Abstract
There is a widespread belief that subjective accounts of disease are key components of measures of disease severity and quality of life. In the present study we have set out to test this hypothesis using visual analogue scales (VAS) for itch, as a subjective measure, and actigraphy as an objective measure. One-hundred and seventeen itchy children and adults (and 25 controls) were studied for clusters of nights (total number 1,654) and actigraphy scores and VAS itch taken daily. Fifty-six percent of the night-to-night variation in actigraphy scores occurred between different individuals, while 44% was intra-subject. Neither age nor sex (children’s or adults’) predicted actigraphy scores, and the only significant predictor of actigraphy score was disease type (p = 0.001, r2 = 0.51). In a multivariate model VAS itch score was not a significant determinant of actigraphy scores for either children or adults (p = 0.26). In order to see if there was a relation between VAS itch and actigraphy within the same patients (rather than between patients), 20 eczema patients wore the actigraph and scored VAS itch nightly for 42 nights. Little relationship was found between the actigraphy score and the VAS itch. Empirical autocorrelation analysis of VAS itch and actigraphy score reveal a clear autocorrelation for subjective VAS scores that was not found for the objective actigraphy score. Our data suggest a dissociation between scratch and perceived or recalled itch. One explanation is that VAS itch scores suffer from considerable anchoring, and context bias, and that their use in measures of disease severity is problematic.
Keywords: actigraphy, pruritus, visual analogue scale, bias
In recent years there has been a considerable growth in interest in subjective measures of disease severity in dermatology (1–3). Much of the focus of this effort has been on attempts to measure the impact of disease on an individual sufferer, accepting that objective accounts may be inadequate to express disease impact. To take an example, the impact of psoriasis affecting, say, 50% of the body area may be greater in a person who likes to regularly swim compared with somebody who does not swim (4). An objective score such as the PASI (5) will not reflect these aspects of disease that are important (4). These arguments are widely acknowledged and accepted (6–8).
Some measures of disease severity or disease impact include individual subjective measures such as itch. An example would be the SCORAD, which includes subjective accounts of itch using a visual analogue scale (VAS) (9). Inclusion of itch in such scores is predicated on the idea that distress due to itch may provide information on suffering that is not fully accounted for by other measures (1, 10).
Measurement of itch presents its own problems (10). Itch is arguably the principal symptom of skin disease (10–12). Like pain, itch is subjective and it is difficult to see how it can be measured except by subjective account. However, much as pain has consequences – acute pain, for instance, may be accompanied by vocalisation, or an increase in heart rate – itch provokes the desire to scratch (10), a behaviour that may be independently verified. A number of strategies have been suggested for how to measure scratch and building on pioneering work by Felix & Shuster (13) we have used wristworn accelerometers or actigraphs (Actiwatch Plus, Cambridge Neurotechnology), worn at night to record scratch as a proxy measure of itch, having validated this technique against infrared videoing of children and manual scoring of scratch movements (14, 15). As compared with earlier work, our focus has been to develop strategies that can be used by subjects in their own homes and that take advantage of digital recording and analytical methods. Others have followed analogous approaches (16–18). Our goal can be considered analogous to the use of peak flow meters to monitor disease severity in asthma, in that we wish to develop a symptom tool that can be used for longitudinal monitoring of disease activity.
One unexpected finding from our previous work on children was that there was little relation between subjective accounts of itch and objective measurements recorded using an actigraph – despite the latter being validated by direct observation of scratch behaviour through infrared videoing of children in bed at night (15). This raises an important issue: assessment, recall and formulation of subjective accounts may be prone to error or artefact (19–22). If this is accepted, then whatever the virtues of subjectively recalled impacts of disease, error remains.
