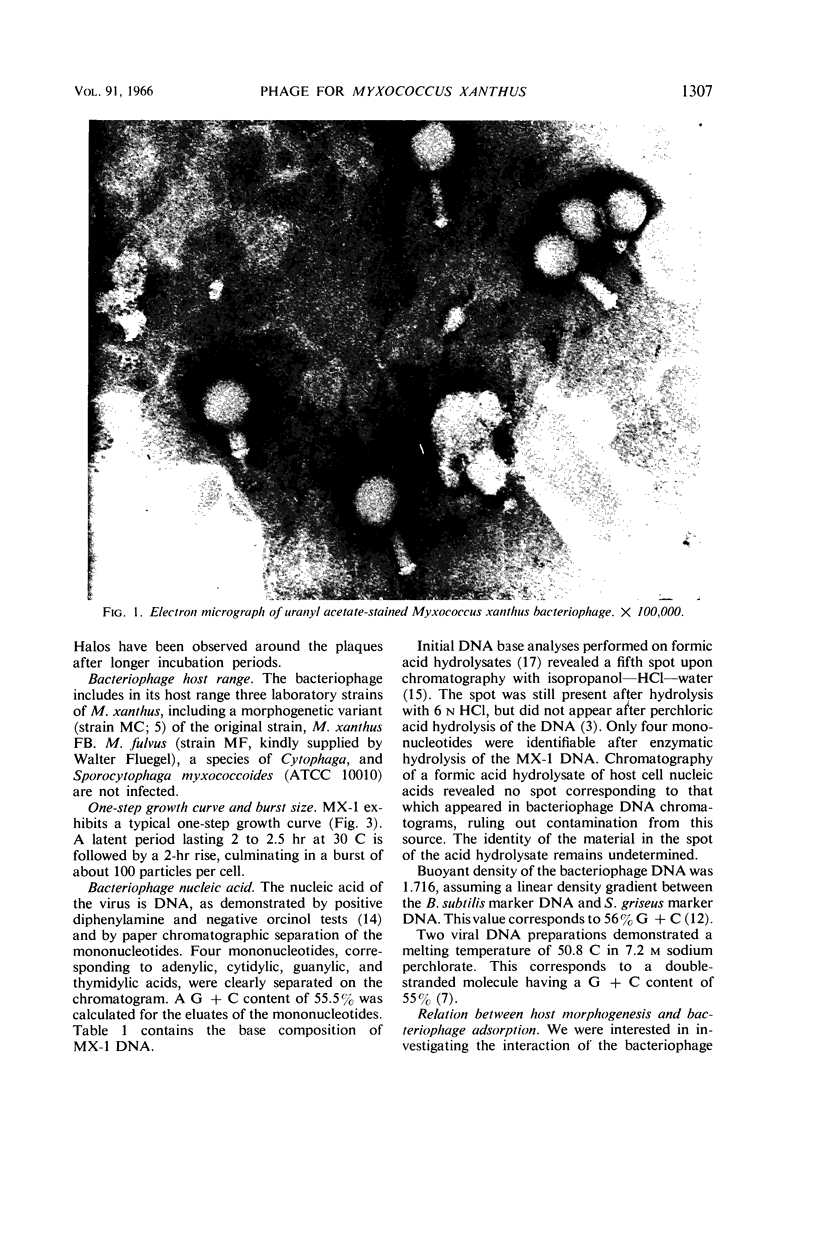
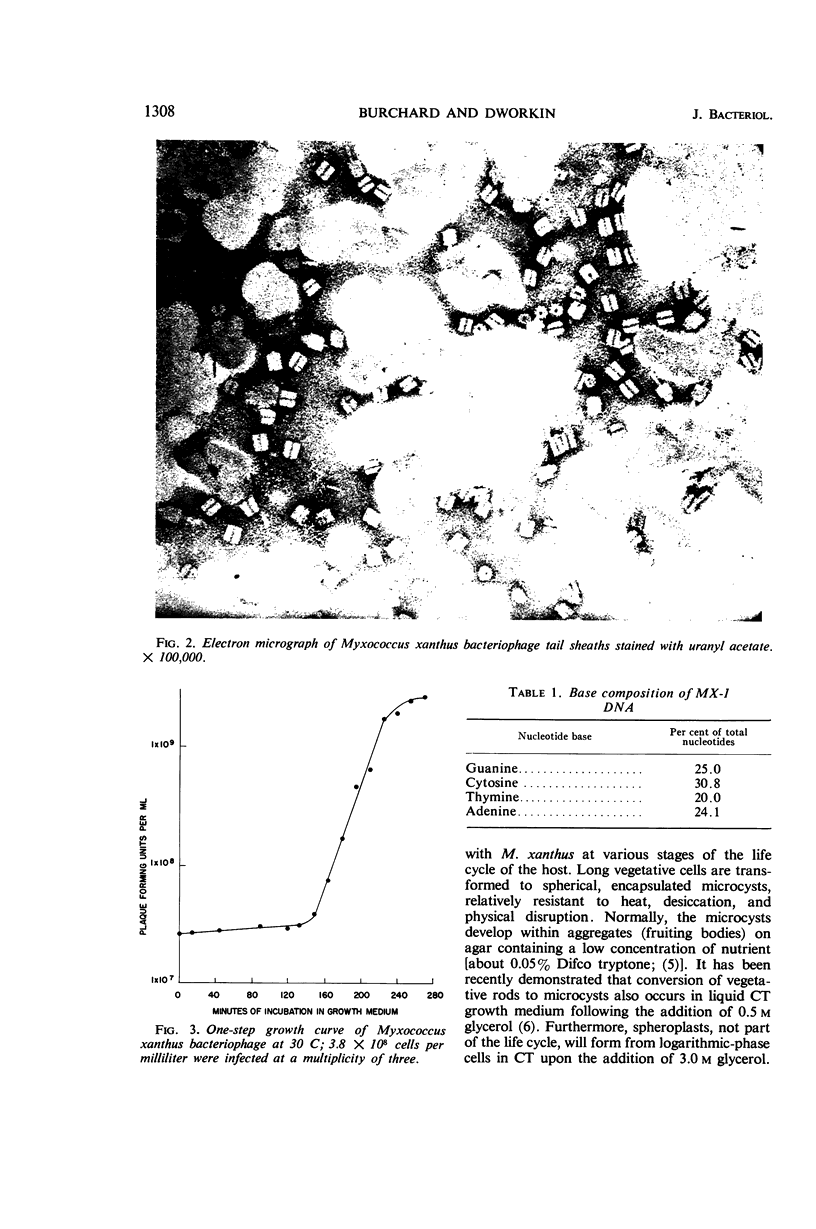
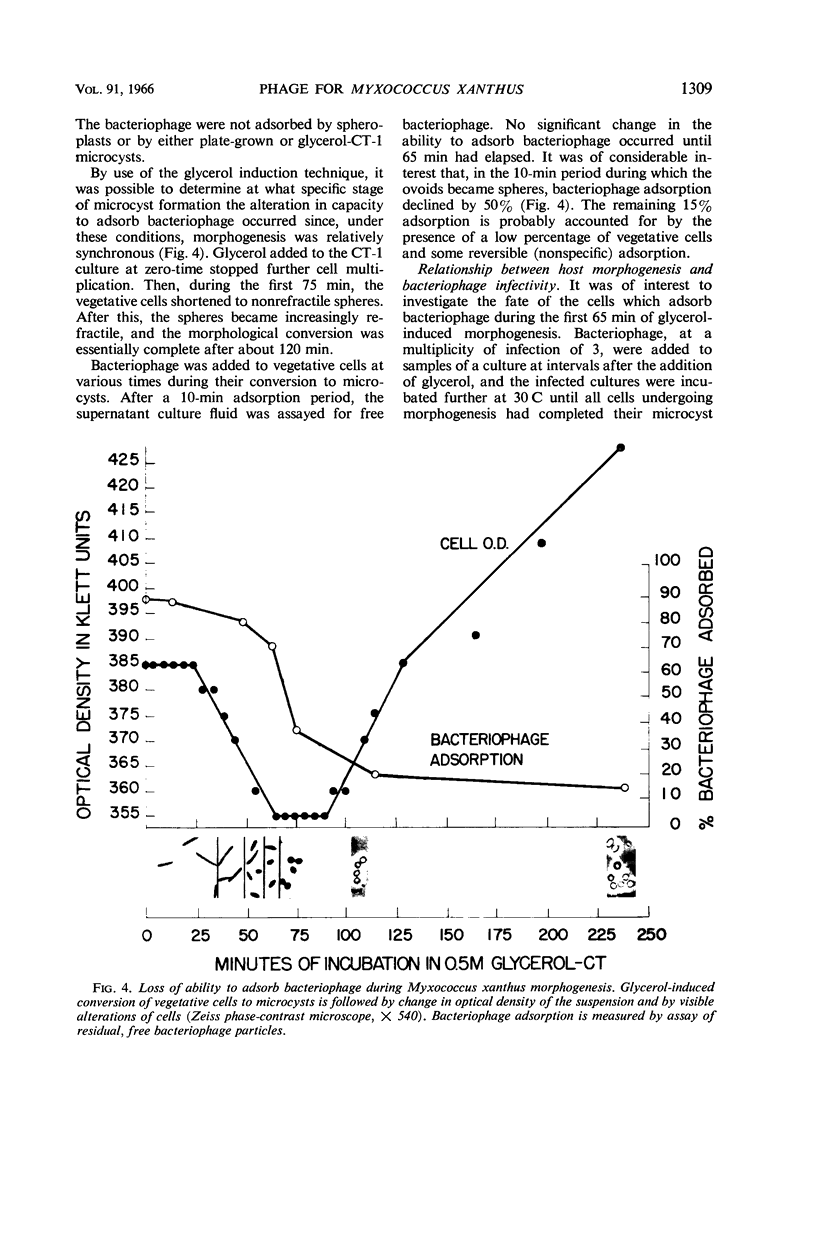
Abstract
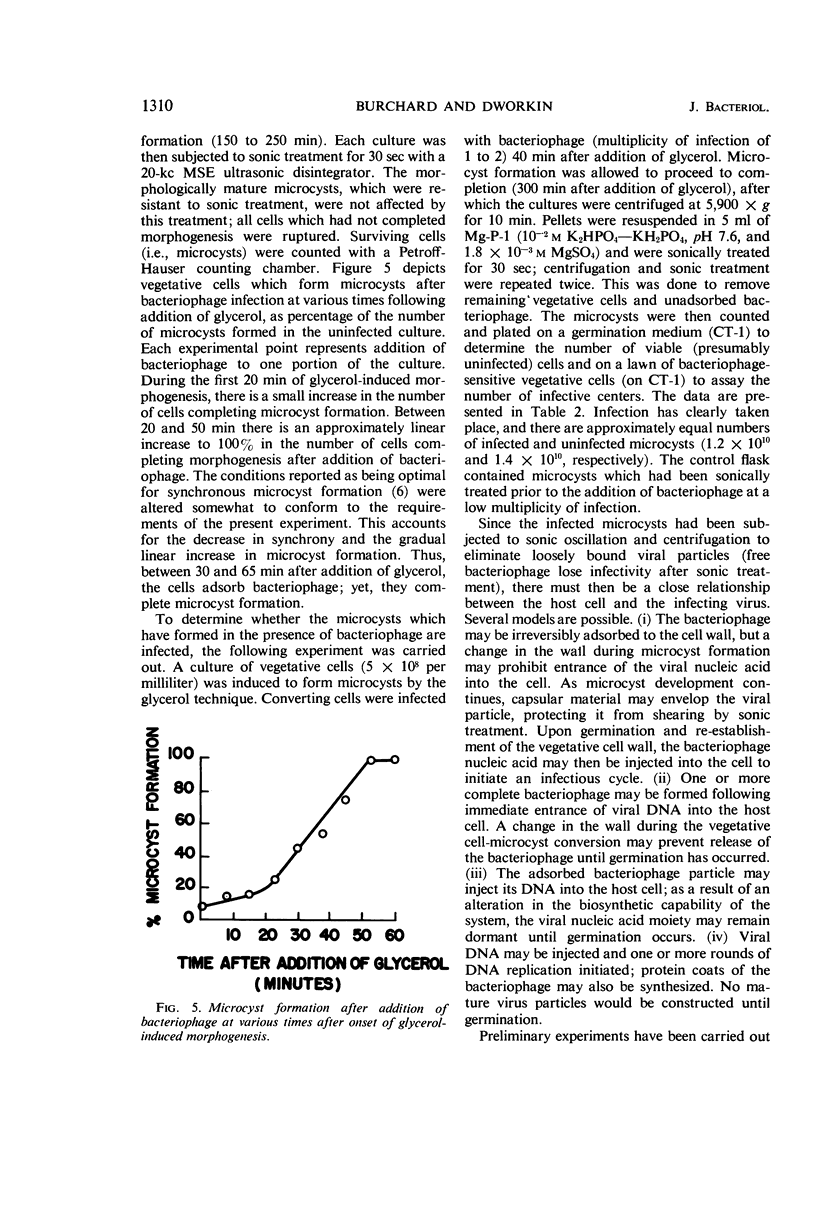
Burchard, Robert P. (University of Minnesota, Minneapolis), and M. Dworkin. A bacteriophage for Myxococcus xanthus: isolation, characterization and relation of infectivity to host morphogenesis. J. Bacteriol. 91:1305–1313. 1966.—A bacteriophage (MX-1) infecting Myxococcus xanthus FBt has been isolated from cow dung. The bacteriophage particle is approximately 175 mμ long. A tail about 100 mμ in length is encased in a contractile sheath and terminates in a tail plate. The head is polyhedral with a width of about 75 mμ. The nucleic acid of the bacteriophage is deoxyribonucleic acid and has a guanine plus cytosine content of 55.5%. The bacteriophage requires 10−3m Ca++ and 10−2m monovalent cation for optimal