Abstract
Objective
To study the prognostic value of antibodies to cyclic citrullinated peptide (anti-CCP) and rheumatoid factor (RF), alone and in combination, in patients with very early synovitis.
Methods
A cross-sectional study was performed in patients with established inflammatory and noninflammatory disease to validate the assay in our unit and confirm previously reported sensitivities and specificities of anti-CCP antibodies. Subsequently, patients with synovitis of ≤ 3 months’ duration were followed for 72 weeks and the ability of anti-CCP antibodies and RF to predict the development of rheumatoid arthritis (RA) and persistent inflammatory arthritis was assessed.
Results
One hundred twenty-four patients were assessed in the initial cross-sectional study. Anti-CCP antibodies and RF were detected by ELISA in only 4% of patients with non-RA inflammatory disease and in no patient with noninflammatory disease. Ninety-six patients with very early synovitis were assessed longitudinally. In these patients with early arthritis, the combination of anti-CCP antibodies and RF had a specificity, positive predictive value (PPV), sensitivity, and negative predictive value (NPV) for a diagnosis of RA of 100%, 100%, 58%, and 88%, respectively. The specificity, PPV, sensitivity, and NPV of this antibody combination for the development of persistent disease-fulfilling classification criteria for RA were 97%, 86%, 63%, and 91%, respectively.
Conclusion
In patients with synovitis of ≤ 3 months’ duration, a combination of anti-CCP antibodies and RF has a high specificity and PPV for the development of persistent RA. This autoantibody combination can be used to identify patients with disease destined to develop RA who may be appropriate for very early intervention.
Keywords: CYCLIC CITRULLINATED PEPTIDE, ANTI-CYCLIC CITRULLINATED PEPTIDE ANTIBODY, RHEUMATOID ARTHRITIS, DIAGNOSIS, EARLY ARTHRITIS, RHEUMATOID FACTOR
Damage to bone and cartilage is usually apparent within the first year of symptoms in patients with rheumatoid arthritis (RA)1,2. Many have extrapolated this to suggest that early therapy will reduce damage by reducing the patient’s cumulative inflammatory burden3-5. In therapeutic studies of “early” intervention (variably defined but usually within the first 2 years of disease onset), “early” aggressive therapy does indeed reduce disease activity while it is being administered6-10, but its ability to modify the underlying course of disease within this timeframe has been disappointing11,12. However, there is some evidence that treatment within the first few months of disease may be qualitatively superior to later therapy13. There is increasing interest in the concept that this very early phase represents a pathologically unique therapeutic window in RA during which intervention may not only control inflammation but also switch off the disease. Such a very early and transient therapeutic window presents the challenge of distinguishing patients with synovitis destined to develop RA (who would benefit from treatment) from those with self-limiting disease or those whose synovitis persists but who do not develop RA. Considerable effort has been applied to identifying predictors of persistence in patients with “early” inflammatory arthritis. In patients with symmetrical peripheral polyarthritis and symptoms of < 6 months’ duration the combination of a positive rheumatoid factor (RF) latex agglutination test and an erythrocyte sedimentation rate (ESR) > 30 mm/h had a specificity and sensitivity of 94% and 69%, respectively, for the prediction of persistence14. In patients with inflammatory arthritis of < 12 months’ duration and involvement of ≥ 2 joints the only significant independent predictor of persistence was a disease duration of > 12 weeks15. In patients with symptoms of < 2 years’ duration, the strongest predictors of persistence were a disease duration of > 6 months and seropositivity for anti-cyclic citrullinated peptide (anti-CCP) antibody16. Predicting persistence, and the development of RA, has not been addressed in patients with synovitis of very short duration.
Autoantibodies directed against citrullinated proteins have been recognized as markers of RA for over 40 years17,18. Since the development of a synthetic CCP for use in an ELISA19 there has been increasing interest in these antibodies as diagnostic and prognostic markers19-21 and in the role of citrullinated peptides in the pathogenesis of RA22. The utility of anti-CCP antibodies for the diagnosis of RA has been assessed in a number of populations, with specificities for RA being > 90% in all studies (90%, 91%, 96%, 98%19,23-25), although with lower sensitivities (66%, 41%, 48%, 43%19,23-25). Recent data suggest that production of anti-CCP antibodies, like RF, may predate the development of RA in at least a third of patients26-28. Such autoantibodies, preceding the onset of symptoms, may be either pathogenic or an epiphenomenon consequent upon subclinical synovial inflammation. Indeed, synovial disease is known to predate clinical manifestations of arthritis in animal models, and histological evidence of synovial inflammation is common in clinically uninvolved joints in patients with RA29,30. Whatever the explanation for their presence preclinically, these antibodies may be a useful predictive marker in very early disease. We undertook a cross-sectional study to confirm previously reported sensitivities and specificities of anti-CCP antibodies in patients with inflammatory and noninflammatory disease, and then a longitudinal study to assess the utility of this antibody in predicting the outcome of very early synovitis (≤ 3 months’ duration).
