Abstract
Purpose of the review
Osteoporosis is a major public health issue resulting in considerable fracture-related morbidity. Although effective treatment exists, adherence to osteoporosis pharmacotherapy is suboptimal and linked to reduced drug effectiveness. Interventions are thus needed to reduce the burden of fractures associated with poor treatment adherence.
Recent findings
Most patients will stop osteoporosis pharmacotherapy, yet the majority who discontinue will reinitiate treatment after an extended gap. The key to improving adherence to osteoporosis pharmacotherapy is to reduce the number and length of gaps in treatment. Multifaceted and individualized interventions may help to improve adherence. New strategies aimed at identifying patients likely to stop therapy may also facilitate the development of targeted interventions.
Summary
Adherence to osteoporosis pharmacotherapy is suboptimal with short periods of persistence and lengthy gaps in therapy. Regular communication regarding the importance of continued therapy is critical. More research to help identify risk profiles of patients likely to become non-adherent, targeted multifaceted interventions to maximize adherence to therapy, and data to support when patients may safely consider a physician directed drug holiday is needed.
Keywords: adherence, compliance, persistence, osteoporosis, measurement
Introduction
Osteoporosis is a major public health issue resulting in considerable fracture-related morbidity [1,2]. Although effective treatment options exist to reduce fracture risk [3,4], adherence to pharmacotherapy is suboptimal and linked to reduced drug effectiveness [5–7,8*] and increased costs [9–11]. Targeted quality improvement interventions are needed to reduce the burden of osteoporosis related to poor drug adherence. In this review, we synthesize recent literature regarding the measurement and prediction of treatment adherence using healthcare utilization data, summarize the main reasons for poor adherence to osteoporosis pharmacotherapy, and briefly review strategies to improve treatment adherence. We highlight new findings and make recommendations for future research.
Using healthcare utilization data to measure adherence
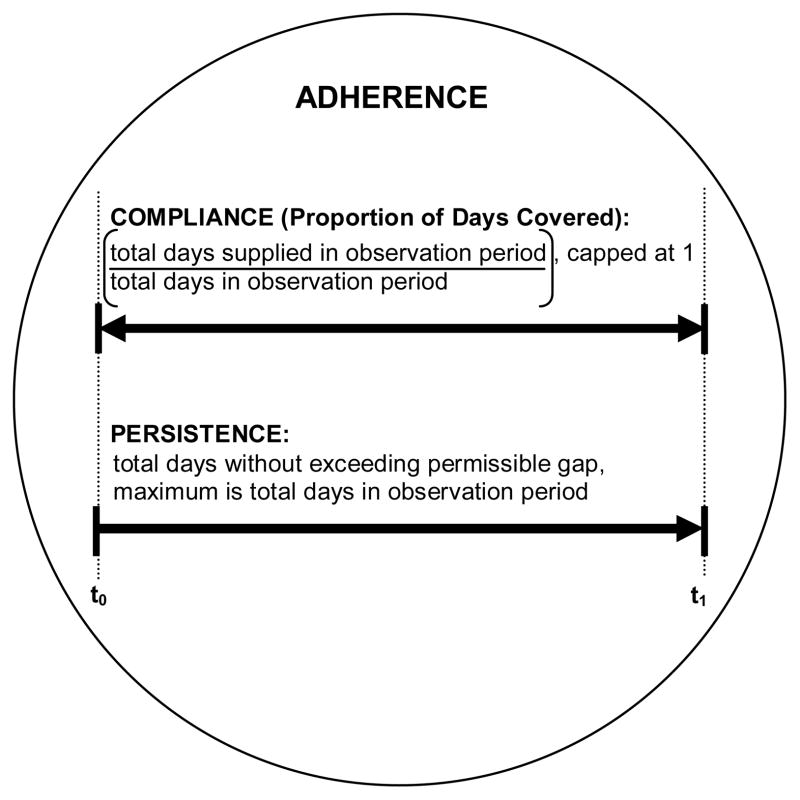
Healthcare utilization (medical and pharmacy claims) data permit assessment of the patterns of drug use and the subsequent impact of adherence to therapy on clinical outcomes. Other common methods to assess adherence to osteoporosis pharmacotherapy include clinician perceptions, and patient self-report [12,13*]. We focus this review on the use of pharmacy claims to measure treatment adherence. Although a pharmacy claim does not guarantee consumption, repeated dispensing over regular intervals is a good proxy for actual adherence to treatment [14,15]. Until recently, there has been a lack of consistency in the use of terminology used to describe adherence to pharmacotherapy using healthcare utilization data [16]. In 2007 and 2008, the International Society of Pharmacoeconomics and Outcomes Research (ISPOR) released suggested reporting standards [17,18]. Adherence to pharmacotherapy is defined by the extent a patient’s behaviour coincides with the prescribed treatment regimen (time, quantity and frequency), and is quantified by measures of compliance and persistence, Figure 1. Although ISPOR recognizes “adherence” and “compliance” as synonyms [17,18], we encourage the use adherence as a general term describing the behaviour, and compliance and persistence as specific measures that quantify adherence to pharmacotherapy [5,12,19,20,21*].
