Abstract
CO2 lasers can be operated at high laser pulse repetition rates for the rapid and precise removal of dental decay. Excessive heat accumulation and peripheral thermal damage is a concern when using high pulse repetition rates. Peripheral thermal damage can adversely impact the mechanical strength of the irradiated tissue, particularly for dentin, and reduce the adhesion characteristics of the modified surfaces. The interpulpal temperature rise was recorded using microthermocouples situated at the roof of the pulp chamber on teeth that were occlusally ablated using a rapidly-scanned CO2 laser operating at 9.3 μm with a pulse duration of 10 to 15 μs and repetition rate of 300 Hz over a 2 min time course. The adhesion strength of laser treated enamel and dentin surfaces was measured for various laser scanning parameters with and without post-ablation acid etching using the single-plane shear test. The mechanical strength of laser-ablated dentin surfaces were determined via the four-point bend test and compared to control samples prepared with 320 grit wet sand paper to simulate conventional preparations. Thermocouple measurements indicated that the temperature remained below ambient temperature if water-cooling was used. There was no discoloration of either dentin or enamel laser treated surfaces, the surfaces were uniformly ablated, and there were no cracks visible. Four-point bend tests yielded mean mechanical strengths of 18.2 N (s.d. = 4.6) for ablated dentin and 18.1 N (s.d. = 2.7) for control (p > 0.05). Shear tests yielded mean bond strengths approaching 30 MPa for both enamel and dentin under certain irradiation conditions. These values were slightly lower than nonirradiated acid-etched control samples. Additional studies are needed to determine if the slightly lower bond strength than the acid-etched control samples is clinically significant. These measurements demonstrate that enamel and dentin surfaces can be rapidly ablated by CO2 lasers with minimal peripheral thermal and mechanical damage and without excessive heat accumulation.
Keywords: enamel, CO2 laser, adhesion, heat accumulation, peripheral thermal damage
Introduction
Several studies have demonstrated that CO2 lasers operating at λ = 9.3 and 9.6 μm wavelengths, which are strongly absorbed by the hydroxyapatite in dental hard tissues, are ideally suited for the efficient ablation of carious dental hard tissue and for surface treatments to increase the resistance to acid dissolution.1, 2, 3, 4, 5, 6, 7, 8, 9 If pulse durations in the range of 5 to 20 μs are used, efficient ablation occurs with minimal peripheral thermal damage.2, 4, 10 CO2 lasers are capable of operating efficiently at high pulse repetition rates and, if used in combination with a laser beam scanning system, they can be used for the precise, efficient, and rapid removal of dental caries. Moreover, they can potentially be manufactured at a relatively low cost. The purpose of this study was to determine whether a CO2 laser scanning ablation system operating with a 300-Hz pulse repetition rate used in conjunction with a water spray can be used to rapidly ablate dental hard tissues without excessive heat accumulation and peripheral mechanical and thermal damage.
Increased potential for thermal damage to the pulp due to heat accumulation from multiple laser pulses delivered in rapid succession is a primary concern when operating at high pulse repetition rates. The temperature rise should not exceed 5.5°C above the physiological temperature in the pulp chamber or pulpal inflammation may occur.11 We hypothesize that heat accumulation can be minimized by rapidly scanning the laser beam over the tooth surface at optimal fluence to ensure efficient ablation and by using a water-spray. In this study, the temperature rise in the interior of the tooth was monitored using microthermocouples embedded in the pulp chamber to determine if the laser energy deposition in the tooth can be offset with air/water cooling.
Excessive heat accumulation during the rapid delivery of a high number of incident laser pulses can also lead to chemical and mechanical changes peripheral to the ablation site that may change the appearance of enamel and dentin, generate cracks and produce chemical and morphological changes that compromise adhesion to those irradiated surfaces. The effect of peripheral thermal damage on adhesion to both dentin and enamel is controversial with a wide range of reported results after irradiation with a variety of lasers including erbium and CO2 lasers, and a variety of postablation surface treatments including etching and mechanical removal of the chemically modified outer layers.
Dentin is particularly susceptible to thermal damage because of the high percentage of collagen, and changes to the collagen are likely to compromise the adhesion to restorative materials.10, 12, 13 Composite bonding to dentin surfaces poses a greater challenge than bonding to enamel due to the added complexity of the collagen matrix. Conventional dentin bonding schemes depend on an acid etchant to remove the smear layer and widen the tubule lumen to increase the penetration of the resin to form resin tags and demineralize the intertubular dentin to form a collagen/resin hybrid layer.14 Such a hybrid zone results in a higher bond strength and tighter seal to reduce microleakage.
The results of adhesion studies involving laser treated dentin have been mixed and some groups report that laser treated surfaces yield similar adhesion characteristics to conventional etching procedures, while other groups report that treatment by the conventional free-running Er: yttrium–aluminum–garnet (YAG) or Er:yttrium scandium gallium garnet (YSGG) lasers yield lower bond strengths even if the surface is acid-etched after laser treatment.15, 16, 17, 18, 19, 20, 21 Studies have also showed mixed results with microleakage.22, 23, 24, 25, 26 The morphological and chemical changes induced in dentin as a result of laser irradiation have also been examined.27, 28, 29, 30, 31, 32, 33 We found that the Er:YAG and Er:YSGG pulses of 150 to 250 μs duration which are used clinically can result in thermal modification of the collagen matrix, reducing the bond strength. When the laser pulse duration was reduced to 35 μs, bond strengths approaching 30 MPa were attainable that were similar to the phosphoric acid etch control samples even when the surfaces were not etched after laser treatment.34 This clearly demonstrates that thermal modification of the dentin can compromise adhesion. Measurements of the thermal emission from tooth surfaces during ablation with Er:YAG, Er:YSGG, and CO2 lasers indicates that the surface temperature at the time of ablation depends on the wavelength and pulse duration.35 The surface temperature is higher for CO2 lasers than for the erbium lasers. This is obviously an advantage for caries inhibition around caries preparation for enamel, but the higher temperatures may result in greater peripheral thermal damage to dentin.
Any potential cracks or microcracks can also reduce the resistance to fracture, and in a recent study we showed that microcracks produced in dentin samples after laser irradiation without supplemental water cooling reduced the bending strength of dentin.36 In order to determine if the increased energy deposition associated with a high laser pulse rate would similarly compromise the structural integrity of dentin, we investigated the effects of rapid ablation using a 300 Hz pulse repetition rate on the mechanical strength of dentin specimens.