MATERIALS AND METHODS
Antibodies to CCP and RF were measured in 221 patients. One hundred twenty-four patients with established inflammatory and noninflammatory diseases were assessed in a cross-sectional study. Patients with established diagnoses were consecutively recruited from either routine rheumatology outpatient clinics or from a hyperlipidemia clinic. Patients had established RA seropositive for RF by latex testing (at presentation and at the time of sample collection; n = 22), established RA seronegative for RF by latex testing (at presentation and time of sample collection; n = 20), Wegener’s granulomatosis (WG; n = 10), systemic lupus erythematosus (SLE; n = 10), ankylosing spondylitis (AS; n = 12), inflammatory bowel disease (IBD: Crohn’s disease, n = 7; ulcerative colitis, n = 3), sarcoidosis (with hilar lymphadenopathy and articular involvement; n = 10), osteoarthritis (OA; n = 10), or hyperlipidemia (with no history of inflammatory disease; n = 20).
Ninety-seven patients were consecutively recruited from the very early inflammatory arthritis clinic at City Hospital, Birmingham, with synovitis of at least one joint and a symptom duration of ≤ 3 months. Patients were followed for up to 18 months. Patients were classified as having RA if they fulfilled 1987 American Rheumatism Association (ARA) criteria at any stage during their followup31. Both list and tree formats were applied, allowing criteria to be satisfied cumulatively. While these criteria have no exclusions, we excluded from the RA category patients with alternative rheumatological diagnoses explaining their inflammatory arthritis. Thus, for example, one patient with polymyositis related arthritis who fulfilled criteria for RA was not included in the RA group. Patients were diagnosed with reactive arthritis (ReA), psoriatic arthritis (PsA), and a number of miscellaneous conditions according to established criteria32-36. The presence of uric acid crystals in synovial fluid was taken as diagnostic of gout. Pseudogout was diagnosed if calcium pyrophosphate crystals were present in the absence of other causes of inflammatory arthritis. Patients were classified as having persistent or resolving synovitis at their final followup. Resolving arthritis was diagnosed if there was no evidence of joint related soft tissue swelling on examination and the patient had not received disease modifying antirheumatic drugs (DMARD) or steroid treatment within the previous 3 months16,37. In contrast, persistent joint related swelling or treatment with DMARD or steroids, at any dose, for inflammatory joint symptoms (within the previous 3 months) defined persistence. The study was approved by the local research ethics committees and all patients gave written informed consent.
IgG anti-CCP antibody was detected using the Diastat™ anti-CCP assay (Axis-Shield Diagnostics Ltd., Dundee, UK) and seropositivity defined as a titer ≥ 10 IU/ml. RF was measured using both a latex agglutination test (RA80 Eiken, Mast Diagnostics, Bootle, UK) and an ELISA (Autostat™ II Total RF; Hycor Biomedical Ltd., Penicuik, UK) in which seropositivity was defined as a titer ≥ 30 IU/ml.
Statistical analysis
For binary variables, groups were compared using the chi-square test. For continuous variables involving 2 groups, groups were compared using the Mann-Whitney test. For continuous variables involving more than 2 groups, groups were compared using the Kruskal-Wallis test; where the difference between groups was statistically significant at the 5% level intergroup comparisons were performed using Dunn’s test. Binary logistic regression analysis was performed using SPSS software.
RESULTS
Cross-sectional study
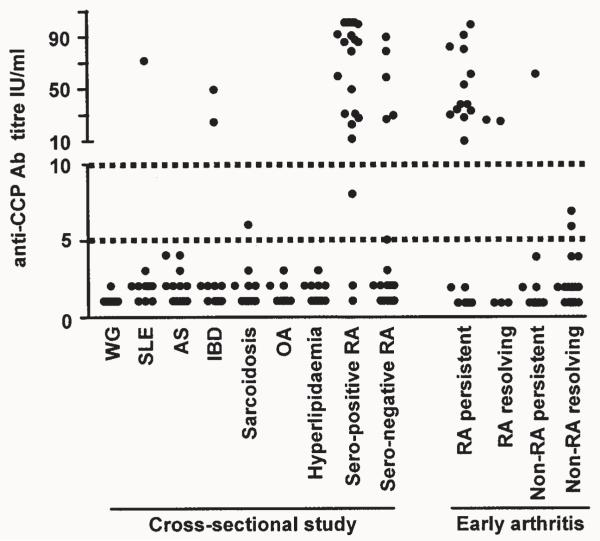
Anti-CCP antibody was positive in 3 of 52 patients (6%) with non-RA inflammatory disease, and was not detected in patients with noninflammatory disease (OA and hyperlipidemia) (Table 1, Figure 1). The percentages of these patients who were seropositive for RF, measured by ELISA, alone and in combination with anti-CCP antibodies are shown in Table 1. Anti-CCP antibody was detected in 86% of patients with established RA seropositive for RF (Table 1, Figure 1). Anti-CCP antibody was detected in 25% of patients with established RA who were seronegative for RF by latex agglutination. In 3 (15%) of these patients, RF was detected by ELISA. Within this population, the specificity and sensitivity of the presence of anti-CCP antibody for a diagnosis of RA were 96% and 57%, respectively. If the cutoff for seropositivity was reduced to 5 IU/ml, the specificity and sensitivity were 95% and 62%, respectively.
