Abstract
p38 mitogen-activated protein kinase (MAPK) acts downstream in the signaling pathway that includes receptor activator of NF-κB (RANK), a powerful inducer of osteoclast formation and activation. We investigated the role of p38 MAPK in parathyroid hormone related protein (PTHrP)-induced osteoclastogenesis in vitro and PTHrP-induced bone resorption in vivo. The ability of FR167653 to inhibit osteoclast formation was evaluated by counting the number of tartrate-resistant acid phosphatase positive multinucleated cells (TRAP-positive MNCs) in in vitro osteoclastgenesis assays. Its mechanisms were evaluated by detecting the expression level of c-Fos and nuclear factor of activated T cells c1 (NFATc1) in bone marrow macrophages(BMMs) stimulated with sRANKL and M-CSF, and by detecting the expression level of osteoprotegerin (OPG) and RANKL in bone marrow stromal cells stimulated with PTHrP in the presence of FR167653. The function of FR167653 on bone resorption was assessed by measuring the bone resorption area radiographically and by counting osteoclast number per unit bone tissue area in calvaria in a mouse model of bone resorption by injecting PTHrP subcutaneously onto calvaria. Whole blood ionized calcium levels were also recorded. FR167653 inhibited PTHrP-induced osteoclast formation and PTHrP-induced c-Fos and NFATc1 expression in bone marrow macrophages, but not the expression levels of RANKL and OPG in primary bone marrow stromal cells treated by PTHrP. Furthermore, bone resorption area and osteoclast number in vivo were significantly decreased by the treatment of FR167653. Systemic hypercalcemia was also partially inhibited. Inhibition of p38 MAPK by FR167653 blocks PTHrP-induced osteoclastogenesis in vitro and PTHrP-induced bone resorption in vivo, suggesting that the p38 MAPK signaling pathway plays a fundamental role in PTHrP-induced osteoclastic bone resorption.
Introduction
Parathyroid hormone related protein (PTHrP), a potent stimulator of osteoclastic bone resorption, was first identified as a causative factor for humoral hypercalcemia of malignancy [1]. A number of clinicopathological and experimental studies have shown that cancer cell-derived PTHrP promotes osteoclastic bone resorption and contributes to the development and progression of cancer metastasis to bone [2]–[5]. PTHrP stimulates osteoclastogenesis by acting on osteoblasts and/or bone marrow stromal cells to increase receptor activator of NF-κB ligand (RANKL) expression and reduce osteoprotegerin (OPG) expression, not by acting directly on osteoclast precursors [6]. Osteoclast precursors that express RANK, a tumor necrosis factor (TNF) receptor family member, recognize RANKL and differentiate into osteoclasts in the presence of macrophage/monocyte colony stimulating factor (M-CSF). OPG is a soluble decoy receptor for RANKL and has the ability to inhibit osteoclastogenesis. Therefore, a relative increase of RANKL versus OPG expression by PTHrP activates osteoclastic bone resorption [7]–[12].
Additionally, a body of evidence suggested that p38 mitogen-activated protein kinase (MAPK) is downstream of the RANK signaling pathway and plays an important role in osteoclast differentiation. The expression of dominant-negative forms of p38 MAPK in RAW264.7 cells inhibited RANKL-induced differentiation of these cells into osteoclasts [13]. Li et al used pyridinylimidazole SB203580, a specific inhibitor of p38 MAPK, to study p38 MAPK function and demonstrated that p38 MAPK is required for osteoclast differentiation [14].
FR167653 was first discovered as a potent inhibitor of TNFα and interleukin (IL)-1β production in lipopolysaccharide-stimulated human monocytes and phytohemagglutinin-M-stimulated human lymphocytes [15], [16]. FR167653 inhibits the activation of p38 MAPK by suppressing the phosphorylation of p38 MAPK, preferentially affecting its α isoform, but not the γ isoform [17]–[19]. FR167653 is effective in treating inflammation, relieving trauma and ischemia-reperfusion injury in vivo [20]–[22]. We previously reported that M-CSF-dependent sRANKL- and TNFα-induced osteoclast formation in primary bone marrow cells, and collagen-induced arthritis in rats were inhibited by FR167653 [23]. However, the relationship of p38 MAPK and PTHrP in osteoclastogenesis and bone resorption is still unclear.
Here we investigate the role of FR167653 on PTHrP-induced osteoclastogenesis, local bone resorption, and hypercalcemia. We found that FR167653 not only blocked osteoclast differentiation induced both by PTHrP and sRANKL with down regulation of c-Fos and NFATc1 in bone marrow macrophages without affecting RANKL and OPG expression in bone marrow stromal cells, but also alleviated bone resorption induced by PTHrP with partial reduction of hypercalcemia in vivo.
Materials and Methods
Animal and reagents
All animal experiments in this study strictly followed a protocol approved by the Institutional Animal Care and Use Committee of Osaka University (approval number: 338). Six-week-old male ddy mice and four- to eight-week-old male BDF1 mice were purchased from Japan SLC (Hamamatsu, Japan) and Oriental Yeast (Tokyo, Japan), respectively. Human recombinant PTHrP (1–34) was purchased from Peptide Institute (Osaka, Japan). M-CSF and soluble recombinant RANKL were purchased from PeproTeck EC (London, UK). FR167653 was provided by Asteras Pharma (Osaka, Japan). c-Fos and actin antibodies were purchased from Cell Signaling Technology (Danvers, USA). NFATc1 antibody was bought from BD Biosciences Pharmingen (Franklin Lakes, USA).