Figure 1.
Quantifying adherence to pharmacotherapy using healthcare utilization data, adapted from prior summaries[18, 25]. Adherence is a general term describing medication taking behaviour and is examined using healthcare utilization data by measurement of compliance and persistence in the observation period. The proportion of days covered (PDC) is also commonly referred to as the medication possession ratio (MPR). We recommend PDC as the standard terminology and measurement of compliance.
t0=start of observation period
t1=end of observation period
Compliance
In the context of osteoporosis pharmacotherapy, most studies have defined good adherence to therapy based on a measure of compliance: medication possession ratio (MPR) of 80% or more [5–7,8*]. When capped at 100%, MPR is synonymous with the proportion of days covered (PDC). PDC is calculated as the total number of days of drug supplied (days covered by drug) in the observation period, divided by the total number of days in the observation period, and capped to 1 or 100%. ISPOR recognizes both MPR and PDC [17], yet does not recommend the use of one term over the other. We believe that “proportion of days covered,” is more intuitive than “medication possession ratio,” because the terminology more clearly describes what is measured, and PDC is consistently capped at 1 or 100%. We therefore use PDC throughout this review and recommend that future studies adopt the term PDC as the standard measure of treatment compliance.
Persistence
Persistence captures the length of time a patient continues with therapy after treatment initiation, and is quantified by the number of days covered by drug without a predefined permissible gap. The permissible gap (grace period) is quantified by a specific time interval in days, by a proportion of the most recently supplied amount of drug, or a combination of the two. Drug discontinuation is therefore identified by an extended number of days following drug coverage without a new dispensing. The permissible gap most frequently used in studies that examine persistence with osteoporosis therapy is 30 days, with other studies allowing gaps of 14 to 120 days [6,12].
Calculating compliance and persistence using claims data – considering immeasurable time
Two main factors impact measurement of compliance and persistence: 1) length of follow-up, and 2) length of permissible gap or “grace period”. Depending on the database utilized, periods of incomplete information may also impact estimated compliance and persistence. For example, many healthcare utilization databases are limited to therapeutics dispensed in community pharmacies [22]. Drugs dispensed in hospital or long-term care may be covered by different drug plans and thus these data may not be available for analysis. Missing periods of drug information, or “immeasurable time” [22], have the potential to underestimate rates of treatment adherence. When relevant, it may be important to adjust for missing periods of drug data when measuring adherence to therapy. For example, to account for potential immeasurable time in hospital when calculating PDC, the number of days in hospital may either be subtracted from the denominator or added to the numerator. Similarly, when estimating treatment persistence based on exceeding a permissible gap in therapy, days in hospital may be subtracted from the observed gap length, or patients may be considered fully covered by drug during the hospitalization.
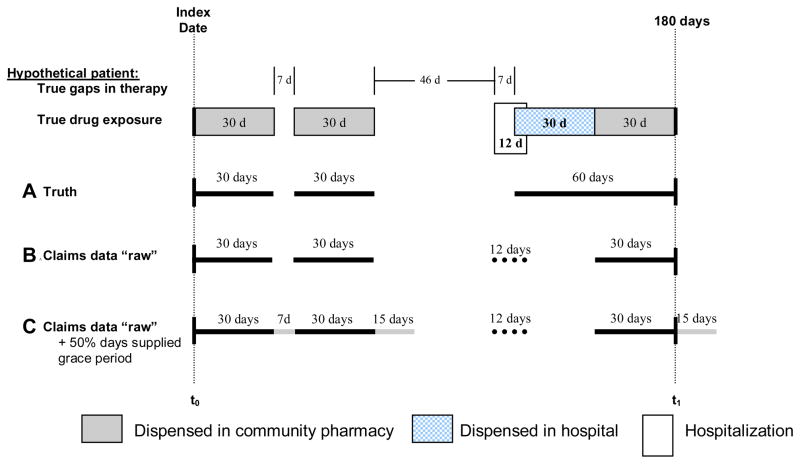
To illustrate the above concepts, we present the hypothetical drug exposure for a sample patient over a 180 day period, Figure 2. During the 180 day period, the patient was dispensed three 30-days supply of drug in a community pharmacy, and one 30-days supply of drug during a 12 day hospitalization. In this example, only drugs dispensed in community pharmacies are tracked by pharmacy claims available for analysis. With a 60-day permissible gap, this patient persisted with therapy over the 180 days, with one short 7-day gap observed between the first and second dispensing, and a subsequent gap of 53 days (46+7) occurring after the second dispensing and before the prescription dispensed in hospital. The patient’s true compliance is measured as a PDC of 67% ([30+30+60]/180, Figure 2A). However, given that one of the 30-days of supplied drug was dispensed in a hospital pharmacy, the estimated compliance and persistence is underestimated when considering only the “raw” pharmacy claims available for analysis. In Table 1, we compare estimated compliance and persistence after varying assumptions underlying each calculation. Permitting no grace period after theoretical drug coverage and ignoring immeasurable days in hospital results in an estimated PDC of 50% ([30+30+30]/180, Figure 2B). Estimated PDC increases to 62% ([30+7+30+15+30]/180, Figure 2C) after allowing for a maximum grace period of 50% days supplied, and to 67% ([82+30]/[180−12], subtracting days in hospital from the denominator) and 69% ([82+30+12]/180, assuming 100% drug coverage during hospitalization and adding days in hospital to the numerator), after also adjusting for immeasurable time in hospital.