Table 1.
Rheumatoid factor (RF) ELISA and anti-CCP antibodies in patients with established inflammatory diseases and with noninflammatory diagnoses.
| Female, % | Age, yrs, median (IQR) |
RF ELISA Positive, % |
Anti-CCP ELISA Positive, % |
RF ELISA Positive and Anti-CCP ELISA Positive, % |
|
|---|---|---|---|---|---|
| WG, n = 10 | 70 | 62 (44.5–77) | 0 | 0 | 0 |
| SLE, n = 10 | 90 | 59 (34.5–74) | 40 | 10 | 10 |
| AS, n = 12 | 17 | 47 (37–50.5) | 0 | 0 | 0 |
| Inflammatory bowel disease, n = 10 |
50 | 62.5 (52–74.5) | 30 | 20 | 10 |
| Sarcoidosis, n = 10 | 30 | 48.5 (37.5–64) | 10 | 0 | 0 |
| OA, n = 10 | 60 | 60.5 (49–72) | 10 | 0 | 0 |
| Hyperlipidemia, n = 20 | 40 | 56 (47–60) | 0 | 0 | 0 |
| Seropositive RA, n = 22 | 57 | 70 (62.5–74) | 100 | 86 | 86 |
| Seronegative RA*, n = 20 | 70 | 62 (53.5–72) | 15 | 25 | 10 |
Seronegative RA defined on the basis of RF tested by latex agglutination. WG: Wegener’s granulomatosis, SLE: systemic lupus erythematosus, AS: ankylosing spondylitis, OA: osteoarthritis; RA: rheumatoid arthritis.
Figure 1.
Anti-CCP antibody titers (IU/ml) in patients with established rheumatological and non-rheumatological diagnoses in the cross-sectional study and in patients with very early inflammatory arthritis. WG: Wegener’s granulomatosis, SLE: systemic lupus erythematosus, AS: ankylosing spondylitis, IBD: inflammatory bowel disease, OA: osteoarthritis, RA: rheumatoid arthritis.
Very early inflammatory arthritis
The outcomes of the 97 patients with very early inflammatory arthritis are shown in Figure 2. Followup was inadequate to determine outcome in one patient, who was excluded from further analysis. The median duration of followup from symptom onset for the remaining patients was 78 weeks (interquartile range, IQR, 74 to 82 weeks). Although all patients were encouraged to attend for followup for 72 weeks, 13 patients with resolving disease did not return for complete followup after their disease had resolved. For these 13 patients, the median duration of followup was 49 weeks (range 6–70 weeks). The baseline characteristics of the 96 patients are shown in Table 2.
Figure 2.
Outcomes of 97 patients with very early inflammatory arthritis. RA: rheumatoid arthritis, PsA: psoriatic arthritis, ReA: reactive arthritis; ARA: American Rheumatism Association, FU: followup..
Table 2.
Baseline characteristics of patients with very early inflammatory arthritis.
| RA | Non-RA | ||||
|---|---|---|---|---|---|
| Persistent | Resolving | Persistent | Resolving | p | |
| N | 19 | 5 | 23 | 49 | |
| Female, % | 58 | 60 | 35 | 41 | NS** |
| Age, yrs* | 67 (59–74) | 56 (44–61) | 37 (25–58) | 41 (32–54) | p = 0.0003† RA persistent vs non-RA persistent p < 0.001 RA persistent vs non-RA resolving p < 0.01 |
| Symptom duration, wks* | 6 (4–9) | 8 (5–12) | 7 (4–10) | 3 (2–7) | p = 0.001† Intergroup comparisons NS |
| Initial CRP, mg/l * | 25 (18–41) | 19 (9–81) | 46 (24–95) | 23 (7–56) | NS† |
| No. swollen joints* | 5 (2–7) | 5 (1–11) | 2 (1–3) | 2 (1–3) | p = 0.0005† RA persistent vs non-RA resolving p < 0.01 |
| No. swollen joint areas* | 3 (2–4) | 5 (1–5) | 2 (1–3) | 1 (1–2.5) | p = 0.001† RA persistent vs non-RA resolving p < 0.01 RA resolving vs non-RA resolving p < 0.01 |
Results given as median (interquartile range) except for RA (Resolving), where median (range) are given.