In summary, the purpose of this study was to determine whether laser ablation of dental hard tissues at high laser pulse repetition rates leads to excessive heat accumulation in the tooth or peripheral thermal and mechanical damage that may reduce the mechanical and adhesive strength after high speed ablation of tooth surfaces with a mechanically scanned CO2 laser. This was accomplished by monitoring the temperature rise in the pulp chamber using microthermocouples to insure the temperature does not exceed 5.5°C, carrying out a series of adhesion studies on laser irradiated enamel and dentin to determine if thermally induced chemical and morphological changes undermine adhesion to composite restorative materials, and mechanical strength tests were conducted on dentin beams irradiated by the laser to determine if microcracks are formed that reduce the mechanical strength.
Materials and Methods
Sample Preparation and Tissue Irradiation Parameters
Noncarious, extracted teeth from patients in the San Francisco bay area were collected with approval from the UCSF Committee on Human Research, cleaned, sterilized with gamma radiation, and stored in a 0.1% thymol solution to preserve tissue hydration and to prevent bacterial growth. Whole teeth were used for the microthermocouple measurements. Human enamel and human dentin blocks, with minimum dimensions of 4 × 4 × 2 mm2, were used for shear bond strength testing. There were a total of 94 enamel samples in 9 groups and 80 dentin samples in 8 groups. The blocks were cut using an Isomet 2000 Buehler (Lake Bluff, Illinois) precision saw and kept well hydrated before ablation. Blocks were polished using 360 carbide grit, and the debris produced was removed by sonication. Beams (1 × 1 × 9 mm) of dentin were used in the dentin mechanical strength study (four-point bend measurements). Two groups were studied, with 10 samples per group. The beams were prepared occlusoapically from facial samples of dentin via 320 grit wet sanding.
A transverse excited atmospheric pressure CO2 laser, Impact 2500 from GSI Lumonics (Rugby, United Kingdom) operating at a wavelength of 9.3 μm was used. The laser was custom modified to produce a Gaussian output beam (single spatial mode) and a pulse duration of between 10 and 15 μs. This laser is capable of high repetition rates up to 500 Hz. A fixed repetition rate of 300 Hz was used for all of these experiments. The laser energy output was monitored using a power meter EPM 1000, Coherent-Molectron (Santa Clara, California), and the Joule meter ED-200 from Gentec (Quebec, Canada). The laser beam was focused to spot diameters of ∼300 μm or 450 μm using a planoconvex ZnSe lens of 125-mm focal length. A razor blade was scanned across the beam to determine the diameter (1/e2) of the laser beam. The laser energy was varied between 14 and 30 mJ per pulse for incident fluence of 9 to 42 J/cm2. Computer-controlled XY galvanometers 6200HM series with MicroMax Series 671 from Cambridge Technology, Inc. (Cambridge) were used to scan the laser beam over sample surfaces. The spacing between each laser spot was varied to produce a varying degree of overlap which influenced the surface roughness. Laser beam scanning rates of 25 and 50 mm/s were investigated. A low volume/low pressure air-actuated fluid spray delivery system consisting of a 780S spray valve, a Valvemate 7040 controller, and a fluid reservoir from EFD, Inc. (East Providence, Rhode Island) was used to provide a uniform spray of fine water mist onto the tooth surfaces at 2 ml/min. The water from the reservoir was at room temperature.
Heat Accumulation Measurements
Type K, 36 gauge, 0.13-mm diameter, 1 m length microthermocouples from Omega Engineering Inc. (Stamford, Connecticut) were placed coronally inside the pulp chamber of extracted human teeth to measure the temperature in the pulp chamber. Thermally conductive paste was used to adhere the thermocouples to the inside of the pulp chamber and maintain thermal contact with the coronal surface of the pulp chamber wall. Radiographs were used to confirm accurate placement of thermocouples. A thermocouple controller, Stanford Research SR630 (Stanford, California) controlled by LABVIEW software (National Instruments, Austin, Texas) was used to record the thermal data. The water spray was activated and the temperature of the tooth was allowed to reach a steady state prior to the start of ablation. The temperature rise in the pulp chamber of each tooth was measured during laser irradiation. The laser was operated at a pulse repetition rate of 300 Hz, and two single pulse energy levels were used, 14 mJ (20 J/cm2) for the first group of 16 teeth and 22 mJ (30 J/cm2) for the second group of 12 teeth. The laser beam was continuously scanned to ablate a 5.0 mm in diameter cylindrical pattern over the occlusal surface of the tooth for a period of 2 min. This 2 min time period was sufficient for the temperature rise in the tooth to reach a steady-state temperature at which the rate of heat deposition was equal to the rate of thermal losses. The laser beam was scanned at a velocity of 0.05 mm/ms in incremental steps of 0.1 mm over a concentric ring pattern with a 2.5-mm maximum radius. The laser beam was directed at the occlusal surface of the teeth (at a 5 mm distance) and the temperature changes in the tooth were monitored over 2 min. A temperature rise of 5.5°C was considered indicative of excessive heat accumulation. The maximum temperature rise after 2 min was recorded for each tooth.
Shear-Bond Test
The adhesive strength of dental composite to laser treated enamel and dentin was determined via a single plane shear-bond test. The bonding resin was single bond along with the Z-250 composite, 3M-ESPE (Minneapolis, Minnesota). Composite was cured using an Elipar Freelight 2 (3M-ESPE). The positive control groups were not irradiated by the laser and they were etched with 35% phosphoric acid, rinsed with water, and gently dried. The negative control groups were neither irradiated by the laser or acid-etched. Subsequently, the bonding resin was applied to all the blocks in two coats, dried, and cured for 20s prior to bonding with composite.
There were three distinct adhesion studies carried out over a 3 yr period and all the groups are listed in Table 1. In the first study, eight dentin groups were investigated and six of the groups were irradiated at a fixed pulse repetition rate of 300 Hz, and the laser beam was scanned at either 25 or 50 mm/s. After laser irradiation, the groups were either acid-etched or not acid-etched before bonding. There was also a comparison between samples scanned at 50 mm/s with and without the water spray since the water-spray is required to prevent thermal damage.
Table 1.
