Abstract
Several of the 28 mammalian transient receptor potential (TRP) channel subunits are expressed throughout the alimentary canal where they play important roles in taste, chemo- and mechanosensation, thermoregulation, pain and hyperalgesia, mucosal function and homeostasis, control of motility by neurons, interstitial cells of Cajal and muscle cells, and vascular function. While the implications of some TRP channels, notably TRPA1, TRPC4, TRPM5, TRPM6, TRPM7, TRPV1, TRPV4, and TRPV6, have been investigated in much detail, the understanding of other TRP channels in their relevance to digestive function lags behind. The polymodal chemo- and mechanosensory function of TRPA1, TRPM5, TRPV1 and TRPV4 is particularly relevant to the alimentary canal whose digestive and absorptive function depends on the surveillance and integration of many chemical and physical stimuli. TRPV5 and TRPV6 as well as TRPM6 and TRPM7 appear to be essential for the absorption of Ca2+ and Mg2+, respectively, while TRPM7 appears to contribute to the pacemaker activity of the interstitial cells of Cajal, and TRPC4 transduces smooth muscle contraction evoked by muscarinic acetylcholine receptor activation. The implication of some TRP channels in pathological processes has raised enormous interest in exploiting them as a therapeutic target. This is particularly true for TRPV1, TRPV4 and TRPA1, which may be targeted for the treatment of several conditions of chronic abdominal pain. Consequently, blockers of these TRP channels have been developed, and their clinical usefulness has yet to be established.
Keywords: Chemosensation, mechanosensation, inflammation, intestinal motility, mechanosensation, pain, taste, TRPA1, TRPV1, TRPV4
INTRODUCTION
The physiological function of the digestive tract depends on the appropriate implementation of a complex set of mechanisms with opposite aims: ingestion and digestion of food and absorption of nutrients and water, on the one hand, as well as defence against toxins, antigens and pathogens and the elimination of indigestible waste, on the other hand. To meet with these challenges, an elaborate system of sensory systems, monitoring processes and feedback mechanisms has been developed, among which transient receptor potential (TRP) channels take a prominent position. Although TRP channels contribute to sensory transduction in every region of the body, they play a particular role in the alimentary canal. This special position derives from their function as molecular sensors for distinct chemical and physical modalities that are rather specific to the digestive system [1,2].
Currently, some 28 different TRP subunit genes have been identified in mammals, comprising 6 families. The TRP channel subunits are made of 6 transmembrane domains with a pore between transmembrane domains 5 and 6 [3]. Members of 5 subunit families, specifically of the vanilloid TRP (TRPV), melastatin TRP (TRPM), ankyrin TRP (TRPA), polycystin TRP (TRPP) and canonical or classical TRP (TRPC) family, are relevant to spice sensing, chemo-, thermo- and/or mechanosensation. This article focusses on recent progress in the understanding of the functional roles of TRP channels in the digestive tract, with particular emphasis on taste, chemical and mechanical nociception, and visceral hyperalgesia. The implications of particular TRP channels in Ca2+ and Mg2+ homeostasis, in the pacemaker activity of the interstitial cells of Cajal (ICCs) and in gastrointestinal (GI) smooth muscle function are also covered.
EXPRESSION AND FUNCTION OF TRP CHANNELS IN THE DIGESTIVE TRACT
TRPV1 channels
Presence of TRPV1 in the gut
As has been extensively reviewed before [2,4,5], the major cellular sources of TRPV1 in the rat, guinea-pig and mouse alimentary canal are spinal and vagal primary afferent neurons. Both in the dorsal root ganglion (DRG) and nodose ganglion cells, TRPV1 is restricted to small and medium-sized somata that are known to give rise to unmyelinated and thinly myelinated fibres. Although virtually all tissues of the body are supplied by TRPV1-positive nerve fibres, TRPV1-immunoreactive fibres are considerably more prevalent in visceral than in somatic afferent neurons. Thus, the majority of nodose ganglion neurons projecting to the stomach and of DRG neurons projecting to the gut express TRPV1 [6-10], while only 32 % of the vagal afferent neurons supplying the mouse jejunum contain TRPV1 [11]. There are both regional and species differences in the chemical coding of primary afferent neurons expressing TRPV1. Thus, calcitonin gene-related peptide (CGRP), substance P, somatostatin and other neuropeptides are messenger molecules characteristic of capsaicin-sensitive TRPV1-positive DRG neurons [2,4,5], while CGRP is absent from vagal afferent neurons containing TRPV1 [11]. Within the rodent and human gut, TRPV1-positive nerve fibres occur in the musculature, enteric nerve plexuses, arterioles and mucosa.
In addition to its prominent location in extrinsic sensory neurons, TRPV1 has also been described in gastrin and parietal cells of the stomach as well as in epithelial cells of the oesophageal, gastric and small intestinal mucosa [12-17]. Some authors have also found expression of TRPV1 in neurons of the guinea-pig, porcine and human enteric nervous system [12,18-20] while other authors failed to confirm this location, given that TRPV1 messenger ribonucleic acid (RNA) disappears from the rat stomach following extrinsic denervation [7]. The chemical coding of TRPV1-positive DRG neurons innervating the mouse gut is distinct from that of enteric neurons [21]. Enteric neurons that issue projections from the rat colon to the spinal cord are likewise TRPV1-negative [22].
Local roles of TRPV1 in the GI tract
Through release of peptide transmitters in the periphery, TRPV1-expressing sensory nerve fibres can modify GI vascular, immune and smooth muscle functions (Figure 1). Following tissue irritation or injury, some of these reactions (e.g., vasodilatation and plasma protein extravasation) contribute to the process of neurogenic inflammation [23]. The neuropeptides involved in the efferent-like mode of operation include CGRP, somatostatin and the tachykinins substance P and neurokinin A [4,24,25]. Administration of capsaicin to the oesophageal, gastric and intestinal mucosa increases mucosal blood flow, a response which is mimicked by exposure to excess acid [26]. The acid- and carbon dioxide-evoked hyperaemia in the oesophageal and duodenal mucosa is inhibited by the TRPV1 antagonist capsazepine, which indicates that acid activates TRPV1 on sensory nerve fibres which release the vasodilator peptide CGRP [27]. Through this type of mechanism TRPV1-positive sensory nerve fibres are able to protect the oesophageal, gastric and intestinal mucosa from a variety of injurious insults [26].
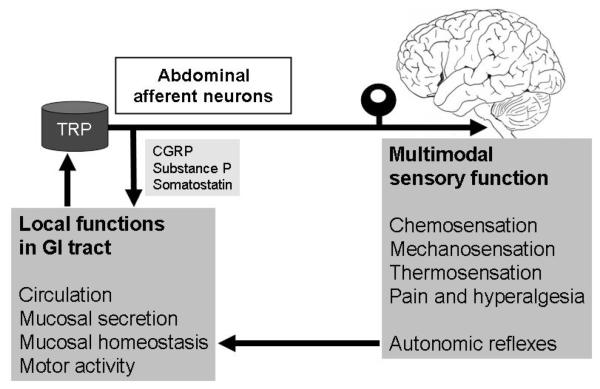
Figure 1.
Dual functional implications of TRP-expressing abdominal afferent neurons. Following stimulation via activation of TRP channels, afferent nerve fibres release neuropeptides from their peripheral endings, whereby they regulate vascular function, secretory processes, mucosal homeostasis and motor activity. Through their afferent function, TRP-expressing neurons contribute to chemosensation, mechanosensation, thermosensation, pain and hyperalgesia and elicit autonomic reflexes.
