore, the aims of this review are to discuss the potential for combining basal insulin with a GLP-1 mimetic, including a review of the evidence presented to date, and to highlight outstanding questions regarding the clinical use of such a combination.
Basal Insulin and GLP-1 Mimetics: Complementary Therapies for Type 2 Diabetes
Basal insulins provide sustained insulin levels for the entire day, providing good control of fasting and interprandial glucose levels; however, compared with short-acting insulins, basal insulins provide limited control of postprandial glucose excursions. Therefore, for individuals that require treatment intensification, current strategies focus on adding a prandial insulin to the existing basal insulin regimen. Adding GLP-1 mimetic-based therapies may offer an alternative approach to adding prandial insulin. GLP-1 is an incretin that is secreted endogenously and targets receptors on pancreatic β-cells to increase insulin secretion rapidly before blood glucose levels reach high levels after a meal.18,19 In terms of diabetes management, native GLP-1 has been considered for the treatment of type 2 diabetes;20 however, its half-life after subcutaneous injection is only 1.5–3 min in humans.21 Several long-acting GLP-1 mimetics have been developed to overcome this limitation, including exenatide and liraglutide as well as others currently under development.
Exenatide (exendin-4; Byetta®, Amylin Pharmaceuticals [San Diego, CA]/Eli Lilly and Co. [Indianapolis, IN]) is a 39-amino acid peptide, based on the exendin-4 protein found in the saliva of the Gila monster lizard, which can activate the GLP-1 receptor.22 Exenatide was approved in the United States in 2005 for the treatment of diabetes. Exenatide has a mean terminal half-life of 2.4 h and is suitable for twice-daily administration, preferably 30–60 min before the first and last meal of the day.23 An extended-release formulation of exenatide suitable for once-weekly administration is also undergoing clinical development.24 Liraglutide (Victoza®, Novo Nordisk, Bagsvaerd, Denmark) is an acetylated analog of GLP-1, with approximately 97% amino acid sequence homology to endogenous GLP-1(7–37). It was approved by the Food and Drug Administration in 2010. Liraglutide has a half-life of approximately 11–15 h in healthy individuals25,26 and is suitable for once-daily administration at any time, independent of meals. Other GLP-1 mimetics currently in clinical development include lixisenatide (sanofi-aventis, paris, France), taspoglutide (Roche, Basel, Switzerland), albiglutide (GlaxoSmithKline, London, UK), LY2189265 (Eli Lilly), and CJC-1134-PC (ConjuChem, Montreal, QC, Canada).27,28
Many studies and reviews have discussed the clinical advantages of GLP-1 mimetics, which will not be repeated in depth here. Nevertheless, it should be emphasized that GLP-1 mimetics provide significant improvements in glycemic control, with a relatively low risk of associated hypoglycemia. It has also been demonstrated that GLP-1 mimetics are associated with moderate, but significant, weight loss. As would be expected from their mechanism of action, GLP-1 mimetics elicit significant improvements in postprandial glucose levels, thus complementing the improvements in fasting blood glucose levels elicited by basal insulins and providing the rationale for a combination of the two treatment types.29 There is some evidence to suggest that exenatide shows better targeting of postprandial glucose excursions than liraglutide. In a 26-week, head-to-head trial of exenatide and liraglutide, the latter was more effective in reducing fasting blood glucose levels, whereas exenatide was more effective against postprandial glucose excursions than liraglutide.30
Clinical Evidence
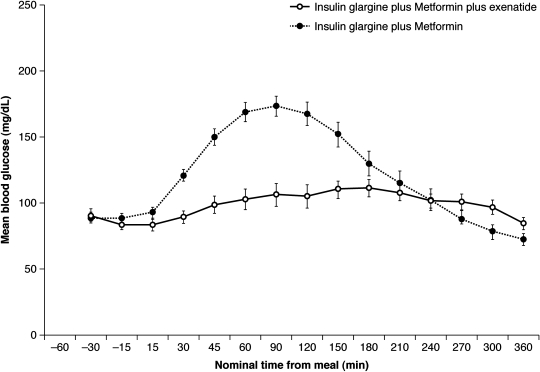
Based on their complementary mechanisms of action, there are increasing reports of the use of basal insulins in combination with GLP-1 mimetics; however, it should be noted that such a practice is not supported by the labeling of any of the current, commercially available GLP-1 mimetics. Kolterman et al.,31 in a 5-day, placebo-controlled crossover study, first showed that the addition of exenatide to once-daily long-acting insulin (with or without oral agents) improved postprandial glucose control in six patients with type 2 diabetes. Table 1 summarizes the clinical data from five subsequent retrospective and prospective studies that have evaluated the efficacy of GLP-1 mimetics in combination with insulin. In a proof-of-concept study by Arnolds et al.,32 patients with diabetes were randomized to one of three groups (insulin glargine plus exenatide, insulin glargine plus sitagliptin, or insulin glargine alone); all patients also received metformin. During the 4-week treatment period, 6-h, postprandial blood glucose excursions were significantly lower with insulin glargine plus exenatide versus insulin glargine alone (Fig. 1). Hemoglobin A1c improved significantly in all three groups, although the study was too short to provide meaningful information in this regard. Weight was unchanged with insulin glargine alone but decreased significantly, albeit slightly, with insulin glargine plus exenatide (−0.9 ± 1.7 kg; P = 0.037). The incidence of hypoglycemia (blood glucose <50 mg/dL) was low in the glargine plus exenatide group, with only two to three events per patient-year. However, the total number of adverse events was higher in the glargine plus exenatide group, compared with both glargine plus sitagliptin and glargine alone groups (62.5% vs. 43.8% and 25.0%, respectively). The majority of events were gastrointestinal disorders, which accounted for 56.3% in the glargine plus exenatide group. By comparison, 19% of patients in the glargine plus sitagliptin group and 16% of patients receiving glargine alone reported gastrointestinal adverse events.