The test groups and associated parameters.
| Groups # (n) | Scan rate | Overlap | Fluence | Water | Etch | Mean (MPa) | Statistical groupsa |
|---|---|---|---|---|---|---|---|
| Dentin set | |||||||
| D1 (10) | 25-mm/s | 1/3 | 18 | H2O | etch | 29.9 (6.4) | 3 |
| D2 (10) | 25-mm/s | 1/3 | 18 | H2O | 14.1 (7.2) | 8,3-6 | |
| D3 (10) | 50-mm/s | 1/3 | 18 | H2O | etch | 21.3 (5.5) | 1,2,4,5 |
| D4 (10) | 50-mm/s | 1/3 | 18 | H2O | 17.3 (3) | 2,3,5,6 | |
| D5 (10) | 50-mm/s | 1/3 | 18 | etch | 14.1 (5.3) | 8,2-4,6 | |
| D6 (10) | 50-mm/s | 1/3 | 18 | 11.3 (5.8) | 8,2,4,5 | ||
| D7 (10) | positive control | etch | 39.0 (5.4) | ||||
| D8 (10) | negative control | 5.1 (8) | 2,5,6 | ||||
| Enamel set 1 | |||||||
| AE1 (10) | 50-mm/s | 1/3 | 20 | H2O | etch | 31.2(2.5) | |
| AE2 (10) | 50-mm/s | 1/3 | 20 | H2O | 5.2(2.4) | ||
| AE3 (10) | positive control | etch | 37 (3.6) | ||||
| Enamel set 2 | |||||||
| BE1 (10) | 30-mm/s | 2/9 | 13 | H2O | etch | 34.1(4.6) | 2,3,6 |
| BE2 (12) | 60-mm/s | 4/9 | 13 | H2O | etch | 34.9(5.1) | 1,3,6 |
| BE3 (12) | 135-mm/s | 1 | 13 | H2O | etch | 33.4(5.5) | 1,2,6 |
| BE4 (10) | 30-mm/s | H-250 | 13 & 42 | H2O | 15.3(3.9) | ||
| BE5 (10) | 30-mm/s | H-500 | 13 & 42 | H2O | 5.8(3.1) | ||
| BE6 (10) | positive control | etch | 36.6(4.9) | 1-3 |
Groups with similar numbers are statistically similar (p>0.05) in each set, D, AE, and BE.
In the second study, there were three groups of enamel samples, a nonirradiated control group that was etched, and two laser irradiated groups that were either acid-etched or not acid-etched. The fluence was 20 J/cm2, the laser beam diameter was 300 μm, and the laser was scanned at a rate of 50 mm/s.
A third adhesion study was carried out on six enamel groups to investigate the influence of the surface roughness/texture on adhesion. The surface topography was varied by modifying the degree of overlap between adjacent laser pulses (surface roughness) and by deliberately drilling a pattern of shallow holes to increase retention. The laser was scanned from point to point at a rate of 300 Hz to produce various degrees of overlap. The incident laser fluence was 13 J/cm2 and the spacing between adjacent laser pulses with a spot size of 450 μm was 100, 200, and 450 μm with water-cooling, and the samples were acid-etched after laser irradiation. An array of small holes 100 μm deep were drilled in two of the groups to serve as retention holes, the surface was first uniformly irradiated using a 100 μm spacing between spots, and then small holes were drilled separated by either 250 or 500 μm using a fluence of 42 J/cm2 with 20 laser pulses per spot. The two groups with retention holes were not acid-etched before bonding. Another positive (acid-etched) control group was also included.
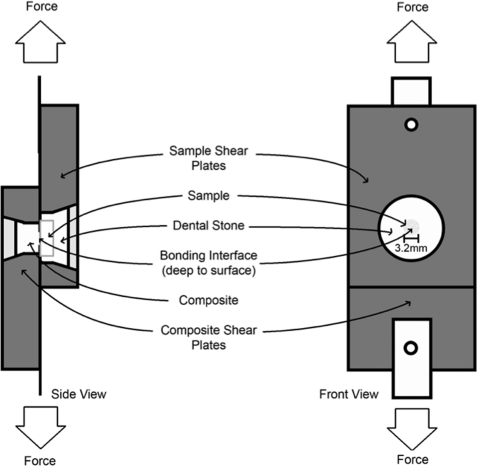
The modified single plane shear test assembly (SPSTA) followed the procedure used by Watanabe et al.37, 38 Figure 1 shows the shear-bond test setup for the SPSTA method. Two aligning plates were used to connect the SPSTA to an Instron testing machine, that recorded measurements in kilograms with the crosshead speed set to 5 mm/min. When the two plates separated, the force level was recorded. The force-failure data (in kilograms) was divided by the surface area of the region and a conversion factor was used to calculate the force in mega-pascals (MPa). Sample groups were compared using one way analysis of variance (ANOVA) with a Tukey post-test. Statistical analysis was carried out using Instat from Graphpad Software (San Diego, California).
Figure 1.
Diagram of the modified single plane shear test assembly.
Four-Point Bend Measurements
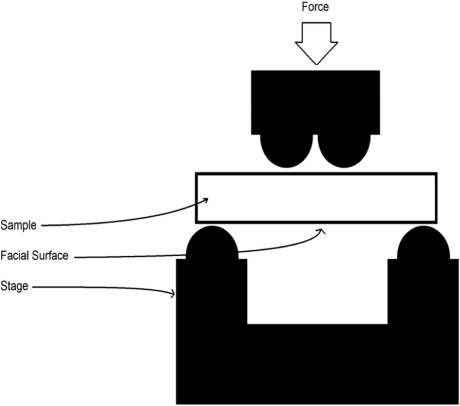
The experimental group of dentin beams (n = 10) were uniformly irradiated on their facial surface. The control group (n = 10) was not irradiated and was wet sanded with 320 grit sand paper to simulate the abrasive properties of a conventional dental hand piece and bur. Next, the beams were placed in a four-point bending apparatus and tested for breaking strength. All testing was performed on a factory-calibrated ELF 3200 mechanical testing machine (EnduraTEC, Minnetonka, Minnesota) in a custom-built four-point bend jig, made from Delrin (Fig. 2). The loading points were spaced 1.8 and 7.2 mm apart; the interface was centered between them. The spacing of the loading points was determined by the size of the dentin beams, which are limited by the size of the human molar tooth. Each beam was positioned so that the irradiated side faced down and the beam was centered between the inner loading points as shown. Bending strengths, σb (in MPa), were computed from the maximum load P (in N), to cause failure, using the standard relationship (ASTM E855/1984):
where a is the spacing (in meters) between upper and lower loading points, b and h are, respectively, the specimen width and thickness (in meters). Four-point bend measurements were only possible for dentin since it is extremely difficult to fabricate similar beams from enamel that are free of cracks.
Figure 2.
Schematic of the four-point bend measurement on the ELF mechanical testing machine. The specimens are beams of dentin with a 1 mm2 cross-section and 9 mm length. Note laser treated area in center of beam.
Optical Microscopy
The surface morphology was examined using at low magnification using a Dino-lite Model Am-2011 (BigC, Torrance, California) digital camera and a Macro zoom 12× microscope from Navitar (Rochester, New York) interfaced to a digital firewire camera. For higher magnification, a Leitz Secolux microscope with 5, 10, 20, 50, and 100× infinity corrected flourite objectives and bright and dark field capability with a maximum magnification of 1000 × (10× eyepieces) interfaced to a digital firewire camera was used to acquire images of sample surfaces.