Primary bone marrow cell culture
Bone marrow cells were collected by removing the tibias and femurs from ddy mice and flushing the bone cavity with serum-free alpha-minimum essential medium (αMEM; Invitrogen, Carlsbad, CA). A sample of 7.5×105 cells in a volume of 0.5 ml/well were cultured in αMEM supplemented with 10% fetal bovine serum (FBS; Equitech-Bio, Kerrville, TX) in the presence of PTHrP (45 ng/ml). Some cultures received an additional treatment of 10 µM, 1 µM or 0.1 µM FR167653. The medium was changed every two days, replacing half of the medium with 0.25 ml fresh medium containing 90 ng/ml PTHrP and 20 µM, 2 µM and 0.2 µM FR167653. After six days of culture, adherent cells were stained for tartrate-resistant acid phosphatase (TRAP), an osteoclast marker enzyme, using a TRAP staining kit (Hokudo, Sapporo, Japan). TRAP-positive multinucleated cells (MNCs) with three or more nuclei were counted as osteoclasts.
M-CSF-dependent bone marrow macrophage (MDBMMs) culture
M-CSF-dependent bone marrow macrophages (MDBMMs) were cultured as described previously [23]–[25]. Briefly, bone marrow cells (5×106) were cultured in the αMEM supplemented with 10% FBS, and M-CSF at the concentration of 100 ng/ml in 100 mm dishes for three days. Then the cells were washed and harvested with 0.02% ethylene diamine tetra-acetate (EDTA) in phosphate-buffered saline, and seeded at a density of 3×105 into 100-mm culture dishes in the presence of M-CSF (100 ng/ml). After three more days of culture, the adherent cells were considered as MDBMMs.
For osteoclast culture, MDBMMs were digested with 0.25% Trypsin-EDTA (Invitrogen) and 1×104/well cells were re-plated into 48-well plates in the presence of sRANKL (100 ng/ml) and M-CSF (100 ng/ml). Some cultures were treated with FR167653. After five more days of culture, adherent cells were fixed and stained for TRAP. TRAP-positive MNCs containing three or more nuclei were counted as osteoclasts.
For the detection of the c-Fos and NFATc1expression, MDBMMs were treated by each concentration of FR167653 for 1 hour. Then, sRANKL and M-CSF were added. After another 24 or 48 hour culture, the cells were collected for RNA extraction and protein extraction.
Real-time polymerase chain reaction
Total RNA was extracted from bone marrow cells (for genes of OPG and RANKL) or bone marrow macrophages (for genes of c-fos and NFATc1) by using the RNeasy Mini Kit (Qiagen, Valencia, CA). RNase-free DNase Set (Qiagen) was used to remove the residual DNA. Five micrograms of RNA were reverse transcribed into cDNA with Ready-To-Go You-Prime First-Strand Beads (Amersham Bioscience, Piscataway, NJ). In order to reduce pipetting error, the final volume of cDNA was reached by adding four volumes RNase-free distilled water to the original cDNA. The resultant product was subjected to real-time PCR.
Real-time PCR was performed in a Light Cycler (Roche Applied Science, Indianapolis, IN) with Light cycler-DNA Master SYBR Green I (Roche Applied Science) and were performed according to the standard protocol recommended by the manufacturer. Reverse transcription was followed by 40 cycles of PCR (denaturation at 95°C for 10 min addition of 1-second at 94°C, 5-second annealing at 58, 60, 61, 59 and 58°C for OPG, RANKL, c-fos, NFATc1 and GAPDH, respecticvely, and 10- second extension at 72°C, and each of optimal fluorescence measurement temperature for 1 second as the amplification and quantification programs, 60–99°C with a heating rate of 0.1°C per second as the melting curve program, and finally, cooling to 40°C.
The primer sequences are as follows:
-
RANKL: sense: 5′-CAC CAT CAG CTG AAG ATA GT-3′
antisense: 5′-CCA AGA TCT CTA ACA TGA CG-3′
-
OPG: sense: 5′-TGGGACCAAAGTGAATGCCGAGA-3′
antisense: 5′-AGCTGCTCTGTGGTGAGGTTC-3′
-
c-fos: sense: 5′-CTGGTGCAGCCCACTCTGGT-3′
antisense: 5′- CTTTCAGCAGATTGGCAATC-3′
-
NFATc1: sense: 5′- CAACGCCCTGACCACCGATA-3′
antisense: 5′- GGCTGCCTTCCGTCTCATAG-3′
-
GAPDH: sense: 5′-ACCACAGTCCATGCCATCAC-3′
antisense: 5′- TCCACCACCCTGTTGCTGTA-3′
Semiquantitative RANKL, OPG, c-fos and NFATc1 expression levels were determined by normalizing with GAPDH expression level.
Western blotting
Cells were washed twice with ice-cold PBS and lysed with a buffer containing 20 mM Tris–HCl, 150 mM NaCl, 1% riton X-100, and inhibitors for proteases and phosphatases [25]. Cell lysates were centrifuged at 10,000 g for 20 min and the supernatants were collected. Twenty micrograms of cellular proteins was resolved by SDS–PAGE and transferred onto nitrocellulose membranes. Membranes were blocked with 5% skim milk in Tris-buffered saline containing 0.1% Tween 20 for 1 h and incubated overnight at 4°C with primary antibodies. Membranes were washed, incubated with appropriate secondary antibodies conjugated to horseradish peroxidase for 1 h, and developed using a chemiluminescence system. Relative protein expression was calculated from band densities obtained in three experiments using UMax PowerLook 1100 scanner (Taiwan) and TotalLab gel image analysis software (Nonlinear Dynamics, New England, UK).