Our aim in the present work was to explore the relation between subjective accounts of itch using visual analogue scales, and scratch assessed objectively using actigraphs. In the process we also wished to address some limitations of our previous work (14,15): rather than examining correlations between subjects, we examined scores for each subject at multiple time points such that within-person correlations between itch and scratch could be examined. Our results are subject to more than one interpretation, but suggest that subjective measures of itch in this particular context may indeed be prone to artefact, perhaps due to anchoring bias (7, 23, 24), and that their uncritical use of them may be misleading.
METHODS
Ethical permission was received from Lothian NHS Ethics Committee for all experiments.
Study 1
Sixty-eight itchy adults and 50 itchy children were recruited. Twelve adult controls and 12 child controls also took part. The subjects were recruited from clinics at the Royal Infirmary of Edinburgh. In so far as was practical, consecutive eczema patients were approached and given information about the study. For inclusion in the study, all participants had to be older than 3 months of age and be able to provide consent or have a parent/guardian willing to do so. Subjects had to have been diagnosed by a Consultant Dermatologist as having a characteristically itchy skin condition and to have complained of an associated symptom of itch. Controls had no evidence of skin disease and reported no symptom of itch. The participants were given time to consider the invitation to take part in the study and then contacted again by telephone. Those who agreed to participate then had an appointment to meet the researcher arranged. Written, informed consent was acquired from all participants and guardians of participants.
Sample sizes were chosen opportunistically, based on availability and what was considered practical in the light of prior experiments (14, 15)
Table I details the subjects and specifies their diagnosis and characteristics. All the child subjects had atopic eczema. The broad groups of diagnoses for adult subjects were: eczema, psoriasis, cholestatic liver disease and pruritus of unknown cause (PUC). A total of 1,654 nights were studied: 1,573 subject nights and 81 control nights.
Table I.
Principal demographic characteristics of the study subjects
| Total n |
Age, years |
Nights studied n |
AD n |
Psoriasis n |
PUC n |
Hepatic itch n |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| F:M ratio | Mean | Range | ||||||||
| Study 1 | Child subjects | 50 | 24:26 | 6.92 | 2–15 | 229 | 50 | 0 | 0 | 0 |
| Child controls | 12 | 10:2 | 10.33 | 6–14 | 42 | N/A | N/A | N/A | N/A | |
| Adult subjects | 67 | 40:27 | 43.5 | 16–78 | 1344 | 30 | 25 | 4 | 8 | |
| Adult controls | 13 | 8:5 | 38.23 | 24–71 | 39 | N/A | N/A | N/A | N/A | |
| Study 2 | Adult subjects | 20 | 9:11 | 40.55 | 16–67 | 761 | 20 | 0 | 0 | 0 |
AD: atopic dermatitis; PUC: pruritus of unknown cause; F: female; M: male. Subjects were classified according to age: child, <16 years; adult, >16 years.
Subjects were requested to wear the actigraph on the wrist of their dominant hand (for consistency’s sake, but also please see reference for further explanation (14)) for three to seven nights. Every day of the study, the participants also completed 10-cm VAS for extent, itch and insomnia. In the case of children, adults (parent/guardian) completed the scores for the participants.
Study 2
Twenty patients were recruited. All were adult eczema patients attending the Royal Infirmary of Edinburgh. For inclusion into the study, the participant had to be 16 years old or over and have atopic dermatitis, as diagnosed by a Consultant Dermatologist. They were approached as described for Study 1 and similarly gave written, informed consent. Eleven females and nine men agreed to participate. Table I gives the subjects’ characteristics. (Although not a designated part of this study, SCORAD scores were available for the volunteers: the median was 34.2 with a range of 9.7 to 67.7).
All subjects were issued with an actigraph and instructed to wear it on the wrist of their dominant hand every night for the next 42 nights. A total of 761 nights were studied (patients forgot to take part on some nights).