For histological examination of peripheral thermal damage, sections 200-μm thick were cut using a linear precision saw, the IsoMet 5000 (Buehler, Lake Buff, Illinois). Polarized light microscopy (PLM) was carried out using a Meiji Techno RZT microscope (Saitama, Japan) with an integrated digital camera, Canon EOS Digital Rebel XT from Canon Inc. (Tokyo, Japan). The sample sections were imbibed in water and examined in the bright field mode with cross polarizers and a red I plate with 550 nm retardation.
Results
Surface Morphological Changes
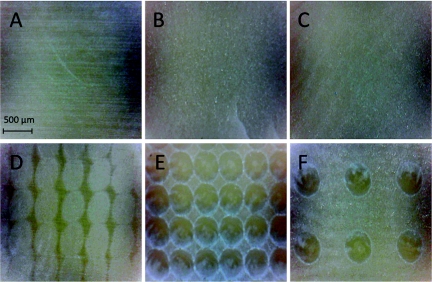
If a water spray was employed, laser irradiated/ablated enamel and dentin surfaces manifested a uniform texture, and there was no discoloration or charring visible indicative of thermal damage on either the enamel or dentin surfaces under both macroscopic and microscopic inspection. Figure 3 shows examples of the human enamel surfaces that were irradiated. The surface treated with the maximum overlap produced a very smooth uniform surface [Fig. 3b] and none of the residual scratches from sample preparation that are visible in the samples before irradiation [Fig. 3a] are visible after laser irradiation. As the spacing between laser pulses was increased, the surface became rougher and more patterned as can be seen in Figs. 3c, 3d for 300 and 450 μm. At 450 μm there is no overlap and the individual laser spots can be clearly distinguished. Enamel surfaces that were prepared with equally spaced retention holes that were spaced 250 and 500 μm apart are shown in Figs. 3e, 3f, respectively.
Figure 3.
Reflected light images of the laser irradiated enamel surfaces and the control samples. (a) no laser irradiation, (b) 100 μm separation between laser spots, (c) 200 μm separation between laser spots, (d) 450 μm separation between laser spots, (e) 100 μm separation between laser spots + holes drilled 250 μm apart, and (f) 100 μm separation between laser spots + holes drilled 500 μm apart.
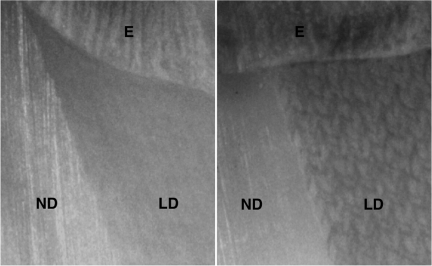
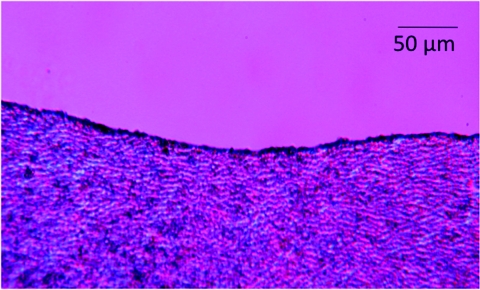
Uniform ablation was also noted for dentin and Fig. 4 shows the surface of a tooth section that was scanned at a rate of 50 mm/s with and without application of a water-spray. The surface is highly uniform with the water spray and there is no discoloration of the dentin surface. A pattern can be seen in the dentin of the sample that was irradiated without water-cooling and there is a slight discoloration. The best way to visualize peripheral thermal damage is to look for changes in birefringence of the collagen caused by thermal damage using polarized light microscopy,2, 10, 13 and Fig. 5 shows a cross section of one of the dentin samples irradiated with a scan rate of 50 mm/s with water cooling. No thermal changes are evident in the polarized light micrograph indicating that the zone of thermal damage is less than 10 μm. The curvature of the enamel surface at the sample surface/edge prevents resolution of any changes smaller than 10 μm.
Figure 4.
Images of dentin after laser irradiation at a scan speed of 50 mm/s with (left) and without (right) water cooling. Normal dentin (ND), laser irradiated dentin (LD) and enamel (E). Note the distinct pattern and discoloration of the irradiated dentin if water-cooling was not used.
Figure 5.
Polarized light micrograph of a thin section of dentin taken across an area irradiated by the laser at 25 mm/s with water-cooling. Note the lack of thermal changes in the dentin.
Heat Accumulation Measurements
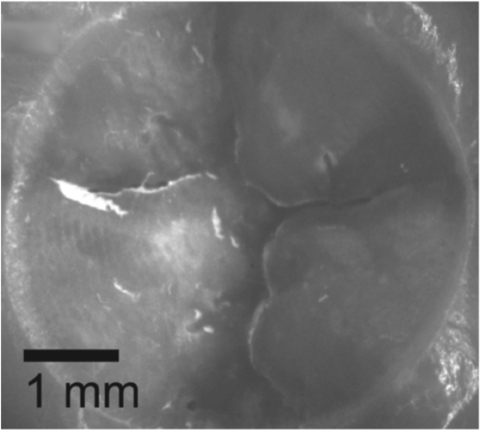
Heat accumulation (thermocouple) measurements indicated that the mean temperature after laser ablation of tooth samples with a pulse energy of 14 mJ (20 J/cm2) was 17.6 ± 0.9°C (n = 16), with a mean change in temperature of 2.0 ± 0.6°C. With a laser pulse energy of 22 mJ (30 J/cm2), the mean temperature after ablation was 19 ± 0.9°C (n = 12), with a mean change in temperature of 3.2 ± 0.8°C. The maximum temperature recorded during ablation remained below the ambient temperature of 21°C for all samples in both groups. Figure 6 shows the occlusal surface of one of the teeth after laser irradiation, the 5 mm area cut by the laser is clearly visible, and the cut is clean and free of any cracks or thermal damage.
Figure 6.
An image of the occlusal surface of one of the teeth after measurement of the temperature rise in the pulp chamber. Note the clean 5 mm in diameter crater cut into the enamel and the large amount of enamel removed.
Adhesion Measurements (Shear Bond Strength)
The results for shear bond testing are tabulated in Table 1. The nonirradiated and acid-etched positive control group (Group AE3) had the highest adhesive strength for the first group of enamel samples. One way ANOVA with a Tukey post-test indicated that there was a significant difference between the three groups (P < 0.05). Visual inspection of group AE1 revealed that failure occurred at the adhesion interface between enamel and bonding agent, as well as cohesively within the bonding agent itself. Group AE2 exhibited only adhesive failure at the enamel-bonding agent interface, while the control group AE3 exhibited both adhesive failure between enamel and bonding agent, as well as cohesive failure and cracking within the bonding agent.