PTHrP-induced bone resorption in an animal model
Male BDF1 mice aged four weeks were injected daily for five days (from day one to five) with PTHrP (18 µg/day; Figure 1). PTHrP was injected into the subcutaneous tissue over the left side of the calvaria twice daily in a volume of 10 µl as described previously [26], [27]. This delivery method produces an exaggerated resorptive response in the calvarial bone, as well as a systemic effect as indicated by hypercalcemia. Animals were housed in an accredited facility and all procedures used in the animal experiments complied with the standards described in the Osaka University Medical School Guidelines for the Care and Use of Laboratory Animal.
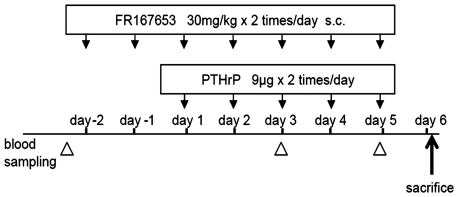
Figure 1. Animal model and treatment protocol.
The PTHrP-induced bone resorption animal model was established by injecting PTHrP (18 µg/day) onto the dorsal surface of the mice calvaria daily for five days (day 1 to 5). Animals in the treatment (n = 7) or the control group (n = 5) were injected with FR167653 (30 mg/kg twice daily) or distilled water, respectively, daily for seven days, initiating from two days before PTHrP injection. 50 µl of whole blood was collected retro-orbitally before treatment, on day three, and on day five to determine whole-blood ionized calcium levels. Mice were sacrificed on day six, 12 hours after the last injection of PTHrP.
Treatment Protocol
All animals were additionally treated with FR167653 or distilled water, injected subcutaneously on the mice back as in the protocol shown in Figure 1. Treatment was initiated two days before PTHrP injection.
Before treatment, 50 µl of whole blood was collected retro-orbitally into heparinized capillary tubes (Radiometer, Copenhagen, Denmark) and basal whole-blood ionized calcium levels were determined with a calcium level analyzer (model ABL505, Radiometer; n = 3 from treatment group and control group, respectively). The treatment group (n = 7) was treated with 30 mg/kg of FR167653 twice daily while the control group (n = 5) was treated with distilled water for seven days (including pretreatment). At day three and five, blood samples were taken three hours after injection of PTHrP retro-orbitally to determine the levels of hypercalcemia. Mice were killed on day six, which was 12 hours after the last injection of PTHrP.
Quantitative analysis of bone resorption
Calvaria were retrieved from sacrificed mice and immersed into neutral buffered formalin. Radiographs were taken with the MX-20 Specimen Radiography System (Faxitron X-ray, Wheeling, IL). The images were scanned into a computer with a flatbed scanner and saved in RGB color format. The quantitative analysis of calvarial bone resorption was processed with an image analysis software package, WinROOF (Mitani, Fukui, Japan). The thresholds were determined by clicking five typical bone resorptive areas.
After decalcification, the anterior and posterior portions of the calvaria were removed just anterior to coronal suture and posterior to lambdoid suture, respectively. The left part of the calvaria was cut into three strips, embedded in paraffin and sectioned. Sections were stained to detect TRAP activity and counterstained with hematoxylin.
Histomorphormetic analysis was performed on the left side of the calvaria to determine the number of osteoclasts present in the bone. The area measured consisted of six visual fields extending from sagittal suture toward the lateral muscle attachment and included all of the bone and marrow spaces. PTHrP-treated calvaria showed a distinct dorsal periosteal pattern of resorption and endosteal resorption. Thus, the osteoclast number represented the sum of periosteal and endosteal measurements. Results were recorded as osteoclast number per unit bone tissue area measured (OcN-BTA). All measurements were made by tracing the section image onto a digitizing platen with the aid of a cameral lucida attachment and WinROOF image analysis software.
Statistical analysis
All analyses were reported as mean ±S.D. Statistical significance was evaluated by Student's t-test or one factor analysis of variance (ANOVA) followed by a Tukey-Kramer post test using a statistical software package, JMP (SAS institute, Cary, NC).
Results
FR167653 inhibits Osteoclast formation in two different cell culture systems
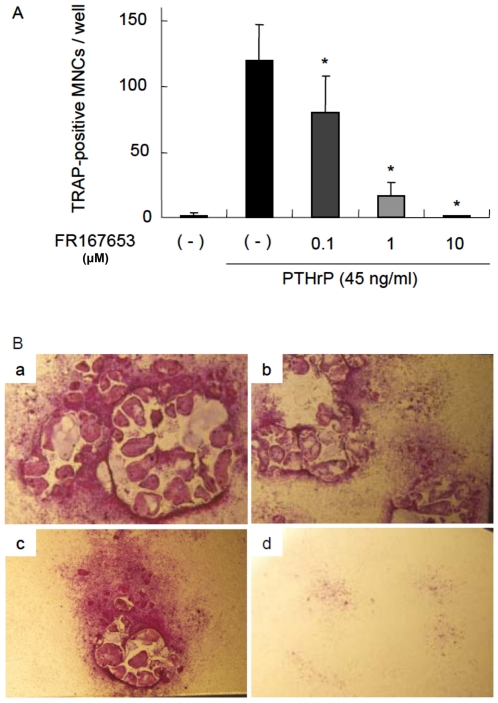
To determine whether FR167653 blocks osteoclastogenesis in vitro, we investigated osteoclastogenesis in a primary bone marrow cells stimulated by PTHrP. As expected, TRAP-positive MNCs (osteoclasts) was formed in primary bone marrow cultures treated with PTHrP. As shown in Figure 2, osteoclast formation induced by PTHrP at day six was inhibited by addition of FR167653 in a dose-dependent manner. At the concentration of 10 µM, FR167653 nearly eliminated osteoclast formation.