Apart from protecting the GI mucosa [26,28,29], TRPV1 activation has been found to exacerbate inflammation in certain models of pancreatitis, ileitis and colitis [4]. In the mouse gastric mucosa, ethanol-induced injury appears to involve TRPV1-mediated release of substance P and subsequent formation of reactive oxygen species [30]. Emerging evidence indicates that TRPV1 contributes to pancreatic islet inflammation associated with type I diabetes and, in addition, plays a role in insulin-dependent glucose regulation, type II diabetes, adipogenesis and obesity [31-34]. The mechanisms whereby stimulation of TRPV1, under some conditions, reduces and, under other conditions, exaggerates tissue inflammation and injury have not yet been fully elucidated. One explanation is that the peptide mediators of TRPV1-positive afferent neurons cover a wide spectrum of actions: Tachykinins facilitate inflammation, while CGRP promotes vasodilatation but not necessarily inflammation, and somatostatin is capable of inhibiting inflammatory processes [24-26].
The messengers released from capsaicin-sensitive afferent nerve endings in the gut act on many targets including the enteric nervous system, GI smooth muscle and epithelium to modify motility and secretion [4,26,35-37]. Tachykinins and adenosine triphosphate stimulate motor activity, while CGRP, vasoactive intestinal polypeptide and nitric oxide account for motor inhibition due to TRPV1 activation [36-38]. Local motor effects mediated by TRPV1 may become operative when the GI tract is disturbed by endogenous or exogenous irritants. For instance, TRPV1 and substance P are involved in the acid-evoked contraction of opossum oesophageal longitudinal muscle [39]. The circular muscle of the isolated human gut is powerfully relaxed by capsaicin, and this effect appears to be mediated by nitric oxide and, to some extent, vasoactive intestinal polypeptide [36]. Despite these motor effects of TRPV1 stimulation in vitro, the motor activity of the small and large intestine of experimental animals is maintained at a physiological level following functional ablation of capsaicin-sensitive afferent neurons [36]. Likewise, no obvious changes in GI motor function have thus far been reported to occur in mice deficient in TRPV1.
Ingestion of capsaicin by humans increases amplitude and velocity of oesophageal pressure waves, decreases proximal gastric tone, inhibits phasic contractions of the proximal stomach and inhibits gastric emptying, but does not significantly alter orocaecal transit time [40,41]. The capsaicin-induced improvement of oesophageal motility has been observed in healthy volunteers as well as in patients with gastro-oesophageal reflux disease [40,42]. The relevance of TRPV1 to GI motor control may be most pronounced under pathopysiological conditions, both locally within the gut and via activation of sympathetic reflexes, resulting in inhibition of GI transit (ileus) following laparotomy or peritoneal irritation [26,36,43]. This contention is also borne out by the observation that the gastroparesis associated with experimental colitis is relieved by TRPV1 blockers [44].
There is some evidence that mucosal functions in the GI tract may be under the direct control of TRPV1 channels expressed by epithelial cells. For instance, acid- or capsaicin-evoked stimulation of epithelial TRPV1 in the feline and human oesophagus induces the formation of platelet-activating factor, which in the in vivo setting may give rise to oesophagitis [16]. Exposure of human isolated antral glands to capsaicin causes release of gastrin and somatostatin, an effect that is thought to be mediated by TRPV1 expressed by gastrin cells and that is blunted by a chilli-rich diet ingested for 3 weeks [17].
Roles of TRPV1 in abdominal pain and hypersensitivity
Activation of TRPV1 on afferent neurons innervating the gut elicits pain in humans and pain-related behaviour in rodents [4,5,45]. The available evidence indicates that TRPV1 may be involved in pain and hyperalgesia throughout the alimentary canal from the mouth to the anus. In addition, TRPV1-expressing vagal afferents in the oesophagus may contribute to the cough evoked by gastro-oesophageal reflux of gastric contents [46]. Intraoesophageal instillation of capsaicin in human volunteers evokes symptoms of heartburn, while the sensitivity to balloon distension or acid exposure remains unchanged [47]. Intragastric administration of capsaicin in humans increases the sensitivity to proximal gastric distension [41], and ingestion of capsaicin capsules induces gastric sensations of pressure, heartburn and warmth [48]. Infusion of capsaicin into the proximal small intestine of human volunteers likewise evokes sensations of pain, cramps, pressure, warmth and nausea [48,49]. The capsaicin-induced sensations are most intense in the duodenum and appear to decrease along the intestine [48] although local capsaicin instillation in ileostomy and colostomy patients is also painful [50,51]. Ingestion of capsaicin for 7 days leads to a decrease in jejunal mechanosensation and to an increase in the jejunal sensitivity to capsaicin [52], and oral chilli intake for 3 days has been reported to increase rectal sensitivity to urgency [53]. In contrast, ingestion of capsaicin (0.25 mg) capsules three times per day for 4 weeks causes desensitization of the duodenum to both acute capsaicin administration and balloon distension [54].
TRPV1 can be sensitized by many proalgesic pathways [4,45,55,56], and there is increasing evidence that GI inflammation causes chemical and mechanical hyperalgesia at least in part by upregulation and sensitization of TRPV1. Nerve growth factor, various inflammatory mediators as well as endocrine factors of the GI mucosa such as 5-hydroxytryptamine (5-HT) are thought to be involved in this process. Importantly, TRPV1 in afferent neurons has also been found to be upregulated in the absence of overt inflammation as is typical of functional GI disorders [2]. This is true for patients with irritable bowel syndrome in which the increased density of TRPV1 in the rectosigmoid colon correlates with pain severity [57]. Non-erosive reflux disease [58], idiopathic rectal hypersensitivity and faecal urgency [20] are other instances of TRPV1 upregulation in the absence of inflammation. Thus, a proportion of patients with functional dyspepsia are more responsive to ingestion of capsaicin capsules than healthy controls [59]. A role of TRPV1 in functional dyspepsia is also suggested by the beneficial effect of a 5 week treatment with capsaicin-containing capsules [60]. Furthermore, the homozygous G315C polymorphism of the TRPV1 gene has been found to be inversely related with symptom severity in functional dyspepsia patients [61]. Patients suffering from diarrhoea-predominant irritable bowel syndrome exhibit hypersensitivity to the painful and burning sensations which chilli elicits in the GI tract [62]. Likewise, TRPV1 has a bearing on post-inflammatory colonic hyperalgesia in rodents, as upregulation of TRPV1 expression and function persists long after the initial inflammatory insult has subsided [63-65].
TRPV1 contributes not only to chemical nociception but also to inflammatory mechanical hyperalgesia in the GI tract. In pelvic afferents innervating the mouse colon, TRPV1 is preferentially expressed by mechanoreceptors that respond to distension with a low frequency of firing, the distension responses of these fibres being sensitized by capsaicin or acidosis [66]. Pelvic afferents innervating the smooth muscle and myenteric plexus of the mouse rectum are likewise sensitive to low-threshold distension and capsaicin [67]. Accordingly, capsaicin enhances the visceromotor response to colorectal distension (an indirect measure of abdominal pain), this effect being inhibited by the TRPV1 blocker SB-705498 [68]. Acute stress exposure of adult rats that have been subjected to maternal separation as neonates causes hypersensitivity to colorectal distension, which is reversed by TRPV1 blockade [69]. Importantly, TRPV1 blockade also prevents the development of mechanical hyperalgesia, that is evoked by repetitive colorectal distension in rats, but does not cause hypoalgesia [70]. The molecular implication of TRPV1 in mechanical pain is not fully understood because the mechanosensitivity of TRPV1 channels awaits to be characterized.
It should not go unnoticed that activation of TRPV1 in the brainstem has an antiemetic effect [71,72], although a pathophysiological role of TRPV1 in emesis has not yet been established.
