Abstract
Background
The most recent strategy for schistosomiasis control in the People's Republic of China aims to reduce the likelihood of environmental contamination of schistosome eggs. Despite considerable progress, it is believed that achievements would be further consolidated with additional intermediate host snail control measures. We provide an empirical framework for discerning the relative contribution of intrinsic effects (density feedback) from other extrinsic drivers of snail population dynamics.
Methods
We set up experiments in two study locations to collect reproduction data of Oncomelania hupensis, the intermediate host snail of Schistosoma japonicum. We applied a set of four population dynamic models that have been widely used to study phenomenological time-series data to examine the properties of demographic density feedback patterns from abundance data. We also contrasted the obtained results with the component feedback of density on survival rate to determine whether adult survival was the principal driver of the demographic feedback observed.
Results
Demographic density feedback models (Ricker- and Gompertz-logistic) accounted for > 99% of Akaike's information criterion model weight, with the Gompertz ranking highest in all O. hupensis population groups. We found some evidence for stronger compensatory feedback in the O. hupensis population from Sichuan compared to a Jiangsu population. Survival rates revealed strong component feedback, but the log-linear relationships (i.e. Gompertz) had less support in the demographic feedback analysis.
Conclusions
Our findings indicate that integrated schistosomiasis control measures must continue to reduce parasite abundance further because intermediate host snail populations tend to grow exponentially at low densities, especially O. hupensis populations in mountainous regions. We conclude that density feedback in adult survival is the principal component contribution to the demographic phenomenon observed in the population fitness (r)-abundance relationship.
Background
In the People's Republic of China (P.R. China), schistosomiasis caused by the blood fluke Schistosoma japonicum has a documented history of more than 2,000 years [1,2]. The first large-scale surveys done in the mid-1950s suggested that the disease was endemic in 12 provinces located along and south of the Yangtze River. More than 10 million people were infected, causing considerable morbidity and even mortality [3,4]. Hence, a national schistosomiasis control programme was launched, placing particular emphasis on the control of the intermediate host snail Oncomelania hupensis, including environmental management and chemical mollusciciding [2-6]. As a result, snail-infested areas have been reduced from approximately 14,320 km2 in the mid-1950s to 3,720 km2 in 2008 [6,7].
Recently, a comprehensive strategy was proposed with the ultimate aim to reduce further the likelihood of contamination of the environment with schistosome eggs. This integrated control strategy consists of health education, access to clean water and adequate sanitation, mechanization of agriculture and fencing of domesticated bovines, along with preventive chemotherapy [8,9]. The main rationale for implementing this new strategy is that schistosomiasis is an environmentally mediated disease, and that it is difficult to eliminate all snail habitats, particularly in lake and marshland regions [10]. However, this new strategy alone does not succeed in eliminating or substantially reducing the incidence of schistosomiasis [11], especially in mountainous regions where suitable snail habitats persist. Additional control measures are needed, such as mollusciciding, which is a time-consuming and costly strategy, because large fluctuations in snail abundance [12,13] can arise from flooding [14,15]. Hence, measures for increasing the effectiveness of mollusciciding, which in turn reduce intermediate host snail abundance and limit the likelihood of re-emergence of schistosomiasis, are required [16].
For the effective control of O. hupensis populations, a fundamental step is a deeper understanding of the snail's intrinsic population dynamics because these properties influence the rate of recovery after withdrawal of snail control [1]. There is, however, a paucity of information describing even basic population dynamics for this species, which severely limits our understanding of the processes of schistosomiasis transmission, and hence hampers the development of effective control approaches. here is a general consensus among ecologists that account must be taken of intrinsic and extrinsic population controls [17] when analysing time-series data, and growing emphasis is placed on determining the degree of interaction between the two aspects driving fluctuations in abundance [18-22]. Intrinsic control normally operates via density feedback whereby component vital rates (e.g. survival and fertility) and/or individual fitness change in response to population density [23]. However, vital rates can respond differently to density changes, meaning that the influence of density on the rate of population growth (termed demographic feedback) as measured by changes in abundance should reflect the net contributions of all component vital rates and extrinsic perturbations [24]. Extrinsic processes include stochastic environmental pressures and human control activities that affect population density, but are not directly affected by population density themselves [22].