Table 1.
Efficacy of Insulin in Combination with Glucagon-Like Peptide-1 Mimetics
| |
|
A1c (%) |
Weight (kg) |
Total insulin dose (U/day) |
|||
|---|---|---|---|---|---|---|---|
| Study | Design | Baseline | Change | Baseline | Change | Baseline | End point |
| Arnolds et al.32 (2010) | Single-center, randomized, open-label, three-way active-comparator, 4-week study. GLAR dose was titrated to FBG ≤100 mg/dL. Grp 1, GLAR+MET+EXE; Grp 2, GLAR+MET+SITA; Grp 3, GLAR+MET. 48 subjects (16 per grp) | Grp 1, 8.4; Grp 2, 7.9; Grp 3, 7.9 | Grp 1, −1.80; Grp 2, −1.49; Grp 3, −1.23 | Grp 1, 94.2; Grp 2, 97.6; Grp 3, 96.3 | Grp 1, −0.9; Grp 2, 0.1; Grp 3, 0.4 | Grp 1, 40.3; Grp 2, 33.4; Grp 3, 32.3 | Grp 1, 41.1; Grp 2, 35.0; Grp 3, 37.9 |
| Buse et al.33 (2011) | Multinational, multicenter, parallel, randomized, placebo-controlled, 30-week study in patients receiving >20 U/day GLAR with or without MET and/or pioglitazone for at least 3 months. Patients with A1c level ≤8% had baseline GLAR dose reduced by 20% for 5 weeks and then titrated to FBG <100 mg/dL. Grp 1, GLAR + EXE; Grp 2, GLAR + placebo | Grp 1, 8.3; Grp 2, 8.5 | Grp 1, −1.74; Grp 2, −1.04 | Grp 1, 95.4; Grp 2, 93.4 | Grp 1, −1.78; Grp 2, 0.96 | Grp 1, 49.5; Grp 2, 47.4 | Change from baseline: Grp 1, 13; Grp 2, 20 |
| Nayak et al.34 (2010) | Single-center, single cohort/non-randomized, open-label, 6/12-mo study. Patients on large insulin doses were identified. Insulin dose and therapies were titrated to encourage weight loss while tolerating submaximal A1c at no worse than baseline or <10% change, whichever is lower, followed by addition of EXE. Up-titration of insulin was encouraged after 3–6 mo of EXE. Patients were followed up for 6 or 12 mo. 160 subjects (57 completed 12 mo of therapy) | 6 mo, 8.8; 12 mo, 9.2 | 6 mo, −0.2; 12 mo, −0.1 | 6 mo, 121.8; 12 mo, 125.2 | 6 mo, −10.7; 12 mo, −12.8 | 6 mo, 144; 12 mo, 159. 96% using ≥2 inj/day; 4% using 1 inj/day | 6 mo, 51; 12 mo, 55. At 6 mo, 37% using ≥2 inj/day; 24% not using insulin |
| Sheffield et al.35 (2008) | Retrospective review of medical records of three private-practice endocrinologists. Patients (n = 124) treated with EXE in combination with insulin for at least 1 year | 8.39 | −0.87 | 111.1 | −5.2 | 96% using basal insulin, 58% using prandial insulin. Dose: Basal, 48; Bolus, 26 | 96% using basal insulin, 32% using prandial insulin. Dose: Basal, 49; Bolus, 17 |
| Viswanathan et al.36 (2007) | Retrospective review of medical records from a single outpatient clinic. Patients (n = 52) with uncontrolled hyperglycemia and weight gain despite oral hypoglycemic agents plus insulin started EXE. Mean follow-up was 26 weeks. 38 patients continued EXE regularly, and 14 discontinued EXE therapy, with a maximum duration of treatment of ≤2 weeks (REF). | EXE, 7.7; REF, 8.4 | EXE, −0.6; REF, 0.0 | EXE, 116.4; REF, 118.0 | EXE, −6.4; REF, +2.4 | EXE: RAI, 50.4; Mix, 72.9; Basal, 58.4 | EXE: RAI, 36.6; Mix, 28.3; Basal, 53.1 |
| Yoon et al.37 (2009) | Retrospective review of medical records from a single university hospital. Patients (n = 188) were treated with insulin in combination with EXE for up to 27 mo, with 157 followed up for 0–6 mo, 116 for 6–12 mo, 77 for 12–18 mo, and 35 for 18–27 mo. | 8.05 | 0–6 mo, −0.66; 6–12 mo, −0.55; 12–18 mo, −0.54; 18–27 mo, −0.54 | 117.8 | 0–6 mo, −2.4; 6–12 mo, −4.3; 12–18 mo, −6.2; 18–27 mo, −5.4 | Total, 99.9; Basal, 62.9; Bolus, 29.4 | % change in basal/bolus: 0–6 mo, −3.9/−33.5; 6–12 mo, −6.3/−25.9; 12–18 mo, −2.3/−29.7; 18–27 mo, −7.2/−55.7 |
EXE, exenatide; FBG, fasting blood glucose; GLAR, insulin glargine; grp, group; inj, injections; MET, metformin; Mix, premixed insulin; mo, months; RAI, rapid-acting insulin; REF, reference group; SITA, sitagliptin.
FIG. 1.
Mean ± SE blood glucose profiles after 4 weeks of treatment. Adapted by permission of the American Diabetes Association from Arnolds et al.32