Thermosensation and thermoregulation by abdominal TRPV1
The fact that TRPV1 is a heat sensor explains why the sensation caused by capsaicin is described as “hot” and “burning”. Although capsaicin and related TRPV1 agonists evoke a thermoregulatory response [73,74], TRPV1 knockout mice have a normal body temperature and do not seem to have a deficit in heat sensing, which is probably due to developmental compensations in heat sensing [2], given that many TRPV1 blockers cause substantial hyperthermia in mice, rats, dogs, monkeys and humans [75-77]. The hyperthermic action of TRPV1 blockers involves cutaneous vasoconstriction and shivering-related thermogenesis, but not warmth-seeking behaviour, and is inhibited by the antipyretic drug acetaminophen [75,76]. Further analysis has shown that the rise of body temperature results from blockade of the proton mode of TRPV1 activation [75,77]. It would appear, therefore, that the body temperature is tonically suppressed by a low tissue pH [77] within the abdominal cavity [75] in which the stomach and colon have an acidic environment [78].
TRPV2 and TRPV3 channels
TRPV2, which is typically activated by temperatures above 52 °C, has been localized to rodent DRG and nodose ganglion neurons supplying the GI tract [79-81]. In contrast to TRPV1, TRPV2 occurs primarily in medium and large diameter afferent neurons [80]. In the rat and guinea-pig small intestine, TRPV2 is not only found in fibres within the muscularis and myenteric plexus but also in myenteric cell bodies [79,80].
TRPV3 is highly expressed by epithelial cells in the nose and tongue [82] as well as in superficial epithelial cells of the mouse distal colon, but not in the superficial epithelial cells of the stomach, duodenum and proximal colon [83]. It likewise occurs in DRG and nodose ganglion neurons, but retrograde labelling failed to identify TRPV3 in vagal afferent neurons supplying the mouse stomach [80,81]. However, the muscle and mucosa of the mouse stomach and small intestine contain TRPV3 messenger RNA [80].
While any implication of TRPV2 and TRPV3 in GI function awaits to be explored, TRPV3 is a sensor for many spices [82] including carvacrol (oregano), eugenol, thymol (thyme), vanillin (vanilla), camphor and menthol (mint) [2,45].
TRPV4 channels
Presence of TRPV4 in the gut
TRPV4 is present in the nodose ganglion, DRG, stomach, small intestine and colon of rodents [80,81,84,85]. Retrograde labelling revealed that vagal afferent neurons projecting to the mouse forestomach express TRPV4 and that, in these neurons, TRPV4 is coexpressed with TRPV1, TRPV2 and TRPA1 to various degrees [80]. TRPV4 has also been localized to mesenteric and pelvic afferent neurons innervating the murine and human intestine [84-86]. The expression of TRPV4 in spinal afferent neurons exhibits a pronounced regional difference, as TRPV4 is particularly abundant in DRG neurons supplying the colon, in which it is coexpressed with CGRP [84-86]. In the human colon, TRPV4 immunoreactivity is found in fine nerve fibres associated with blood vessels in the submucosa and serosa, while the myenteric plexus, the circular and the longitudinal smooth muscle are largely negative [84]. In the mouse colon, TRPV4 also occurs in brush-bordered epithelial cells, but not in mucus-secreting epithelial cells, as well as in unidentified cells of the submucosal and muscular layers [85].
Functional implications of TRPV4 in the gut
The sensory modalities of TRPV4 include strong acidosis, hypo-osmolarity, warmth, mechanical stimuli such as distension of the gut, 5,6-epoxyeicosatrienoic acid (an endogenously formed metabolite of arachidonic acid), and the synthetic phorbol ester 4-α-phorbol 12,13-didecanoate [87]. In the colon, TRPV4 has turned out to play a major role in mechanical pain and hyperalgesia. TRPV4 agonists excite colonic afferent neurons [86] and enhance the mechanosensory responses of colonic serosal and mesenteric afferent nerve fibres, while the mechanosensitivity of these high threshold afferents is substantially attenuated in TRPV4 knockout mice [84]. The TRPV4 agonist-induced sensitization of colonic afferent nerve fibres goes hand in hand with mechanical hyperalgesia [85,86]. Vice versa, TRPV4 knockout or intravertebral pretreatment of mice with small interfering RNA (siRNA) specific to TRPV4 reduces the visceromotor response to colonic distension in the noxious range [84,85]. This specific contribution to high threshold mechanosensory function makes TRPV4 currently the only nociceptor-specific TRP channel in the gut [5].
TRPV4 and protease-activated receptor-2 (PAR-2) are colocalized in spinal afferent neurons, in which these two entities interact with each other in causing mechanical hyperalgesia in the mouse colon [85,86]. Thus, the excitatory effect of TRPV4 stimulation on firing of colonic afferents is increased by a PAR-2 agonist [86], and a subnociceptive dose of a PAR-2 agonist is able to sensitize colonic afferents to a subnociceptive dose of a TRPV4 agonist [85]. Activation of PAR-2 likewise evokes discharge of action potentials in colonic afferent nerve fibres and enhances the visceromotor response to colorectal distension. These effects of PAR-2 agonism are absent in TRPV4 knockout mice as well as after pretreatment with TRPV4 siRNA [85,86]. It follows that TRPV4 is involved in mechanical nociception under basal conditions and mediates the mechanical hyperalgesia evoked by PAR-2 agonism. However, this interaction is not confined to PAR-2, given that 5-HT and histamine can enhance the expression of TRPV4 in DRG neurons projecting to the mouse colon and are able to enforce the ability of TRPV4 agonism to induce mechanical hyperalgesia [88]. Importantly, the colonic hypersensitivity to colorectal distension evoked by 5-HT and histamine is attenuated by TRPV4 siRNA [88].
As TRPV4 is upregulated in inflammatory bowel disease, and serosal blood vessels in tissues obtained from patients with active colitis are more densely innervated by TRPV4-positive nerve fibres than in healthy controls [84] it would appear that TRPV4 is a novel therapeutic target in patients with abdominal pain.
TRPV5 and TRPV6 channels
TRPV5 and TRPV6 are typically expressed in epithelia including the mucosa of the small intestine where, in particular, TRPV6 is involved in Ca2+ absorption [89]. Both channels are targets of calciotropic hormone regulation, including vitamin D and parathyroid hormone. TRPV5 knockout mice upregulate their intestinal TRPV6 expression to compensate for the negative Ca2+ balance caused by the loss of TRPV5-mediated Ca2+ reabsorption in the kidney [89]. In contrast, TRPV6 knockout mice do not display any compensatory mechanism, which results in secondary hyperparathyreodism [89].
TRPA1 channels
Presence of TRPA1 in the digestive system
TRPA1 is expressed by trigeminal ganglion, nodose ganglion and DRG neurons [80,81,90-96]. In the DRG neurons, especially in those projecting to the gut, TRPA1 is preferentially expressed by small and medium diameter cell bodies in which it is colocalized with TRPV1 and CGRP. Similarly, TRPA1 on nodose ganglion neurons in the guinea-pig is primarily associated with small to medium diameter somata and co-expressed with PAR-2 [95]. Within the GI tract, TRPA1 has been found in the stomach, small intestine and colon of the rat and mouse, the major source being peripheral nerve fibres of DRG and nodose ganglion neurons [80,93,94,96,97]. In addition, TRPA1 occurs in neurons of the human and mouse enteric nervous system [91,97], in 5-hydroxytryptamine (5-HT)-releasing enterochromaffin cells, and in cholecystokinin-releasing endocrine cells of the human, rat and mouse GI mucosa [98,99]. In the dog, TRPA1 is strongly expressed in the stomach, pancreas, small intestine and colon [100].
