Abstract
In vivo investigations have confirmed the beneficial effects of hydrophilic, cationic Mn(III) -based catalytic antioxidants in models of oxidative stress. We hypothesized the octyl porphyrin, MnTnOct-2-PyP5+, a lipophilic but equally potent antioxidant, would be more efficacious in reducing oxygen and glucose deprivation (OGD)-induced cell death. Using a cell culture model of rat mixed neuronal/glial cells, we investigated the effect of MnTnOct-2-PyP5+ on the OGD-induced cell death as compared to the effects of widely studied hydrophilic analogues MnTE-2-PyP5+ and MnTDE-2-ImP5+ P and a standard compound, dizocilpine (MK-801). Cell death was evaluated at 24 hours using lactate dehydrogenase (LDH) release, 3-(4,5-dimethyltiazol-2-yl) -2,5 diphenyltetrazolium bromide(MTT) and propidium iodide staining. At lower concentrations, all three porphyrins reduced cell death as compared to cultures exposed to OGD alone. When the cultures were exposed to MnTnOct-2-PyP5+ before OGD but not during the deprivation, it was a very efficacious compound as judged by LDH release. MnTnOct-2-PyP5+ becomes less efficacious if the exposure was prolonged. While no extensive toxicity was seen with MnTnOct-2-PyP5+ P , the effects observed, though, might have been the result of the interplay of efficacy and toxicity leading to a diminished protectiveness.
Keywords: MnTE-2-PyP5+ (AEOL10113), MnTnOct-2-PyP5+, MnTDE-2-ImPP5+ (AEOL10150), neuronal/glial cell culture, oxygen and glucose deprivation (OGD)
Introduction
Oxidative stress is a key intracellular pathological condition that mediates neuronal death in the presence of oxygen and glucose deprivation (OGD) [1]. Recent pharmacological advances have allowed for the design of different SOD mimics such as Mn salen derivatives [2], Mn cyclic polyamines [3], nitroxide [4], MitoQ series of compounds [5,6], and Mn porphyrins [7–10]. Most of them scavenge/reduce peroxynitrite, though, also with different efficacy, Mn porphyrins being the most efficacious log kred ≥ 7.5 [7–10]. We have shown that Mn(III) ortho isomeric positively charged N-alkylpyridyl- or N,N’-dialkylimidazolylporphyrins (MnTalkyl-2-PyP5+, MnTDalkyl-2-ImP5+) are among the most effective synthetic antioxidants in scavenging both superoxide and peroxynitrite [11,12]. They offer remarkable protection in all diseases that have oxidative stress in common including central nervous system injuries such as ALS, cancer, diabetes, morphine tolerance [13–18]. MnTE-2-PyP5+ and MnTDE-2-ImP5+ were beneficial in middle cerebral artery occlusion models when given as late as 6h after the insult [19].
We have recently reported that if catalytic potency in scavenging superoxide and peroxynitrite is maintained, but lipophilicity is increased, the efficacy in vivo increases tremendously [20]. We increased lipophilicity by increasing the length of the N-alkylpyridyl chains from 1 to 8 carbon atoms which increased liopophilicity up to 9-fold [Table 1] [20]. Thus, MnTnHex-2-PyP5+ that is 4.3-fold more lipophilic than MnTE-2-PyP5+, is 120-fold more protective; it is efficacious in several models of oxidative stress (ALS, E. coli, kidney ischemia/reperfusion, morphine tolerance, radioprotection) at lowest level (0.05 mg/kg) among synthetic antioxidants tested[14,21–24]
Table 1.
The properties of Mn porphyhrins: the ability to catalyze dismutation of O2•− (kcat) , the redox ability, i.e. the metal centered redox potential (E1/2), and the lipophilicity as given by the ratio of the compound and solvent path on TLC silica plates (solvent KNO3sat H2O:H2O: acetonitrile= 1:1+8).
| Compound | logk cat | E1/2, mV vs NHE | Rf |
|---|---|---|---|
| MnTE-2-PyP5+ a | 7.76 | +228 | 0.13 |
| MnTDE-2-ImP5+ b | 7.83 | +346 | 0.17 |
| MnTnHex-2-PyP5+ a | 7.48 | +314 | 0.57 |
| MnTnOct-2-PyP5+ a | 7.71 | +367 | 0.80 |
ref Batinic-Haberle et al, Dalton 2002
ref Batinic-Haberle et al Dalton 2004
Herein for the first time we tested the octyl analogue, MnTnOct-2-PyP5+ (Figure 1, Table 1} in a mammalian system of oxidative stress. MnTnOct-2-PyP5+ was as effective as hexyl in protecting SOD-deficient E. coli to grow aerobically [21]. Both hexyl and octyl porphyrins have partial micellar character and are thus toxic at higher doses. Yet the TD50 determined for mice to be 12.5 mg/kg (subcutaneously) is 250 higher than its effective dose of 0.05 mg/kg while that ratio is 15 with MnTE-2-PyP5+ allowing thus wider therapeutic window with MnTnHex-2-PyP5+ [Moen Panni unpublished]. The same is likely valid with MnTnOct-2-PyP5+; therefore this compound may be regarded a prospective therapeutic also.