All subjects were issued with dated sheets showing the VAS for itch, disease extent and insomnia. All of the subjects had a ‘ballot’ box and they were instructed to post each day’s scoring sheet into the ballot box immediately after completing it. The sheet for day 0 was completed and deposition demonstrated to the researcher by the participant. On (or as near to) day 21 of the study, the participant and researcher met so that the actigraph could be checked for full functionality. On (or as near to) day 42 of the study, the researcher met with the participant to collect the actigraph and download its data, and to collect the completed forms – all, it was expected, to be contained in the ballot box.
Actigraphy
The digital accelerometer used was the Actiwatch Plus (Cambridge Neurotechnology, Cambridge, United Kingdom). This instrument detects and measures movement by utilising a piezo-electric accelerometer that logs the integration of intensity, amount and duration of movement in three axes. The Actiwatch senses and logs 32 times/s and a summation value is available for the measurement epoch. Epoch length was set at one minute. The Actiwatch is supplied with a proprietary reader that enables downloading to a computer. The digital accelerometer output was exported to software (Excel; Microsoft Ltd, Seattle, USA) for analysis. The nocturnal score was a simple sum of all the scores logged between 1 am and 5 am (a time range shown in previous unpublished studies to exclude most extraneous ambulatory movement).
Statistical analyses
Data was managed using Excel (Microsoft Ltd, Seattle, USA.) and exported to ‘R’ v2.9 running on a Mac OS 10.6 for all statistical analyses (25). In order to differentiate between adults completing their own subjective symptom scores and child subjects – who required an adult to complete them for them – analyses which involved examination of VAS itch scores were conducted separately for those under and over 16 years old. Actiwatch scores are non-normally distributed and were log-transformed (14, 15). For studies comparing disease groups, where data were available for more than one night, mean scores were used.
Within- and between-night analyses were performed by ANOVA using person as a factor (26). Following univariate analyses, accelerometer scores were modelled using linear regression with sex and disease treated as factors and age and VAS as continuous variables. Non-significant terms were removed from the model and levels of each factor compared using pairwise Student’s t-tests with Holm’s correction (25,26).
For Study 2, VAS itch and log actigraph scores were modelled using a mixed-effect model with person as the unit using the nmle package in ‘R’. Since this does not take full account of the correlation structure, the autocorrelation function in ‘R’ (‘acf’) was used and moving model averages compared using ANOVA. Detailed exploration of different lag periods or different classes of time series models was not performed. Graphical representation of the autocorrelation structure shows to what extent a score on day n, is correlated with scores on subsequent successive days (n+1, n+2, n+3…).
RESULTS
Study 1
Determinants of actigraphy scores
For the majority of subjects, readings from three or more nights were available. Fifty-six percent of the variation was between-person and 44% within-person (i.e. due to variation from night to night for the same person) (ANOVA). Means for each subject were calculated for subsequent analyses. Children (subjects <16 years old) and adults (>16 years) were examined separately. The total data set is shown in Fig. 1.
Fig. 1.
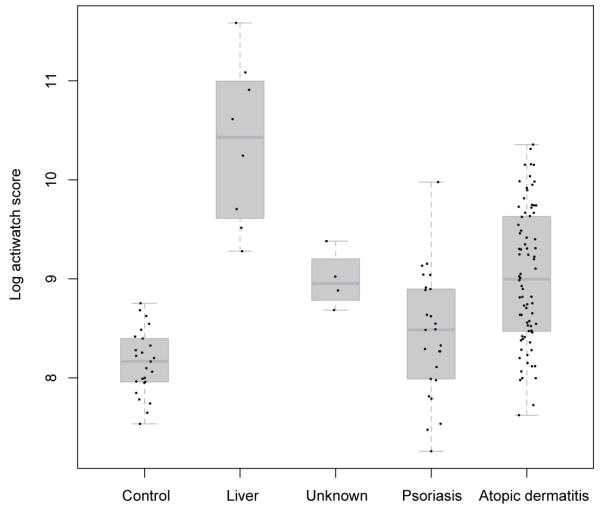
Study 1. Mean overnight actigraphy score for all participants (children and adults) separated by diagnosis: control, liver (= hepatic itch), unknown (= pruritus of unknown cause), psoriasis and atopic dermatitis.