Previous research pertaining to O. hupensis focused mainly on the environmental conditions correlated with snail abundance, and hence particular emphasis was placed on elucidating extrinsic influences. Similar to mosquito vector species [25-27], snail population dynamics can also exhibit strong density feedback, and the form and relative strength of feedback might differ markedly among populations given the strong genetic differentiation observed in intermediate host snails [28,29]. Failure to take intrinsic dynamics into account can lead to an over-estimation of the medium- to long-term effectiveness of density control methods, such as mollusciciding or habitat modification [23].
Here we provide an empirical framework for discerning the relative contributions of intrinsic drivers of snail population dynamics for a better understanding of the eco-epidemiology and control of schistosomiasis. We first examine the evidence for, strength and form of phenomenological (demographic) density feedback operating in focal snail populations based on a series of biological experiments under quasi-field conditions. We also test whether the intrinsic dynamics of O. hupensis follow a Gompertz-like compensatory feedback, which is consistent with organisms having high turn-over rates such as insects [30,31]. The Gompertz model and its analogues express population growth (r) or vital rates such as survival (s) as a negative log-linear relationship with density, with high r or s at low densities which decline rapidly as population size increases and then tapers to an asymptote [23]. Examining the patterns of component feedback in snail survival provides insight into the principal drivers of the strength and form of the demographic response. The relevant elements derived from the intrinsic model will provide an ecologically based evidence to formulate cost-effective control strategies towards schistosomiasis elimination, and to ensure the lowest transmission risk or density of O. hupensis [23].
Methods
Study site
To deepen our understanding of intrinsic effects on O. hupensis abundance patterns, snail reproduction experiments were done in two different settings: a marshland at Zhang Jiagang, Jiangsu province (119° 30' E longitude and 31° 50' N latitude) and a mountainous region in Pujiang county, Sichuan province (103° 24' E, 30° 18' N) (Figure 1).
Figure 1.
Study sites in Jiangsu and Sichuan provinces, People's Republic of China.
Snail experiments
In September 2008, we collected O. hupensis from each study site, noting the sex ratio (male:female) from a random sample of 500 snails which was close to 1:1 at both sites. In late 2008, we marked randomly selected adult snails from each study site (on the shell, using waterproof marker pens), and placed them into individual 0.5-m2 cages with soil covering the bottom (Figure 2). According to long-term experience with snail surveillance as part of the national schistosomiasis control programme, densities fluctuate between 10 and 800 snails per m2 [32,33]. We therefore adjusted experimental snail densities from 2 to 1,000 (i.e. 2, 4, 6, 8, 10, 20, 40, 80, 100, 150, 200, 400, 800 and 1000) per 0.5-m2. In those cages where 40 snails or less were kept, we placed equal numbers of male and female snails into individual cages to avoid potential sex biases. We kept snail cages at approximately 'natural' soil moisture conditions throughout the year to mimic normal variation. We kept the first generation of snails in cages until late June because the period of peak egg production of O. hupensis is around March and April, and generally lasts until June. In June 2009, we counted the snails and removed the marked adults (both dead and alive). We estimated the second generation of snails by counting them in November 2009.
Figure 2.
Schematic diagram of a snail-raising cage. The frame of the cage is made of crude wire mesh. Inner components of the frame are covered by nylon yarn silk with a mesh size of 40 μm, thus preventing the leaking of snail eggs.
In late September 2009, we repeated the experiment until the following year. We randomly selected adult snails in the second experiment from the new generation of snails obtained in the first experiment. We assembled the data obtained from the two experiments and subjected them to a pooled analysis.