Figure 2. FR167653 inhibits TRAP-positive osteoclast formation in PTHrP-treated bone marrow cells.
A. Bone marrow cells were treated with PTHrP (45 ng/ml) and FR167653 for six days. TRAP-positive MNCs with three or more nuclei were counted as osteoclasts. n = 9. *: p<0.001 versus the group treated only with PTHrP. B. TRAP staining for bone marrow cells stimulated with PTHrP (45 ng/ml). a: no treatment. b: FR167653 (0.1 µM). c: FR167653 (1 µM). d: FR167653 (10 µM). TRAP-positive MNCs appear as red cells with clear peripheries.
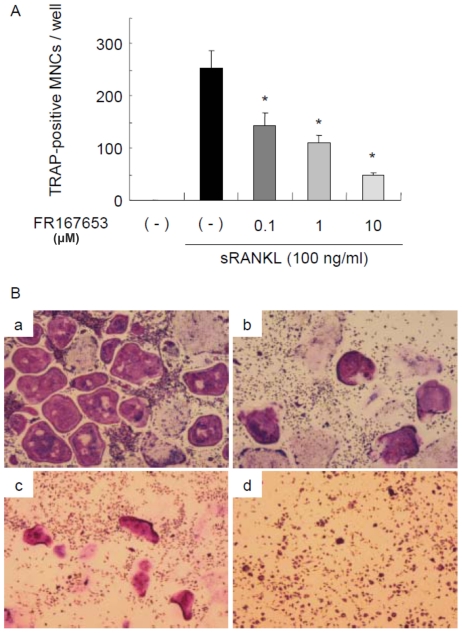
Unlike bone marrow cells, MDBMM cells are a homogeneous bone marrow macrophage population with a contamination by stromal cells of less than 1 in 1,000 cells. As shown in Figure 3, MDBMM cells formed TRAP-positive osteoclasts when treated with RANKL together with M-CSF even in the absence of stromal cells. FR167653 added to the cultures significantly inhibited RANKL-induced osteoclast formation in a dose-dependent manner. These data suggest that FR167653 acts directly on osteoclast precursors to inhibit osteoclast formation.
Figure 3. Effects of FR167653 on osteoclast formation in M-CSF-dependent bone marrow macrophages treated with sRANKL.
A. Osteoclast formation assay using M-CSF-dependent bone marrow macrophages (MDBMMs) prepared as described in the Materials and Methods section. TRAP-positive MNCs with three or more nuclei were counted as osteoclasts. n = 6. *: p<0.001 versus the group treated only with sRANKL. B. TRAP staining for MDBMMs stimulated by sRANKL (100 ng/ml). a: No treatment. b: FR167653 (0.1 µM). c: FR167653 (1 µM). d: FR167653 (10 µM).
OPG and RANKL expression levels in bone marrow cells were not affected by FR167653 treatment
To further elucidate the mechanism of FR167653 inhibition of PTHrP-induced osteoclastogenesis, we examined the effects of FR167653 on the OPG and RANKL expression in bone marrow stromal cells.
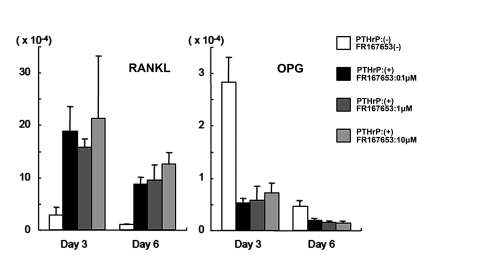
Bone marrow stromal cells were obtained by culturing primary bone marrow cells for ten days. Almost all non-adherent or loosely attached hematopoietic cells in the primary bone marrow cell cultures were removed by pipetting each time when the medium was changed. Expression of RANKL and OPG mRNA in bone marrow stromal cells was increased or decreased, respectively, within three days of PTHrP treatment. The increased expression even lasted 6 days post-treatment. Even at a dose of 10 µM, FR167653 has no detectable effect on the PTHrP-mediated expression of RANKL and OPG mRNA (Figure 4). Taken together, these results further suggest that FR167653 acts directly on osteoclast precursors (rather than on stromal cells or osteoblasts) in its role as an inhibitor of osteoclast formation.
Figure 4. FR167653 does not affect OPG or RANKL expression by PTHrP-treated stromal cells.
Bone marrow stromal cells were seeded into six-well plates and treated with PTHrP (90 ng/ml) and FR167653 at various concentrations for three and six days. Real-time PCR was used to determine the OPG or RANKL gene expression levels. The results are shown as mean ±S.D. of three independent experiments. Expression of RANKL and OPG mRNA was increased or decreased, respectively, within three days of PTHrP treatment, and lasted 6 days post-treatment. The expression level of RANKL and OPG did not changed by adding 1 µM or 10 µM FR167653 (P>0.05).
Expression of c-Fos and NFATc1 in osteoclast precursors (MDBMMs) were inhibited by FR167653
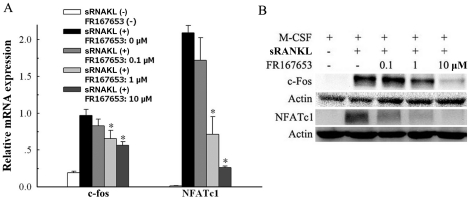
In order to investigate the mechanism of FR167653 on osteoclast formation in osteocalst precursor cells, we detected the expression of c-Fos and NFATc1 in bone marrow macrophage cells by western blotting and RT-PCR following the FR167653 treatments. As shown in Figure 5, the mRNA expression levels of c-fos and NFATc1 were dramatically increased when treated with sRANKL and these effects were inhibited by FR167653 in a dose-dependent manner(p<0.05; Figure 5A). Similarly, c-Fos and NFATc1 protein levels were decreased in the presence of FR167653 dose-dependently (Figure 5B).