The mean bond strengths for the second set of enamel samples irradiated using different scanning parameters designed to influence the surface morphology are also shown in Table 1. The three laser groups with varying surface roughness (laser spot separation) and acid etching had bond strengths exceeding 30 MPa, and even though the mean bond strength of the control group was higher than the laser treated sample groups, they were statistically similar (P > 0.05). The sample group with the closely spaced retention holes that were spaced 250 μm apart (Group BE4) had a significantly higher mean bond strength than the holes spaced 500 μm apart (Group BE5), and the enamel samples prepared without retention holes (Group AE2) or the negative nonetched enamel control samples from previous studies (2.7±2.3 MPa, n = 12)39 and (2.1±1.8 MPa, n = 10)40.
The shear bond strengths for the eight dentin sample groups are also listed in Table 1. The shear bond strength for the positive control group (Group D7) was very high, 39.0 MPa versus only 5.1 MPa for the nonetched negative control group (Group D8). The highest bond strengths for the laser irradiated samples were for the acid-etched groups with water cooling, 29.9 (Group D1) and 21.3 (Group D3) for the 25 mm/s and the 50 mm/s groups, respectively. The scan rate did not make a significant difference in bond strength. The nonetched laser irradiated samples had a lower bond strength than the acid-etched laser irradiated samples, and a much higher bond strength than the nonirradiated nonetched (negative control sample). The laser irradiated dentin groups without water-cooling had lower bond strengths. The laser irradiated groups with acid-etching and water cooling did have significantly lower bond strengths than the acid-etched positive control samples.
Mechanical Strength Measurements (Four Point Bend)
The four-point bend tests on dentin samples yielded mean mechanical strengths of 18.2 ± 4.6 N (n = 10) after laser irradation and 18.1 ± 2.7 N (n = 10) for the control. An unpaired t-test indicated that there was no significant difference between the two groups (P > 0.05).
Discussion
The principal concern during laser irradiation at high pulse repetition rates is the possibility of thermal damage to the vital pulpal tissues at the center of the tooth. A temperature rise of 5.5°C can lead to pulpal necrosis.11 Thermocouple temperature measurements show that it is feasible to offset the temperature rise in the tooth during ablation in tooth occlusal surfaces with a water spray at laser pulse repetition rates as high as 300 Hz. Although the laser is capable of higher pulse rates of 400 and 500 Hz, 300 Hz is sufficient for high-speed ablation, and operation of the laser has been less stable at 400 and 500 Hz. In a recent clinical safety study carried out with this same laser, the pulse repetition rate was limited to 50 Hz and an incident fluence of 20 J/cm2 (12 to 14 mJ per pulse) was employed, and there were no observable detrimental effects on the pulpal tissues at this lower rate of energy deposition.41 Wigdor and Walsh5 and Wigdor et al.42 investigated the effect of a 9.6 μm CO2 laser with longer 60 μs laser pulses on the pulps of vital canine and human teeth. Energy levels of 2 and 3 W (90 and 136 Hz) were delivered using a circular scanner with no apparent inflammation or vascular changes.
The rate of energy deposition with 300 Hz (22 mJ per pulse) in this study approached 6.6 W, which is comparable to the rates of energy deposition for the erbium laser being used clinically that utilize much higher single pulse energies—up to 300 mJ—but lower repetition rates of 10 to 50 Hz. It is advantageous to employ a laser that can be operated at higher laser pulse repetition rates since lower energy pulses and smaller spot sizes can be used to provide more selective removal. Even though carious tissue is removed at higher rates than sound tissue, it would be most efficient to control the laser scanner by computer and use either image guidance43 or acoustic/spectral feedback44 to achieve the highest selectivity.
When a water spray was applied there was no visible thermal damage to either enamel or dentin surfaces and the ablated surfaces were more uniform than in prior studies. The adhesion results of the shear bond test indicated that the enamel shear bond strength with postablation acid etching was high and exceeded 30 MPa. The control acid-etched samples had a slightly higher, statistically significant (P < 0.05) mean bond strength in the first adhesion study, but the results were statistically similar in a second more extensive comparison (Groups BE1-BE6). We did not anticipate low bond strengths ∼5 MPa, six times lower, for the laser irradiated samples that were not acid-etched,45 since much higher bond strengths were measured without acid etching in two previous studies using a 9.6 μm CO2 laser with a slightly shorter laser pulse of 5 to 8 μs duration. Those bond strengths were 18.2±7.4 MPa, n = 10 with a water spray and 15.6 ±4.3, n = 10 and 18.5 ± 4.2, n = 9 without a water spray.39, 40 Drummond et al.46 compared the shear bond strength of sealants for enamel that was acid etched and laser etched with a 9.6 μm CO2 laser with 60 μs laser pulses and observed that the acid-etched bond strength was twice the laser etched bond strength. Their results are consistent with our previous studies and are higher than the results in this study. Application of a primer increased the bond strength of both groups in their study. One major difference between these studies and the previous work is that the laser beam was scanned over the enamel surface producing a more highly uniform smooth surface. In previous studies the surface manifested a higher surface roughness that was assumed to be responsible for the increased bond strength. This hypothesis provided the rationale for our second enamel adhesion study to investigate the influence of the surface roughness on adhesion. However, varying the scanning parameters to vary the surface roughness failed to produce a significant difference in adhesive strength. The repetition rate in our previous 9.6 μm studies was low; only 10 Hz versus 300 Hz for this study, which can account for the difference. However, no thermal damage was apparent on the samples in this study, moreover the peripheral thermal damage should have been quite high in the two separate 9.6 μm CO2 laser studies in which water-cooling was not used, yet mean bond strengths of 15 and 18 MPa were achieved without acid etching.39, 40 Furthermore, we did not observe a significant reduction in the bond strength of dentin upon reducing the laser scanning rate from 50 to 25 mm/s, even though dentin is more sensitive to thermal damage than enamel.39, 40 Additional studies at varying pulse repetition rates may be needed to resolve this issue.
The outer few micrometers of enamel and dentin are thermally transformed from a carbonated hydroxyapatite mineral phase into a more acid resistant purer phase hydroxyapatite. This is advantageous since it increases resistance to acid dissolution and tooth decay, however it likely reduces the effectiveness of acid etching. The increased acid resistance rendered to enamel surfaces is an important advantage in using the CO2 laser for cutting since those areas now have a greater resistance to tooth decay.1, 47, 48, 49 It is not so important for dentin since laser irradiation and thermal modification is not as effective in inhibiting demineralization on dentin surfaces due to the high collagen content.50 The temperatures required for thermal transformation of the carbonated hydroxyapatite are sufficiently high to destroy the collagen—dentin is ∼50% collagen by volume—causing subsequent contraction of the dentin and the formation of cracks in the modified layer.50, 51, 52 It is desirable to achieve suitably high adhesive strength without having to remove the acid resistant enamel layer, since that thermally modified layer is likely to inhibit the formation of secondary caries. There may be a set of optimum laser irradiation parameters and acid etching conditions that can produce the desired surface roughness/morphology needed for adhesion while maintaining the increased resistance to acid dissolution. Further studies are needed utilizing different laser scanning parameters along with modification of the acid etching conditions to test this hypothesis. Another possibility is that the lower bond strength combined with a thin layer of modified enamel or dentin may result in equivalent performance with respect to resistance to microleakage and secondary caries. It is not clear to what degree the phosphoric acid etch has removed the acid resistant modified enamel layer and dissolution studies are needed to determine the acid resistance of such surfaces.