The outcomes of a larger-scale and longer term prospective, randomized study of exenatide in combination with insulin glargine were recently reported by Buse et al.33 In this study, patients with type 2 diabetes receiving insulin glargine with metformin and/or pioglitazone and an A1c level of 7.1–10.5% were randomized to receive exenatide (n = 137) or placebo (n = 122) in addition to their existing treatment. Over 30 weeks of treatment, A1c levels were lower in both treatment groups but significantly lower with exenatide versus placebo (P < 0.001). Weight decreased by 1.8 kg with insulin glargine plus exenatide and increased by 1.0 kg with insulin glargine plus placebo. The number of hypoglycemic events per patient-year was comparable between the groups (P = 0.49), but the incidence of gastrointestinal adverse events, such as nausea, vomiting, and diarrhea, was substantially higher in patients receiving exenatide, in line with previous controlled trials of GLP-1 mimetics. Significantly more patients in the glargine plus exenatide group discontinued the study owing to adverse events (9% vs. 1% for placebo, P < 0.01). The total insulin dose increased in both treatment arms. but the increase was significantly lower for patients receiving exenatide. It is interesting to note that this study was performed in a population with an extended duration of disease (mean duration of diabetes, 12 years) and receiving high doses of insulin at baseline (mean insulin dose, 48 U/day).33 Because patients in the early stages of diabetes do not usually receive high doses of insulin, one can assume that these individuals are at a more advanced stage of diabetes, suggesting that the combination of basal insulin and a GLP-1 mimetic may be effective across the spectrum of disease severity, even at a later stage of disease evolution.
The remaining studies published in full to date were either prospective observational studies34 or retrospective analyses of patient records.35–37 These studies consistently showed improvements in A1c and body weight by combining exenatide with insulin, with or without oral antidiabetes drugs, and with duration of therapy of up to 2 years. However, it must be noted that the studies were generally small (n = 52–188) and did not contain a control group, with exception of the study by Viswanathan et al.,36 which included a reference group of individuals who discontinued exenatide because of insurance, personal, or economic reasons. The improvements in A1c and body weight observed in these studies with the combination of exenatide and insulin were offset by increased reports of gastrointestinal side effects, which led to the discontinuation of a small number of patients in each of the studies.34–37 Hypoglycemia was also reported in a small number of patients in each of these studies, although the majority of cases were mild and did not require hospitalization.
In the prospective, observational study by Nayak et al.,34 the A1c change from baseline to 6 or 12 months was small, being only −0.2% and −0.1%, respectively, but weight loss was more marked at −10.7 and −12.8 kg, respectively. These findings reflect the strategy used in that single-center study, as the objective seemed to focus on maximizing weight loss rather than targeting glycemic control. In that study, down-titration of the insulin dose was encouraged to aid weight loss during the first 6 months of exenatide therapy. Hence, the mean insulin dose decreased from 144 U/day to 51 U/day at 6 months, and 24% of patients stopped using insulin altogether. Thus, although the approach used in that study offered good improvements in body weight and a small improvement in systolic (but not diastolic) blood pressure, these positive effects were unfortunately offset by a lack of improvement in glycemic control, and it is unclear whether clinically relevant improvements in other risk factors occurred.