Children
Linear regression of log actigraphy scores showed no effect of age (p = 0.67) or sex (p = 0.365). As expected, scores were higher for subjects with atopic dermatitis than controls: mean log actigraphy score 9.02 vs. 8.29 (p = 0.021). VAS itch was not a significant predictor in either univariate (p = 0.261) or multivariate (p = 0.284) models. Fig. 2 shows the relation between actigraphy and VAS itch scores (the slope of the regression line shown is not significantly different from 0).
Fig. 2.
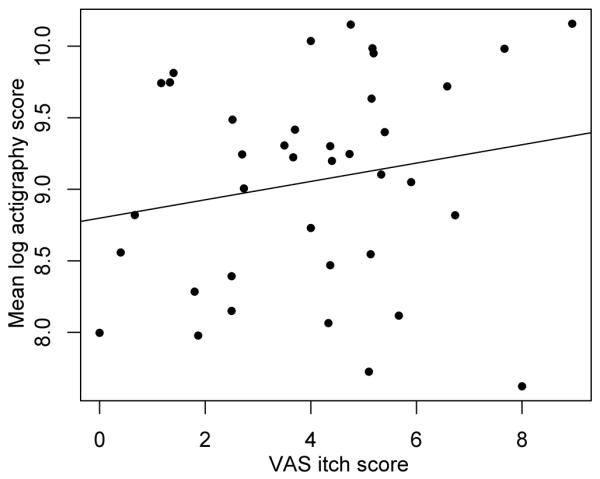
Study 1. Regression analysis of children’s mean log overnight actigraphy score and visual analogue scale (VAS) itch score. A ‘best-fit’ regression line (slope not significantly different from 0) is shown.
Adults
Sex and age had no effect on actigraphy scores. Mean log actigraphy scores were 10.4, 8.95, 8.43, 8.99 and 8.14 in patients with liver disease, atopic dermatitis, psoriasis, pruritus of unknown cause and controls respectively. Measures of uncorrected effect can be seen in Fig. 1, and a matrix of Holm-corrected pairwise Student’s t-tests performed following linear regression using pooled error is shown in Table II. As can be seen, scores were significantly higher for subjects with liver disease compared with other disease groups and controls. Scores for subjects with atopic dermatitis, but not those with psoriasis, were significantly higher than in controls.
Table II.
Summary of principal differences between actigraphy scores for adults by diagnostic group. Pairwise comparisons are made using Student’s t-tests with pooled error following linear regression. Mean log scores are: control, 8.14; liver, 10.4; PUC, 8.99; psoriasis, 8.43 and AD, 8.95.
| Control | Liver | PUC | Psoriasis | |
|---|---|---|---|---|
| Liver | < 0.0001 | – | – | – |
| PUC | 0.0782 | 0.0034 | – | – |
| Psoriasis | 0.3564 | 0.0001 | 0.3033 | – |
| AD | 0.0015 | 0.0001 | 0.8884 | 0.0184 |
PUC: pruritus of unknown cause; AD: atopic dermatitis.
Mean VAS itch scores were 6.64, 4.83, 4.68 and 2.53 in patients with liver disease, psoriasis, atopic dermatitis and patients with itch of unknown cause, respectively. The only significant difference between these groups was between those with liver disease and those with itch of unknown cause (p = 0.04; Student’s t-test). The spread of the values within each group was large.
In univariate analyses both diagnosis (p < 0.001) and VAS itch (p = 0.042) were significant predictors of actigraphy scores, but the r2 value for VAS itch was only 0.06 (r2 for diagnostic category = 0.51). Once diagnosis was entered into the regression equation, VAS itch was no longer significant (p = 0.27). The relation, by diagnostic group, between VAS itch score and actigraphy is shown in Fig. 3 (best-fit lines are shown, but their slopes do not differ significantly from 0).