Figure 5. The effects of FR167653 on sRANKL-induced c-Fos and NFATc1 expression level in M-CSF-dependent bone marrow macrophages (MDBMMs).
Bone marrow macrophages were treated with 100 ng/ml M-CSF and 100 ng/ml sRANKL without or with FR67653 at the indicated concentration. After 24 h incubation, cells were harvested and subjected to RT-PCR (A). For western blot analysis (B), the cells were incubated for 48 h. Expression of c-Fos and NFATc1 in both mRNA and protein levels were increased following the sRANKL treatment. This enhancement was inhibited by FR167653 in a dose dependent manner. The results are shown as mean ±S.D. of three independent experiments. *: p<0.05 versus the group treated only with sRANKL.
FR167653 reduces PTHrP-induced bone resorption in vivo
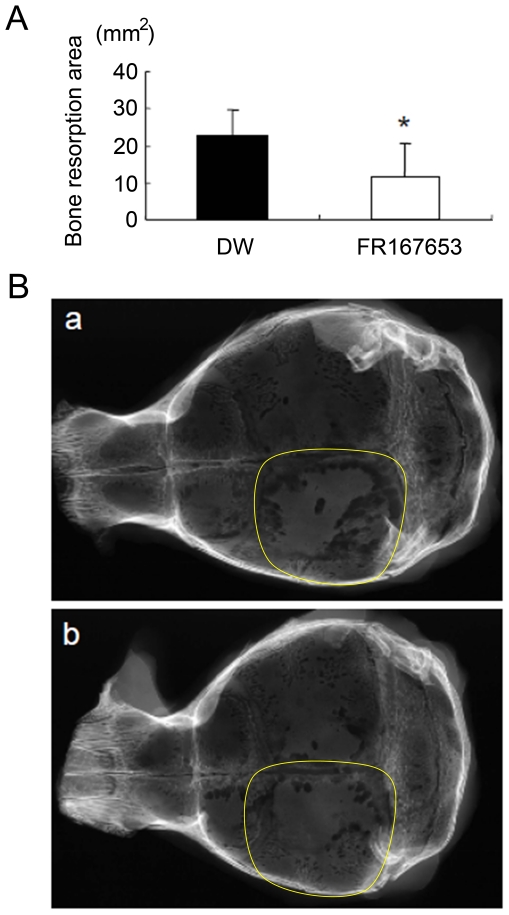
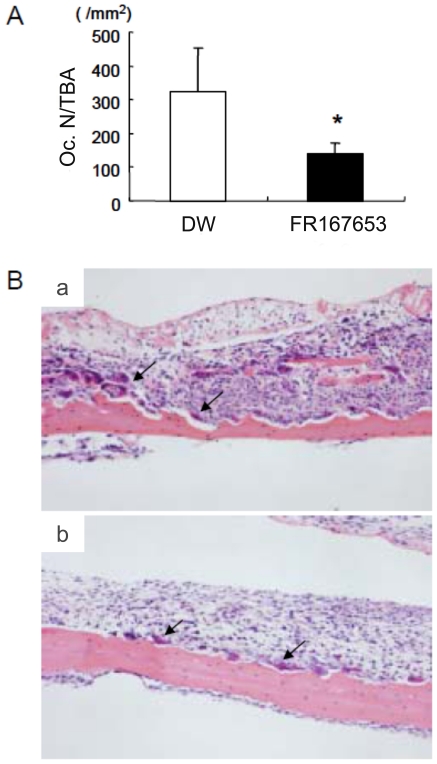
We next examined the effects of FR167653 on PTHrP-induced bone resorption in vivo. Animal models of bone resorption were established by direct injection of PTHrP onto the dorsal surface of mice calvaria. Radiographic evidence of bone resorption comprises zones of increased radiolucency in the calvaria. Histologically, these changes are associated with significant periosteal bone resorption on the dorsal calvarial surface and increased marrow spaces, accompanied with increased numbers of osteoclasts. FR167653 significantly reduced the radiographic bone resorption areas (p<0.05; Figure 6) and osteoclast number (as determined on histological sections) (p<0.005, Figure 7).
Figure 6. The effect of FR167653 on PTHrP-induced bone resorption.
The calvarial bone resorption was measured radiographically on the left side (area marked by yellow line) where treated by direct subcutaneous injection of PTHrP. A. Treatment by FR167653 reduced the bone resorption area (*: P<0.005). DW:distilled water. B. Representative X-rays of mouse calvaria with PTHrP-induced bone resorption. a: control group (treated with DW). b: FR167653 treatment group.
Figure 7. The effects of FR167653 on osteoclast number in calvaria from PTHrP-treated animals.
A. Osteoclast number per unit bone area (OcN-BTA) was measured histologically as described in the Materials and Methods section. Treatment of FR167653 reduces the number of osteoclasts (*: P<0.005). B. TRAP staining and counterstain with hematoxylin on calvaria sections. a: Histological section of animals treated with DW. b: Histological section of animals treated with FR167653. Osteoclasts are indicated by arrowheads.