Analysis
Demographic density feedback
Although there are many potential mathematical simplifications of complex population dynamics in time-series analysis (e.g. see references [34,35]), we used an a priori model-building strategy to arrive at a set of four population dynamics models commonly used to describe phenomenological time-series data [23,30]. Density-independent models assume constant growth without the influence of density. We applied two density-independent models: (i) random walk with a long-term growth rate (intercept) rm = 0 (RW; equation 1) and (ii) exponential growth with a constant (non-zero) value of rm (EX; equation 2). In contrast, density-feedback models consider r as a linear function of Nt; we used a stochastic form of the Ricker-logistic model (RL; equation 3), and a stochastic Gompertz-logistic model with log-transformed Nt and long-term, average carrying capacity K (GL; equation 4).
| (1) |
| (2) |
| (3) |
| (4) |
where Nt = snail population size at time t, r = realized population rate of change, rm = maximum rate of population change, K = carrying capacity and process error εt~ Normal(0, σ2). We fitted all models on the basis of maximum-likelihood using linear regression.
Component density feedback
We applied analogues to the EX, RL and GL models for snail survival rate (s) analysis. We did not test a zero-intercept model analogous to the demographic feedback RW model because there is not an a priori expectation of a particular constant, density-independent survival rate [30]. These contrast the three competing hypotheses of density-independent survival (equation 5), survival declining linearly with density (equation 6), and survival declining log-linearly with density (equation 7), respectively. We used the logit transformation to normalise the s response prior to fitting the three linear models:
| (5) |
| (6) |
| (7) |
where nl = number of live snails, nd = number of dead snails, ni = initial number of snails (nl + nd), sm = the mean or maximum survival rate (intercept), β= coefficient (slope) of the abundance (ni) parameter and ε = process error (as described above).
Model comparison
To rank all models, we calculated Akaike's information criterion corrected for small sample sizes (AICc) [36,37], the difference between the model's AICc and that of the top-ranked model i (Δi, ΔAICc; equation 8), and the relative model weights (wi; equation 9) [37]. Thus, the strength of evidence (wAICc) for any particular model varied from 0 (no support) to 1 (complete support) relative to the entire model set of M candidate models. We did all analyses using the open-source R statistical program, version 2.12.1 [38].
| (8) |
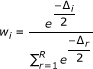 |
(9) |
We also estimated each model's goodness of fit using the percentage of deviance explained (%DE) relative to the null (intercept-only) model.
Results
Breeding patterns of O. hupensis
We collected the first and second generations of O. hupensis snails over the two study years (2009 and 2010) in both sites (Jiangsu and Sichuan provinces) in June and November, respectively, and recorded snail abundances and mortalities. There was a strong compensatory feedback of density on reproduction for both marshland and mountainous-area snails (Figure 3). Pooling all data, the combined evidence for density feedback models was > 99% for O. hupensis, based on the sum of the wAICc for the Ricker-logistic and Gompertz-logistic models, with most support for the Gompertz (Table 1).
Figure 3.
Four population dynamics models examining the relationship between population growth rate (r) and density for the pooled data (upper), Jiangsu (middle) and Sichuan (lower) populations. See also Table 1. Dotted line, random walk (RW); dashed line, exponential model (EX); dot-dash line, Ricker-logistic (RL); solid line, Gompertz-logistic (GL). See text for details.
Table 1.
Contrasting four demographic density feedback models for O.hupensis snail density
| Setting | Model | AICc | ΔAICc | wAICc | %DE |
|---|---|---|---|---|---|
| Overall | RW | 252.568 | 72.111 | 2.1939E-16 | 0.0 |
| EX | 234.944 | 54.487 | 1.4731E-12 | 30.2 | |
| RL | 197.414 | 16.957 | 0.0002 | 66.1 | |
| GL | 180.457 | 0.000 | 0.9998 | 75.1 | |
| Jiangsu | RW | 122.090 | 46.979 | 5.8346E-11 | 0.0 |
| EX | 97.919 | 22.808 | 1.0344E-05 | 61.2 | |
| RL | 80.208 | 5.097 | 0.0725 | 81.2 | |
| GL | 75.111 | 0.000 | 0.9275 | 84.3 | |
| Sichuan | RW | 131.071 | 42.189 | 6.8969E-10 | 0.0 |
| EX | 129.474 | 40.593 | 1.5323E-09 | 13.6 | |
| RL | 106.567 | 17.685 | 0.0001 | 66.3 | |
| GL | 88.882 | 0.000 | 0.9999 | 82.5 |
RW, random walk; EX, exponential growth; RL, Ricker-logistic; GL, Gompertz-logistic; %DE, % deviance explained (goodness of fit). Highest-ranked models are shown in boldface
Model strength of evidence based on Akaike's information criterion corrected for small samples (AICc).