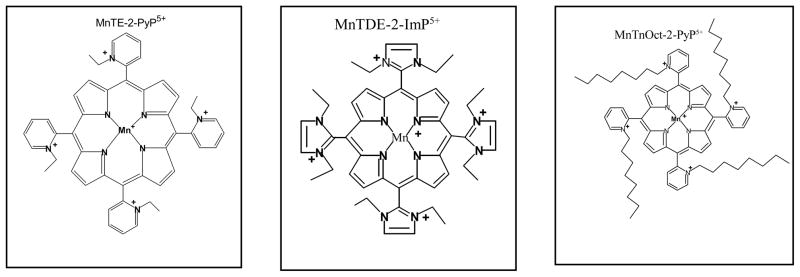
Figure 1.
Schematic drawings of cationic ortho N Mn(III) -alkylpyridyl- and diortho N,N’-imidazolylporphyrin-based catalytic antioxidants MnTE-2-PyP5+ (AEOL10113) and MnTDE-2-ImP5+ (AEOL 10150) in addition to an even more lipophilic but equally potent antioxidant, the octyl porphyrin, MnTnOct-2-PyP5+ .
The enhanced bioavailability of MnTnOct-2-PyP5+ should promote improved neuronal survival as compared to hydrophilic compounds with fewer carbon atoms in the alkylpyridyl chains [20, 25, 26]. This hypothesis was tested in mixed neuronal/glial cortical cell cultures exposed to oxygen and glucose deprivation.
Methods
All animal procedures were approved by the Duke University Animal Care and Use Committee.
Preparation of mixed neuronalglial cell cultures
Mixed neuronal/glial cultures were prepared from fetal Sprague-Dawley (Harlan Sprague Dawley, Inc. Indianapolis, IN) rat brains at 18 days of gestation as previously described [wrong ref]. Brains were harvested from 10–15 pups and dissected to separate cortex from meninges and subcortical structures using anatomical landmarks. Cortices were pooled and minced into 2 mm3 pieces in a buffered salt solution (BSS; Hanks Balanced Salt Solution [Life Technologies, Gaithersburg, MD] supplemented with 20 mM HEPES buffer (pH 7.4, containing 0.25% trypsin [Life Technologies]). The tissue was incubated for 20 min at 37° C in a 5% CO2/95% room air atmosphere, then washed twice with ice-cold, glutamine-free minimum essential medium (MEM; Life Technologies) containing 15 mM glucose, 5% fetal bovine serum (Gibco Diagnostics, Inc., Madison, WI), 5% horse serum (GIBCO), and 1% DNase-I (Sigma Chemical Co., St. Louis, MO, U.S.A.). Tissue pieces were dissociated by trituration through a fire-polished 9” Pasteur pipette. The resultant suspension was centrifuged at 50 g for 10 min, the supernatant discarded, and the pellet resuspended in growth medium (MEM supplemented with 15 mM glucose, 5% fetal bovine serum, and 5% horse serum). The dissociated cells were plated to achieve a confluent monolayer (4 x 105 cells per well for neuronal/glial cultures) on poly-D-lysine coated, 24-well culture plates (Falcon 3047; Becton Dickinson Co., Lincoln Park, NJ). Cultures were maintained undisturbed at 37 °C in a humidified 5% CO2/balance room air atmosphere for 10–14 days prior to use. Previous studies performed under identical culture conditions demonstrated cell types in 10 day old cultures are 54 ± 4% neurons and 46 ± 7% glia as determined by immunohistochemical staining for cell specific cytoskeletal filaments (neurofilament-160 for neurons and glial fibrillary acidic protein for astrocytes)[27].
Preparation of Mn porphyrins
All compounds studied are synthesized as previously reported in details [7,20,28]. Particular attention was paid to their purification, especially to a longer alkyl-chain analogue, MnTnOct-2-PyP5+P , which was additionally purified using ultrafilter 500-cutoff.
Dose-response
Based on SOD-deficient E. coli study, a dose response curve was determined using 3, 10, 30 and 100 μM of MnTE-2-PyP5+ and MnTDE-2-ImPP 5+ [21]. Identical concentrations were chosen for the MnTnOct-2-PyP5+. All compounds were diluted in glucose-free phosphate buffered saline (PBS: 7.65 g NaCl, 0.724 g Na2OH4 and 0.21 g K2PO4, pH 7.4) to provide the desired concentrations and immediately used for experimentation.
Oxygen and glucose deprivation
The original glucose containing media was removed from all treatment groups and replaced with a glucose-free phosphate buffered saline (PBS: 7.65 g NaCl, 0.724 g Na2OH4 and 0.21 g K2PO4, pH 7.4). All media changes were followed by a wash with PBS. No serum was included in the glucose-free PBS. In the oxygen and glucose deprivation phase, the media was washed with PBS and changed to hypoxic, glucose-free PBS. The glucose free/hypoxic PBS was prepared by passing the PBS through a microbubbler apparatus containing the hypoxic (94% N2/6% CO2, pH 7.37 +/− 0.4) gas mixture. The 94% N2/6% CO2 gas mixture was used to maintain the previous incubating conditions without oxygen and with minimal change in pH. The hypoxic, glucose-free PBS was then applied in a thin layer (enough to cover the cells) to the cell culture dishes. Dishes exposed to hypoxia were then placed in a small, 3-liter, airtight experimental hypoxia chamber (Billups-Rothenberg; San Diego, CA) with inflow and outflow connectors. The experiments were conducted in a constant 37ºC environment by placing the chambers in a water-jacketed incubator. The gaseous environment was controlled by the delivery of all gas via a heater humidifier (Fisher-Paykel; Laguna Hills, CA) servo-controlled to 37 °C via the inflow adapter of the chamber. Delivered and end-tidal concentrations of oxygen were monitored using a gas analyzer (Datex Instruments Corporation, Tewksbury, MA) and maintained at <0.2% [29].