The results from the study by Nayak et al.34 are in accord with those of Hirsch et al.,38 who reported that the current use of a GLP-1 mimetic in combination with insulin seems to be driven by patient characteristics. In that study, patients prescribed exenatide in combination with insulin were more likely to weigh >113 kg and have a body mass index of >40 kg/m2, a Charlson Comorbidity Index of ≥2, and a baseline A1c of >9%. This is a notoriously difficult patient population to treat because of the severity of disease and the dangers of exacerbating their obesity with intensive therapy to reach glycemic targets. In these patients, the prospect of improved control in the absence of weight gain is highly desirable. However, considering the etiology of diabetes and the likelihood of severe β-cell dysfunction in patients with such severe disease, one may ask whether a basal–bolus regimen and marked lifestyle changes may be more appropriate.
It must also be acknowledged that there was no consistency in insulin use in any of the studies because they included patients using basal insulin alone, basal plus prandial insulin, or premixed insulin. These differences limit the ability to conclude which insulin regimen would be most effective when used in combination with a GLP-1 mimetic. Nevertheless, in all studies that included the use of prandial or premixed insulin, the doses of prandial insulin were decreased. In contrast, the dosage of basal insulin generally remained constant, supporting the hypothesis that GLP-1 mimetics would be particularly suitable for use in combination with basal insulins.
Unanswered Questions and Future Studies
The data from the proof-of-concept study by Arnolds et al.32 are interesting, but there is a clear need to evaluate the benefits of a basal insulin in combination with a GLP-1 mimetic in the absence of oral antidiabetes agents. There is also the question of how to adjust insulin and GLP-1 mimetic doses when used in combination. Exenatide is normally administered at a dose of 5 μg for approximately 1 month to improve tolerability and then increased to 10 μg; this was the dosing regimen used in combination with insulin glargine in the study by Buse et al.33 However, in another study, exenatide was continued at 5 μg throughout the observation period,36 whereas in the proof-of-concept study, the dose of exenatide was increased after 2 weeks,32 rather than the 1 month recommended by the label. Therefore, studies should confirm whether the current dosing requirements are still appropriate, or whether lower doses should be used to manage the risk of adverse events, including gastrointestinal events, when combined with insulin. In terms of the insulin dose, all of the studies showed small reductions in dosage, particularly in the first 6 or 12 months of therapy.37 Unfortunately, because of the designs of the study published in full to date, it is not possible to gauge how GLP-1 mimetics affect the basal or prandial components of insulin therapy. One study has indicated that the insulin glargine dose can be up-titrated during exenatide therapy.33 One may speculate that prandial doses would need to be down-titrated to avoid excess postprandial hypoglycemia.
All of the studies published to date have focused on twice-daily exenatide; this is not surprising given the more extensive clinical experience with this drug. In addition to the twice-daily administration, it is also important to determine whether similar improvements in glycemic control are possible with once-daily or once-weekly GLP-1 mimetics. Regimens that require fewer injections are likely to reduce the burden of treatment administration associated with basal–bolus insulin regimens and may therefore increase treatment satisfaction. Indeed, patient convenience and treatment satisfaction should be included in the outcomes of future studies assessing basal insulin and GLP-1 mimetic combinations. Furthermore, two of the main limitations associated with basal–bolus and premixed regimens are weight gain and hypoglycemia; it is, therefore, important to assess all three outcomes in future studies.
Identifying populations that would most benefit from the combination of basal insulin and GLP-1 mimetic combinations is also an area for study. The current treatment paradigm for type 2 diabetes essentially focuses on the progression from lifestyle interventions to pharmacotherapy with metformin and basal insulin, followed by further intensification with addition of prandial insulin. Because a GLP-1 mimetic could theoretically be used in combination with basal insulin, rather than intensifying basal insulin to a basal–bolus or premixed regimen, the efficacy of this approach should be determined. Further studies would be helpful to determine whether the addition of GLP-1 mimetics to basal insulin offer greater or comparable improvements in glycemic control to basal–bolus or premixed insulin regimens.
GLP-1 mimetics potentiate endogenous insulin secretion, and it is conceivable that they may have limited efficacy in patients with long-standing diabetes and limited β-cell functional capacity. Future studies should focus on patients with earlier-stage disease, who are more likely to have good residual β-cell function. For patients with higher A1c, the addition of a prandial insulin may be more effective than the use of GLP-1 mimetics, and head-to-head studies of GLP-1 mimetics versus prandial insulin in patients already on basal insulin would be of interest to better understand which patients would benefit most from each strategy.