Groups compared using chi-square test.
Groups compared using Kruskal-Wallis test, where the difference between groups was statistically significant at the 5% level, intergroup comparisons were performed using Dunn’s test. NS: not significant.
Twenty-four (25%) of the patients with very early inflammatory arthritis were classified as having RA at some point during followup and 72 (75%) were not. Of those 24 fulfilling criteria for RA, 19 (79%) had persistent disease and 5 (21%) had disease that resolved during followup. Of these 5 patients, 3 fulfilled classification criteria for RA by tree and list formats, one by list format alone, and one by tree format alone. Two of these 5 patients were seropositive for both anti-CCP antibodies and RF by latex agglutination and ELISA. One had presented with an 8 week history of wrist synovitis, which had resolved at 4 week followup (without steroid treatment) and remained in remission at 72 weeks (anti-CCP antibody titer 26 IU/ml). The other had a 6 week history of wrist, knee and ankle synovitis, was treated with intraarticular and intramuscular steroid, and remained in remission at 72 weeks (anti-CCP antibody titer 27 IU/ml). During followup, 4 of the 5 patients (80%) with resolving RA received steroid therapy (usually intraarticular). In this study, we assessed the value of seropositivity for RF, as well as anti-CCP antibody, in predicting the development of RA. Determining the prognostic value of a variable (e.g., RF) when the presence of that variable contributes to the determination of outcome (e.g., classification of a patient as having RA) can lead to a circular argument. However, if RF is excluded from the classification criteria, 17 of 19 patients with persistent RA and 3 of 5 patients with resolving RA are still classified as having RA.
Of the 72 patients with non-RA inflammatory arthritis, 23 had persistent disease (unclassified, n = 14; PsA, n = 4; arthritis related to IBD, n = 2; polymyositis, n = 1; dermatomyositis, n = 1; and Behçet’s disease, n = 1) and 49 patients had resolving disease (unclassified, n = 33; ReA, n = 6; gout, n = 6; pseudogout, n = 1; PsA, n = 2; and IBD related arthritis, n = 1). Only one patient in the non-RA group was seropositive for anti-CCP antibodies. This patient had PsA diagnosed on the basis of bilateral knee synovitis with psoriasis, nail pitting, and onycholysis (anti-CCP antibody titer 62 IU/ml). No patient with non-RA disease was seropositive for both RF (measured using either ELISA or latex agglutination) and anti-CCP antibodies. During followup, 20 of the 49 patients (41%; unclassified, n = 10; ReA, n = 5; crystal arthritis, n = 3; PsA, n = 1; and IBD related arthritis, n = 1) with resolving non-RA inflammatory arthritis received steroid therapy (usually intraarticular).
The specificity, positive predictive value (PPV), sensitivity, and negative predictive value (NPV) of RF detected by ELISA for the development of disease-fulfilling classification criteria for RA were 94%, 79%, 63%, and 88%, respectively (Table 3). Results were very similar for RF detected by latex testing (specificity, PPV, sensitivity, and NPV: 96%, 83%, 63%, and 88%, respectively). The presence of anti-CCP antibody alone had a greater specificity (99%) and PPV (94%) (Table 3). When the presence of anti-CCP antibody was combined with RF detected by ELISA, the specificity and PPV for RA were 100%, although with a sensitivity reduced to 58% (Table 3). Results were identical for the combination of RF detected by latex agglutination and anti-CCP antibody.
Table 3.
Sensitivity, specificity, positive (PPV), and negative predictive values (NPV) of seropositivity for RF by ELISA and anti-CCP antibodies in patients with very early inflammatory arthritis.