As performed previously [13], cultures were exposed to conditions of oxygen and glucose deprivation for 2 h. The exposure was terminated by removing the PBS, adding the original media for 24 hrs, and then analyzing for cell death and viability as described below. Control cells were incubated in PBS in a normoxic incubator for the same time period as the experimental group.
LDH release
Cellular injury was assessed 24 hours after oxygen and glucose deprivation by measuring the amount of LDH released into overlying medium by damaged cells. In brief, a 200-μl sample of culture medium was added to a polystyrene cuvette containing 10 mM lactate and 5 μmol of NAD (B-nicotinamide adenine dinucleotide; Sigma, St. Louis, Mo.) in 2.75 ml of 50 mM glycine buffer pH 9.2 at 24° C. LDH activity was determined from the initial rate of reduction of NAD+ as calculated using a linear least square curve fit of the temporal changes in fluorescence signal from the cuvette (340 nm excitation, 450 nm emission) and expressed in units of enzymatic activity (nmol of lactate converted to pyruvate per min). Analysis was performed on a fluorescence spectrophotometer (Perkin Elmer Model LS50B; Bodenseewerk GmbH, Uberlinger, Germany).
Cell viability assay
Twenty-four hours after treatment, the neuronal/glial cell cultures were incubated with 250 μg/ml 3-(4, 5-dimethyltiazol-2-yl) -2, 5 diphenyltetrazolium bromide (MTT, Sigma)) for 30 min at 37° C [30]. MTT is absorbed into cells and transformed into formazan by mitochondrial succinate dehydrogenase. Accumulation of formazan directly reflects the activity of mitochondria, which functions as an indirect measurement of cell viability. Sodium dodecyl sulfate in dilute hydrochloric acid was used to dissolve the purple formazan product. After the precipitate in each well was resuspended on a microplate mixer for 10 min, an optical density (OD) reading at 540 nm was measured using an ELISA plate reader. A dose response curve was determined and cell viability measured as absorbance was compared to control and cell cultures exposed to oxygen and glucose deprivation.
Propidium iodide assay
Cells were exposed to a final concentration of 40 μg/ml of propidium iodide dissolved in BSS for 10 min. After washing with BSS, the cells were fixed with 4% paraformaldehyde for 10 min, followed by another washing with BSS. The cells were then stored in 300 μl of BSS. All staining procedures were done at room temperature. The cultures were then viewed under an inverted fluorescent microscope at 20x magnification with the observer blinded to treatment condition. Three fields were chosen in each separate well (10 wells for each experimental condition) for cell count determination per experiment. Dead cells were defined as those visibly stained by propidium iodide, which signals membrane disruption [27].
Statistical Analysis
Data were compared by one-way analysis of variance. When indicated by a significant F ratio, post hoc testing was performed by use of Scheffe’s test. Values are reported as mean ± SD. A P value < 0.05 was considered significant. Statistical analysis was performed using StatView 5.0 (SAS; Cary, NC).
Results
The protection afforded against oxygen and glucose deprivation-induced cell death was afforded with all compounds (Figures 2–4,6). Differences observed relate to the levels of those compounds and the type of assay used. All the compounds are more beneficial if the pretreatment of the cells before oGD was performed (Figure 2 vs Figure 3). The most effective compound as judged by LDH assay (when treatment with compound was done before and during deprivation) was MnTE-2-PyPP 5+ (Figure 3), while MnTDE-2-ImP5+ was the most protective judged by propidium iodide assay (Figure 5). MnTnOct-2-PyP5+ P was efficacious in LDH assay when cell were treated prior to stress only; during prolonged exposure potential toxicity counterbalanced the protectiveness (Figure 2).
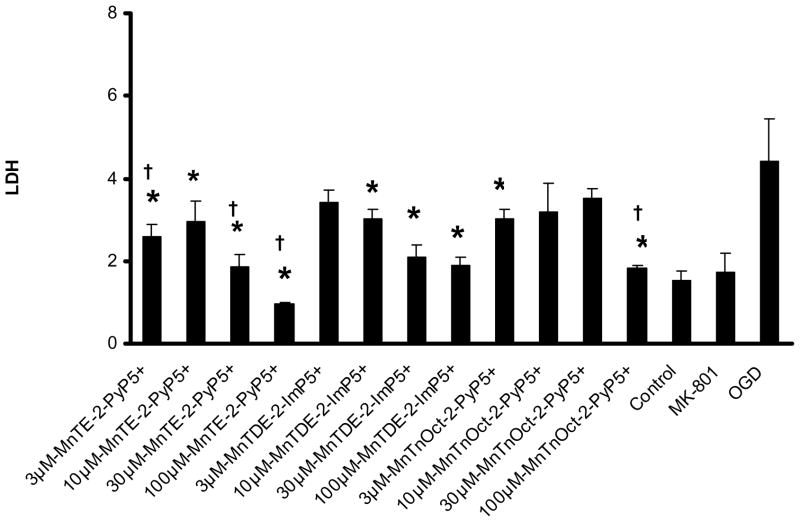
Figure 2. LDH dose response curve: 30 min pretreatment prior to exposure to oxygen and glucose deprivation.