The cost-effectiveness of the regimens should also be addressed. Prescription costs for a basal insulin–GLP-1 mimetic combination are likely to be relatively high; however, there is the potential that indirect healthcare cost savings achieved through improvements in glycemic control, reduced risk of hypoglycemia and vascular complications, and the potential for weight loss may offset the direct costs of medication. Table 2 summarizes several studies that are now underway, or have been recently completed, and should provide some insight into some of these, and other questions, and the results of these studies are eagerly awaited.
Table 2.
Ongoing Studies
| Study/phasea | Status | Intervention | Background therapy | Primary outcome | Planned number of patients | Study duration (weeks) | Planned completion date |
|---|---|---|---|---|---|---|---|
| NCT00873223, Phase I | C | LIRA | DET | Effects of LIRA on pharmacokinetics of DET after 12 weeks of therapy | 33 | 12 | September 2009 |
| NCT00667732, Phase IV | C | EXE vs. placebo | GLAR + MET | Change in A1c from baseline to 8, 32, and 58 weeks | 75 | 32 | October 2009 |
| NCT00560417, Phase III | C | LIS-PS vs. GLAR | OADs + EXE | Difference in change in A1c from baseline between LIS-PS and GLAR | 341 | 24 | December 2009 |
| NCT01006889, Phase IV | O | EXE | DET | Effect on hepatic steatosis (assessed noninvasively by MRS) of replacing RAI with EXE while maintaining bedtime DET in well-controlled patients with T2DM and NAFLD | 24 | 6 months | March 2010 |
| NCT00856986, Phase III | O | LIRA + DET vs. LIRA | MET | Change in A1c from baseline | 990 | 26 (with 26-week extension) | April 2010 |
| NCT00866658, Phase III | C | LIXI vs. placebo | Basal insulin ± SU | Change in A1c from baseline | 300 | 24 | June 2010 |
| NCT01076842, Phase IV | R | DET vs. EXE vs. DET + EXE | OADs | Change in A1c from baseline | 75 | 24 | January 2011 |
| NCT00715624, Phase III | O | LIXI vs. placebo | Basal insulin ± MET | Change in A1c from baseline | 450 | 24 | March 2011 |
| NCT00976391, Phase III | O | ALB vs. LIS tid | GLAR ± OADs | Change in A1c from baseline | 500 | 26 | March 2011 |
| NCT01140893, Phase II/III | Pre-rec | EXE vs. placebo | CSII | Change in A1c from baseline | 110 | 6 months | May 2011 |
| NCT00960661, Phase III | R | EXE vs. LIS tid | GLAR + MET | Difference in change in A1c from baseline between EXE and LIS | 740 | 30 | August 2011 |
Studies are ordered by estimated completion date.
Registration number at clinicaltrials.gov.
ALB, albiglutide; C, completed; CSII, continuous subcutaneous insulin infusion; DET, insulin detemir; EXE, exenatide; GLAR, insulin glargine; LIRA, liraglutide; LIS, insulin lispro; LIS-PS, insulin lispro protamine suspension; LIXI, lixisenatide; MET, metformin; MRS, magnetic resonance imaging and spectroscopy; NAFLD, non-alcoholic fatty liver disease; O, ongoing; OAD, oral antidiabetes drug; Pre-rec, pre-recruitment (study is not yet open for participant recruitment); R, recruiting; RAI, rapid-acting insulin; SITA, sitagliptin; SU, sulfonylurea; tid, three times daily; T2DM, type 2 diabetes mellitus.
Conclusions
Preliminary evidence reviewed here suggests that the combination of a basal insulin with a GLP-1 mimetic is a potentially attractive treatment strategy for some patients with type 2 diabetes. Most of the studies to date have considered the combination in obese patients with very poor glycemic control, but the optimal target population remains to be determined. In terms of the sequence for introduction, the American Diabetes Association/European Association for the Study of Diabetes consensus algorithm currently recommends that both basal insulin and GLP-1 mimetics are appropriate for patients failing lifestyle changes and metformin therapy, although basal insulin (or a sulfonylurea) is preferred, based on efficacy and the wealth of clinical experience.1 GLP-1 mimetics are advocated for special populations where hypoglycemia may be particularly hazardous or where weight gain is a concern.1 The majority of patients will receive basal insulin first, and future studies should evaluate adding a GLP-1 mimetic for patients with postprandial glucose levels poorly controlled on basal insulin and oral agents.