The slope of r versus log (Nt) (Gompertz-logistic) relationship for the pooled O. hupensis population was -0.796 (standard error [SE] = 0.084) (equation 10). The slopes of the relationship were -0.502 (SE = 0.082) and -1.133 (SE = 0.115) for the Jiangsu and Sichuan subspecies, respectively (equations 11 and 12), suggesting a stronger compensatory feedback mechanism in the Sichuan subspecies (Figure 3).
| (10) |
| (11) |
| (12) |
The effective population growth rates of mountainous snails in low-density groups are about 3-4 times higher than those observed in the marshland.
The survival probability of the snails in higher-density groups was lower than that in lower-density groups, and there was strong evidence for either a Ricker-like (linear) or Gompertz-like (log-linear) decline in logit-transformed survival rate for both generations and both study populations (Table 2 and Figure 4). However, the first generation demonstrated more linear decline, while the second generation had a more log-linear decline in survival with density (Figure 4). These results suggest that adult survival is one of the principal component vital rates driving the demographic feedback patterns.
Table 2.
Contrasting three models of O.hupensis snail survival rate as a function of density: density-independent (DI), linear decline (DDL) and log-linear decline (DDLL)
| Setting | Generation | Model | AICc | ΔAICc | wAICc | %DE |
|---|---|---|---|---|---|---|
| Jiangsu | G1 | DI | 30.148 | 12.297 | 0.0022 | 0.0 |
| DDL | 17.851 | 0.000 | 0.9792 | 67.2 | ||
| DDLL | 25.763 | 7.912 | 0.0187 | 42.3 | ||
| G2 | DI | 41.111 | 1.165 | 0.2866 | 0.0 | |
| DDL | 41.828 | 1.883 | 0.2002 | 16.9 | ||
| DDLL | 39.946 | 0.000 | 0.5132 | 27.4 | ||
| Sichuan | G1 | DI | 45.009 | 4.368 | 0.0682 | 0.0 |
| DDL | 40.641 | 0.000 | 0.6053 | 42.2 | ||
| DDLL | 41.876 | 1.235 | 0.3265 | 36.9 | ||
| G2 | DI | 47.623 | 5.387 | 0.0602 | 0.0 | |
| DDL | 48.007 | 5.771 | 0.0497 | 18.9 | ||
| DDLL | 42.236 | 0.000 | 0.8901 | 46.3 |
DDL, linear density effect; DDLL, log-linear density effect; DI, density-independent; %DE, % deviance explained (goodness of fit). Highest-ranked models are shown in boldface.
Mode1 strength of evidence based on Akaike's information criterion corrected for small samples (AICc).
Figure 4.

Three contrasting models of O. hupensis survival rate (s) as a function of density: density-independent (DI - dashed line), linear decline (DDL - dotted line) and log-linear decline (DDLL - solid line). See also Table 2. Upper panels: Jiangsu population (left: first generation; right: second generation); lower panels: Sichuan population (left: first generation; right: second generation).