A dose response curve was determined at 24 hours after oxygen and glucose deprivation, using previously in vivo dosing (3, 10, 30 and 100 μM) of MnTE-2-PyP5+ and MnTDE-2-ImP5+ P . The same doses were used for the MnTnOct-2-PyP5+ compound. These conditions were compared to control, oxygen and glucose deprivation. * statisitical difference (p<0.005) from oxygen and glucose deprivation (OGD); † statisitically equal (p>0.999) to control.
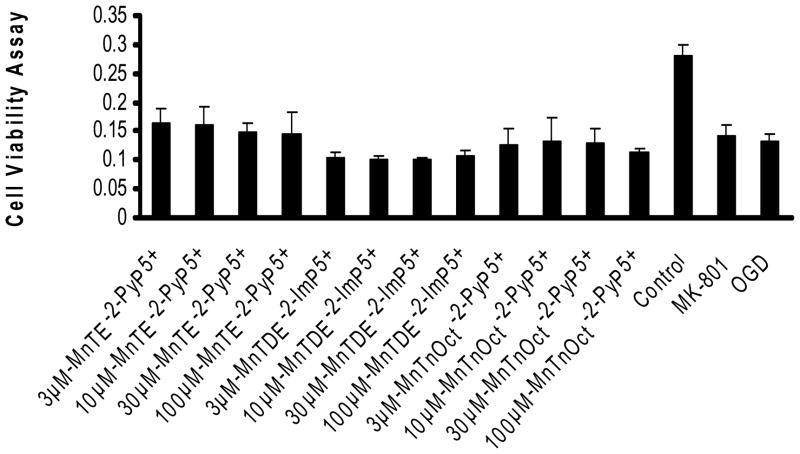
Figure 4. Cell viability/MTT assay: 30 min pretreatment prior to exposure to oxygen and glucose deprivation with continued exposure during 2 hours of oxygen and glucose deprivation.
Cell viability was measured 24 hours after exposure to the experimental conditions as noted previously. Cell viability is a linear relationship with absorbance and was measured at 540nm. Data demonstrates a decrease in absorbance/cell viability with 2 hours of oxygen and glucose deprivation as compared to control. Though some experimental conditions demonstrate improved viability as compared to oxygen and glucose deprivation, it was not statistically significant. Ten wells were used for each experimental condition. * statisitical difference (p<0.005) from oxygen and glucose deprivation (OGD); † statisitically equal (p>0.999) to control.
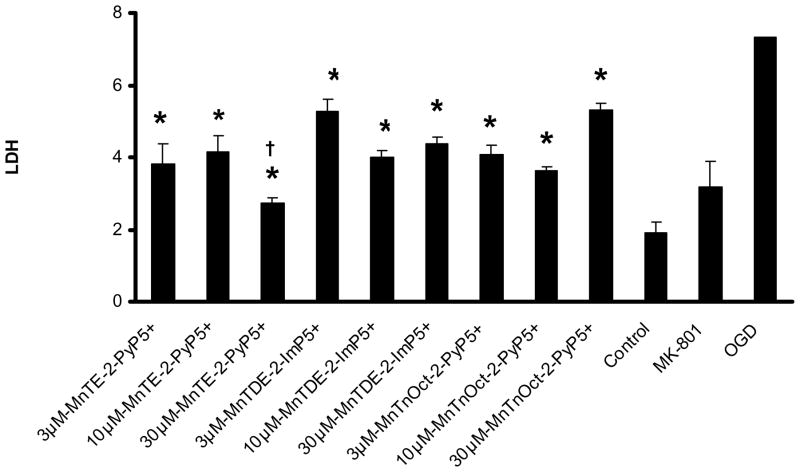
Figure 3. LDH dose response curve: 30 min pretreatment prior to exposure to oxygen and glucose deprivation with continued exposure during 2 hours of oxygen and glucose deprivation.
A dose response curve was determined at 24 hours after exposure to oxygen and glucose deprivation, using previously in vivo dosing (3, 10, 30 and 100 μM) of MnTE-2-PyP5+ and MnTDE-2-ImPP 5+. . The same doses were used for the MnTnOct-2-PyP5+. These conditions were compared to control, oxygen and glucose deprivation alone and 10μM MK801. Six wells were used for each condition. * statisitical difference (p<0.005) from oxygen and glucose deprivation (OGD); † statisitically equal (p>0.999) to control.