adsorption. Grown on vegetative cells of M. xanthus FBt at 30 C in 2% Casitone medium, the bacteriophage has a latent period of 120 min and a burst size of approximately 100. Host range studies indicate that three strains of M. xanthus including a morphogenetic mutant are sensitive to the bacteriophage, whereas M. fulvus, Cytophaga, Sporocytophaga myxococcoides, and a fourth strain of M. xanthus are not. Of the two cellular forms characteristic of the Myxococcus life cycle, the bacteriophage infect only the vegetative cells; they do not adsorb to microcysts. Ability to adsorb bacteriophage is lost between 65 and 75 min after initiation of the relatively synchronous conversion of vegetative cells to microcysts. The bacteriophage does not adsorb to spheroplasts. After the appearance of visible morphogenesis and before the loss of bacteriophage receptor sites, addition of bacteriophage results in the formation of microcysts which give rise to infective centers only upon germination. The possibility that the infected microcysts are harboring intact bacteriophages has been eliminated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANACKER R. L., ORDAL E. J. Study of a bacteriophage infecting the myxobacterium Chondrococcus columnaris. J Bacteriol. 1955 Dec;70(6):738–741. doi: 10.1128/jb.70.6.738-741.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DWORKIN M., GIBSON S. M. A SYSTEM FOR STUDYING MICROBIAL MORPHOGENESIS: RAPID FORMATION OF MICROCYSTS IN MYXOCOCCUS XANTHUS. Science. 1964 Oct 9;146(3641):243–244. doi: 10.1126/science.146.3641.243. [DOI] [PubMed] [Google Scholar]
- DWORKIN M. Nutritional requirements for vegetative growth of Myxococcus xanthus. J Bacteriol. 1962 Aug;84:250–257. doi: 10.1128/jb.84.2.250-257.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEIDUSCHEK E. P. On the factors controlling the reversibility of DNA denaturation. J Mol Biol. 1962 Jun;4:467–487. doi: 10.1016/s0022-2836(62)80103-x. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGASANIK B., VISCHER E., DONIGER R., ELSON D., CHARGAFF E. The separation and estimation of ribonucleotides in minute quantities. J Biol Chem. 1950 Sep;186(1):37–50. [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- WYATT G. R., COHEN S. S. The bases of the nucleic acids of some bacterial and animal viruses: the occurrence of 5-hydroxymethylcytosine. Biochem J. 1953 Dec;55(5):774–782. doi: 10.1042/bj0550774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WYATT G. R. The purine and pyrimidine composition of deoxypentose nucleic acids. Biochem J. 1951 May;48(5):584–590. doi: 10.1042/bj0480584. [DOI] [PMC free article] [PubMed] [Google Scholar]