Figure 2.
Example drug exposure and adherence for a hypothetical patient.
A. Truth: dark solid lines=true consumption
B. Claims data “raw”: dark solid lines=drug use identified through pharmacy claims, dotted line=days in hospital identified through medical claims.
C. Claims data “raw” +50% days supplied grace period: dark solid lines=drug use identified through raw pharmacy claims, light solid lines=coverage added by applying a maximum 50% days supplied grace period to all gaps, dotted line=days in hospital identified through medical claims.
t0=start of observation period, t1=end of observation period; calculations only consider drug coverage during the observation period
Table 1.
Calculating compliance and persistence under various assumptions for the sample patient depicted in Figure 2
| Compliance (Proportion of Days Covered) | % | Persistence (60 day permissible gap) | |||
|---|---|---|---|---|---|
| No. days drug supplied (numerator) | No. days observed (denominator) | No. days in largest gap | No. days persistedc | ||
| Truth (Figure 2A) | 120 | 180 | 66.7 | (46+7)=53 | 180 |
| Claims data “raw” (Figure 2B) | |||||
| ignoring days in hospital | 90 | 180 | 50.0 | (53+30)=83 | 67 |
| subtract days in hospital (n=12)a | 90 | 168 | 53.5 | (83−12)=71 | 67 |
| 100% covered in hospital (n=12)b | 102 | 180 | 56.7 | 46 | 180 |
| 50% days supplied grace period (Figure 2C) | |||||
| ignoring days in hospital | 112 | 180 | 62.0 | (46−15+7+30)=68 | 82 |
| subtract days in hospital (n=12)a | 112 | 168 | 66.7 | (68−12)=56 | 180 |
| 100% covered in hospital (n=12)b | 124 | 180 | 68.9 | (46−15)=31 | 180 |
Proportion of days covered (PDC)=(number of days of drug supplied in the observation period/number of days in the observation period), capped at 1 or 100%.
PDC: corrected for immeasurable time in hospital by subtracting 12 days from denominator; persistence: subtract days in hospital (12 days) from gap length
PDC: corrected for immeasurable time in hospital by adding 12 days to numerator; persistence: subtract days in hospital (12 days) from gap length
defined by the number of days covered by drug before a gap of more than 60 days
Our hypothetical example highlights how subtle differences in assumptions to account for periods of immeasurable time can impact measurement of compliance and persistence. However, immeasurable time during a short acute hospitalization may be less relevant than during long-term care. Depending on the purpose of the analysis and data utilized, authors may ignore immeasurable time, adjust for immeasurable time or censor analyses at the start of immeasurable time (e.g., on admission date). A recent paper stopped follow-up time during hospitalization and until 4 weeks after discharge when calculating adherence to bisphosphonates [23]. Immeasurable time is database specific, and thus decisions regarding how to adjust for immeasurable time should be based on the data utilized and the purpose of the analysis.
The length of follow-up and grace period applied can also greatly impact compliance and persistence measurement. For example, healthcare utilization data in Ontario, Canada (1999–2004) identified that extending the grace period between refills of oral bisphosphonates from 50% to 300% of days supplied increased the 1-year persistence from 49% to 76%, and 5-year persistence from 17% to 41% [24]. In the same study, considering any bisphosphonate dosing within the 180 days prior to the end of 5-year follow-up identified 64% as persisting with therapy to 5 years. Differences in assumptions underlying measurement of compliance and persistence makes it difficult to compare results between studies [16].