Treatment with FR167653 has the effect on the cotnrol of PTHrP-induced systemic hypercalcemia
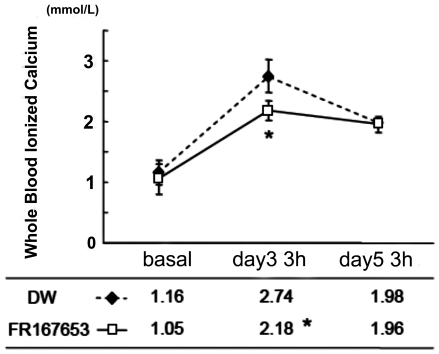
PTHrP is one of the causative factors of the hypercalcemia that is associated with malignancy, since PTHrP can induce systemic bone resorption. Furthermore, extensive local bone resorption is to some extent a contributory cause of hypercalcemia. For this reason, we monitored whole-blood ionized calcium levels in our experimental system. The level of whole-blood ionized calcium was increased at three hours after PTHrP injection on days 3 and 5 compared to day 1. When additionally treated with FR167653, the whole-blood ionized calcium concentration was significantly decreased at day 3, but not day 5. This suggest the functional involvement of p38 MAPK in PTHrP-induced systemic hypercalcemia (Figure 8).
Figure 8. The effect of FR167653 on whole-blood ionized calcium levels in PTHrP-treated mice.
PTHrP induces a significant elevation of whole-blood ionized calcium levels in mice within three hours of injection with PTHrP (at day three and five). The whole-blood ionized calcium levels are lower in the FR167653-treated group than in the DW-treated group (control group) at day three (*: P<0.05). By day five, however, there was no significant difference between two groups.
Discussion
There are several reports about p38 MAPK inhibitor, SB203580, inhibits osteoclast differentiation in RAW264 induced by RANKL [28] or in bone marrow cells induced by 1α, 25- (OH)2 D3 and prostaglandin E2 (PGE2) [14]. Previously, we reported that M-CSF-dependent sRANKL- and TNFα-induced osteoclast formation in primary bone marrow cells, and collagen-induced arthritis in rats were inhibited by FR167653 [23]. Here we further show that FR167653, a selective inhibitor of p38 MAPK, strongly inhibits osteoclast differentiation in PTHrP-treated bone marrow cell cultures.
We also investigated the mechanism of FR167653 involved in the inhibition of osteoclast formation. Most osteoclastgenesis inducers are thought to stimulate osteoclast formation via up-regulation of RANKL and down-regulation of OPG gene expression as mediated by osteoblast or bone marrow stromal cells [25], [29]. In our study, however, even the highest concentration of FR167653 that was tested (10 µM) has no effect on RANKL and OPG expression by PTHrP-stimulated primary bone marrow stromal cells. These data suggest that p38 MAPK is not involved in the regulation of PTHrP-mediated bone resorption-related functions of osteoblasts, including regulation of RANKL and OPG expression. This is consistent with previous results that SB203580 has no effect on RANKL and OPG expression by primary osteoblast stimulated with 1α, 25- (OH)2D3 and PGE2 [14]. Together, the data suggest that p38 MAPK is involved in the differentiation of osteoclast precursors, rather than in the function of supporting cell such as osteoblasts or bone marrow stromal cells.
However, the current study found that the up-regulation of c-Fos and NFATc1expression treated with RANKL in osteoclast precursor cells (MDBMMs) were inhibited by FR167653 in a dose-dependent manner in both mRNA and protein levels. This strongly suggested the inhibition of osteoclast differentiation by FR167653 is involved in regulating c-Fos and NFATc1 expression in osteoclast precursor cells. The similar results were reported by using SB203580 [30]. These findings, together with the present study, suggest that p38 MAPK-mediated signal propagation down to c-Fos and NFATc1 are of fundamental importance for the differentiation of osteoclast precursors into osteoclasts.
In our animal experiments, local injection of PTHrP on calvaria caused significant bone resorption and systemic hypercalcemia, and increased osteoclast number. Treatment with FR167653 successfully reduced bone resorption and osteoclast number in calvaria, and eventually protected the calvaria from serious osteoclastic dystruction induced by locally administered PTHrP. However, some relieving effect on hypercalcemia was observed. In our hands, inhibition of p38 MAPK by FR167653 significantly decreased the whole-blood calcium level on day 3. However, there is no difference in blood calcium level between the FR167653 treatment group and the control group at day 5.
It was reported that recombinant human OPG is effective in inhibiting bone resorption and hypercalcemia induced by PTHrP in vivo [27]. Treatment with chimeric form of OPG in combination with PTHrP (20 µg/day) maintained whole blood ionized calcium levels within the normal range (∼1.20 mmol/l). Even though FR167653 was given subcutaneously, it should have act systemically. The concentration of calcium in extracellular fluids is under control by a complex homeostatic system strictly that includes the parathyroid glands, kidneys, bones, and intestines [31], [32]. An increase of extracellular calcium concentration is sensed by a calcium-sensing receptor (CaR) in the plasma membrane of parathyroid gland cells and the kidney which in turn affects parathyroid hormone, calcitonin, and 1, 25 (OH) 2D3 secretion. Although intracellular signaling systems regulated by the CaR are still in obscurity, recent evidence suggests that MAPK pathways are activated by CaR [33], [34]. For example, calcium homeostasis by angiotensin II in adrenal glomerulosa cells is mediated by activation of p38 MAPK pathways [35]. These observations may help us to understand the surprisingly small effect of p38 MAPK pathway blockade on regulating hypercalcemia induced by PTHrP because the inhibition of p38 MAPK pathway may block CaR signaling pathway as well, which in turn causes reduced reaction to extracellular Ca2+ in tissues that express CaR. The detailed mechanism for why inhibition of p38 MAPK partially prevents hypercalcemia even though it is effective in reducing bone resorption merits further studies.