Functional implications of TRPA1 in the alimentary canal
TRPA1 was originally identified as a cold-activated channel but later turned out to be a multimodal sensor for various spices and an extensive list of irritant chemicals. Most of these chemicals are reactive electrophiles which need to be recognized and avoided because of their potential to damage tissues through modification of nucleic acids, proteins and other biomolecules [101]. Its sensory modalities place TRPA1 specifically in a position (i) to taste spicy compounds present in mustard, horseradish, wasabi, garlic, onion, cinnamon, ginger, oregano, wintergreen and clove, (ii) to detect toxic environmental stimuli such as nicotine, formaldehyde, acrolein, iodoacetamide and methyl p-hydroxybenzoate, an antibacterial added to food, drug and cosmetic products, and (iii) to survey the alimentary canal for potentially deleterious conditions arising from the presence of alkalosis, H2S, oxidative insults (4-hydroxy-2-nonenal, H2O2, acetaldehyde). In addition, there is evidence that TRPA1 contributes to mechanosensation in the gut.
TRPA1 agonists such as allyl isothiocyanate (AITC) excite vagal afferent C-fibres, but not Aδ-fibres, in the guinea-pig oesophagus [102] and colonic afferent neurons in the mouse [96]. Intracolonic administration of AITC and other TRPA1 agonists to rodents activates spinal afferent neurons, as shown by c-Fos expression in the spinal cord [96,103], and causes symptoms of pain as deduced from the observation of a visceromotor response [92]. These nociceptive reactions to TRPA1 stimulation in the gut are inhibited by the TRPA1 blocker HC-030031 [103], by intrathecal pretreatment with a TRPA1 antisense oligodeoxynucleotide [92] and TRPA1 gene knockout [93,96]. In addition to stimulating afferent neurons, TRPA1 agonists sensitize colonic afferents to mechanical stimulation and enhance the visceromotor response to colorectal distension, these effects being prevented by TRPA1 gene knockout or HC-030031 [93,96].
In the mouse GI tract, TRPA1 is required for normal mechano- and chemosensory function in distinct subsets of vagal, splanchnic and pelvic afferent neurons [93]. Spike discharges in response to punctate mechanical stimulation in splanchnic afferents with receptive fields in the mesentery and serosa and in pelvic afferents with receptive fields in the mucosa, muscularis and serosa are attenuated in TRPA1 knockout mice, while the response of pelvic afferents to muscle stretch remains unabated [93]. Despite these findings there is at present uncertainty as to whether TRPA1 contributes to normal mechanonociception in the colon, given that the visceromotor response to noxious colorectal distension has been reported to be reduced [93] or to remain unchanged [96] in TRPA1 knockout mice. In contrast, there is pharmacological evidence that in the rat stomach TRPA1 plays a role in mechanical nociception, because the pseudoaffective pain reaction (contraction of the acromiotrapezius muscle) to gastric distension is attenuated by a TRPA1 antisense oligodeoxynucleotide and HC-030031 [94].
Increasing evidence indicates that TRPA1 contributes to the mechanical hypersensitivity that is associated with GI inflammation. Thus, colitis induced by trinitrobenzene sulfonic acid (TNBS) in rats causes upregulation of TRPA1 in DRG neurons innervating the rat colon and enhances the visceromotor response to colorectal distension, this effect being reduced by intrathecal pretreatment with a TRPA1 antisense oligodeoxynucleotide [92]. Likewise, the effect of TNBS-induced colitis to enhance spinal neuron activation and the visceromotor pain reaction due to colorectal distension is absent in TRPA1 knockout mice [96]. This observation is consistent with the ability of TNBS-induced colitis to amplify the ability of TRPA1 agonists to sensitize mechanosensitive afferents in the splanchnic and pelvic nerves [93].
The inflammation-evoked gain in TRPA1 function is likely to depend on several proinflammatory mediators including bradykinin and endogenous PAR-2 agonists. Pharmacological and gene knockout experiments indicate that TRPA1 mediates the bradykinin-induced mechanical sensitization of splanchnic afferents innervating the colonic serosa of the mouse as well as the bradykinin-evoked mechanical hypersensitivity of vagal afferent neurons of the guinea-pig oesophagus, while the activation of vagal and splanchnic afferents by bradykinin is independent of TRPA1 [93,102]. Mast cell activation and activation of PAR-2 are further stimuli that cause mechanical hypersensitivity of vagal afferent neurons in the guinea-pig oesophagus via a TRPA1-dependent mechanism [95]. Similarly, the ability of a PAR-2 agonist to enhance the visceromotor response to colorectal distension is abrogated by TRPA1 gene deletion [96], while the effect of a PAR-2 agonist to stimulate colonic afferents in the splanchnic nerves is unaltered in TRPA1 knockout mice [93].
In addition to mechanical hypersensitivity, TRPA1 contributes to inflammation-evoked chemical hypersensitivity in the gut. Induction of mild colitis with dextran sulphate sodium causes colonic hypersensitivity to AITC as revealed by increased expression of c-Fos in the mouse spinal cord, this effect being prevented by HC-030031 [103]. Similarly, TNBS-induced colitis in the rat amplifies the visceromotor response to intracolonic AITC in a TRPA1 antisense oligodeoxynucleotide-sensitive manner [92]. TNBS-evoked colitis is associated with an upregulation of TRPA1 in DRG neurons supplying the colon [92], an effect that may involve neurotrophic factors formed under conditions of inflammation [91].
Within the GI tract, activation of TRPA1 has been found to modify both epithelial and muscular functions. Thus, stimulation of TRPA1 on the mouse neuroendocrine cell line STC-1 causes cell activation and release of cholecystokinin [98], and activation of TRPA1 on rat enterochromaffin cells leads to release of 5-HT [99]. In vivo, AITC has a protective effect against experimentally induced gastric lesions in the rat, an effect that appears to involve endogenous prostaglandins [104].
The effects of TRPA1 stimulation on GI motor activity are region- and species-dependent. Although intracolonic AITC (2.5 %) does not alter compliance of the mouse colon in vivo [96], isolated segments of the mouse colon, but not small intestine, contract in response to AITC, the reponse being depressed by tetrodotoxin [97]. In the guinea-pig ileum, AITC is able to evoke contraction via a mechanism that involves 5-HT and activation of 5-HT3 receptors, which suggests that the AITC-induced release of 5-HT from enterochromaffin cells has an impact on the control of intestinal motility [99]. While the effect of TRPA1 stimulation in the intestine is to facilitate motor activity, gastric emptying in the rat in vivo is delayed by TRPA1 agonists via an action involving 5-HT and 5-HT3 receptors [105]. Administration of TRPA1 agonists to the dog in vivo stimulates motor activity in the gastric antrum and jejeunum and elicits giant migrating contractions in the colon [100]. The prokinetic effects of TRPA1 agonists could be utilized in the therapy of motor stasis in the gut, given that in mice AITC is able to antagonize atonic constipation induced by clonidine as well as spastic constipation induced by loperamide [106].
The emerging pathophysiological implications of TRPA1 suggest that TRPA1 blockers hold therapeutic potential. TRPA1 blockers such as HC-030031 have already been characterized, and it awaits to be explored whether interference with TRPA1 disturbs temperature homeostasis, as it has been found with TRPV1 blockers.
TRPM4 and TRPM5 channels
TRPM4 and TRPM5 messenger RNA is abundantly expressed in the human digestive system [107]. Of particular note is that TRPM5 occurs not only on the receptor cells of the lingual taste buds but also on chemosensory cells throughout the gut. Thus, TRPM5 is present in the stomach, small intestine and colon of mice and humans, the channel occurring in solitary brush (also termed tufted or caveolated) cells at the surface epithelium of the small and large intestine and in endocrine cells of the duodenal glands [103,107,108-112]. In addition, TRPM5 is present in pancreatic islets of the mouse in which it is expressed by the insulin-secreting β cells [113].