Considering drug overlap and switching
We simplified our hypothetical example in Figure 2 by studying adherence to a single drug with no overlap in drug prescriptions. In most cases when prescriptions for the same drug or drug class overlap, patients are assumed to have refilled early, and completed the first prescription before starting the second prescription. A recent study truncated to a maximum number of 180 days supplied when adding days of drug overlap [25*]. The decision whether or not to truncate to a maximum number of days supplied based on prescription overlap may depend on the data utilized [26*], and therefore clarity in describing methods and rationale is important [17].
Switching between drugs or regimens may also complicate decisions to account for drug overlap. For example, many patients switch between osteoporosis drugs due to adverse effects, and thus it may not be appropriate to add overlap days when patients switch between drugs. However, the argument of no overlap after only a regimen change--e.g., from daily to weekly alendronate--weakens as a patient may use their extra daily medication if they are delayed in picking up a future weekly dispensing. Careful consideration is thus important when deciding upon methods to account for prescription overlap.
Adherence to concurrent medications
Little information is available regarding adherence to concurrent osteoporosis medications, such as concurrent adherence to an oral bisphosphonate and a selective estrogen receptor modulator. Standard methods for measuring concurrent adherence have recently been proposed and grouped into prescription-based and interval-based approaches [25*]. These recommended reporting standards may be useful to examine adherence to concurrent osteoporosis therapies.
Treatment reinitiation after an extended gap
Most publications that have examined adherence to osteoporosis pharmacotherapy have considered only the initial treatment episode. However, an under-reported finding is that many patients who discontinue pharmacotherapy return to treatment after an extended gap. For example, although 67% of older low-income adults in Pennsylvania who started osteoporosis pharmacotherapy between 1996 and 2002 experienced an extended gap of 60 or more days, 30% reinitiated treatment within 6-months and 50% within 2 years of drug discontinuation [27]. Data from Australia recently identify that among veterans taking oral bisphosphonates between April 2001 and 2007, 19% experienced one extended gap of more than 105 days between prescriptions, and 13% experienced 2 or more periods of extended gaps in treatment [26*]. These data highlight the importance of reducing the length of, and number of gaps in osteoporosis pharmacotherapy to help improve treatment adherence.
Predicting poor adherence using healthcare utilization data
Recent evidence suggests that healthcare utilization data may be useful to identify patients likely to become non-adherent to pharmacotherapy [23,28*,29*,30,31]. First, longer delays in filling a prescription predicts poor compliance (PDC<80%) [30], and non-persistence [28*]. Second, longer gap lengths between prescription refills within the first 3 months of treatment initiation predicts treatment discontinuation [31]. Third, a shorter length of persistence after treatment initiation is negatively associated with treatment reinitiation [23,27]. Fourth, poor compliance to treatment for other asymptomatic conditions predicts poor compliance to bisphosphonate therapy [29*]. Collectively, these findings suggest that healthcare utilization data may become a valuable resource for the early identification of patients likely to discontinue osteoporosis treatment. In addition to healthcare utilization data, patient responses regarding their concerns, need and medication affordability may predict non-adherence [32*,33]. More research to develop risk profiles, or prognostic indices of poor treatment adherence may help to identify patients for targeted adherence interventions.
Improving adherence to osteoporosis pharmacotherapy
Commonly reported barriers to osteoporosis treatment adherence include: actual and perceived side effects, dosing complexity, medication costs, lack of perceived need for therapy, poor perceptions regarding treatment effectiveness, poor patient-provider relationship, little patient involvement in treatment decision making and lack of treatment follow-up [21*,34*,35,36]. Evidence suggests that patients regularly reassess their perceived need for treatment against barriers to continued therapy [21*,36,37]. Strategies that enhance patient-provider communication and treatment follow-up may thus help to improve treatment adherence [38*].
First, patients who feel comfortable with their physicians are more likely to trust the diagnosis, accept a prescribed treatment, and return to their doctor to discuss medication problems [34*,38*]. Healthcare providers play a key role in shaping perceptions of fracture risk and osteoporosis drug effectiveness [34*,39*,40]. However, many patients fail to associate fracture with a diagnosis of osteoporosis [39*,40], and patients underestimate the extent of bone loss identified by bone mineral density testing [41]. Improved patient understanding of bone quality and need for pharmacotherapy is therefore critical [21*,34*,35,37]. Second, early treatment follow-up facilitates adherence by addressing adverse drug effects and problems with dosing complexity [34]. In fact, drug switching; between drugs or drug regimens; improves compliance to osteoporosis pharmacotherapy [12,23,34*,42,43].