Traditionally, intensification has been achieved by moving to a basal plus regimen (basal insulin plus a single short-acting insulin injection) or with premixed insulin preparations. For many patients these strategies are successful, but some patients may find that intensive insulin therapy increases the burden of care because of an increased risk of hypoglycemia and weight gain.39–41 Basal insulin in combination with a GLP-1 mimetic may provide improvements in glycemic control with the benefit of better weight management, avoidance of hypoglycemia, and increased treatment satisfaction. However, the addition of a GLP-1 mimetic may also increase the incidence of gastrointestinal side effects in some patients. Clinicians and patients must remember that the combination of an insulin analog and a GLP-1 mimetic remains off-label, and there are still many questions to be answered. Studies are now underway, or have recently been completed, to help provide answers to these questions, and their findings should help inform optimal management of type 2 diabetes in the future.
Acknowledgments
Editorial support for this article was provided by Huw Jones, Ph.D. of Medicus International and was funded by sanofi-aventis.
Author Disclosure Statement
R.P. is an employee of sanofi-aventis.
References
- 1.Nathan DM. Buse JB. Davidson MB. Ferrannini E. Holman RR. Sherwin R. Zinman B. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blickle JF. Hancu N. Piletic M. Profozic V. Shestakova M. Dain MP. Jacqueminet S. Grimaldi A. Insulin glargine provides greater improvements in glycaemic control vs. intensifying lifestyle management for people with type 2 diabetes treated with OADs and 7–8% A1c levels. The TULIP study. Diabetes Obes Metab. 2009;11:379–386. doi: 10.1111/j.1463-1326.2008.00980.x. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch IB. Yuan H. Campaigne BN. Tan MH. Impact of prandial plus basal vs basal insulin on glycemic variability in type 2 diabetic patients. Endocr Pract. 2009;15:343–348. doi: 10.4158/EP08308.ORR. [DOI] [PubMed] [Google Scholar]
- 4.Janka HU. Plewe G. Riddle MC. Kliebe-Frisch C. Schweitzer MA. Yki-Jarvinen H. Comparison of basal insulin added to oral agents versus twice-daily premixed insulin as initial insulin therapy for type 2 diabetes. Diabetes Care. 2005;28:254–259. doi: 10.2337/diacare.28.2.254. [DOI] [PubMed] [Google Scholar]
- 5.Swinnen SG. Snoek FJ. Dain MP. DeVries JH. Hoekstra JB. Holleman F. Rationale, design, and baseline data of the insulin glargine (Lantus) versus insulin detemir (Levemir) Treat-To-Target (L2T3) study: a multinational, randomized noninferiority trial of basal insulin initiation in type 2 diabetes. Diabetes Technol Ther. 2009;11:739–743. doi: 10.1089/dia.2009.0044. [DOI] [PubMed] [Google Scholar]
- 6.Yki-Järvinen H. Kauppinen-Mäkelin R. Tiikkainen M. Vähätalo M. Virtamo H. Nikkilä K. Tulokas T. Hulme S. Hardy K. McNulty S. Hänninen J. Levänen H. Lahdenperä S. Lehtonen R. Ryysy L. Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetologia. 2006;49:442–451. doi: 10.1007/s00125-005-0132-0. [DOI] [PubMed] [Google Scholar]
- 7.Fonseca V. Bell DS. Berger S. Thomson S. Mecca TE. A comparison of bedtime insulin glargine with bedtime neutral protamine Hagedorn insulin in patients with type 2 diabetes: subgroup analysis of patients taking once-daily insulin in a multicenter, randomized, parallel group study. Am J Med Sci. 2004;328:274–280. doi: 10.1097/00000441-200411000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Gerstein HC. Yale JF. Harris SB. Issa M. Stewart JA. Dempsey E. A randomized trial of adding insulin glargine vs. avoidance of insulin in people with Type 2 diabetes on either no oral glucose-lowering agents or submaximal doses of metformin and/or sulphonylureas. The Canadian INSIGHT (Implementing New Strategies with Insulin Glargine for Hyperglycaemia Treatment) Study. Diabet Med. 2006;23:736–742. doi: 10.1111/j.1464-5491.2006.01881.x. [DOI] [PubMed] [Google Scholar]
- 9.Holman RR. Farmer AJ. Davies MJ. Levy JC. Darbyshire JL. Keenan JF. Paul SK. Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med. 2009;361:1736–1747. doi: 10.1056/NEJMoa0905479. [DOI] [PubMed] [Google Scholar]