Discussion
First experiences with implementing a new integrated control strategy for schistosomiasis control in P.R. China are promising [8,9,11]. However, we speculate that control efforts could be further enhanced by rigorously implementing intermediate host-snail suppression until snail densities are below a pre-set detection threshold. Our models revealed a strong feedback mechanism, whereby extremely low-density habitats following control can in fact produce the largest subsequent pulses of snails, thus potentially continuing or even exacerbating schistosomiasis outbreaks [39]. Hence, snail control, in concert with the new comprehensive schistosomiasis control strategy [7], would also act to suppress natural fluctuations of the disease in humans and animals, especially in areas of low prevalence. There is a need to continue suppression given that in lake districts, fishermen and river-transportation workers regularly come into contact with contaminated freshwater bodies (schistosome eggs and, most importantly, schistosome cercariae, which are the infective stages), and thus perpetuate local outbreaks. In mountainous regions, buffalo (Bubalus bubalis) act as the major definitive host of S. japonicum, and are still used as the core farming animal in agriculture, and hence cannot be replaced immediately by more modern mechanical farming equipment. It follows that the persistence of suitable snail habitats, and hence intermediate host snails, calls for sustained control via chemical mollusciciding and environmental management.
A better understanding of the population dynamics of intermediate host snails holds promise to render snail control more effective. With regard to the work presented here, the following conclusions can be drawn. First, we showed that effective mollusciciding must reduce snail density to less than two specimens per 0.5 m2 because even at these low densities, populations can increase quickly to over 50 times initial abundance given low initial competition among individuals. Second, the only molluscicide currently recommended by the World Health Organization is niclosamide [40]. At present in P.R. China, the most commonly used formula of molluscicide is 50% wettable powder of niclosamide ethanolamine salt (Bayluscide) [41]. A recent systematic review showed that snail mortality is around 88% using a 50% wettable powder of niclosamide ethanolamine salt post spraying for 15 days [42]. There is considerable concern that, if mollusciciding is only attempted once by spraying niclosamide in the field, the few surviving snails will engender a rapid re-bound in the months to come. We therefore recommend applying molluscicide at least twice yearly and, if financial and technical resources allow, even more frequently.
Although the population growth dynamic of snails in both marshland and mountainous regions followed the same pattern, we observed some notable differences. For example, the slope of the regression model for O. hupensis in the mountainous areas is considerably greater than that for the marshland (-1.133 versus -0.502), which translates to the prediction that mountainous snails are likely more sensitive to variation in density than marshland strains at low population sizes. As a result, O. hupensis from mountainous regions seem more elusive to control than marshland snails because of the high rebound rate expected subsequent to mollusciciding. Compared to marshland O. hupensis, our findings suggest that snail control in mountainous regions must be implemented with utmost vigilance to reduce population rate of increase that tends constantly towards exponential growth at low densities. If possible, environmental management in mountainous regions should be recommended with the ultimate goal to eliminate suitable snail habitats. Recent work indicated that schistosomiasis is re-emerging in mountainous regions, perhaps explained by the overall high rate of snail rebound [39].
Although we could not test the component contribution of reproduction explicitly given the confounding effects of a semelparous, annual-breeding species (i.e. a generation lasts only until the next breeding cycle), it is clear that adult survival contributes a large component to the demographic response. We also found that the decline in survival with density was greatest and linear for the first generation, but weaker and log-linear for the second (Figure 4). This might arise from differing environmental conditions experienced by each generation, or differential mortality patterns, or a combination of both. Thus, examining patterns of density feedback across a wider range of experimental conditions will further enhance our understanding of snail rebound potential following control.
Of course, snail population size is not solely a product of density feedback. In addition to intrinsic influences, the distribution, extent and density of O. hupensis depend on a complex interaction of different environmental factors, of which temperature, rainfall and flooding are the three most important [43]. Future modelling must consider these factors and the potential influence of environmental changes (e.g. climate change) on long-term predictions of the cost-effectiveness of control interventions.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
GJY conceived the study and wrote the first version of the manuscript. XNZ, LPS, FW, BZ and DCQ helped in the field experiments in Jiangsu and Sichuan provinces. CJAB contributed to data analysis. XNZ, JU and CJAB revised the manuscript. All of authors read, contributed to, and approved the final version of the manuscript.
Contributor Information
Guo-Jing Yang, Email: guojingyang@hotmail.com.
Xiao-Nong Zhou, Email: xiaonongzhou1962@gmail.com.
Le-Ping Sun, Email: lepingsun@yahoo.com.cn.
Feng Wu, Email: wufengwx302@yahoo.com.cn.