FR167653 has been used in several inflammatory disease models and no obvious adverse events were observed [15]–[19]. In our previous in vivo study [23], we found no significant side effects with daily treatments of 32 mg/kg, a dose found to be safe and effective in other inflammation models as well [36]. In the present study, we also found that there is no significant side effects when twice daily treatment with 30 mg/kg FR167653 for about 1 week. Long-term injection of FR167653 may lead to toxic events; indeed, one study demonstrated that FR167653 treatment increased plasma creatine and lactate dehydrogenase levels in rats [37]. Clearly, the potentially adverse effects of FR167653, including its modulation of calcium homeostasis, need to be studied extensively.
In conclusion, our data indicate that a potent p38 MAPK inhibitor, FR167653, blocks PTHrP-induced osteoclastogenesis in vitro, and bone resorption and hypercalcemia in vivo. Our results indicate that the responses of other tissues or organs to the p38 MAPK inhibitor may affect calcium homeostasis. This study provides a plausible explanation and target for PTHrP-induced osteoclastogenesis, which will help us to understand the mechanism of bone resorption-related diseases.
Acknowledgments
We thank Dr. Noriyuki Tsumaki for his helpful discussion. We also thank Miss Kanae Asai and Miss Mizuki Nakata for their excellent technical assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology, Japan and by the Ministry of Health, Labor and Welfare, Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Reference
- 1.Wysolmerski JJ, Broadus AE. Hypercalcemia of malignancy: the central role of parathyroid hormone-related protein. Annu Rev Med. 1994;45:189–200. doi: 10.1146/annurev.med.45.1.189. [DOI] [PubMed] [Google Scholar]
- 2.Guise TA, Yin JJ, Taylor SD, Kumagai Y, Dallas M, et al. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Invest. 1996;98:1544–1549. doi: 10.1172/JCI118947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, et al. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest. 1999;103:197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mundy GR, Yoneda T. Bisphosphonates as anticancer drugs. N Engl J Med. 1998;339:398–400. doi: 10.1056/NEJM199808063390609. [DOI] [PubMed] [Google Scholar]
- 5.Coleman RE, Lipton A, Roodman GD, Guise TA, Boyce BF, et al. Metastasis and bone loss: advancing treatment and prevention. Cancer Treat Rev. 2010;36:615–620. doi: 10.1016/j.ctrv.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas RJ, Guise TA, Yin JJ, Elliott J, Horwood NJ, et al. Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology. 1999;140:4451–4458. doi: 10.1210/endo.140.10.7037. [DOI] [PubMed] [Google Scholar]
- 7.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 8.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 10.Jimi E, Furuta H, Matsuo K, Tominaga K, Takahashi T, et al. The cellular and molecular mechanisms of bone invasion by oral squamous cell carcinoma. Oral Dis. 2011;17:462–468. doi: 10.1111/j.1601-0825.2010.01781.x. [DOI] [PubMed] [Google Scholar]
- 11.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, et al. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anandarajah AP. Role of RANKL in bone diseases. Trends Endocrinol Metab. 2009;20:88–94. doi: 10.1016/j.tem.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto M, Sudo T, Saito T, Osada H, Tsujimoto M. Involvement of p38 mitogen-activated protein kinase signaling pathway in osteoclastogenesis mediated by receptor activator of NF-kappa B ligand (RANKL). J Biol Chem. 2000;275:31155–31161. doi: 10.1074/jbc.M001229200. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Udagawa N, Itoh K, Suda K, Murase Y, et al. p38 MAPK-mediated signals are required for inducing osteoclast differentiation but not for osteoclast function. Endocrinology. 2002;143:3105–3113. doi: 10.1210/endo.143.8.8954. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto N, Sakai F, Yamazaki H, Nakahara K, Okuhara M. Effect of FR167653, a cytokine suppressive agent, on endotoxin-induced disseminated intravascular coagulation. Eur J Pharmacol. 1996;314:137–142. doi: 10.1016/s0014-2999(96)00537-7. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto N, Sakai F, Yamazaki H, Sato N, Nakahara K, et al. FR167653, a dual inhibitor of interleukin-1 and tumor necrosis factor-alpha, ameliorates endotoxin-induced shock. Eur J Pharmacol. 1997;327:169–174. doi: 10.1016/s0014-2999(97)89657-4. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi S, Keto Y, Fujita T, Uchiyama T, Yamamoto A. FR167653, a p38 mitogen-activated protein kinase inhibitor, prevents Helicobacter pylori-induced gastritis in Mongolian gerbils. J Pharmacol Exp Ther. 2001;296:48–56. [PubMed] [Google Scholar]
- 18.Cau J, Favreau F, Zhang K, Febrer G, de la Motte GR, et al. FR167653 improves renal recovery and decreases inflammation and fibrosis after renal ischemia reperfusion injury. J Vasc Surg. 2009;49:728–740. doi: 10.1016/j.jvs.2008.09.056. [DOI] [PubMed] [Google Scholar]