Fig. 3.
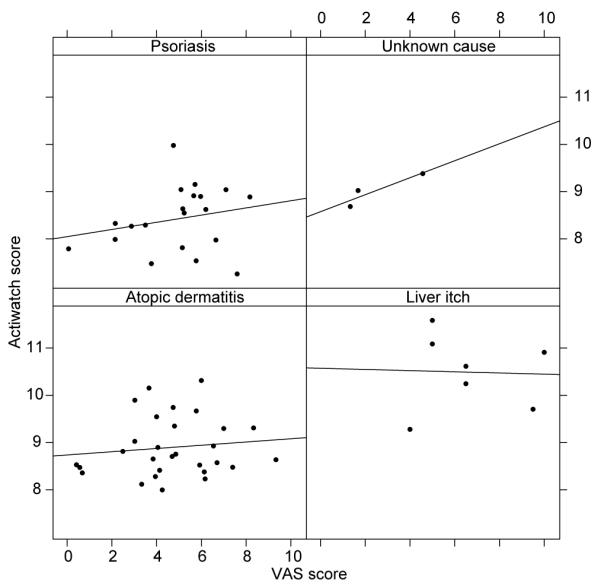
Study 1. Regression analysis of adults’ Actiwatch mean log overnight score and visual analogue scale (VAS) itch score separated by diagnostic group: psoriasis, unknown cause (= pruritus of unknown cause), atopic dermatitis and liver itch (= hepatic itch.) ‘Best-fit’ regression lines are shown (none of whose slopes is significantly different from 0).
Study 2
In a different cohort of 20 adults with eczema, actigraphy and VAS itch scores were recorded over 42 nights. Scatter plots of VAS itch against actigraphy are shown for each of the twenty persons in Fig. 4. It can be seen that there appears to be little relation between actigraphy and VAS itch scores. Because scores from night to night for the same person cannot be assumed to be independent, we initially modelled the data using a mixed-effect model with person as the grouping factor. In order to take account of the correlation structure, we then compared this model (using ANOVA) with a simple moving average model using the corARMA function (25, 26) with a delay of 2 or 3 days. This model provided a better fit to the data (p < 0.001). The empirical autocorrelation structure for VAS itch is shown in Fig. 5, with a dashed line representing statistical significance (p = 0.001) plotted. The autocorrelation bar crossing the p = 0.001 dashed line indicates that the data on successive days are highly correlated. This is most evident for lags of 1 to 3 days (the correlation on day 0 is of course perfect as the same figure is being compared), but the stepwise diminution of the height of the autocorrelation bar reflects a diminution of the correlation from any one day (n) over subsequent successive days (n+1, n+2, n+3…). No pattern for autocorrelation was seen for actigraphy scores (data not shown).
Fig. 4.
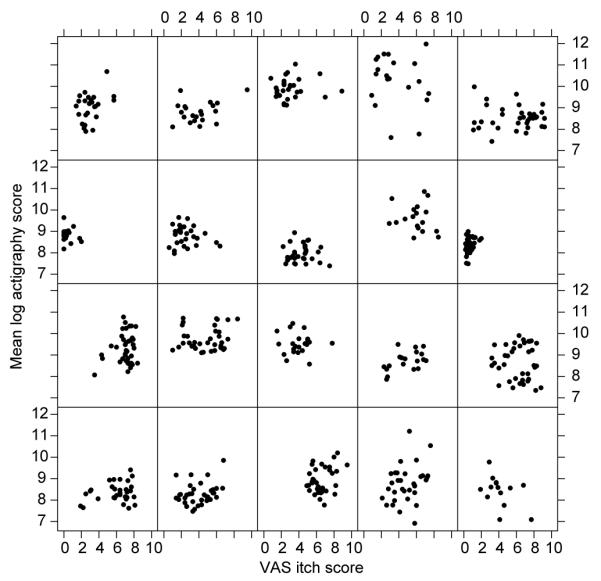
Study 2. Individual scatter plots of visual analogue scale (VAS) itch against mean log overnight actigraphy score for every participant.