We also experimented with drilling small retention holes 100 to 200 μm deep in the enamel with the laser. These surfaces were not acid-etched prior to bonding. There was no significant increase in bond strength for the lower hole density (500 μm spacing) but the bond strength increased threefold for the higher hole density (250 μm spacing) which indicates that surface patterning can increase the bond strength without acid etching. This is encouraging because this approach provides a means of increasing the bond strength without removal of the modified enamel layer that has increased resistance to acid dissolution.
Dentin could also be rapidly removed by a CO2 laser operating at 9.3 μm with a pulse duration of 10 to 15 μs with minimal peripheral thermal damage. Peripheral thermal damage was not evident under examination using polarized light microscopy indicating that the zone of thermal damage is less than the minimal resolvable thickness ∼10 μm. We have previously demonstrated that CO2 laser pulses delivered under similar ablative conditions thermally modify a thin layer of enamel around the incision, converting it to a more acid resistant mineral phase even when water-cooling is used.47 Since the enamel is modified under similar irradiation conditions, we must assume that there is also some peripheral thermal modification of dentin even though the layer is too thin to be observed using PLM. The width of thermal damage/modification must be very small, i.e., <10 μm and is less than what we have observed in previous studies using longer laser pulses.10 This indicates that the heat accumulation due to the high pulse repetition rate, 300 Hz in this case, can be successfully offset by rapidly scanning the laser beam. Scanning rates of 25 and 50 mm/s were both successful in minimizing thermal damage, we did not explore lower scanning speeds. Such scanning rates are not excessive and can easily be employed in vivo.
One area of concern was that the bond strength of laser irradiated dentin was significantly lower than the bond strength of the positive control, the conventional preparation with 35% phosphoric acid etch. However, the mean shear bond strength was 39.0 MPa for the positive control which is extremely high. In our last dentin adhesion study using exactly the same adhesion model, the mean shear bond strength of the positive control was only 30.7 MPa, and the mean shear bond strength in that previous study for surfaces treated by Er:YAG lasers with pulse durations of 0.5 and 20 to 30 μs were 26.3 and 28.8, respectively, with water-cooling.34
The respective bond strengths of dentin to composite for the etched and water-cooled irradiated groups were significantly higher than the negative control, which indicates that etching following ablation is beneficial. Albeit, the bond strengths of the laser ablated groups were all significantly lower than the positive control. It is not clear that there is a clinical significance to the lower bond strength of the CO2 laser treated surfaces versus the positive control since the bond strengths approached 30 MPa, which is quite high. Other more sophisticated studies involving restoration longevity and microleakage are needed to better compare adhesion to conventional and laser-prepared surfaces. For example, most adhesion studies, including this one, report bond-strengths after only 24 h, and the bond strength deteriorates with time and it is not clear whether the longevity of bonds to laser-treated surfaces would be similar to nontreated surfaces.
Thermal modification of dentin can lead to the formation of microcracks, and in a previous study we demonstrated that laser irradiation conditions that caused peripheral thermal damage produced a decrease in the resistance to fracture.36 Rectangular dentin samples 1 × 1 × 9 mm were used for the four point bend measurement. It is not possible to produce similarly sized samples made of human enamel due to the dimensions and geometry of the human tooth, and therefore we did not carry out similar measurements on enamel. The bending strength was not significantly different for the laser irradiated samples which indicates that the minor thermal effects from the laser does not compromise the mechanical strength.
In conclusion, these results suggest that dental hard tissues can be rapidly ablated with a mechanically scanned CO2 laser at high pulse repetition rates without excessive heat accumulation in the tooth or peripheral thermal damage that produce no significant reduction in the tissue's mechanical strength or a major reduction of adhesive strength to restorative materials.
Acknowledgments
This work was supported by NIH/NIDCR Grant Nos. R01-DE19631 and T32 DE0073060. The authors would also like to thank Larry Watanabe for his contributions.
References
- Featherstone J. D. B., Barrett-Vespone N. A., Fried D., Kantorowitz Z., and Lofthouse J., “CO2 laser inhibition of artificial caries-like lesion progression in dental enamel,” J. Dent. Res. 77(6), 1397–1403 (1998). 10.1177/00220345980770060401 [DOI] [PubMed] [Google Scholar]
- Fan K., Bell P., and Fried D., “The rapid and conservative ablation and modification of enamel, dentin and alveolar bone using a high repetition rate TEA CO2 laser operating at λ = 9.3 μm,” J. Biomed. Opt. 11(6), 064008 (2006). 10.1117/1.2401151 [DOI] [PubMed] [Google Scholar]
- Fried D., Glena R. E., Featherstone J. D. B., and Seka W., “Multiple pulse irradiation of dental hard tissues at CO2 wavelengths,” Proc. SPIE 2394, 41–50 (1995). 10.1117/12.207455 [DOI] [Google Scholar]
- Fried D., Murray M. W., Featherstone J. D. B., Akrivou M., Dickenson K. M., and Duhn C., “Dental hard tissue modification and removal using sealed TEA lasers operating at λ = 9.6 μm,” J. Biomed. Opt. 6(2), 231–238 (2001). 10.1117/1.1344192 [DOI] [PubMed] [Google Scholar]
- Wigdor H. A. and J. T.Walsh, Jr., “Histologic analysis of the effect on dental pulp of a 9.6-μm CO2 laser,” Lasers Surg. Med. 30(4), 261–266 (2002). 10.1002/lsm.10051 [DOI] [PubMed] [Google Scholar]
- Ertl T. and Muller G., “Hard tissue ablation with pulsed CO2 lasers,” Proc. SPIE 1880, 176–181 (1993). 10.1117/12.148321 [DOI] [Google Scholar]
- Krapchev V. B., Rabii C. D., and Harrington J. A., “Novel CO2 laser system for hard tissue ablation,” Proc. SPIE 2128, 341–348 (1994). 10.1117/12.184916 [DOI] [Google Scholar]
- Moshonov J., Stabholz A., Bar-Hilel R., and Peretz B., “Chemical analysis and surface morphology of enamel and dentin following 9.6mu CO2 laser irradiation versus high speed drilling,” J. Dent. 33(5), 427–432 (2005). 10.1016/j.jdent.2004.11.005 [DOI] [PubMed] [Google Scholar]