| Diagnosis | RF ELISA Positive |
Anti-CCP ELISA Positive |
RF ELISA Positive and Anti-CCP ELISA Positive |
RF ELISA Positive or Anti-CCP ELISA Positive |
|
|---|---|---|---|---|---|
| RA, n = 24 | Persistent, n = 19 | 13 | 13 | 12 | 14 |
| Resolving, n = 5 | 2 | 2 | 2 | 2 | |
| Non-RA, n = 72 | Persistent, n = 23 | 0 | 1 | 0 | 1 |
| Resolving, n = 49 | 4 | 0 | 0 | 4 | |
| RA (n = 24) vs | Sensitivity, % | 63 | 63 | 58 | 67 |
| Non-RA (n = 72) | Specificity, % | 94 | 99 | 100 | 93 |
| PPV, % | 79 | 94 | 100 | 76 | |
| NPV, % | 88 | 89 | 88 | 89 | |
| RA persistent | Sensitivity, % | 68 | 68 | 63 | 74 |
| (n = 19) vs other | Specificity, % | 92 | 96 | 97 | 91 |
| (n = 77) | PPV, % | 68 | 81 | 86 | 67 |
| NPV, % | 92 | 93 | 91 | 93 |
If patients fulfilling classification criteria for RA and whose disease persisted were analyzed independently and compared with all other patients, the combination of seropositivity for RF detected by ELISA and anti-CCP antibodies again had the highest specificity and PPV for the development of persistent RA (97% and 86%, respectively), with a sensitivity and NPV of 63% and 91%, respectively (Table 3). Results again were identical for the combination of RF detected by latex agglutination and anti-CCP antibodies. The specificity and PPV of anti-CCP antibody alone were 96% and 81% with a sensitivity and NPV of 68% and 93% (Table 3). If the cutoff for seropositivity for anti-CCP antibodies was reduced to 5 IU/ml (Figure 1), the specificity and PPV of anti-CCP antibodies alone for the prediction of persistent RA were 94% and 72% with a sensitivity and NPV of 68% and 92%, respectively.
We assessed the relationship between a number of other baseline characteristics and patient outcome (Table 2). Patients with persistent RA had a median of 5 swollen joints (IQR 2–7) and 3 swollen joint areas (IQR 2-4) at presentation. These were significantly greater than in patients with non-RA resolving disease (Table 2). In addition, patients with persistent RA had significantly more swollen joints and more swollen joint areas at initial presentation compared with all other patients combined (Mann-Whitney test, p = 0.002 and p = 0.01, respectively). Patients with persistent RA were also significantly older than patients with non-RA persistent and non-RA resolving disease (Table 2) and significantly older than patients in the 3 other groups combined (Mann-Whitney test, p < 0.001). The symptom duration at presentation in patients who developed persistent RA was no different from that of patients in the other 3 groups (Table 2). When patients with persistent RA were compared with all other patients together, again there was no significant difference in symptom duration (Mann-Whitney test, p = 0.07).
We used binary logistic regression analysis to determine which baseline characteristics were independently predictive of the development of persistent RA. The baseline characteristics chosen for analysis are shown in Table 4. We included criteria that form part of the 1987 ARA classification criteria for RA. While univariate analysis revealed that age, arthritis of ≥ 3 joint areas, arthritis of hand joints, and seropositivity for both RF and anti-CCP antibodies were all significantly associated with the development of persistent RA, binary logistic regression analysis revealed that only age, symmetrical arthritis, and seropositivity for both RF and anti-CCP antibodies were significant independent predictors of the development of persistent RA (Table 4). Seropositivity for both RF and anti-CCP antibody was the most significant independent predictor in this analysis. Taking this analysis as model 1, we repeated it, replacing seropositivity for both RF and anti-CCP antibody with: seropositivity for RF alone (model 2); seropositivity for anti-CCP antibody alone (model 3); and 3 separate variables, namely seropositivity for RF, seropositivity for anti-CCP antibody, and seropositivity for both RF and anti-CCP antibody (model 4). By determining the −2 log likelihood of each of these models we were able to assess how these anti-body variables compared for prediction of the development of persistent RA. The −2 log likelihood for model 4 was 37.7. This was significantly better than that for model 2, which used RF alone (−2 log likelihood 45.5; p < 0.05). However, there was no statistically significant difference between model 4 and models 1 and 3 (−2 log likelihood 41.2 and 38.2, respectively).
Table 4.
The predictive value of a range of baseline characteristics for the development of persistent RA in patients with very early inflammatory arthritis. Results of binary logistic regression analysis.
| RA Persistent, | Other, | Univariate Analysis |
Binary Logistic Regression Analysis | ||
|---|---|---|---|---|---|
| n = 19 | n = 77 | p | OR (95% CI)* | p | |
| Age, yrs, median (IQR) | 67 (59–74) | 41 (30–56) | < 0.001 | 1.08 (1.01–1.2) | 0.02 |
| Female, % | 58 | 40 | 0.26 | 1.5 (0.3–8.7) | 0.65 |
| Symptom duration > 6 weeks, % |
47 | 38 | 0.55 | 0.4 (0.05–3.4) | 0.41 |
| EMS ≥ 1 h, % | 53 | 34 | 0.09 | 1.03 (0.1–7.4) | 0.98 |
| Arthritis of ≥ 3 joint areas, % |
58 | 27 | 0.02 | 1.7 (0.2–12.2) | 0.62 |
| Arthritis of wrist or MCP or PIP joints, % |
79 | 36 | 0.002 | 5.3 (0.7–42.1) | 0.11 |
| Symmetrical arthritis, % | 37 | 21 | 0.27 | 10.2 (1.1–96.2) | 0.04 |
| Subcutaneous nodules, % | 5 | 3 | 0.53 | 0.5 (0.01–24) | 0.72 |
| RF ELISA positive and anti-CCP ELISA positive, % |
63 | 3 | < 0.001 | 79.6 (7.5–849.9) | < 0.001 |
For age, the odds ratio is per year-increase in age. EMS: early morning stiffness. MCP: metacarpophalangeal. PIP: proximal interphalangeal.