Fig. 5.
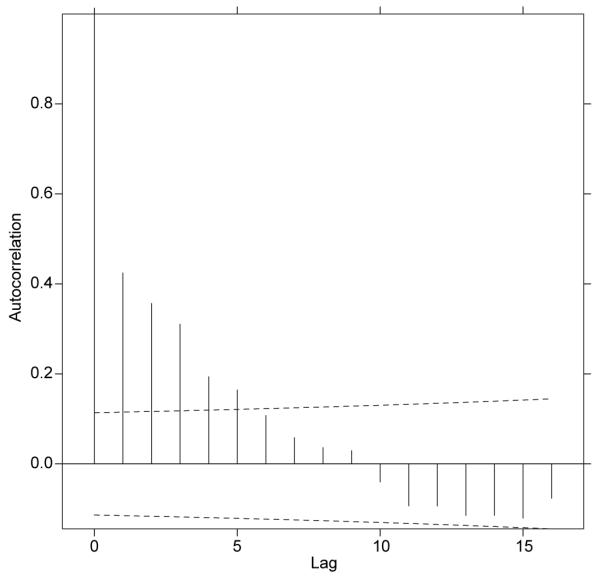
Study 2. Graph demonstrating the autocorrelation structure of grouped individuals’ visual analogue scale itch scores. Dashed line: statistical significance (p = 0.001) (autocorrelation lines crossing this bar indicate statistical significance).
DISCUSSION
The current work builds upon previous studies and in large part confirms that the relation between objective measures of scratch and VAS itch is poor. Before discussion, some limitations of the work are highlighted.
The work was based on those attending secondary care, and therefore was probably skewed towards patients with more severe disease, as reflected by the SCORAD scores. Patients were volunteers and, since the study involved a significant amount of time and contact with the investigators, the study group may not be typical of the reference population. We obviously were not able to control prospectively the severity of disease during the study period – if patient groups at the extreme end of the scale for symptom severity had been used, it is possible different conclusions might have been made, although the SCORAD scores for the eczema group were as high or higher than in many therapeutic trials.
In study 1 we have shown that there is no obvious relation between itch assessed using VAS scores and actigraphy scores for either children with atopic dermatitis or adults with a range of diagnoses (see Fig 2 and 3. Previous work has validated actigraphy against direct observation of children scratching at night, and both scratching and restlessness are correlated with each other, and higher in those with atopic dermatitis than in controls (14, 15). Itch-related behaviour at night is not as stereotyped as when awake, and actigraphy is, we believe, a useful practical assay for scratch when compared with the time-consuming nature of direct observation of video recordings. Although we analysed children and adults separately, we saw no influence of sex or age on actigraphy scores. The key determinant of differences in actigraphy scores was diagnostic group, with those with liver disease the most severely affected. This assumes, however, that restlessness due to other factors differing between diagnostic groups is not confounding our scores – an assumption that needs to be tested. Although VAS itch scores differed between the different diagnostic groups, the differences were largely non-significant, again emphasising a disconnect between VAS scores and actigraphy.
In study 1 we showed that approximately 60% of the recorded variation was between-subject and approximately 40% within-subject. This fact emphasises that nocturnal movements vary from night to night within any one subject, and of course limits the power of actigraphy scores for experimental studies unless repeated sampling is carried out. It is quite possible that other factors – night of the week and work patterns – contribute to this within-person variation, as well as variation in the disease that is causing the scratch. We have not specifically examined these factors.