In the taste buds of the tongue, the Ca2+-gated TRPM5 channel contributes to the signal transduction of sweet, umami and bitter tastants [108,114]. These tastants bind to G-protein-coupled taste receptors which initiate a second-messenger signalling cascade that leads to an increase in the intracellular Ca2+ concentration which gates TRPM5 and causes depolarization of the taste cells and transmission to gustatory afferent neurons [115]. The expression of TRPM5 in chemosensory cells of the GI tract is thought to indicate that TRPM5 may exert taste functions throughout the alimentary canal and contribute to the surveillance of the chemical composition in its lumen. In keeping with this role, the expression of TRPM5 in the upper small intestine is inversely correlated with the blood glucose concentration in type 2 diabetes subjects [112]. In the pancreatic islets TRPM5 regulates the glucose-dependent release of insulin, given that the effect of glucose to release insulin is significantly reduced in TRPM5 knockout mice, which causes an impaired glucose tolerance in these animals [113]. In addition, TRPM5-positive taste cells in the intestine may play local effector roles as they release β-endorphin into the intestinal lumen when they are exposed to hyperosmolar solutions or glucose [111].
TRPM6 and TRPM7 channels
TRPM6 occurs along the gut, and both TRPM6 and TRPM7 have been found in the mouse colon [107,116]. Apart from epithelial cells, TRPM7 is found in ICCs of the myenteric plexus and circular muscle throughout the GI tract of mice and humans [117,118]. TRPM6 and TRPM7 possess an atypical α-kinase domain and are involved in Mg2+ homeostasis, given that genetic and functional data indicate that both channel kinases are involved in the cellular Mg2+ absorption in the colonic mucosa and in the reabsorption of Mg2+ in the kidney [119]. Hypomagnesaemia associated with dietary Mg2+ restriction in mice causes upregulation of TRPM6 expression both in the intestine and kidney [116]. TRPM6 and TRPM7 can form heteromeric ion channels, but there is still controversy as to whether TRPM6 can also function on its own and TRPM7 is required for trafficking of TRPM6 to the plasma membrane [119].
Electrophysiological, molecular biological, and immunohistochemical experiments indicate that TRPM7 contributes to the nonselective cation current that underlies the pacemaker activity of ICCs in the GI tract [117]. Thus, the electrophysiological and pharmacological properties of the nonselective cation current in ICCs of the mouse are identical to those of TRPM7, and the pacemaker activity of the ICCs is inhibited by TRPM7-specific siRNA [117]. TRPM7 expressed on ICCs is also likely to contribute to pacemaker activity in the human gut, since slow waves in the isolated muscle of the human small and large intestine are attenuated by blockade of TRPM7 channels with La3+ [118].
TRPM8 channels
TRPM8 is expressed by a distinct population of primary afferent neurons originating in the nodose ganglion and DRG as well as by the papillae of the tongue [80,81,90]. Unlike that of TRPA1, the colocalization of TRPM8 with TRPV1 is limited [81,90,94]. Within the gut, TRPM8 has been localized to the muscle of the rat stomach and colon [120] as well as to the liver [107]. The expression of TRPM8 in the mouse stomach and small intestine remains controversial, given that both positive [80] and negative [97] results have been reported.
TRPM8 is activated by temperatures in the cool to cold range as well as by various chemical entities including menthol, icilin, geraniol, L-carvone, isopulegol and linalool. Thus, TRPM8 may play a chemosensory role in the alimentary canal, although its precise functional implications in the gut await to be investigated. In the mouse colon, menthol induces a long lasting relaxation of smooth muscle [97], but it remains questionable if this motor effect involves TRPM8 because the presence of this TRP channel in the gut has not unequivocally been established [80,97].
TRPP2 channels
TRPP2 and the related PKD1 polycystin L3 (PKD1L3), which is required for the formation of functional TRPP2 channels, are expressed by a select class (type III) of taste chemoreceptor cells of the mouse tongue, which are distinct from those mediating sweet, umami and bitter tastes [121-123]. Although several members of the TRPV, TRPA and TRPC channel subfamilies are sensitive to alterations in extracellular pH [78], genetic and functional studies corroborate that TRPP2 is the sour taste receptor [121-123]. Targeted ablation of TRPP2-expressing taste receptor cells results in a specific and total loss of sour taste transduction, whereas responses to sweet, umami, bitter and salty tastants remain unchanged [121].
TRPC channels
TRPC channels are widely distributed in the GI tract, the cellular sources being vagal afferent neurons, enteric neurons, smooth muscle cells, and ICCs. TRPC1, TRPC3, TRPC5 and TRPC6 occur in nodose ganglion neurons in which TRPC1 and TRPC6 are, at least in part, coexpressed with TRPV1 and TRPA1, given that systemic pretreatment of rats with a neurotoxic dose of capsaicin causes a loss of TRPV1, TRPA1, TRPC1 and TRPC6 from vagal afferent neurons [81]. All seven types of TRPC channels appear to occur in the murine stomach [124]. Within the murine and canine gut, TRPC4, TRPC6 and TRPC7 as well as splice variants of TRPC4 and TRPC7 are present in the GI and vascular smooth muscle, TRPC4 being the most abundant subunit [117,125-128]. The guinea-pig enteric nervous system expresses TRPC1, TRPC3, TRPC4 and TRPC6, which are differentially distributed to the myenteric and submucosal plexuses [129]. While TRPC1 is widely distributed to myenteric neurons with cholinergic and nitrergic phenotypes as well as to submucosal cholinergic and noncholinergic secretomotor neurons, TRPC3 is restricted to submucosal neurons containing neuropeptide Y [129]. TRPC4 and TRPC6 are likewise preferentially expressed by noncholinergic secretomotor neurons of the submucosal plexus [129].
The roles of TRPC channels in digestive function are incompletely understood. While TRPC4 does not seem to contribute to the basal pattern of pacemaker activity in the ICCs [127], TRPC4, TRPC5 and TRPC6 operate as downstream effectors of GI muscarinic acetylcholine receptors. For instance, TRPC5 and TRPC6 are activated by cholinergic stimulation of ICCs and smooth muscle in the murine stomach and intestine, and the nonselective cation current evoked by muscarinic receptor stimulation is abolished in TRPC4 knockout mice [117,127,128]. TRPC4 activity appears to account for more than 80 % of the muscarinic receptor-induced cation current in intestinal smooth muscle, while the residual current is mediated by TRPC6 [128].
CONCLUSIONS
The digestive tract contains several TRP channels which play essential roles in taste, chemo- and mechanosensation, thermoregulation, pain and hyperalgesia, mucosal function and homeostasis, control of motility by neurons, ICCs and muscle cells, and vascular function. The polymodal chemo- and mechanosensory function of TRPA1, TRPM5, TRPV1 and TRPV4 is particularly relevant to the alimentary canal whose digestive and absorptive function depends on the surveillance and integration of many chemical and physical stimuli. TRPV5 and TRPV6 as well as TRPM6 and TRPM7 appear to be essential for the absorption of Ca2+ and Mg2+, respectively, while TRPM7 appears to contribute to the pacemaker activity of ICCs, and TRPC4 transduces smooth muscle contraction evoked by muscarinic acetylcholine receptor activation.