DISCUSSION
The ability to predict the development of RA in patients with very early inflammatory arthritis is important if therapy is to be targeted at such patients. Anti-CCP antibody has been widely reported to have a high specificity for established RA. In patients presenting with “recent” onset synovitis (duration ≤ 24 months), the specificity and sensitivity of anti-CCP antibody for the diagnosis of RA were 96% and 48%, respectively, compared with 91% and 54%, respectively, for IgM RF19. A requirement for both anti-CCP antibody and RF increased the specificity to 98%, while reducing the sensitivity to 39%. Results were similar in patients with inflammatory arthritis of up to 12 months’ duration, where the presence of anti-CCP antibody had a specificity and sensitivity of 91% and 41%, although patients with synovitis of < 6 weeks’ duration were excluded from this study24. Excluding patients with very early synovitis from studies predicting outcome is not necessarily helpful, as it is precisely these patients who may benefit most from early aggressive therapy.
This is the first study that has assessed the utility of anti-CCP antibodies in patients with very early inflammatory arthritis. Our study has a number of limitations. First, the number of patients is relatively small. Second, it is possible that treatment may have had an effect on the development of sufficient ARA criteria necessary to classify patients as having RA. Thus, for example, 17 of the 23 patients with non-RA persistent arthritis had been treated with a second-line antirheumatic drug or oral prednisolone. In theory, some of these patients may have developed criteria to allow classification as RA had these medications not been commenced. In addition, 4 of 5 patients with resolving RA and 20 of 49 patients with resolving non-RA inflammatory arthritis were treated with steroid (usually by the intraarticular route) with the steroid injection having been given > 3 months before the final clinical assessment. Again, it is possible that this therapy may have switched off the disease and prevented the development of RA, although currently there is little evidence to support such a role for steroid.
We found that seropositivity for a combination of RF and anti-CCP antibody was associated with a specificity and PPV of 100% for the diagnosis of RA. However, 21% of patients fulfilling classification criteria for RA had disease that resolved on followup. The utility of the 1987 ARA criteria in early disease has been questioned by many38. Indeed, in patients with early inflammatory polyarthritis, satisfaction of these classification criteria at baseline had a PPV for disease persistence at 3 years of only 82% by tree format and 85% by list format39. From a pragmatic view-point, clinicians are interested in predicting the development of persistent disease fulfilling criteria for RA. Analyzing such patients separately, the specificity, PPV, sensitivity, and NPV of a combination of seropositivity for RF and anti-CCP antibody for the prediction of persistent RA in our population were 97%, 86%, 63%, and 91%, respectively. These results are similar to those from other studies of patients with synovitis within the first few years of disease. This combination of autoantibodies is apparent in some patients with other established inflammatory diseases. However, in patients with very early synovitis it can be used with high specificity and PPV to identify those destined to develop RA who may be appropriate for very early intervention. Binary logistic regression analysis, including a range of baseline clinical variables and the combination of RF and anti-CCP antibodies, revealed that seropositivity for these antibodies was the strongest independent predictor of the development of persistent RA.
In our population RF alone had a relatively high specificity for the subsequent diagnosis of RA. Most other studies have reported specificities of between 80% and 90%. For example, the specificity of RF for a subsequent diagnosis of RA was 87% in a study of patients with synovitis of < 1 year duration24 and was 88% in the Austrian very early arthritis cohort40. However, in our population, combining RF with a measurement of anti-CCP antibodies afforded some additional specificity. This may be important if the presence of these autoantibodies is to influence treatment decisions in very early synovitis, where minimizing exposure of patients who are not going to develop RA to potentially toxic therapies is desirable.
Acknowledgments
Supported by the Arthritis Research Campaign (ARC) and the Dudley Group of Hospitals NHS Trust R&D Directorate.