Potential strategies to improve adherence to osteoporosis pharmacotherapy include improving patient-provider relationships and increased treatment monitoring through regular follow-up, clinical testing and reminder systems [34*,38*,44*,45]. Providing patients with educational material alone does not improve treatment adherence [44*,46]. Instead, multifaceted and individualized approaches with regular follow-up are needed [34*,38*,44*,45]. A new intensive intervention trial designed to improve adherence to osteoporosis pharmacotherapy is currently underway [47*]. The intervention involves patient education and 10 scheduled motivational interviews over a 12-month period, and thus looks promising. We await with anticipation the impact of the intervention on treatment adherence, as well as results of the cost-effectiveness analysis [47*].
Dosing regimen
Evidence supports better adherence to weekly versus daily osteoporosis therapy [12]. However, there is little evidence to conclude whether differences in adherence exist between monthly and weekly regimens [20, 48]. New treatment options, such as annual zoledronic acid infusion and forthcoming semi-annual denosumab will change the landscape of osteoporosis treatment adherence. Nonetheless, safety concerns and costs may prohibit the rapid uptake of these new therapies, and thus for now, better strategies to improve adherence to daily, weekly and monthly regimens are important.
Duration of therapy
The focus of this paper has been on measuring and improving adherence to osteoporosis pharmacotherapy. However, in some cases, a physician directed drug holiday from oral bisphosphonate treatment may be appropriate [49,50]. Recent non-experimental evidence suggests that women highly adherent to bisphosphonate with PDC ≥80% at 2 years, or ≥66% at 3 years had similar subsequent 1-year hip fracture risk compared to patients who continued pharmacotherapy after 2 or 3 years respectively [51*]. Better understanding of bisphosphonate treatment patterns and evidence to support if, when, how long and among which patients a physician directed drug holiday may be appropriate are needed.
Conclusion
Adherence to osteoporosis pharmacotherapy is suboptimal with short periods of persistence and lengthy gaps in therapy. Patients regularly reassess their need for treatment within the broader context of their quality of life. Regular follow-up is therefore critical, particularly if patients may be at high risk for poor adherence. More research to help identify risk profiles of patients likely to become non-adherent, targeted multifaceted interventions to maximize adherence to therapy, and data to support when patients may safely consider a physician directed drug holiday is needed.
Acknowledgments
Dr Cadarette holds a Canadian Institutes of Health Research New Investigator Award in the Area of Aging and Osteoporosis. Authors acknowledge Mary Elias, BSc and Milica Nikitovic, BSc who contributed with thoughtful discussions and manuscript review.
References and recommended reading
- 1.U.S. Department of Health and Human Services. Bone health and osteoporosis: a report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Office of the Surgeon General; 2004. [Google Scholar]
- 2.Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 3.MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2008;148:197–213. doi: 10.7326/0003-4819-148-3-200802050-00198. [DOI] [PubMed] [Google Scholar]
- 4.Khosla S. Increasing options for the treatment of osteoporosis [editorial] N Engl J Med. 2009;361:818–820. doi: 10.1056/NEJMe0905480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siris ES, Selby PL, Saag KG, et al. Impact of osteoporosis treatment adherence on fracture rates in North America and Europe. Am J Med. 2009;122:S3–S13. doi: 10.1016/j.amjmed.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Rabenda V, Hiligsmann M, Reginster JY. Poor adherence to oral bisphosphonate treatment and its consequences: a review of the evidence. Expert Opin Pharmacother. 2009;10:2303–2315. doi: 10.1517/14656560903140533. [DOI] [PubMed] [Google Scholar]
- 7.Imaz I, Zegarra P, Gonzalez-Enriquez J, et al. Poor bisphosphonate adherence for treatment of osteoporosis increases fracture risk: systematic review and meta-analysis. Osteoporos Int. 2010 doi: 10.1007/s00198-009-1134-4. [DOI] [PubMed] [Google Scholar]