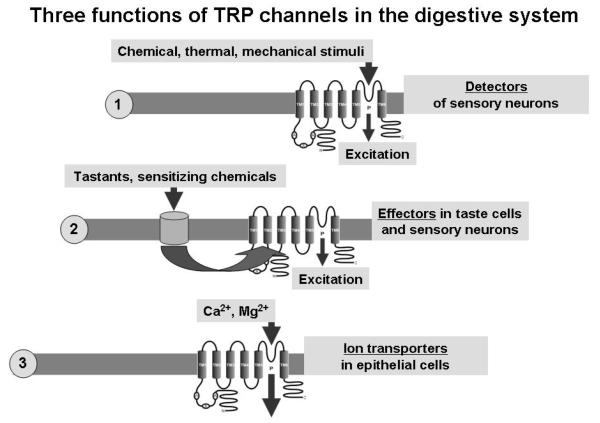
As is evident from the available information, TRP channels in the digestive system can function in at least three different capacities: (i) as molecular sensors (detectors or primary transducers) of chemical and physical stimuli, (ii) as downstream or secondary transducers (or effectors) of cell activation induced by G protein-coupled receptors or other ion channels, and (iii) as ion transport channels (Figure 2). The secondary transducer roles place TRP channels in a position to orchestrate many physiological and pathological cell processes. This holds true for the sensitization of afferent nerve fibres to several modalities in response to inflammatory mediators. A secondary transducer role is also typical of TRPM5 in its taste function and of TRPC channels in their role to control smooth muscle responsiveness to acetylcholine.
Figure 2.
Schematic diagram illustrating 3 different roles of TRP channels in the digestive system: (i) their role as molecular sensors (detectors or primary transducers) of chemical and physical stimuli, (ii) their role as downstream or secondary transducers (effectors) of cell activation induced by G protein-coupled receptors or other ion channels, and (iii) their role as ion transport channels.
The implication of TRP channels in physiological and pathological processes within the alimentary canal, the changes in TRP channel function associated with GI inflammation and hyperalgesia, and the emergence of TRP channelopathies [87] have raised enormous interest in the therapeutic exploitation of these molecular entities. This is in particular true for TRPV1, TRPV4 and TRPA1 which may be targeted for the treatment of inflammatory and functional abdominal pain syndromes. Consequently, many blockers of TRPV1 and, more recently, TRPA1 have been developed, but their therapeutic efficacy and safety in GI disorders have not yet been established. Interference with TRP channels is a challenging therapeutic approach [2,45,130], given that these channels seem to play many important physiological roles within and outside the GI system.
ACKNOWLEDGEMENTS
Work performed in the author’s laboratory was supported by the Zukunftsfonds Steiermark (grant 262) and the Austrian Science Funds (FWF grant L25-B05).
REFERENCES
- [1].Bandell M, Macpherson LJ, Patapoutian A. From chills to chilis: mechanisms for thermosensation and chemesthesis via thermoTRPs. Curr. Opin. Neurobiol. 2007;17:490–497. doi: 10.1016/j.conb.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Holzer P. TRPV1: a new target for treatment of visceral pain in IBS? Gut. 2008;57:882–884. doi: 10.1136/gut.2008.149724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Clapham DE, Julius D, Montell C, Schultz G. International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol. Rev. 2005;57:427–450. doi: 10.1124/pr.57.4.6. [DOI] [PubMed] [Google Scholar]
- [4].Holzer P. TRPV1 and the gut: from a tasty receptor for a painful vanilloid to a key player in hyperalgesia. Eur. J. Pharmacol. 2004;500:231–241. doi: 10.1016/j.ejphar.2004.07.028. [DOI] [PubMed] [Google Scholar]
- [5].Blackshaw LA, Brierley SM, Hughes PA. TRP channels: new targets for visceral pain. Gut. 2010;59:126–135. doi: 10.1136/gut.2009.179523. [DOI] [PubMed] [Google Scholar]
- [6].Robinson DR, McNaughton PA, Evans ML, Hicks GA. Characterization of the primary spinal afferent innervation of the mouse colon using retrograde labelling. Neurogastroenterol. Motil. 2004;16:113–124. doi: 10.1046/j.1365-2982.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- [7].Schicho R, Florian W, Liebmann I, Holzer P, Lippe IT. Increased expression of TRPV1 receptor in dorsal root ganglia by acid insult of the rat gastric mucosa. Eur. J. Neurosci. 2004;19:1811–1818. doi: 10.1111/j.1460-9568.2004.03290.x. [DOI] [PubMed] [Google Scholar]
- [8].Brierley SM, Carter R, Jones W, Xu L, Robinson DR, Hicks GA, Gebhart GF, Blackshaw LA. Differential chemosensory function and receptor expression of splanchnic and pelvic colonic afferents in mice. J. Physiol. (London) 2005;567:267–281. doi: 10.1113/jphysiol.2005.089714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hwang SJ, Oh JM, Valtschanoff JG. Expression of the vanilloid receptor TRPV1 in rat dorsal root ganglion neurons supports different roles of the receptor in visceral and cutaneous afferents. Brain Res. 2005;1047:261–266. doi: 10.1016/j.brainres.2005.04.036. [DOI] [PubMed] [Google Scholar]
- [10].Christianson JA, McIlwrath SL, Koerber HR, Davis BM. Transient receptor potential vanilloid 1-immunopositive neurons in the mouse are more prevalent within colon afferents compared to skin and muscle afferents. Neuroscience. 2006;140:247–257. doi: 10.1016/j.neuroscience.2006.02.015. [DOI] [PubMed] [Google Scholar]
- [11].Tan LL, Bornstein JC, Anderson CR. Neurochemical and morphological phenotypes of vagal afferent neurons innervating the adult mouse jejunum. Neurogastroenterol. Motil. 2009;21:994–1001. doi: 10.1111/j.1365-2982.2009.01322.x. [DOI] [PubMed] [Google Scholar]
- [12].Nozawa Y, Nishihara K, Yamamoto A, Nakano M, Ajioka H, Matsuura N. Distribution and characterization of vanilloid receptors in the rat stomach. Neurosci. Lett. 2001;309:33–36. doi: 10.1016/s0304-3940(01)02021-3. [DOI] [PubMed] [Google Scholar]
- [13].Kato S, Aihara E, Nakamura A, Xin H, Matsui H, Kohama K, Takeuchi K. Expression of vanilloid receptors in rat gastric epithelial cells: role in cellular protection. Biochem. Pharmacol. 2003;66:1115–1121. doi: 10.1016/s0006-2952(03)00461-1. [DOI] [PubMed] [Google Scholar]
- [14].Faussone-Pellegrini MS, Taddei A, Bizzoco E, Lazzeri M, Vannucchi MG, Bechi P. Distribution of the vanilloid (capsaicin) receptor type 1 in the human stomach. Histochem. Cell Biol. 2005;124:61–68. doi: 10.1007/s00418-005-0025-9. [DOI] [PubMed] [Google Scholar]
- [15].Kechagias S, Botella S, Petersson F, Borch K, Ericson AC. Expression of vanilloid receptor-1 in epithelial cells of human antral gastric mucosa. Scand. J. Gastroenterol. 2005;40:775–782. doi: 10.1080/00365520510015782. [DOI] [PubMed] [Google Scholar]
- [16].Cheng L, de la Monte S, Ma J, Hong J, Tong M, Cao W, Behar J, Biancani P, Harnett KM. HCl-activated neural and epithelial vanilloid receptors (TRPV1) in cat esophageal mucosa. Am. J. Physiol. 2009;297:G135–G143. doi: 10.1152/ajpgi.90386.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ericson A, Nur EM, Petersson F, Kechagias S. The effects of capsaicin on gastrin secretion in isolated human antral glands: before and after ingestion of red chilli. Dig. Dis. Sci. 2009;54:491–498. doi: 10.1007/s10620-008-0400-1. [DOI] [PubMed] [Google Scholar]