- 10.Massi Benedetti M. Humburg E. Dressler A. Ziemen M. A one-year, randomised, multicentre trial comparing insulin glargine with NPH insulin in combination with oral agents in patients with type 2 diabetes. Horm Metab Res. 2003;35:189–196. doi: 10.1055/s-2003-39080. [DOI] [PubMed] [Google Scholar]
- 11.Riddle MC. Rosenstock J. Gerich J. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080–3086. doi: 10.2337/diacare.26.11.3080. [DOI] [PubMed] [Google Scholar]
- 12.Rosenstock J. Schwartz SL. Clark CM., Jr Park GD. Donley DW. Edwards MB. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care. 2001;24:631–636. doi: 10.2337/diacare.24.4.631. [DOI] [PubMed] [Google Scholar]
- 13.Monnier L. Colette C. Dunseath GJ. Owens DR. The loss of postprandial glycemic control precedes stepwise deterioration of fasting with worsening diabetes. Diabetes Care. 2007;30:263–269. doi: 10.2337/dc06-1612. [DOI] [PubMed] [Google Scholar]
- 14.Peter R. Dunseath G. Luzio SD. Chudleigh R. Choudhury SR. Owens DR. Relative and absolute contributions of postprandial and fasting plasma glucose to daytime hyperglycaemia and HbA(1c) in subjects with Type 2 diabetes. Diabet Med. 2009;26:974–980. doi: 10.1111/j.1464-5491.2009.02809.x. [DOI] [PubMed] [Google Scholar]
- 15.Owens DR. Adding a single dose of insulin glulisine to basal insulin glargine plus oral antihyperglycemic drug therapy improves glycemic control in type 2 diabetes: a 6-month proof-of-concept study [abstract] Diabetes. 2009;58:A122. [Google Scholar]
- 16.Miller ME. Bonds DE. Gerstein HC. Seaquist ER. Bergenstal RM. Calles-Escandon J. Childress RD. Craven TE. Cuddihy RM. Dailey G. Feinglos MN. Ismail-Beigi F. Largay JF. O'Connor PJ. Paul T. Savage PJ. Schubart UK. Sood A. Genuth S. The effects of baseline characteristics, glycaemia treatment approach, glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444. doi: 10.1136/bmj.b5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Action to Control Cardiovascular Risk in Diabetes Study Group: Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ebert R. Creutzfeldt W. Gastrointestinal peptides and insulin secretion. Diabetes Metab Rev. 1987;3:1–26. doi: 10.1002/dmr.5610030101. [DOI] [PubMed] [Google Scholar]
- 19.Kreymann B. Williams G. Ghatei MA. Bloom SR. Glucagon-like peptide-1 7–36: a physiological incretin in man. Lancet. 1987;2:1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- 20.Gutniak MK. Linde B. Holst JJ. Efendic S. Subcutaneous injection of the incretin hormone glucagon-like peptide 1 abolishes postprandial glycemia in NIDDM. Diabetes Care. 1994;17:1039–1044. doi: 10.2337/diacare.17.9.1039. [DOI] [PubMed] [Google Scholar]
- 21.Vilsboll T. Agerso H. Krarup T. Holst JJ. Similar elimination rates of glucagon-like peptide-1 in obese type 2 diabetic patients and healthy subjects. J Clin Endocrinol Metab. 2003;88:220–224. doi: 10.1210/jc.2002-021053. [DOI] [PubMed] [Google Scholar]
- 22.Goke R. Fehmann HC. Linn T. Schmidt H. Krause M. Eng J. Goke B. Exendin-4 is a high potency agonist and truncated exendin-(9–39)-amide an antagonist at the glucagon-like peptide 1-(7–36)-amide receptor of insulin-secreting beta-cells. J Biol Chem. 1993;268:19650–19655. [PubMed] [Google Scholar]
- 23.Datapharm Communications: Summary of Product Characteristics: Byetta 5 micrograms solution for injection, prefilled pen. Byetta 10 micrograms solution for injection, prefilled pen. www.medicines.org.uk/emc/medicine/19257#PHARMACOLOGICAL_PROPS. 2010. [Dec 21;2010 ]. www.medicines.org.uk/emc/medicine/19257#PHARMACOLOGICAL_PROPS
- 24.Malone J. Trautmann M. Wilhelm K. Taylor K. Kendall DM. Exenatide once weekly for the treatment of type 2 diabetes. Expert Opin Investig Drugs. 2009;18:359–367. doi: 10.1517/13543780902766802. [DOI] [PubMed] [Google Scholar]
- 25.Agerso H. Jensen LB. Elbrond B. Rolan P. Zdravkovic M. The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia. 2002;45:195–202. doi: 10.1007/s00125-001-0719-z. [DOI] [PubMed] [Google Scholar]
- 26.Elbrond B. Jakobsen G. Larsen S. Agerso H. Jensen LB. Rolan P. Sturis J. Hatorp V. Zdravkovic M. Pharmacokinetics, pharmacodynamics, safety, and tolerability of a single-dose of NN2211, a long-acting glucagon-like peptide 1 derivative, in healthy male subjects. Diabetes Care. 2002;25:1398–1404. doi: 10.2337/diacare.25.8.1398. [DOI] [PubMed] [Google Scholar]