- 8•.Wilkes MM, Navickis RJ, Chan WW, Lewiecki EM. Bisphosphonates and osteoporotic fractures: a cross-design synthesis of results among compliant/persistent postmenopausal women in clinical practice versus randomized controlled trials. Osteoporos Int. 2010 doi: 10.1007/s00198-009-0991-1. In press. [DOI] [PubMed] [Google Scholar]; This meta-analysis differentiates itself from other recent systematic reviews by comparing result that study the impact of adherence to bisphosphonates on fracture risk using non-experimental designs, to results from randomized trials. Results suggest that patients who adhere to bisphosphonate therapy benefit to a similar extent as patients treated in randomized trials
- 9.Hiligsmann M, Rabenda V, Gathon HJ, et al. Potential Clinical and Economic Impact of Nonadherence with Osteoporosis Medications. Calcif Tissue Int. 2010 doi: 10.1007/s00223-009-9329-4. [DOI] [PubMed] [Google Scholar]
- 10.Danese MD, Badamgarav E, Bauer DC. Effect of adherence on lifetime fractures in osteoporotic women treated with daily and weekly bisphosphonates. J Bone Miner Res. 2009;24:1819–1826. doi: 10.1359/jbmr.090506. [DOI] [PubMed] [Google Scholar]
- 11.Cotte FE, Fautrel B, De Pouvourville G. A Markov model simulation of the impact of treatment persistence in postmenopausal osteoporosis. Med Decis Making. 2009;29:125–139. doi: 10.1177/0272989X08318461. [DOI] [PubMed] [Google Scholar]
- 12.Kothawala P, Badamgarav E, Ryu S, et al. Systematic review and meta-analysis of real-world adherence to drug therapy for osteoporosis. Mayo Clinic Proceedings. 2007;82:1493–1501. doi: 10.1016/S0025-6196(11)61093-8. [DOI] [PubMed] [Google Scholar]
- 13•.Copher R, Buzinec P, Zarotsky V, et al. Physician perception of patient adherence compared to patient adherence of osteoporosis medications from pharmacy claims. Curr Med Res Opin. 2010;26:777–785. doi: 10.1185/03007990903579171. [DOI] [PubMed] [Google Scholar]; Despite a low response rate (22%), this paper provides some evidence to suggest that physicians overestimate their patients’ adherence to osteoporosis pharmacotherapy. Results thus reinforce the importance of patient-provider communication when designing strategies to improve adherence to therapy
- 14.Hansen RA, Kim MM, Song L, et al. Comparison of methods to assess medication adherence and classify nonadherence. Ann Pharmacother. 2009;43:413–422. doi: 10.1345/aph.1L496. [DOI] [PubMed] [Google Scholar]
- 15.Curtis JR, Westfall AO, Allison J, et al. Agreement and validity of pharmacy data versus self-report for use of osteoporosis medications among chronic glucocorticoid users. Pharmacoepidemiol Drug Safety. 2006;15:710–718. doi: 10.1002/pds.1226. [DOI] [PubMed] [Google Scholar]
- 16.Cramer JA, Silverman SL, Gold DT. Methodological considerations in using claims databases to evaluate persistence with bisphosphonates for osteoporosis. Curr Med Res Opin. 2007;23:2369–2377. doi: 10.1185/030079907X226311. [DOI] [PubMed] [Google Scholar]
- 17.Peterson AM, Nau DP, Cramer JA, et al. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10:3–12. doi: 10.1111/j.1524-4733.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 18.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 19.Warriner AH, Curtis JR. Adherence to osteoporosis treatments: room for improvement. Curr Opin Rheumatol. 2009;21:356–362. doi: 10.1097/BOR.0b013e32832c6aa4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gold DT, Trinh H, Safi W. Weekly versus monthly drug regimens: 1-year compliance and persistence with bisphosphonate therapy. Curr Med Res Opin. 2009;25:1831–1839. doi: 10.1185/03007990903035604. [DOI] [PubMed] [Google Scholar]
- 21•.Schousboe JT, Dowd BE, Davison ML, Kane RL. Association of medication attitudes with non-persistence and non-compliance with medication to prevent fractures. Osteoporos Int. 2010 doi: 10.1007/s00198-009-1141-5. [DOI] [PubMed] [Google Scholar]; In addition to the importance of patient beliefs regarding their need for and concerns about therapy, authors identify the importance of self-efficacy in predicting treatment non-adherence. Authors also suggest that non-compliance may be associated with unintentional behaviours related to forgetting to take medication, whereas nonpersistence is likely an intentional act resulting from a conscious decision to stop treatment
- 22.Suissa S. Immeasurable time bias in observational studies of drug effects on mortality. Am J Epidemiol. 2008;2008:329–335. doi: 10.1093/aje/kwn135. [DOI] [PubMed] [Google Scholar]
- 23.Zambon A, Baio G, Mazzaglia G, et al. Discontinuity and failures of therapy with bisphosphonates: joint assessment of predictors with multi-state models. Pharmacoepidemiol Drug Saf. 2008;17:260–269. doi: 10.1002/pds.1530. [DOI] [PubMed] [Google Scholar]
- 24.Melo M, Qiu F, Sykora K, et al. Persistence with bisphosphonate therapy in older people. J Am Geriatr Soc. 2006;54:1015–1016. doi: 10.1111/j.1532-5415.2006.00758.x. [DOI] [PubMed] [Google Scholar]