- [18].Poonyachoti S, Kulkarni-Narla A, Brown DR. Chemical coding of neurons expressing delta- and kappa-opioid receptor and type I vanilloid receptor immunoreactivities in the porcine ileum. Cell Tissue Res. 2002;307:23–33. doi: 10.1007/s00441-001-0480-0. [DOI] [PubMed] [Google Scholar]
- [19].Anavi-Goffer S, Coutts AA. Cellular distribution of vanilloid VR1 receptor immunoreactivity in the guinea-pig myenteric plexus. Eur. J. Pharmacol. 2003;458:61–71. doi: 10.1016/s0014-2999(02)02653-5. [DOI] [PubMed] [Google Scholar]
- [20].Chan CL, Facer P, Davis JB, Smith GD, Egerton J, Bountra C, Williams NS, Anand P. Sensory fibres expressing capsaicin receptor TRPV1 in patients with rectal hypersensitivity and faecal urgency. Lancet. 2003;361:385–391. doi: 10.1016/s0140-6736(03)12392-6. [DOI] [PubMed] [Google Scholar]
- [21].Tan LL, Bornstein JC, Anderson CR. Distinct chemical classes of medium-sized transient receptor potential channel vanilloid 1-immunoreactive dorsal root ganglion neurons innervate the adult mouse jejunum and colon. Neuroscience. 2008;156:334–343. doi: 10.1016/j.neuroscience.2008.06.071. [DOI] [PubMed] [Google Scholar]
- [22].Suckow SK, Caudle RM. Identification and immunohistochemical characterization of colospinal afferent neurons in the rat. Neuroscience. 2008;153:803–813. doi: 10.1016/j.neuroscience.2008.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24:739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- [24].Maggi CA. Tachykinins and calcitonin gene-related peptide (CGRP) as co-transmitters released from peripheral endings of sensory nerves. Prog. Neurobiol. 1995;45:1–98. doi: 10.1016/0301-0082(94)e0017-b. [DOI] [PubMed] [Google Scholar]
- [25].Pintér E, Helyes Z, Szolcsányi J. Inhibitory effect of somatostatin on inflammation and nociception. Pharmacol. Ther. 2006;112:440–456. doi: 10.1016/j.pharmthera.2006.04.010. [DOI] [PubMed] [Google Scholar]
- [26].Holzer P. Neural emergency system in the stomach. Gastroenterology. 1998;114:823–839. doi: 10.1016/s0016-5085(98)70597-9. [DOI] [PubMed] [Google Scholar]
- [27].Akiba Y, Ghayouri S, Takeuchi T, Mizumori M, Guth PH, Engel E, Swenson ER, Kaunitz JD. Carbonic anhydrases and mucosal vanilloid receptors help mediate the hyperemic response to luminal CO2 in rat duodenum. Gastroenterology. 2006;131:142–152. doi: 10.1053/j.gastro.2006.04.018. [DOI] [PubMed] [Google Scholar]
- [28].Massa F, Sibaev A, Marsicano G, Blaudzun H, Storr M, Lutz B. Vanilloid receptor (TRPV1)-deficient mice show increased susceptibility to dinitrobenzene sulfonic acid induced colitis. J. Mol. Med. 2006;84:142–146. doi: 10.1007/s00109-005-0016-2. [DOI] [PubMed] [Google Scholar]
- [29].Martelli L, Ragazzi E, di Mario F, Martelli M, Castagliuolo I, Dal Maschio M, Palù G, Maschietto M, Scorzeto M, Vassanelli S, Brun P. A potential role for the vanilloid receptor TRPV1 in the therapeutic effect of curcumin in dinitrobenzene sulphonic acid-induced colitis in mice. Neurogastroenterol. Motil. 2007;19:668–674. doi: 10.1111/j.1365-2982.2007.00928.x. [DOI] [PubMed] [Google Scholar]
- [30].Gazzieri D, Trevisani M, Springer J, Harrison S, Cottrell GS, Andre E, Nicoletti P, Massi D, Zecchi S, Nosi D, Santucci M, Gerard NP, Lucattelli M, Lungarella G, Fischer A, Grady EF, Bunnett NW, Geppetti P. Substance P released by TRPV1-expressing neurons produces reactive oxygen species that mediate ethanol-induced gastric injury. Free Radic. Biol. Med. 2007;43:581–589. doi: 10.1016/j.freeradbiomed.2007.05.018. [DOI] [PubMed] [Google Scholar]
- [31].Razavi R, Chan Y, Afifiyan FN, Liu XJ, Wan X, Yantha J, Tsui H, Tang L, Tsai S, Santamaria P, Driver JP, Serreze D, Salter MW, Dosch HM. TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell. 2006;127:1123–1135. doi: 10.1016/j.cell.2006.10.038. [DOI] [PubMed] [Google Scholar]
- [32].Gram DX, Ahrén B, Nagy I, Olsen UB, Brand CL, Sundler F, Tabanera R, Svendsen O, Carr RD, Santha P, Wierup N, Hansen AJ. Capsaicin-sensitive sensory fibers in the islets of Langerhans contribute to defective insulin secretion in Zucker diabetic rat, an animal model for some aspects of human type 2 diabetes. Eur. J. Neurosci. 2007;25:213–223. doi: 10.1111/j.1460-9568.2006.05261.x. [DOI] [PubMed] [Google Scholar]
- [33].Zhang LL, Yan Liu D, Ma LQ, Luo ZD, Cao TB, Zhong J, Yan ZC, Wang LJ, Zhao ZG, Zhu SJ, Schrader M, Thilo F, Zhu ZM, Tepel M. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ. Res. 2007;100:1063–1070. doi: 10.1161/01.RES.0000262653.84850.8b. [DOI] [PubMed] [Google Scholar]
- [34].Suri A, Szallasi A. The emerging role of TRPV1 in diabetes and obesity. Trends Pharmacol. Sci. 2008;29:29–36. doi: 10.1016/j.tips.2007.10.016. [DOI] [PubMed] [Google Scholar]
- [35].Boudaka A, Wörl J, Shiina T, Neuhuber WL, Kobayashi H, Shimizu Y, Takewaki T. Involvement of TRPV1-dependent and -independent components in the regulation of vagally induced contractions in the mouse esophagus. Eur. J. Pharmacol. 2007;556:157–165. doi: 10.1016/j.ejphar.2006.11.005. [DOI] [PubMed] [Google Scholar]
- [36].Barthó L, Benkó R, Holzer-Petsche U, Holzer P, Undi S, Wolf M. Role of extrinsic afferent neurons in gastrointestinal motility. Eur. Rev. Med. Pharmacol. Sci. 2008;12(Suppl. 1):21–31. [PubMed] [Google Scholar]
- [37].De Man JG, Boeckx S, Anguille S, de Winter BY, de Schepper HU, Herman AG, Pelckmans PA. Functional study on TRPV1-mediated signalling in the mouse small intestine: involvement of tachykinin receptors. Neurogastroenterol. Motil. 2008;20:546–556. doi: 10.1111/j.1365-2982.2007.01064.x. [DOI] [PubMed] [Google Scholar]
- [38].Matsumoto K, Kurosawa E, Terui H, Hosoya T, Tashima K, Murayama T, Priestley JV, Horie S. Localization of TRPV1 and contractile effect of capsaicin in mouse large intestine: high abundance and sensitivity in rectum and distal colon. Am. J. Physiol. 2009;297:G348–G360. doi: 10.1152/ajpgi.90578.2008. [DOI] [PubMed] [Google Scholar]
- [39].Paterson WG, Miller DV, Dilworth N, Assini JB, Lourenssen S, Blennerhassett MG. Intraluminal acid induces oesophageal shortening via capsaicin-sensitive neurokinin neurons. Gut. 2007;56:1347–1352. doi: 10.1136/gut.2006.115881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gonzalez R, Dunkel R, Koletzko B, Schusdziarra V, Allescher HD. Effect of capsaicin-containing red pepper sauce suspension on upper gastrointestinal motility in healthy volunteers. Dig. Dis. Sci. 1998;43:1165–1171. doi: 10.1023/a:1018831018566. [DOI] [PubMed] [Google Scholar]