REFERENCES
- 1.McGonagle D, Conaghan PG, O’Connor P, et al. The relationship between synovitis and bone changes in early untreated rheumatoid arthritis: a controlled magnetic resonance imaging study. Arthritis Rheum. 1999;42:1706–11. doi: 10.1002/1529-0131(199908)42:8<1706::AID-ANR20>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 2.Conaghan PG, O’Connor P, McGonagle D, et al. Elucidation of the relationship between synovitis and bone damage: A randomized magnetic resonance imaging study of individual joints in patients with early rheumatoid arthritis. Arthritis Rheum. 2003;48:64–71. doi: 10.1002/art.10747. [DOI] [PubMed] [Google Scholar]
- 3.Emery P, Salmon M. Early rheumatoid arthritis: time to aim for remission? Ann Rheum Dis. 1995;54:944–7. doi: 10.1136/ard.54.12.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Dell JR. Treating rheumatoid arthritis early: a window of opportunity? Arthritis Rheum. 2002;46:283–5. doi: 10.1002/art.10092. [DOI] [PubMed] [Google Scholar]
- 5.Boers M. Understanding the window of opportunity concept in early rheumatoid arthritis. Arthritis Rheum. 2003;48:1771–4. doi: 10.1002/art.11156. [DOI] [PubMed] [Google Scholar]
- 6.van der Heide A, Jacobs JW, Bijlsma JW, et al. The effectiveness of early treatment with “second-line” antirheumatic drugs. A randomized, controlled trial. Ann Intern Med. 1996;124:699–707. doi: 10.7326/0003-4819-124-8-199604150-00001. [DOI] [PubMed] [Google Scholar]
- 7.Boers M, Verhoeven AC, Markusse HM, et al. Randomised comparison of combined step-down prednisolone, methotrexate and sulphasalazine with sulphasalazine alone in early rheumatoid arthritis. Lancet. 1997;350:309–18. doi: 10.1016/S0140-6736(97)01300-7. [DOI] [PubMed] [Google Scholar]
- 8.Mottonen T, Hannonen P, Leirisalo-Repo M, et al. FIN-RACo Trial Group Comparison of combination therapy with single-drug therapy in early rheumatoid arthritis: a randomised trial. Lancet. 1999;353:1568–73. doi: 10.1016/s0140-6736(98)08513-4. [DOI] [PubMed] [Google Scholar]
- 9.Bathon JM, Martin RW, Fleischmann RM, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000;343:1586–93. doi: 10.1056/NEJM200011303432201. [DOI] [PubMed] [Google Scholar]
- 10.Genovese MC, Bathon JM, Martin RW, et al. Etanercept versus methotrexate in patients with early rheumatoid arthritis: two-year radiographic and clinical outcomes. Arthritis Rheum. 2002;46:1443–50. doi: 10.1002/art.10308. [DOI] [PubMed] [Google Scholar]
- 11.Landewe RB, Boers M, Verhoeven AC, et al. COBRA combination therapy in patients with early rheumatoid arthritis: long-term structural benefits of a brief intervention. Arthritis Rheum. 2002;46:347–56. doi: 10.1002/art.10083. [DOI] [PubMed] [Google Scholar]
- 12.Verstappen SM, Jacobs JW, Bijlsma JW, et al. Five-year followup of rheumatoid arthritis patients after early treatment with disease-modifying antirheumatic drugs versus treatment according to the pyramid approach in the first year. Arthritis Rheum. 2003;48:1797–807. doi: 10.1002/art.11170. [DOI] [PubMed] [Google Scholar]
- 13.Mottonen T, Hannonen P, Korpela M, et al. Delay to institution of therapy and induction of remission using single-drug or combination disease-modifying antirheumatic drug therapy in early rheumatoid arthritis. Arthritis Rheum. 2002;46:894–8. doi: 10.1002/art.10135. [DOI] [PubMed] [Google Scholar]
- 14.Tunn EJ, Bacon PA. Differentiating persistent from self-limiting symmetrical synovitis in an early arthritis clinic. Br J Rheumatol. 1993;32:97–103. doi: 10.1093/rheumatology/32.2.97. [DOI] [PubMed] [Google Scholar]
- 15.Green M, Marzo-Ortega H, McGonagle D, et al. Persistence of mild, early inflammatory arthritis: the importance of disease duration, rheumatoid factor, and the shared epitope. Arthritis Rheum. 1999;42:2184–8. doi: 10.1002/1529-0131(199910)42:10<2184::AID-ANR20>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 16.Visser H, le Cessie S, Vos K, Breedveld FC, Hazes JM. How to diagnose rheumatoid arthritis early: a prediction model for persistent (erosive) arthritis. Arthritis Rheum. 2002;46:357–65. doi: 10.1002/art.10117. [DOI] [PubMed] [Google Scholar]
- 17.Nienhuis RL, Mandema E. A new serum factor in patients with rheumatoid arthritis; the antiperinuclear factor. Ann Rheum Dis. 1964;23:302–5. doi: 10.1136/ard.23.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Majka DS, Holers VM. Can we accurately predict the development of rheumatoid arthritis in the preclinical phase? Arthritis Rheum. 2003;48:2701–5. doi: 10.1002/art.11224. [DOI] [PubMed] [Google Scholar]