- 25•.Choudhry NK, Shrank WH, Levin RL, et al. Measuring concurrent adherence to multiple related medications. Am J Manag Care. 2009;15:457–464. [PMC free article] [PubMed] [Google Scholar]; Authors recommend standards for measuring adherence to concurrent medications with useful figures to conceptualize recommendations
- 26•.Roughead EE, Ramsay E, Priess K, et al. Medication adherence, first episode duration, overall duration and time without therapy: the example of bisphosphonates. Pharmacoepidemiol Drug Saf. 2009;18:69–75. doi: 10.1002/pds.1687. [DOI] [PubMed] [Google Scholar]; This paper emphasizes that reporting only the first episode of bisphosphonate use may underestimate treatment duration since extended gaps is therapy are common. Authors recommend efforts to reduce the length of gaps in therapy rather than focusing on persistence with initial therapy
- 27.Brookhart MA, Avorn J, Katz JN, et al. Gaps in treatment among users of osteoporosis medications: the dynamics of noncompliance. Am J Med. 2007;120:251–256. doi: 10.1016/j.amjmed.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 28•.Yu AP, Yu YF, Nichol MB, Gwadry-Sridhar F. Delay in filling the initial prescription for a statin: a potential early indicator of medication nonpersistence. Clin Ther. 2008;30:761–774. doi: 10.1016/j.clinthera.2008.04.015. [DOI] [PubMed] [Google Scholar]; This study used a novel method to estimate “dispensation delay” in filling an index statin prescription by calculating the number of days between dispensing and the most recent physician or hospital visit. Longer dispensation delay was found to predict poor adherence to therapy
- 29•.Curtis JR, Xi J, Westfall AO, et al. Improving the prediction of medication compliance: the example of bisphosphonates for osteoporosis. Med Care. 2009;47:334–341. doi: 10.1097/MLR.0b013e31818afa1c. [DOI] [PMC free article] [PubMed] [Google Scholar]; Results from this paper suggest that pharmacy claims history of non-adherence to chronic medications for asymptomatic conditions may help to identify patients likely to become non-adherence to osteoporosis pharmacotherapy
- 30.Mabotuwana T, Warren J, Harrison J, Kenealy T. What can primary care prescribing data tell us about individual adherence to long-term medication?-comparison to pharmacy dispensing data. Pharmacoepidemiol Drug Saf. 2009;18:956–964. doi: 10.1002/pds.1803. [DOI] [PubMed] [Google Scholar]
- 31.Hansen RA, Dusetzina SB, Dominik RC, Gaynes BN. Prescription refill records as a screening tool to identify antidepressant non-adherence. Pharmacoepidemiol Drug Saf. 2010;19:33–37. doi: 10.1002/pds.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32•.McHorney CA. The Adherence Estimator: a brief, proximal screener for patient propensity to adhere to prescription medications for chronic disease. Curr Med Res Opin. 2009;25:215–238. doi: 10.1185/03007990802619425. [DOI] [PubMed] [Google Scholar]; This paper reports on the development and initial validation of a 3-item screener designed to predict treatment adherence. The screener may be useful to help identify patients likely to become non-adherent; however, further validation is required. More efforts to develop prognostic indices of treatment non-adherence are welcomed
- 33.McHorney CA, Victor Spain C, Alexander CM, Simmons J. Validity of the adherence estimator in the prediction of 9-month persistence with medications prescribed for chronic diseases: a prospective analysis of data from pharmacy claims. Clin Ther. 2009;31:2584–2607. doi: 10.1016/j.clinthera.2009.11.030. [DOI] [PubMed] [Google Scholar]
- 34•.Lau E, Papaioannou A, Dolovich L, et al. Patients’ adherence to osteoporosis therapy: exploring the perceptions of postmenopausal women. Can Fam Physician. 2008;54:394–402. [PMC free article] [PubMed] [Google Scholar]; Using focus group methods, authors identify 6 key factors that influence treatment adherence, and recommend strategies to improve treatment adherence to therapy. Results also emphasize that interventions to improve adherence may need to be individualized
- 35.Kamatari M, Koto S, Ozawa N, et al. Factors affecting long-term compliance of osteoporotic patients with bisphosphonate treatment and QOL assessment in actual practice: alendronate and risedronate. J Bone Miner Metab. 2007;25:302–309. doi: 10.1007/s00774-007-0768-6. [DOI] [PubMed] [Google Scholar]
- 36.McHorney CA, Schousboe JT, Cline RR, Weiss TW. The impact of osteoporosis medication beliefs and side-effect experiences on non-adherence to oral bisphosphonates. Curr Med Res Opin. 2007;23:3137–3152. doi: 10.1185/030079907X242890. [DOI] [PubMed] [Google Scholar]