- 19.Yoshinari D, Takeyoshi I, Koibuchi Y, Matsumoto K, Kawashima Y, et al. Effects of a dual inhibitor of tumor necrosis factor-alpha and interleukin-1 on lipopolysaccharide-induced lung injury in rats: involvement of the p38 mitogen-activated protein kinase pathway. Crit Care Med. 2001;29:628–634. doi: 10.1097/00003246-200103000-00029. [DOI] [PubMed] [Google Scholar]
- 20.Nishikori T, Irie K, Suganuma T, Ozaki M, Yoshioka T. Anti-inflammatory potency of FR167653, a p38 mitogen-activated protein kinase inhibitor, in mouse models of acute inflammation. Eur J Pharmacol. 2002;451:327–333. doi: 10.1016/s0014-2999(02)02238-0. [DOI] [PubMed] [Google Scholar]
- 21.Furuichi K, Wada T, Iwata Y, Sakai N, Yoshimoto K, et al. Administration of FR167653, a new anti-inflammatory compound, prevents renal ischaemia/reperfusion injury in mice. Nephrol Dial Transplant. 2002;17:399–407. doi: 10.1093/ndt/17.3.399. [DOI] [PubMed] [Google Scholar]
- 22.Hou Z, Yanaga K, Kamohara Y, Eguchi S, Tsutsumi R, et al. A new suppressive agent against interleukin-1beta and tumor necrosis factor-alpha enhances liver regeneration after partial hepatectomy in rats. Hepatol Res. 2003;26:40–46. doi: 10.1016/s1386-6346(02)00334-0. [DOI] [PubMed] [Google Scholar]
- 23.Nishikawa M, Myoui A, Tomita T, Takahi K, Nampei A, et al. Prevention of the onset and progression of collagen-induced arthritis in rats by the potent p38 mitogen-activated protein kinase inhibitor FR167653. Arthritis Rheum. 2003;48:2670–2681. doi: 10.1002/art.11227. [DOI] [PubMed] [Google Scholar]
- 24.Azuma Y, Kaji K, Katogi R, Takeshita S, Kudo A. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J Biol Chem. 2000;275:4858–4864. doi: 10.1074/jbc.275.7.4858. [DOI] [PubMed] [Google Scholar]
- 25.Huang H, Chang EJ, Ryu J, Lee ZH, Lee Y, et al. Induction of c-Fos and NFATc1 during RANKL-stimulated osteoclast differentiation is mediated by the p38 signaling pathway. Biochem Biophys Res Commun. 2006;351:99–105. doi: 10.1016/j.bbrc.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Boyce BF, Aufdemorte TB, Garrett IR, Yates AJ, Mundy GR. Effects of interleukin-1 on bone turnover in normal mice. Endocrinology. 1989;125:1142–1150. doi: 10.1210/endo-125-3-1142. [DOI] [PubMed] [Google Scholar]
- 27.Morony S, Capparelli C, Lee R, Shimamoto G, Boone T, et al. A chimeric form of osteoprotegerin inhibits hypercalcemia and bone resorption induced by IL-1beta, TNF-alpha, PTH, PTHrP, and 1, 25(OH)2D3. J Bone Miner Res. 1999;14:1478–1485. doi: 10.1359/jbmr.1999.14.9.1478. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto M, Sudo T, Maruyama M, Osada H, Tsujimoto M. Activation of p38 mitogen-activated protein kinase is crucial in osteoclastogenesis induced by tumor necrosis factor. FEBS Lett. 2000;486:23–28. doi: 10.1016/s0014-5793(00)02231-6. [DOI] [PubMed] [Google Scholar]
- 29.Brandstrom H, Jonsson KB, Ohlsson C, Vidal O, Ljunghall S, et al. Regulation of osteoprotegerin mRNA levels by prostaglandin E2 in human bone marrow stroma cells. Biochem Biophys Res Commun. 1998;247:338–341. doi: 10.1006/bbrc.1998.8783. [DOI] [PubMed] [Google Scholar]
- 30.Horwood NJ, Elliott J, Martin TJ, Gillespie MT. Osteotropic agents regulate the expression of osteoclast differentiation factor and osteoprotegerin in osteoblastic stromal cells. Endocrinology. 1998;139:4743–4746. doi: 10.1210/endo.139.11.6433. [DOI] [PubMed] [Google Scholar]
- 31.Magno AL, Ward BK, Ratajczak T. The calcium-sensing receptor: a molecular perspective. Endocr Rev. 2011;32:3–30. doi: 10.1210/er.2009-0043. [DOI] [PubMed] [Google Scholar]
- 32.Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81:239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- 33.Tfelt-Hansen J, MacLeod RJ, Chattopadhyay N, Yano S, Quinn S, et al. Calcium-sensing receptor stimulates PTHrP release by pathways dependent on PKC, p38 MAPK, JNK, and ERK1/2 in H-500 cells. Am J Physiol Endocrinol Metab. 2003;285:E329–337. doi: 10.1152/ajpendo.00489.2002. [DOI] [PubMed] [Google Scholar]
- 34.Morgan R, Fairfax B, Pandha HS. Calcium insensitivity of FA-6, a cell line derived from a pancreatic cancer associated with humoral hypercalcemia, is mediated by the significantly reduced expression of the Calcium Sensitive Receptor transduction component p38 MAPK. Mol Cancer. 2006;5:51. doi: 10.1186/1476-4598-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Startchik I, Morabito D, Lang U, Rossier MF. Control of calcium homeostasis by angiotensin II in adrenal glomerulosa cells through activation of p38 MAPK. J Biol Chem. 2002;277:24265–24273. doi: 10.1074/jbc.M110947200. [DOI] [PubMed] [Google Scholar]
- 36.Wada T, Furuichi K, Sakai N, Iwata Y, Yoshimoto K, et al. A new anti-inflammatory compound, FR167653, ameliorates crescentic glomerulonephritis in Wistar-Kyoto rats. J Am Soc Nephrol. 2000;11:1534–1541. doi: 10.1681/ASN.V1181534. [DOI] [PubMed] [Google Scholar]
- 37.Gardiner SM, Kemp PA, March JE, Bennett T. Influence of FR167653, an inhibitor of TNF-alpha and IL-1, on the cardiovascular responses to chronic infusion of lipopolysaccharide in conscious rats. J Cardiovasc Pharmacol. 1999;34:64–69. doi: 10.1097/00005344-199907000-00011. [DOI] [PubMed] [Google Scholar]