- Mullejans R., Eyrich G., Raab W. H., and Frentzen M., “Cavity preparation using a superpulsed 9.6-microm CO2 laser–a histological investigation,” Lasers Surg. Med. 30(5), 331–336 (2002). 10.1002/lsm.10063 [DOI] [PubMed] [Google Scholar]
- Dela Rosa A. A., Sarma A. V., Le C. Q., Jones R. S., and Fried D., “Peripheral thermal and mechanical damage to dentin with microsecond and sub-microsecond 9.6 μm, 2.79 μm, and 0.355 μm laser pulses,” Lasers Surg. Med. 35, 214–228 (2004). 10.1002/lsm.20090 [DOI] [PubMed] [Google Scholar]
- Zach L. and Cohen G., “Pulp response to externally applied heat,” Oral. Surg., Oral. Med., Oral. Pathol. 19, 515–530 (1965). 10.1016/0030-4220(65)90015-0 [DOI] [PubMed] [Google Scholar]
- Sheth K. K., Staninec M., Sarma A. V., and Fried D., “Selective targeting of protein, water and mineral in dentin using UV and IR pulsed lasers: The effect on the bond strength to composite restorative materials,” Lasers Surg. Med. 35, 245–253 (2004). 10.1002/lsm.20102 [DOI] [PubMed] [Google Scholar]
- Lee C., Ragadio J., and Fried D., “Influence of wavelength and pulse duration on peripheral thermal and mechanical damage to dentin and alveolar bone,” Proc. SPIE 3910, 193–203 (2000). 10.1117/12.380827 [DOI] [Google Scholar]
- Marshall G. W., Marshall S. J., Kinney J. H., and Balooch M., “The dentin substrate: structure and properties related to bonding,” J. Dent. 25, 441–458 (1997). 10.1016/S0300-5712(96)00065-6 [DOI] [PubMed] [Google Scholar]
- Keller U. and Hibst R., “Effects of Er:YAG laser on enamel bonding of composite materials,” Proc. SPIE 1880, 163–165 (1993). 10.1117/12.148319 [DOI] [Google Scholar]
- Shahabi S., Brockhurst P. J., and Walsh L. J., “Effect of tooth-related factors on the shear bond strengths obtained with CO2 laser conditioning of enamel,” Aust. Dent. J. 42(2), 81–84 (1997). 10.1111/j.1834-7819.1997.tb00101.x [DOI] [PubMed] [Google Scholar]
- Altshuler G. B., Belikov A. V., Vlasova S. N., and Erofeev A. V., “The research of seal materials adhesion to walls of cavity in enamel and dentine formation by Er-laser radiation,” Proc. SPIE 2327, 101–112 (1994). 10.1117/12.197596 [DOI] [Google Scholar]
- Cooper L. F., Myers M. L., Nelson D. G. A., and Mowery A. S., “Shear strength of composite resin bonded to laser pretreated dentin,” J. Prosth. Dent. 60, 45–49 (1988). 10.1016/0022-3913(88)90348-4 [DOI] [PubMed] [Google Scholar]
- Visuri S. R., Gilbert J. L., and Walsh J. T., “Shear test of composite bonded to dentin: Er:YAG laser vs. dental handpiece preparations,” Proc. SPIE 2394, 223–227 (1995). 10.1117/12.207445 [DOI] [Google Scholar]
- Martinez-Insua A., Da Silva Dominguez L., Rivera F. G., and Santana-Penin U. A., “Differences in bonding to acid-etched or Er:YAG-laser-treated enamel and dentin surfaces,” J. Prosthet. Dent. 84(3), 280–288 (2000). 10.1067/mpr.2000.108600 [DOI] [PubMed] [Google Scholar]
- Ceballos L., Toledano M., Osorio R., Tay F., and Marshall G. W., “Bonding of Er:YAG laser treated dentin,” J. Dent. Res. 81(4), 119–122 (2002). 10.1177/154405910208100207 [DOI] [PubMed] [Google Scholar]
- Hossain M., Nakamura Y., Yamada Y., Murakami Y., and Matsumoto K., “Microleakage of composite resin restoration in cavities prepared by Er,Cr:YSGG laser irradiation and etched bur cavities in primary teeth,” J. Clin. Pediatr. Dent. 26(3), 263–268 (2002). [DOI] [PubMed] [Google Scholar]
- Ceballos L., Osorio R., Toledano M., and Marshall G. W., “Microleakage of composite restorations after acid or Er-YAG laser cavity treatments,” Dent. Mater. 17(4), 340–346 (2001). 10.1016/S0109-5641(00)00092-0 [DOI] [PubMed] [Google Scholar]
- Yazici A. R., Frentzen M., and Dayangac B., “In vitro analysis of the effects of acid or laser etching on microleakage around composite resin restorations,” J. Dent. 29(5), 355–361 (2001). 10.1016/S0300-5712(01)00027-6 [DOI] [PubMed] [Google Scholar]
- Roebuck E. M., Saunders W. P., and Whitters C. J., “Influence of Erbium:YAG laser energies on the microleakage of Class V resin-based composite restorations,” Am. J. Dent. 13(5), 280–284 (2000). [PubMed] [Google Scholar]
- Palma Dibb R. G., Milori Corona S. A., Borsatto M. C., Ferreira K. C., Pereira Ramos R., and Pecora J. Djalma, “Assessing microleakage on class V composite resin restorations after Er:YAG laser preparation varying the adhesive systems,” J. Clin. Laser Med. Surg. 20(3), 129–133 (2002). 10.1089/104454702760090209 [DOI] [PubMed] [Google Scholar]
- Dostalova T., Jelinkova H., Kresja O., and Hamal K., “Evaluation of the surface changes in enamel and dentin due to possiblity of thermal overheating induced by Er:YAG laser radiation,” Scanning Microsc. 10, 285–291 (1996). [PubMed] [Google Scholar]
- Ishizaka Y., Eguro T., Maeda T., and Tanaka K., “Effects of Er:YAG laser irradiation on human dentin:Polarizing microscopic, light microscopic and microradiographic observations and FTIR analysis,” Lasers Surg. Med. 31, 171–176 (2002). 10.1002/lsm.10061 [DOI] [PubMed] [Google Scholar]
- Schein M. T., Bocangel J. S., Nogueira G. E. C., and Schein P. A. L., “SEM evaluation of the interaction pattern between dentin and resin after cavity preparation using the Er:YAG laser,” J. Dent. 31, 127–135 (2003). 10.1016/S0300-5712(03)00003-4 [DOI] [PubMed] [Google Scholar]
- Keller U. and Hibst R., “Experimental studies of the application of the Er:YAG laser on dental hard substances: II. Light microscopic and SEM investigations,” Lasers Surg. Med. 9, 345–351 (1989). 10.1002/lsm.1900090406 [DOI] [PubMed] [Google Scholar]
- Freiberg R. J. and Cozean C. D., “Pulsed erbium laser ablation of hard dental tissue: the effects of atomized water spray vs water surface film,” Proc. SPIE 4610, 74–84 (2002). 10.1117/12.469306 [DOI] [Google Scholar]
- Fried D., Ashouri N., Breunig T. M., and Shori R. K., “Mechanism of water augmentation during IR laser irradiation of dental enamel,” Lasers Surg. Med. 31, 186–193 (2002). 10.1002/lsm.10085 [DOI] [PubMed] [Google Scholar]
- Fried D., Zuerlein M. J., Le C. Q., and Featherstone J., “Thermal and chemical modification of dentin by 9-11 μm CO2 laser pulses of 5-100-μs duration,” Lasers Surg. Med. 3, 275–282 (2002). 10.1002/lsm.10100 [DOI] [PubMed] [Google Scholar]
- Le C. Q., Staninec M., and Fried D., “The influence of pulse duration on the bond strength of dentin to composite after Er:YAG laser irradiation,” in Lasers in Dentistry XI, Vol. 5687, pp. 151–156 (2005).