In study 2 we had a chance to look in more detail at the relation between VAS itch and actigraphy over time. Again, we saw little convincing relation, and examination of the autocorrelation shows a very different pattern for actigraphy and VAS itch. The VAS itch scores clearly show an effect of lag, and the question is: why? We asked our subject to post their scores in an attempt to minimise the filling out of results in batches, and to limit knowledge of the score on one day influencing the score on the next day. This strategy is not foolproof and electronic diaries or other methods of continuous sampling would perhaps provide a better approach (27, 28). We suspect that the presence of the temporal pattern for VAS itch scores (and not actigraphy) is more easily explained by subjects anchoring their scores based on recollection of previous scoring (7, 23). Whether or not this is indeed the correct interpretation, coupled with the results of study 1, our data suggest that the use of VAS scores for itch may be limited in their utility as a subjective measure of disease activity in the context of the chronic diseases we studied. We suspect that objective scores such as actigraphy – however imperfect – deserve more attention for monitoring disease activity.
ACKNOWLEDGEMENT
This work was supported by the Wellcome Trust (grant number 076754).
REFERENCES
- 1.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 2.Both H, Essink-Bot ML, Busschbach J, Nijsten T. Critical review of generic and dermatology-specific health-related quality of life instruments. J Invest Dermatol. 2007;127:2726–2739. doi: 10.1038/sj.jid.5701142. [DOI] [PubMed] [Google Scholar]
- 3.Chen SC. Dermatology quality of life instruments: sorting out the quagmire. J Invest Dermatol. 2007;127:2695–2696. doi: 10.1038/sj.jid.5701176. [DOI] [PubMed] [Google Scholar]
- 4.Rees JL. Trials, evidence, and the management of patients with psoriasis. J Am Acad Dermatol. 2003;48:135–143. doi: 10.1067/mjd.2003.19. [DOI] [PubMed] [Google Scholar]
- 5.Fredriksson T, Pettersson U. Severe psoriasis – oral therapy with a new retinoid. Dermatologica. 1978;157:238–244. doi: 10.1159/000250839. [DOI] [PubMed] [Google Scholar]
- 6.Nord E. Cost-value analysis in health care: Making sense out of QALYs. Cambridge University Press; Cambridge: 1999. [Google Scholar]
- 7.Bjorner JB, Fayers P. Self-rated health. In: Fayers P, Hays R, Idler E, editors. Assessing quality of life in clinical trials: methods and practice. Oxford University Press; Oxford: 2005. pp. 309–323. [Google Scholar]
- 8.Basra MK, Fenech R, Gatt RM, Salek MS, Finlay AY. The Dermatology Life Quality Index 1994-2007: a comprehensive review of validation data and clinical results. Br J Dermatol. 2008;159:997–1035. doi: 10.1111/j.1365-2133.2008.08832.x. [DOI] [PubMed] [Google Scholar]
- 9.Stalder JF, Taieb A, Atherton DJ, Bieber T, Bonifazi E, Broberg A, et al. Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology. 1993;186:23–31. doi: 10.1159/000247298. [DOI] [PubMed] [Google Scholar]
- 10.Wahlgren CF. Measurement of itch. Semin Dermatol. 1995;14:277–284. doi: 10.1016/s1085-5629(05)80048-3. [DOI] [PubMed] [Google Scholar]
- 11.Shuster S, Fisher GH, Harris E, Binnell D. The effect of skin disease on self image [proceedings] Br J Dermatol. 1978;99(Suppl 16):18–19. [PubMed] [Google Scholar]
- 12.Rees JL, Laidlaw A. Pruritus: more scratch than itch. Clin Exp Dermatol. 1999;24:490–493. doi: 10.1046/j.1365-2230.1999.00541.x. [DOI] [PubMed] [Google Scholar]