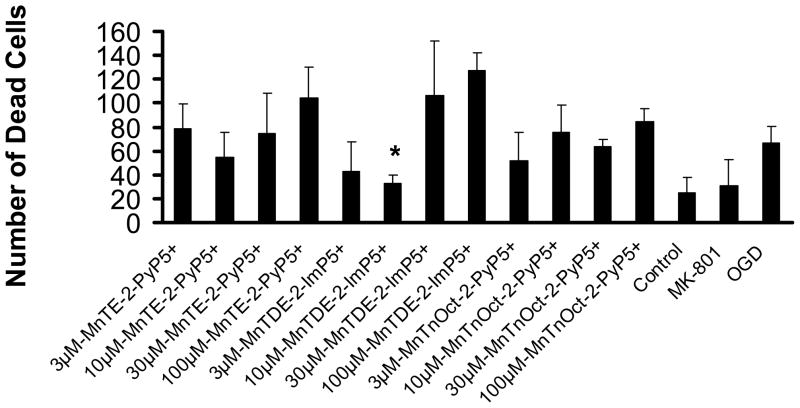
Figure 5. Cell death/propidium iodide assay: 30 min pretreatment prior to exposure to oxygen and glucose deprivation with continued exposure during 2 hours of oxygen and glucose deprivation.
The number of dead cells was counted in three fields in each well and expressed as mean+/− standard deviation. Ten wells were examined for each experimental condition. * statisitical difference (p<0.005) from oxygen and glucose deprivation (OGD); † statisitically equal (p>0.999) to control.
LDH analysis
Two experiments were used to determine neuronal/glial cell death using LDH analysis. These experiments are the following: 1) 30 min pretreatment with the Mn porphyrins prior to oxygen and glucose exposure (Figure 2). 2); and 30 min pretreatment with continuation of the treatment during the 2h of oxygen and glucose deprivation (Figure 3). In both experiments, the conditions provided by 2h of oxygen and glucose deprivation were statistically different than control and 10 μM MK801 to give the study a power of 1.0. Six wells were used for all conditions in both experiments.
In the first experiment, all compounds at all concentrations statistically decreased oxygen and glucose deprivation-induced LDH release (p<0.001), except for 3μM MnTDE-2-ImP5+, 10 and 30 μM of the MnTnOct-2-PyP5+. When compared to control conditions, LDH release was similar in the 100 μM MnTE-2-PyP5+, and MnTnOct-2-PyP5+, and 3 and 30 μM MnTE-2-PyP5+ groups (p> 0.999).
In the second experiment, when compared to control conditions, 30μM MnTE-2-PyP5+ (p=0.296) demonstrated similar LDH release. When compared to cells stressed by oxygen and glucose deprivation, statistical significance was achieved with 3, 10 and 30 μM MnTnOct-2-PyP5+, MnTE-2-PyP5+ and MnTDE-2-ImP5+ P ,
Cell Viability Assay
Two hours of oxygen and glucose deprivation provided a (p<0.001) statistical difference in cell viability to fulfill a power of 1.0 for this assay (Figure 4). Ten wells for each condition were used for this assay. None of the conditions, including 10 μM MK801, afforded statistically significant improved cell viability when compared to the cells that underwent oxygen and glucose deprivation alone.
Propidium Iodide Staining
Ten cell culture wells were used for each of the experimental conditions to provide a power of 1.0 (Figure 5). When compared to control, the number of dead cells was statistically different (p<0.001). Compared to oxygen and glucose deprivation alone, 10 μM MK801 and 10 μM of MnTDE-2-ImP5+ P , afforded significant protection against cell death (p<0.001). Cell death was enhanced with 30 and 100μM concentrations of MnTDE-2-ImP5+. The other conditions had no statistically significant effect. There was a trend towards protection with low, 3 μM MnTnOct-2-PyPP 5+, indicating that this compound is likely very effective at much lower doses where toxicity will not counterbalance benefit.
Discussion
Superoxide dismutases are first lines of defense maintaining steady state levels of superoxide, thus all other reactive species formed down stream, particularly peroxynitrite also. MnSOD (SOD2) is one of the four major superoxide dismutases and is distributed in the mitochondrial matrix [wrong refs, must come some review refs maybe from Fridovich] and abundant in neural tissue. Under pathological conditions endogenous SOD may not be able to offer sufficient protection. Thus, exogenous antioxidants may be beneficial. Several different classes have been studied, metalloporhyrins being among the most effective ones [2–10, 31].
We have developed structure-activity relationship for Mn porphyrins. Based on structural activity relationship it became obvious that the most potent compounds must have Mn site sufficiently electron-deficient for thermodynamic facilitation of the O2•− dismutation. The efficacy to remove superoxide parallels the efficacy to reduce peroxynitrite (12). Further, the respective compounds must bear positive charges close to the Mn site needed for the electrostatic facilitation for the approach of negatively charged O2•− and ONOO− [8,10]. The proper lipophilicity and the size of molecule are also critical for in vivo bioavailabity, i.e. efficacy [14, 20–24, 32, 33]. Finally the toxicity needs to be modulated as well [32].
Our most potent compounds showed efficacy in nearly any model of oxidative stress tested, including central nervous system injuries [13–18, 20–24]. MnTE-2-PyP5+ and MnTDE-2-ImP5+ P have demonstrated neuroprotection in stroke model and are associated with a decrease in aconitase inactivation, 8-hydroxyguanine formation and cytokine expression [13,19]. Based on the remarkable in vivo efficacy, the lipophilic MnTnHex-2-PyP5+ is presently our most promising drug. Although it suffers from micellar-based toxicity at higher dose its toxicity to efficacy ratio is 250 and 17-fold better than for its more hydrophilic analogue, MnTE-2-PyP5+. Herein, for the first time we tested the octyl analogue, MnTnOct-2-PyP5+ on efficacy and toxicity. It is 1.4-fold more lipophilic than MnTnHex-2-PyP5+ compound.