Bo Zhong, Email: zhongbo1968@163.com.
Dong-Chuan Qiu, Email: qiudongchuan@163.com.
Jürg Utzinger, Email: Juerg.Utzinger@unibas.ch.
Corey JA Bradshaw, Email: corey.bradshaw@adelaide.edu.au.
Acknowledgements
This project received financial support from the UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) (A70530), Shanghai S & T Project (11XD1405400), and the National S & T Major Program (2008ZX10004-011). CJAB is partially funded by the Australian Academy of Science.
References
- Zhou XN, Wang LY, Chen MG, Wu XH, Jiang QW, Chen XY, Zheng J, Utzinger J. The public health significance and control of schistosomiasis in China - then and now. Acta Trop. 2005;96:97–105. doi: 10.1016/j.actatropica.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Utzinger J, Zhou XN, Chen MG, Bergquist R. Conquering schistosomiasis in China: the long march. Acta Trop. 2005;96:69–96. doi: 10.1016/j.actatropica.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Mao CP, Shao BR. Schistosomiasis control in the People's Republic of China. Am J Trop Med Hyg. 1982;31:92–99. [PubMed] [Google Scholar]
- Maegraith B. Schistosomiasis in China. Lancet. 1958;271:208–214. doi: 10.1016/s0140-6736(58)90686-x. [DOI] [PubMed] [Google Scholar]
- Chen MG, Feng Z. Schistosomiasis control in China. Parasitol Int. 1999;48:11–19. doi: 10.1016/S1383-5769(99)00004-5. [DOI] [PubMed] [Google Scholar]
- Zhou XN, Bergquist R, Leonardo L, Yang GJ, Yang K, Sudomo M, Olveda R. Schistosomiasis japonica: control and research needs. Adv Parasitol. 2010;72:145–178. doi: 10.1016/S0065-308X(10)72006-6. [DOI] [PubMed] [Google Scholar]
- Hao Y, Yi DH, Zhang XF, Xiong JJ, Yuan WZ, Hu SJ, Wu XH, Zhu R, Guo JG, Huang XB. et al. Assessment report on infection control of schistosomiasis in China, 2008. Chin J Schisto Cont. 2009;21:457–463. [Google Scholar]
- Wang LD, Guo JG, Wu XH, Chen HG, Wang TP, Zhu SP, Zhang ZH, Steinmann P, Yang GJ, Wang SP. et al. China's new strategy to block Schistosoma japonicum transmission: experiences and impact beyond schistosomiasis. Trop Med Int Health. 2009;14:1475–1483. doi: 10.1111/j.1365-3156.2009.02403.x. [DOI] [PubMed] [Google Scholar]
- Wang LD, Chen HG, Guo JG, Zeng XJ, Hong XL, Xiong JJ, Wu XH, Wang XH, Wang LY, Xia G. et al. A strategy to control transmission of Schistosoma japonicum in China. N Engl J Med. 2009;360:121–128. doi: 10.1056/NEJMoa0800135. [DOI] [PubMed] [Google Scholar]
- Zhu R, Gray DJ, Thrift AP, Williams GM, Zhang Y, Qiu DC, Zheng F, Li YS, Guo J, Zhu HQ. et al. A 5-year longitudinal study of schistosomiasis transmission in Shian village, the Anning River valley, Sichuan province, the People's Republic of China. Parasit Vectors. 2011;4:43. doi: 10.1186/1756-3305-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray DJ, McManus DP, Li YS, Williams GM, Bergquist R, Ross AG. Schistosomiasis elimination: lessons from the past guide the future. Lancet Infect Dis. 2010;10:733–736. doi: 10.1016/S1473-3099(10)70099-2. [DOI] [PubMed] [Google Scholar]
- Zhou XN, Yang GJ, Yang K, Wang XH, Hong QB, Sun LP, Malone JB, Kristensen TK, Bergquist NR, Utzinger J. Potential impact of climate change on schistosomiasis transmission in China. Am J Trop Med Hyg. 2008;78:188–194. [PubMed] [Google Scholar]
- Yang GJ, Vounatsou P, Zhou XN, Tanner M, Utzinger J. A potential impact of climate change and water resource development on the transmission of Schistosoma japonicum in China. Parassitologia. 2005;47:127–134. [PubMed] [Google Scholar]