- 13.Felix R, Shuster S. A new method for the measurement of itch and the response to treatment. Br J Dermatol. 1975;93:303–312. doi: 10.1111/j.1365-2133.1975.tb06496.x. [DOI] [PubMed] [Google Scholar]
- 14.Benjamin K, Waterston K, Russell M, Schofield O, Diffey B, Rees JL. The development of an objective method for measuring scratch in children with atopic dermatitis suitable for clinical use. J Am Acad Dermatol. 2004;50:33–40. doi: 10.1016/s0190-9622(03)02480-0. [DOI] [PubMed] [Google Scholar]
- 15.Bringhurst C, Waterston K, Schofield O, Benjamin K, Rees JL. Measurement of itch using actigraphy in pediatric and adult populations. J Am Acad Dermatol. 2004;51:893–898. doi: 10.1016/j.jaad.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 16.Ebata T, Iwasaki S, Kamide R, Niimura M. Use of a wrist activity monitor for the measurement of nocturnal scratching in patients with atopic dermatitis. Br J Dermatol. 2001;144:305–309. doi: 10.1046/j.1365-2133.2001.04019.x. [DOI] [PubMed] [Google Scholar]
- 17.Bender B, Leung S, Leung D. Actigraphy assessment of sleep disturbance in patients with atopic dermatitis: An objective life quality measure. J Allergy Clin Immunol. 2003;111:598–602. doi: 10.1067/mai.2003.174. [DOI] [PubMed] [Google Scholar]
- 18.Hon KL, Lam MC, Leung TF, Kam WY, Lee KC, Li MC, et al. Nocturnal wrist movements are correlated with objective clinical scores and plasma chemokine levels in children with atopic dermatitis. Br J Dermatol. 2006;154:629–635. doi: 10.1111/j.1365-2133.2006.07213.x. [DOI] [PubMed] [Google Scholar]
- 19.Redelmeier DA, Kahneman D. Patients’ memories of painful medical treatments: real-time and retrospective evaluations of two minimally invasive procedures. Pain. 1996;66:3–8. doi: 10.1016/0304-3959(96)02994-6. [DOI] [PubMed] [Google Scholar]
- 20.Redelmeier DA, Katz J, Kahneman D. Memories of colonoscopy: a randomized trial. Pain. 2003;104:187–194. doi: 10.1016/s0304-3959(03)00003-4. [DOI] [PubMed] [Google Scholar]
- 21.Broderick JE, Schwartz JE, Vikingstad G, Pribbernow M, Grossman S, Stone AA. The accuracy of pain and fatigue items across different reporting periods. Pain. 2008;139:146–157. doi: 10.1016/j.pain.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stone AA, Broderick JE, Kaell AT, DelesPaul PA, Porter LE. Does the peak-end phenomenon observed in laboratory pain studies apply to real-world pain in rheumatoid arthritics? J Pain. 2000;1:212–217. doi: 10.1054/jpai.2000.7568. [DOI] [PubMed] [Google Scholar]
- 23.Kahneman D, Tversky A, editors. On the reality of cognitive illusions. Psychol Review. 1996;103:582–586. doi: 10.1037/0033-295x.103.3.582. [DOI] [PubMed] [Google Scholar]
- 24.Kahneman D, Tversky A. Choices, Values and Frames. Cambridge University Press and the Russell Sage Foundation; New York: 2000. Evaluation by Moments: Past and Future. [Google Scholar]
- 25.R Development Core Team . R: A language and environment for statistical computing [Internet] R Foundation for Statistical Computing; Vienna, Austria: 2009. Available from: http://www.R-project.org. [Google Scholar]
- 26.Crawley MJ. The R Book. John Wiley and Sons Ltd; Chichester, England: 2007. [Google Scholar]
- 27.Kahneman D, Krueger AB, Schkade DA, Schwarz N, Stone AA. A survey method for characterizing daily life experience: The day reconstruction method. Science. 2004;306:1776–1780. doi: 10.1126/science.1103572. [DOI] [PubMed] [Google Scholar]
- 28.Langan SM, Bourke JF, Silcocks P, Williams HC. An exploratory prospective observational study of environmental factors exacerbating atopic eczema in children. Br J Dermatol. 2006;154:979–980. doi: 10.1111/j.1365-2133.2006.07153.x. [DOI] [PubMed] [Google Scholar]