- Fried D., Visuri S. R., Featherstone J. D. B., Seka W., Glena R. E., Walsh J. T., McCormack S. M., and Wigdor H. A., “Infrared radiometry of dental enamel during Er:YAG and Er:YSGG laser irradiation,” J. Biomed Opt. 1(4), 455–465 (1996). 10.1117/12.250668 [DOI] [PubMed] [Google Scholar]
- Staninec M., Meshkin N., Manesh S. K., Ritchie R. O., and Fried D., “Weakening of dentin from cracks resulting from laser irradiation,” Dent. Mat. 25(4), 520–525 (2009). 10.1016/j.dental.2008.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe L. G., Marshall G. W., and Marshall S. J., “Dentin shear strength: Effect of tubule orientation and intratooth location,” Dent. Mater. 12, 109–115 (1996). 10.1016/S0109-5641(96)80077-7 [DOI] [PubMed] [Google Scholar]
- Watanabe L. G., Marshall G. W., and Marshall S. J., “Variables influence on shear bond strength testing to dentin,” Advanced Adhesive Dentistry, 3rd International Kuraray Symposium, Granada International Symposium, pp. 75–90 (1999).
- Staninec M., Gardner A. K., Le C. Q., Sarma A. V., and Fried D., “Adhesion of composite to enamel and dentin surfaces irradiated by IR laser pulses of 0.5-35 micros duration,” J. Biomed. Mater. Res., Part B: Appl. Biomater. 79(1), 193–201 (2006). 10.1002/jbm.b.30530 [DOI] [PubMed] [Google Scholar]
- Staninec M., Xie J., Le C. Q., and Fried D., “Influence of an optically thick water layer on the bond-strength of composite resin to dental enamel,” Lasers Surg. Med. 33, 264–269 (2003). 10.1002/lsm.10229 [DOI] [PubMed] [Google Scholar]
- Staninec M., Darling C. L., Goodis H. E., Pierre D., Cox D. P., Fan K., Larson M., Parisi R., Hsu D., Manesh S. K., Ho C., Hosseini M., and Fried D., “Pulpal effects of enamel ablation with a microsecond pulsed λ = 9.3-μm CO2 laser,” Lasers Surg. Med. 41, 256–263 (2009). 10.1002/lsm.20748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigdor H., Walsh J. T., and Mostofi R., “The effect of the CO2 laser (9.6 μm) on the dental pulp in humans,” Proc. SPIE 3910, 158–163 (2000). 10.1117/12.380822 [DOI] [Google Scholar]
- Tao Y.-C. and Fried D., “Near-infrared image-guided laser ablation of dental decay,” J. Biomed. Opt. 14, 1–6 (2009). 10.1117/1.3253390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K. H. and Fried D., “Selective removal of dental composite using a rapidly scanned carbon dioxide laser,” Proc. SPIE 7884, 1–7 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen D., Lee C., Staninec M., and Fried D., “High-speed scanning ablation of dental hard tissues with a λ = 9.3-μm CO2 laser: Heat accumulation and peripheral thermal damage,” Proc. SPIE 7549, 754907 (2010). 10.1117/12.849334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond J. L., Wigdor H. A., Walsh J. T., Fadavi S., and Punwani I., “Sealant bond strengths of CO2 laser-etched versus acid-etched bovine enamel,” Lasers Surg. Med. 27(2), 111–118 (2000). [DOI] [PubMed] [Google Scholar]
- Can A. M., Darling C. L., Ho C. M., and Fried D., “Non-destructive assessment of inhibition of demineralization in dental enamel irradiated by a λ = 9.3-μm CO2 laser at ablative irradiation intensities with PS-OCT,” Lasers Surg. Med. 40, 342–349 (2008). 10.1002/lsm.20633 [DOI] [PubMed] [Google Scholar]
- Featherstone J. D. B., Barrett-Vespone N. A., Fried D., Kantorowitz Z., Lofthouse J., and Seka W., “Rational choice of CO2 laser conditions for inhibition of caries progression,” Proc. SPIE 2394, 57–67 (1995). 10.1117/12.207457 [DOI] [Google Scholar]
- Fried D., Featherstone J. D., Le C. Q., and Fan K., “Dissolution studies of bovine dental enamel surfaces modified by high-speed scanning ablation with a λ = 9.3 μm TEA CO2 laser,” Lasers Surg. Med. 38(9), 837–845 (2006). 10.1002/lsm.20385 [DOI] [PubMed] [Google Scholar]
- Le C. Q., Fried D., and Featherstone J. D. B., “Lack of dentin acid resistance following 9.3 um CO2 laser irradiation,“Proc. SPIE 6843, 68430J (2008). 10.1117/12.778801 [DOI] [Google Scholar]
- Manesh S. K., Darling C. L., and Fried D., “Nondestructive assessment of dentin demineralization using polarization-sensitive optical coherence tomography after exposure to fluoride and laser irradiation,” J. Biomed. Mater. Res., Part B: Appl. Biomater 90(2), 802–812 (2009). 10.1002/jbm.b.31349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried D., Zuerlein M. J., Featherstone J. D. B., and Machule D., “Thermal and chemical modification of dentin by pulsed CO2 laser irradiation at 9 to 11 μm,” Proc. SPIE 2973, 94–101 (1997). 10.1117/12.273576 [DOI] [Google Scholar]