- Zhou XN, Lin DD, Yang HM, Chen HG, Sun LP, Yang GJ, Hong QB, Brown L, Malone JB. Use of Landsat TM satellite surveillance data to measure the impact of the 1998 flood on snail intermediate host dispersal in the lower Yangtze River Basin. Acta Trop. 2002;82:199–205. doi: 10.1016/S0001-706X(02)00011-6. [DOI] [PubMed] [Google Scholar]
- Lin DD, Zhou XN, Liu Y, Sun L, Hu F, Yang G, Hong Q. Prediction of snail habitats in the marshland around Poyang Lake affected by flood in 1998 using remote sensing. Chin J Schisto Cont. 2002;14:119–121. [Google Scholar]
- Standley CJ, Adriko M, Arinaitwe M, Atuhaire A, Kazibwe F, Fenwick A, Kabatereine NB, Stothard JR. Epidemiology and control of intestinal schistosomiasis on the Sesse Islands, Uganda: integrating malacology and parasitology to tailor local treatment recommendations. Parasit Vectors. 2010;3:64. doi: 10.1186/1756-3305-3-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchin P. Population regulation: old arguments and a new synthesis. New York: Academic Press; 1995. [Google Scholar]
- Bjørnstad ON, Grenfell BT. Noisy clockwork: time series analysis of population fluctuations in animals. Science. 2001;293:638–643. doi: 10.1126/science.1062226. [DOI] [PubMed] [Google Scholar]
- Clark F, Brook BW, Delean S, Akçakaya HR, Bradshaw CJA. The theta-logistic is unreliable for modelling most census data. Meth Ecol Evol. 2010;1:253–262. [Google Scholar]
- de Little SC, Bradshaw CJA, McMahon CR, Hindell MA. Complex interplay between intrinsic and extrinsic drivers of long-term survival trends in southern elephant seals. BMC Ecology. 2007;7:3. doi: 10.1186/1472-6785-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sæther BE. Environmental stochasticity and population dynamics of large herbivores: a search for mechanisms. Trends Ecol Evol. 1997;12:143–149. doi: 10.1016/S0169-5347(96)10068-9. [DOI] [PubMed] [Google Scholar]
- Yang GJ, Bradshaw CJA, Whelan PI, Brook BW. Importance of endogenous feedback controlling the long-term abundance of tropical mosquito species. Popul Ecol. 2008;50:293–305. doi: 10.1007/s10144-008-0082-8. [DOI] [Google Scholar]
- Turchin P. Complex population dynamics: a theoretical/empirical synthesis. Princeton: Princeton University Press; 2003. [Google Scholar]
- Münster-Swendsen M, Berryman A. Detecting the causes of population cycles by analysis of R-functions: the spruce needle-miner, Epinotia tedella, and its parasitoids in Danish spruce plantations. Oikos. 2005;108:495–502. doi: 10.1111/j.0030-1299.2005.13747.x. [DOI] [Google Scholar]
- Kunkel KE, Novak RJ, Lampman RL, Gu W. Modeling the impact of variable climatic factors on the crossover of Culex restauns and Culex pipiens (Diptera: culicidae), vectors of West Nile virus in Illinois. Am J Trop Med Hyg. 2006;74:168–173. [PubMed] [Google Scholar]
- Russell RC. Seasonal abundance and age composition of two populations of Culex annulirostris (Diptera: Culicidae) at Darwin, Northern Territory, Australia. J Med Entomol. 1986;23:279–285. doi: 10.1093/jmedent/23.3.279. [DOI] [PubMed] [Google Scholar]
- Su T, Webb JP, Meyer RP, Mulla MS. Spatial and temporal distribution of mosquitoes in underground storm drain systems in Orange county, California. J Vector Ecol. 2003;28:79–89. [PubMed] [Google Scholar]