The efficacy observed is comparable to or less than that of other two Mn porphyrins with fewer carbon atoms in the alkyl chains. The effects observed were dependent upon the assay and the concentrations used. MTT assay was not able to distinguish between compounds. In the LDH assay, where treatment only prior to oxygen and glucose deprivation was done, 100 μM MnTnOct-2-PyP5+ was efficacious, decreasing the injury of cells to control levels. The protective effects of MnTnOct-2-PyP5+ were lesser than expected as judged by propidium iodide and with LDH assay (where pretreatment and treatment during deprivation was performed), likely hampered by the toxicity involved when cell exposure to the drug was prolonged; i.e. the effects of MnTnOct-2-PyP5+ observed might have resulted from the interplay of efficacy and toxicity.
Still, given the very sensitive nature of neurons, the toxic effects, if any, are significantly lower than we expected. That is a motivating observation with respect to the future studies on the evaluation of the utility of MnTnOct-2-PyP5+ as a neuroprotective agent. In all 4 assays, MnTE-2-PyP5+ and MnTDE-2-ImP5+ P were of comparable efficacy. Judged by LDH assay the former was a better performer while based upon the propidium iodide assay the latter was performing better. All compounds show signs of toxicity at higher doses. Our findings are consistent with those obtained previously by Sheng et al. on the effect of MnTDE-2-ImPP 5+ in mixed neuronal/glial cell cultures exposed to 2 hr of oxygen and glucose deprivation.
The failure of compounds to show protective effects by MTT assay when compared to propidium iodide may be in part related to the different nature of these two assays one being extracellular (LDH) and the other intracellular (MTT) [24]. With MTT assay a trend towards protection of cells was observed with MnTE-2-PyP5+. Oxygen and glucose deprivation induced mitochondrial aconitase activation [1, 13, 14], may have provided greater insight into the protection afforded by MnTnOct-2-PyP5+ at the mitochondrial level. Our data (33, 34) indicate that MnTE-2-PyP5+ accumulates in all organs, brain included and in mitochondria and nucleus (Spasojevic, Tse, Piganelli unpublished). It is highly likely that octyl analogue distributes at greater extent in all organs and subcellular compartments. Our most recent data that have shown the remarkable benefit of lipophilic Mn porphyrins in vivo in animal models, justify studies that are in progress addressing their organ and cellular distribution.
Acknowledgments
LWF thanks the Multidisciplinary Neuroprotection Laboratories at Duke University Medical Center (NIH Grants T32 GM08600-09 and RO1 GM067139-03); IBH acknowledges the support by the National Institutes of Health (IR21-ESO/3682) and the National Institutes for Allergy and Infectious Diseases (U19AI067798) grants; IS thanks NIH/NCI Duke Comprehensive Cancer Center Core Grant (5-P30-CA14236-29); the authors wish to thank Ms. Candace Berryman for her editorial assistance.
Abbreviations
- MnTE-2-PyP5+
Mn(III) mesotetrakis(N-ethylpyridinium-2-yl)porphyrin, EPyP and MnTDE-2-ImPP 5+ Mn(III) mesotetrakis(N,N’-diethylpyridinium-2-yl)porphyrin, DEImP
- MnTnOct-2-PyP5+
Mn(III) mesotetrakis(N-n-octylpyridinium-2-yl)porphyrin, OctPyP
- LDH
lactate dehydrogenase
- MTT
3-(4,5-dimethyltiazol-2-yl) -2,5 diphenyltetrazolium bromide
- SOD
superoxide dismutase
References
- 1.Li QY, Pedersen C, Day BD, Patel M. Dependence of excitotoxic neurodegeneration on mitochondrial aconitase inactivation. J of Neurochem. 2001;78:746–755. doi: 10.1046/j.1471-4159.2001.00457.x. [DOI] [PubMed] [Google Scholar]
- 2.Van Empel VPM, Bertrand AT, Van Oort RJ, Van der Nagel R, Engelen M, Van Rijen HV, Doevendans PA, Crijns HJ, Ackerman SL, Sluiter W, De Windt LJ. EUK_8, a superoxide dismutase and catalase mimetic, reduces cardiac oxidative stress and ameliorates pressure overload-induced heart failure in the harlequin mouse mutant. J Am Coll Cardiol. 2006;48:8245–832. doi: 10.1016/j.jacc.2006.02.075. [DOI] [PubMed] [Google Scholar]