- 19.Schellekens GA, Visser H, de Jong BA, et al. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000;43:155–63. doi: 10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Kroot EJ, de Jong BA, van Leeuwen MA, et al. The prognostic value of anti-cyclic citrullinated peptide antibody in patients with recent-onset rheumatoid arthritis. Arthritis Rheum. 2000;43:1831–5. doi: 10.1002/1529-0131(200008)43:8<1831::AID-ANR19>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Meyer O, Labarre C, Dougados M, et al. Anticitrullinated protein/peptide antibody assays in early rheumatoid arthritis for predicting five year radiographic damage. Ann Rheum Dis. 2003;62:120–6. doi: 10.1136/ard.62.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill JA, Southwood S, Sette A, Jevnikar AM, Bell DA, Cairns E. Cutting edge: the conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC Class II molecule. J Immunol. 2003;171:538–41. doi: 10.4049/jimmunol.171.2.538. [DOI] [PubMed] [Google Scholar]
- 23.Lee DM, Schur PH. Clinical utility of the anti-CCP assay in patients with rheumatic diseases. Ann Rheum Dis. 2003;62:870–4. doi: 10.1136/ard.62.9.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldbach-Mansky R, Lee J, McCoy A, et al. Rheumatoid arthritis associated autoantibodies in patients with synovitis of recent onset. Arthritis Res. 2000;2:236–43. doi: 10.1186/ar93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jansen AL, van der Horst-Bruinsma IE, van Schaardenburg D, van de Stadt RJ, de Koning MH, Dijkmans BA. Rheumatoid factor and antibodies to cyclic citrullinated peptide differentiate rheumatoid arthritis from undifferentiated polyarthritis in patients with early arthritis. J Rheumatol. 2002;29:2074–6. [PubMed] [Google Scholar]
- 26.Kurki P, Aho K, Palosuo T, Heliovaara M. Immunopathology of rheumatoid arthritis. Antikeratin antibodies precede the clinical disease. Arthritis Rheum. 1992;35:914–7. doi: 10.1002/art.1780350810. [DOI] [PubMed] [Google Scholar]
- 27.Rantapaa-Dahlqvist S, de Jong BA, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–9. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 28.Nielen MM, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50:380–6. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- 29.Kraan MC, Versendaal H, Jonker M, et al. Asymptomatic synovitis precedes clinically manifest arthritis. Arthritis Rheum. 1998;41:1481–8. doi: 10.1002/1529-0131(199808)41:8<1481::AID-ART19>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 30.Soden M, Rooney M, Cullen A, Whelan A, Feighery C, Bresnihan B. Immunohistological features in the synovium obtained from clinically uninvolved knee joints of patients with rheumatoid arthritis. Br J Rheumatol. 1989;28:287–92. doi: 10.1093/rheumatology/28.4.287. [DOI] [PubMed] [Google Scholar]
- 31.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 32.Kingsley G, Sieper J. Third International Workshop on Reactive Arthritis. 23-26 September 1995, Berlin, Germany. Report and abstracts. Ann Rheum Dis. 1996;55:564–84. doi: 10.1136/ard.55.8.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gladman DD, Shuckett R, Russell ML, Thorne JC, Schachter RK. Psoriatic arthritis (PSA) — an analysis of 220 patients. Q J Med. 1987;62:127–41. [PubMed] [Google Scholar]
- 34.The International Study Group for Behcet’s disease Evaluation of diagnostic (‘classification’) criteria in Behcet’s disease — towards internationally agreed criteria. Br J Rheumatol. 1992;31:299–308. [PubMed] [Google Scholar]
- 35.Bohan A, Peter JB. Polymyositis and dermatomyositis. N Engl J Med. 1975;292:344–7. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 36.Bohan A, Peter JB. Polymyositis and dermatomyositis. N Engl J Med. 1975;292:403–7. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 37.Harrison BJ, Symmons DP, Brennan P, Barrett EM, Silman AJ. Natural remission in inflammatory polyarthritis: issues of definition and prediction. Br J Rheumatol. 1996;35:1096–100. doi: 10.1093/rheumatology/35.11.1096. [DOI] [PubMed] [Google Scholar]
- 38.Symmons DP, Hazes JM, Silman AJ. Cases of early inflammatory polyarthritis should not be classified as having rheumatoid arthritis. J Rheumatol. 2003;30:902–4. [PubMed] [Google Scholar]
- 39.Harrison BJ, Symmons DP, Barrett EM, Silman AJ. The performance of the 1987 ARA classification criteria for rheumatoid arthritis in a population based cohort of patients with early inflammatory polyarthritis. J Rheumatol. 1998;25:2324–30. [PubMed] [Google Scholar]
- 40.Machold KP, Stamm TA, Eberl GJM, et al. Very recent onset arthritis — clinical, laboratory and radiological findings during the first year of disease. J Rheumatol. 2002;29:2278–87. [PubMed] [Google Scholar]