- Zhao QP, Jiang MS, Littlewood DT, Nie P. Distinct genetic diversity of Oncomelania hupensis, intermediate host of Schistosoma japonicum in mainland China as revealed by ITS sequences. PLoS Negl Trop Dis. 2010;4:e611. doi: 10.1371/journal.pntd.0000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SZ, Wang YX, Yang K, Liu Q, Wang Q, Zhang Y, Wu XH, Guo JG, Bergquist R, Zhou XN. Landscape genetics: the correlation of spatial and genetic distances of Oncomelania hupensis, the intermediate host snail of Schistosoma japonicum in mainland China. Geospat Health. 2009;3:221–231. doi: 10.4081/gh.2009.222. [DOI] [PubMed] [Google Scholar]
- Brook BW, Bradshaw CJ. Strength of evidence for density dependence in abundance time series of 1198 species. Ecology. 2006;87:1445–1451. doi: 10.1890/0012-9658(2006)87[1445:SOEFDD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- McKinney ML. Extinction vulnerability and selectivity: combining ecological and paleontological views. Ann Rev Ecol Syst. 1997;28:495–516. doi: 10.1146/annurev.ecolsys.28.1.495. [DOI] [Google Scholar]
- Huang QN, Tang LL, Jiang XG, Chen Z, Zhou XN. Retrieving eco-environment factors relevant to Oncomelania snail distribution based on QuickBird image. Chin J Parasitol Parasit Dis. 2007;25:304–309. [PubMed] [Google Scholar]
- Yang K, Zhou XN, Wu XH, Steinmann P, Wang XH, Yang GJ, Utzinger J, Li HJ. Landscape pattern analysis and Bayesian modeling for predicting Oncomelania hupensis distribution in Eryuan county, People's Republic of China. Am J Trop Med Hyg. 2009;81:416–423. [PubMed] [Google Scholar]
- Hall BH, Cummins C. TSP 5.0 reference manual, 2005. http://www.tspintl.com accessed: 10 January 2011.
- Yamamura K, Yokozawa M, Nishimori M, Ueda Y, Yokosuka T. How to analyze long-term insect population dynamics under climate change: 50-year data of three insect pests in paddy fields. Popul Ecol. 2006;48:31–48. doi: 10.1007/s10144-005-0239-7. [DOI] [Google Scholar]
- Akaike H. In: Proceedings of the Second International Symposium on Information Theory. Petrov BN, Csaki F, editor. Budapest, Hungary; 1973. Information theory as an extension of the maximum likelihood principle; pp. 267–281. [Google Scholar]
- Burnham KP, Anderson DR. Model selection and multimodal inference: a practical information-theoretic approach. New York: Springer-Verlag; 2002. [Google Scholar]
- R Development Core Team. R Foundation for Statistical Computing. Vienna, Austria; 2011. R: a language and environment for statistical computing. [Google Scholar]
- Liang S, Yang CH, Zhong B, Qiu DC. Re-emerging schistosomiasis in hilly and mountainous areas of Sichuan, China. Bull World Health Organ. 2006;84:139–144. doi: 10.2471/BLT.05.025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. The role of mollusciciding in schistosomiasis control. Geneva: World Health Organization; 1983. pp. 72–73. [Google Scholar]
- Bao JG, Zhang GH, Wu WD, Zhang SQ, Zhang XY, Lv DB, Fang GR, Wang FF, Zhu L, Dai QJ. Snail control effect of 4%powder of niclosamide tthanolamine salt in field trial. Parasit Infect Dis. 2004;2:163–165. [Google Scholar]
- Yang GJ, Li W, Sun LP, Wu F, yang K, Huang YX, Zhou XN. Molluscicidal efficacies of different formulations of niclosamide: result of meta-analysis of Chinese literature. Parasit Vectors. 2010;3:84. doi: 10.1186/1756-3305-3-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao CP. Biology of schistosome and control of schistosomiasis. Beijing: People's Health Press; 1990. [Google Scholar]