- 37.Cadarette SM, Gignac MA, Jaglal SB, et al. Measuring patient perceptions about osteoporosis pharmacotherapy. BMC Res Notes. 2009;2:133. doi: 10.1186/1756-0500-2-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38•.Zolnierek KB, Dimatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Med Care. 2009;47:826–834. doi: 10.1097/MLR.0b013e31819a5acc. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review highlights through meta-analysis, the critical importance of patient-provider communication in improving adherence to therapy
- 39•.Giangregorio L, Dolovich L, Cranney A, et al. Osteoporosis risk perceptions among patients who have sustained a fragility fracture. Patient Educ Couns. 2009;74:213–220. doi: 10.1016/j.pec.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; Although limited by low response rate (29% response rate, 49% participation rate), the mix of quantitative and qualitative methods complement other studies and results help to better understand the importance of patient-physician communication and patient perceptions of risk on treatment adherence
- 40.Sale JE, Beaton DE, Sujic R, Bogoch ER. ‘If it was osteoporosis, I would have really hurt myself.’ Ambiguity about osteoporosis and osteoporosis care despite a screening programme to educate fragility fracture patients. J Eval Clin Pract. 2010 doi: 10.1111/j.1365-2753.2009.01176.x. [DOI] [PubMed] [Google Scholar]
- 41.Cadarette SM, Beaton DE, Gignac MAM, et al. Minimal error in self-report of having had DXA, but self-report of its results was poor. J Clin Epidemiol. 2007;60:1306–1311. doi: 10.1016/j.jclinepi.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 42.Kertes J, Dushenat M, Vesterman JL, et al. Factors contributing to compliance with osteoporosis medication. Isr Med Assoc J. 2008;10:207–213. [PubMed] [Google Scholar]
- 43.Ideguchi H, Ohno S, Takase K, et al. Outcomes after switching from one bisphosphonate to another in 146 patients at a single university hospital. Osteoporos Int. 2008;19:1777–1783. doi: 10.1007/s00198-008-0618-y. [DOI] [PubMed] [Google Scholar]
- 44•.Gleeson T, Iversen MD, Avorn J, et al. Interventions to improve adherence and persistence with osteoporosis medications: a systematic literature review. Osteoporos Int. 2009;20:2127–2134. doi: 10.1007/s00198-009-0976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; This systematic review of interventions designed to improve osteoporosis adherence highlights the importance of follow-up interactions and the role of patient counseling in helping to improve treatment adherence
- 45.Schlenk EA, Bernardo LM, Organist LA, et al. Optimizing medication Adherence in Older Patients: A Systematic Review. J Clin Outcomes Manag. 2008;15:595–606. [PMC free article] [PubMed] [Google Scholar]
- 46.Shu AD, Stedman MR, Polinski JM, et al. Adherence to osteoporosis medications after patient and physician brief education: post hoc analysis of a randomized controlled trial. Am J Manag Care. 2009;15:417–424. [PMC free article] [PubMed] [Google Scholar]
- 47•.Solomon DH, Gleeson T, Iversen M, et al. A blinded randomized controlled trial of motivational interviewing to improve adherence with osteoporosis medications: design of the OPTIMA trial. Osteoporos Int. 2010;21:137–144. doi: 10.1007/s00198-009-0951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes an intensive motivational interviewing trial designed to improve adherence to osteoporosis pharmacotherapy that is currently underway. Detailed rationale and methods may help to inform other intervention strategies
- 48.Cotte FE, Fardellone P, Mercier F, et al. Adherence to monthly and weekly oral bisphosphonates in women with osteoporosis. Osteoporos Int. 2010;21:145–155. doi: 10.1007/s00198-009-0930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geusens P. Bisphosphonates for postmenopausal osteoporosis: determining duration of treatment. Curr Osteoporos Rep. 2009;7:12–17. doi: 10.1007/s11914-009-0003-6. [DOI] [PubMed] [Google Scholar]
- 50.Watts NB, Chines A, Olszynski WP, et al. Fracture risk remains reduced one year after discontinuation of risedronate. Osteoporos Int. 2008;19:365–372. doi: 10.1007/s00198-007-0460-7. [DOI] [PubMed] [Google Scholar]
- 51•.Curtis JR, Westfall AO, Cheng H, et al. Risk of hip fracture after bisphosphonate discontinuation: implications for a drug holiday. Osteoporos Int. 2008;19:1613–1620. doi: 10.1007/s00198-008-0604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper used a non-experimental “real-world” study design and found that patients who persistent with bisphosphonate therapy with PDC≥80% after 2 or PDC≥66% after 3 years, may consider taking a 1-year drug holiday without increasing their risk for hip fracture. These data therefore corroborate recent trial extension evidence, yet future research to support and clarify these findings is important