- 3.Salvemini D, Wang Z-Q, Zweier JL, Samouilov A, Macarthur H, Misko TP, Curie MG, Cuzzocrea S, Sikorski JA, Ruley DP. A nonpeptidyl mimic of superoxide dismutase with therapeutic activity ion rats. Science. 1999;286:304–306. doi: 10.1126/science.286.5438.304. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein S, Samuni A, Hideg K, Merenyi G. Structure-activity relationship of cyclic nitroxides as SOD mimics and scavengers of nitrogen dioxide and carbonate radicals. J Phys Chem A. 2006;110:3679–3685. doi: 10.1021/jp056869r. [DOI] [PubMed] [Google Scholar]
- 5.James AM, Cocheme HM, AJ, Murphy MP. Interactions ofSmith, R. mitochondria-targeted and untargeted ubiquinones with the mitochondrial respiratory chain and reactive oxygen species. J Biol Chem. 2005;28:21295–21312. doi: 10.1074/jbc.M501527200. [DOI] [PubMed] [Google Scholar]
- 6.Smith RAJ, Porteous CM, Ganes AM, Murphy MP. Delivery of bioactive molecules to mitochondria. Proc Natl Acad Sci. 2003;100:5407–5412. doi: 10.1073/pnas.0931245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batini -Haberle I, Benov L, Spasojevi I, Hambright P, Crumbliss AL, Fridovich I. The relationship between redox potentials, proton dissociation constants of pyrrolic nitrogens, and in vitro and in vivo superoxide dismutase activities of Manganese(III) and Iron(III) cationic and anionic porphyrins. Inorg Chem. 1999;38:4011–4022. [Google Scholar]
- 8.Rebouças JS, Spasojevi I, Batini -Haberle I. Pure manganese(III) 5,10,15,20-tetrakis(4-benzoic acid)porphyrin (MnTBAP) is not a superoxide dismutase mimic in aqueous systems: A case of structure-activity relationship as a watchdog mechanism in experimental therapeutics and biology. J Inorg Biol Chem. 2008;13:289–302. doi: 10.1007/s00775-007-0324-9. [DOI] [PubMed] [Google Scholar]
- 9.Spasojevi I, Batini -Haberle I, Stevens RD, Hambright P, Thorpe AN, Grodkowski J, Neta P, Fridovich I. Manganese(III) biliverdin IX dimethyl ester: A powerful catalytic scavenger of superoxide employing the Mn(III)/Mn(IV) redox couple. Inorg Chem. 2001;40:726–739. doi: 10.1021/ic0004986. [DOI] [PubMed] [Google Scholar]
- 10.Spasojevic I, Batinic-Haberle I, Reboucas JS, Idemori YM, Fridovich I. Electrostatic Contribution in the Catalysis of O2•− Dismutation by Superoxide Dismutase Mimics. J Biol Chem. 2003;278:6831–6837. doi: 10.1074/jbc.M211346200. [DOI] [PubMed] [Google Scholar]
- 11.Ferrer-Sueta G, Batinic-Haberle I, Spasojevic I, Fridovich I. Peroxynitrite Scavanging by Manganese (III) Meso-Tetrakis-(N-methylpyridyl)Porphyrins. Chem Res Toxicol. 1999;12:442–449. doi: 10.1021/tx980245d. [DOI] [PubMed] [Google Scholar]
- 12.Ferrer-Sueta G, Vitturi D, Batinic-Haberle I, Fridovich I, Goldstein S, Czaspki G, Radi R. Reactions of manganeses porphyrins with peroxynitrite and carbonate radical ion. J Biol Chem. 2008;278:27432–27438. doi: 10.1074/jbc.M213302200. [DOI] [PubMed] [Google Scholar]
- 13.Sheng H, Enghild JJ, Patel M, Batinic-Haberle I, Calvi C, Day BJ, Pearlstein RD, Crapo JD, Warner DS. Effects of metalloporphyrin catalytic antioxidants in experimental brain ischemia. Free Radical Biology and Medicine. 2002;33:942–961. doi: 10.1016/s0891-5849(02)00979-6. [DOI] [PubMed] [Google Scholar]
- 14.Gauter-Fleckenstein B, Fleckenstein K, Owzar K, Jian C, Batinic-Haberle I, Vujaskovic Z. Comparison of two Mn porphyrin-based mimics of superoxide-dismutase (SOD) in pulmonary radioprotection. Free Radic Biol Med. 2007 doi: 10.1016/j.freeradbiomed.2007.10.058. published on-line Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheng H, Batinic-Haberle I, Warner DS. Catalytic Antioxidants as Novel Pharmacologic Approaches to Treatment of Ischemic Brain Injury. Drug News and Perspectives. 2002;15:654–665. doi: 10.1358/dnp.2002.15.10.740236. [DOI] [PubMed] [Google Scholar]
- 16.Piganelli JD, Flores SC, Cruz C, Koepp J, Young R, Bradley B, Kachadourian R, Batinic-Haberle I, Haskins K. A Metalloporphyrin Superoxide Dismutase Mimetic (SOD Mimetic) Inhibits Autoimune Diabetes. Diabetes. 2002;51:347–355. doi: 10.2337/diabetes.51.2.347. [DOI] [PubMed] [Google Scholar]