- 27.Madsbad S. Kielgast U. Asmar M. Deacon C. Torekov SS. Holst JJ An overview of once-weekly GLP-1 receptor agonists—available efficacy, safety data, perspectives for the future. Diabetes Obes Metab 2011 Jan 5. [Epub ahead of print]. [DOI] [PubMed]
- 28.Thorkildsen C. Neve S. Larsen BD. Meier E. Petersen JS. Glucagon-like peptide 1 receptor agonist ZP10A increases insulin mRNA expression and prevents diabetic progression in db/db mice. J Pharmacol Exp Ther. 2003;307:490–496. doi: 10.1124/jpet.103.051987. [DOI] [PubMed] [Google Scholar]
- 29.Brodows RG. Qu Y. Johns D. Kim D. Holcombe JH. Quantifying the effect of exenatide and insulin glargine on postprandial glucose excursions in patients with type 2 diabetes. Curr Med Res Opin. 2008;24:1395–1397. doi: 10.1185/030079908x297268. [DOI] [PubMed] [Google Scholar]
- 30.Buse JB. Rosenstock J. Sesti G. Schmidt WE. Montanya E. Brett JH. Zychma M. Blonde L. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 31.Kolterman OG. Buse JB. Fineman MS. Gaines E. Heintz S. Bicsak TA. Taylor K. Kim D. Aisporna M. Wang Y. Baron AD. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2003;88:3082–3089. doi: 10.1210/jc.2002-021545. [DOI] [PubMed] [Google Scholar]
- 32.Arnolds S. Dellweg S. Clair J. Dain MP. Nauck MA. Rave K. Kapitza C. Further improvement in postprandial glucose control with addition of exenatide or sitagliptin to combination therapy with insulin glargine and metformin: a proof-of-concept study. Diabetes Care. 2010;33:1509–1515. doi: 10.2337/dc09-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buse JB. Bergenstal RM. Glass LC. Heilmann CR. Lewis MS. Kwan AY. Hoogwerf BJ. Rosenstock J. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2011;154:103–112. doi: 10.7326/0003-4819-154-2-201101180-00300. [DOI] [PubMed] [Google Scholar]
- 34.Nayak UA. Govindan J. Baskar V. Kalupahana D. Singh BM. Exenatide therapy in insulin-treated type 2 diabetes and obesity. QJM. 2010;103:687–694. doi: 10.1093/qjmed/hcq112. [DOI] [PubMed] [Google Scholar]
- 35.Sheffield CA. Kane MP. Busch RS. Bakst G. Abelseth JM. Hamilton RA. Safety and efficacy of exenatide in combination with insulin in patients with type 2 diabetes mellitus. Endocr Pract. 2008;14:285–292. doi: 10.4158/EP.14.3.285. [DOI] [PubMed] [Google Scholar]
- 36.Viswanathan P. Chaudhuri A. Bhatia R. Al-Atrash F. Mohanty P. Dandona P. Exenatide therapy in obese patients with type 2 diabetes mellitus treated with insulin. Endocr Pract. 2007;13:444–450. doi: 10.4158/EP.13.5.444. [DOI] [PubMed] [Google Scholar]
- 37.Yoon NM. Cavaghan MK. Brunelle RL. Roach P. Exenatide added to insulin therapy: a retrospective review of clinical practice over two years in an academic endocrinology outpatient setting. Clin Ther. 2009;31:1511–1523. doi: 10.1016/j.clinthera.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 38.Hirsch I. Silberman C. Calingaert B. Patient factors associated with GLP-1 analog use with, without insulin in type 2 diabetes (T2D): results from a large cohort analysis [abstract] Diabetes. 2010;59:601-P. [Google Scholar]
- 39.Redekop WK. Koopmanschap MA. Stolk RP. Rutten GE. Wolffenbuttel BH. Niessen LW. Health-related quality of life and treatment satisfaction in Dutch patients with type 2 diabetes. Diabetes Care. 2002;25:458–463. doi: 10.2337/diacare.25.3.458. [DOI] [PubMed] [Google Scholar]
- 40.Davis RE. Morrissey M. Peters JR. Wittrup-Jensen K. Kennedy-Martin T. Currie CJ. Impact of hypoglycaemia on quality of life and productivity in type 1 and type 2 diabetes. Curr Med Res Opin. 2005;21:1477–1483. doi: 10.1185/030079905X61929. [DOI] [PubMed] [Google Scholar]
- 41.Bode BW. Testa MA. Magwire M. Hale PM. Hammer M. Blonde L. Garber A. Patient-reported outcomes following treatment with the human GLP-1 analogue liraglutide or glimepiride in monotherapy: results from a randomized controlled trial in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12:604–612. doi: 10.1111/j.1463-1326.2010.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]