- 17.Moeller BJ, Batinic-Haberle I, Spasojevic I, Rabbani ZN, Anscher MS, Vujaskovic Z, Dewhirst MW. Effects of a catalytic metalloporphyrin antioxidant on tumor radioresponsiveness. Int J Rad Oncol Biol Phys. 2005;63:545–552. doi: 10.1016/j.ijrobp.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 18.Sompol P, Ittarat W, Tangpong J, Chen Y, Doubinskaia I, Batinic-Haberle I, Mohammad Abdul H, Butterfield A, St Clair DK. Alzheimer’s disease: An insight into the mechanisms of oxidative stress-mediated mitochondrial injury. Neuroscience. 2008 doi: 10.1016/j.neuroscience.2008.01.044. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackensen GB, Patel M, Sheng H, Calvi C, Batinic-Haberle I, Day BJ, Liang LP, Fridovich I, et al. Neuroprotection from delayed post-ischemic administration of a metalloporphyrin catalytic antioxidant. J Neurosci. 2001;21:4582–4592. doi: 10.1523/JNEUROSCI.21-13-04582.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Batinic-Haberle I, Spasojevic I, Stevens RD, Hambright P, Fridovich IJ. Manganese(III) Meso Tetrakis Ortho N-alkylpyridylporphyrins. Synthesis, Characterization and Catalysis of O2•− Dismutation. Chem Soc, Dalton Trans. 2002:2689–2696. [Google Scholar]
- 21.Okado-Matsumoto A, Batinic-Haberle I, Fridovich I. Complementation of SOD-deficient Escherichia Coli by manganese porphyrin mimics of superoxide dismutase activity. Free Radic Biol Med. 2004;37:401–10. doi: 10.1016/j.freeradbiomed.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 22.Saba H, Batini -Haberle I, Munusamy S, Mitchell T, Lichti C, Megyesi J, MacMillan-Crow LA. Manganese porphyrin reduces renal injury and mitochondrial damage during ischemia/reperfusion. Free Radic Biol Med. 2007;42:1571–1578. doi: 10.1016/j.freeradbiomed.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crow J. MnTnHex-2-PyP5+ in ALS. unpublished. [Google Scholar]
- 24.Batibic-Haberle I, Ndengele MM, Cuzzocrea S, Reboucas JS, Matuscak GM, St Clair DK, Dewhirst MW, Spasojevic I, Salvemini D. Inhibition of morphine tolerance and suppression of tumor angiogenesis: Dual action of Mn porphyrin-based drugs in cancer therapy and pain management. AACR; San Diego: 2008. [Google Scholar]
- 25.Leinenweber SB, Sheng H, Lynch JR, Batinic-Haberle I, Laskowitz DT, Crapo JD, Pearlstein RD, Warner DS. Effects of a Manganese(III) Porphyrin Catalytic Antioxidant in a Murine Model of Closed Head Injury. Eur J Pharmacol. 2006;531:126–132. doi: 10.1016/j.ejphar.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 26.Dugan LL, Gabrielsen JK, Yu SP, Lin TS, Choi DW. Buckminsterfullerenol free radical scavengers reduce excitotoxic and apoptotic cell death of cultured cortical neurons. Neurobiol Dis. 1996;3:129–135. doi: 10.1006/nbdi.1996.0013. [DOI] [PubMed] [Google Scholar]
- 27.Wise-Faberowski, Aono M, Pearlstein Warner DS. Apoptosis is not enhancedR, in primary mixed neuronal/glial cultures protected by isoflurane against N-methyl-d-aspartate excitotoxicity. Anesth Analg. 2004;99:1708–14. doi: 10.1213/01.ANE.0000136474.35627.FF. [DOI] [PubMed] [Google Scholar]
- 28.Batinic-Haberle I, Spasojevic I, Stevens RD, Hambright P, Neta P, Okado-Matsumoto A, Fridovich I. New Class of Potent Catalysts of O2•− Dismutation. Mn(III) methoxyethylpyridyl- and methoxyethylimidazolylporphyrins. J Chem Soc Dalton Trans. 2004:1696–1702. doi: 10.1039/b400818a. [DOI] [PubMed] [Google Scholar]
- 29.Wise-Faberowski, Sumners C, Raizada M. Oxygen and glucose deprivation-induced neuronal apoptosis is attenuated by halothane and isoflurane. Anesthesia and Analgesia. 2001;93:1281–7. doi: 10.1097/00000539-200111000-00051. [DOI] [PubMed] [Google Scholar]
- 30.Koh SH, Noh MY, Kim SH. Amyloid-beta-induced neurotoxicity is reduced by inhibition of glycogen synthase kinase-3. Brain Res. 2008;1188:254–62. doi: 10.1016/j.brainres.2007.10.064. [DOI] [PubMed] [Google Scholar]
- 31.Warner DS, Sheng H, Batinic-Haberle I. Oxidants, Antioxidants, and the Ischemic Brain. J Exp Biology. 2004;207:3221–3231. doi: 10.1242/jeb.01022. [DOI] [PubMed] [Google Scholar]
- 32.Spasojevic I, Chen Y, Noel TJ, Fan P, Zhang L, Reboucas JS, St Clair DK, Batinic-Haberle I. Potent redox modulator of oxidative stress, MnTE-2-PyP5+. Pharmacokinetics in mouse plasma, liver, kidney, spleen, lung, heart and brain. Free Radic Biol Med. 2008 doi: 10.1016/j.freeradbiomed.2008.05.015. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spasojevic I, Yumin C, Noel T, Yu I, Pole MP, Zhang L, Zhao Y, St Clair DK, Batinic-Haberle I. Mn porphyrin-based SOD mimic, MnTE-2-PyP5+ targets mouse heart mitochondria. Free Radic Biol Med. 2007;42:1193–1200. doi: 10.1016/j.freeradbiomed.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]