- [41].Lee KJ, Vos R, Tack J. Effects of capsaicin on the sensorimotor function of the proximal stomach in humans. Aliment. Pharmacol. Ther. 2004;19:415–425. doi: 10.1046/j.1365-2036.2004.01823.x. [DOI] [PubMed] [Google Scholar]
- [42].Grossi L, Cappello G, Marzio L. Effect of an acute intraluminal administration of capsaicin on oesophageal motor pattern in GORD patients with ineffective oesophageal motility. Neurogastroenterol. Motil. 2006;18:632–636. doi: 10.1111/j.1365-2982.2006.00793.x. [DOI] [PubMed] [Google Scholar]
- [43].Holzer P, Lippe IT, Holzer-Petsche U. Inhibition of gastrointestinal transit due to surgical trauma or peritoneal irritation is reduced in capsaicin-treated rats. Gastroenterology. 1986;91:360–363. doi: 10.1016/0016-5085(86)90569-x. [DOI] [PubMed] [Google Scholar]
- [44].De Schepper HU, De Man JG, Ruyssers NE, Deiteren A, Van Nassauw L, Timmermans JP, Martinet W, Herman AG, Pelckmans PA, De Winter BY. TRPV1 receptor signaling mediates afferent nerve sensitization during colitis-induced motility disorders in rats. Am. J. Physiol. 2008;294:G245–G253. doi: 10.1152/ajpgi.00351.2007. [DOI] [PubMed] [Google Scholar]
- [45].Holzer P. The pharmacological challenge to tame the transient receptor potential vanilloid-1 (TRPV1) nocisensor. Br. J. Pharmacol. 2008;155:1145–1162. doi: 10.1038/bjp.2008.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kollarik M, Brozmanova M. Cough and gastroesophageal reflux: Insights from animal models. Pulm. Pharmacol. Ther. 2009;22:130–134. doi: 10.1016/j.pupt.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kindt S, Vos R, Blondeau K, Tack J. Influence of intra-oesophageal capsaicin instillation on heartburn induction and oesophageal sensitivity in man. Neurogastroenterol. Motil. 2009;21:1032–e82. doi: 10.1111/j.1365-2982.2009.01332.x. [DOI] [PubMed] [Google Scholar]
- [48].Hammer J, Vogelsang H. Characterization of sensations induced by capsaicin in the upper gastrointestinal tract. Neurogastroenterol. Motil. 2007;19:279–287. doi: 10.1111/j.1365-2982.2007.00900.x. [DOI] [PubMed] [Google Scholar]
- [49].Schmidt B, Hammer J, Holzer P, Hammer HF. Chemical nociception in the jejunum induced by capsaicin. Gut. 2004;53:1109–1116. doi: 10.1136/gut.2003.029793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Drewes AM, Schipper KP, Dimcevski G, Petersen P, Gregersen H, Funch-Jensen P, Arendt-Nielsen L. Gut pain and hyperalgesia induced by capsaicin: a human experimental model. Pain. 2003;104:333–341. doi: 10.1016/s0304-3959(03)00039-3. [DOI] [PubMed] [Google Scholar]
- [51].Arendt-Nielsen L, Schipper KP, Dimcevski G, Sumikura H, Krarup AL, Giamberardino MA, Drewes AM. Viscero-somatic reflexes in referred pain areas evoked by capsaicin stimulation of the human gut. Eur. J. Pain. 2008;12:544–551. doi: 10.1016/j.ejpain.2007.08.010. [DOI] [PubMed] [Google Scholar]
- [52].Hammer J. Effect of repeated capsaicin ingestion on intestinal chemosensation and mechanosensation. Aliment. Pharmacol. Ther. 2006;24:679–686. doi: 10.1111/j.1365-2036.2006.03022.x. [DOI] [PubMed] [Google Scholar]
- [53].Gonlachanvit S, Fongkam P, Wittayalertpanya S, Kullavanijaya P. Red chili induces rectal hypersensitivity in healthy humans: possible role of 5HT-3 receptors on capsaicin-sensitive visceral nociceptive pathways. Aliment. Pharmacol. Ther. 2007;26:617–625. doi: 10.1111/j.1365-2036.2007.03396.x. [DOI] [PubMed] [Google Scholar]
- [54].Führer M, Hammer J. Effect of repeated, long term capsaicin ingestion on intestinal chemo- and mechanosensation in healthy volunteers. Neurogastroenterol. Motil. 2009;21:521–527. e7. doi: 10.1111/j.1365-2982.2008.01227.x. [DOI] [PubMed] [Google Scholar]
- [55].Geppetti P, Trevisani M. Activation and sensitisation of the vanilloid receptor: role in gastrointestinal inflammation and function. Br. J. Pharmacol. 2004;141:1313–1320. doi: 10.1038/sj.bjp.0705768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat. Rev. Drug Discov. 2007;6:357–372. doi: 10.1038/nrd2280. [DOI] [PubMed] [Google Scholar]
- [57].Akbar A, Yiangou Y, Facer P, Walters JR, Anand P, Ghosh S. Increased capsaicin receptor TRPV1 expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut. 2008;57:923–929. doi: 10.1136/gut.2007.138982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Bhat YM, Bielefeldt K. Capsaicin receptor (TRPV1) and non-erosive reflux disease. Eur. J. Gastroenterol. Hepatol. 2006;18:263–270. doi: 10.1097/00042737-200603000-00006. [DOI] [PubMed] [Google Scholar]
- [59].Hammer J, Führer M, Pipal L, Matiasek J. Hypersensitivity for capsaicin in patients with functional dyspepsia. Neurogastroenterol. Motil. 2008;20:125–133. doi: 10.1111/j.1365-2982.2007.00997.x. [DOI] [PubMed] [Google Scholar]
- [60].Bortolotti M, Coccia G, Grossi G, Miglioli M. The treatment of functional dyspepsia with red pepper. Aliment. Pharmacol. Ther. 2002;16:1075–1082. doi: 10.1046/j.1365-2036.2002.01280.x. [DOI] [PubMed] [Google Scholar]
- [61].Tahara T, Shibata T, Nakamura M, Yamashita H, Yoshioka D, Hirata I, Arisawa T. Homozygous TRPV1 315C influences the susceptibility to functional dyspepsia. J. Clin. Gastroenterol. 2010;44:e1–e7. doi: 10.1097/MCG.0b013e3181b5745e. [DOI] [PubMed] [Google Scholar]
- [62].Gonlachanvit S, Mahayosnond A, Kullavanijaya P. Effects of chili on postprandial gastrointestinal symptoms in diarrhoea predominant irritable bowel syndrome: evidence for capsaicin-sensitive visceral nociception hypersensitivity. Neurogastroenterol. Motil. 2009;21:23–32. doi: 10.1111/j.1365-2982.2008.01167.x. [DOI] [PubMed] [Google Scholar]
- [63].Eijkelkamp N, Kavelaars A, Elsenbruch S, Schedlowski M, Holtmann G, Heijnen CJ. Increased visceral sensitivity to capsaicin after DSS-induced colitis in mice: spinal cord c-Fos expression and behavior. Am. J. Physiol. 2007;293:G749–G757. doi: 10.1152/ajpgi.00114.2007. [DOI] [PubMed] [Google Scholar]
- [64].Jones RC, Otsuka E, Wagstrom E, Jensen CS, Price MP, Gebhart GF. Short-term sensitization of colon mechanoreceptors is associated with long-term hypersensitivity to colon distention in the mouse. Gastroenterology. 2007;133:184–194. doi: 10.1053/j.gastro.2007.04.042. [DOI] [PubMed] [Google Scholar]
- [65].Winston J, Shenoy M, Medley D, Naniwadekar A, Pasricha PJ. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology. 2007;132:615–627. doi: 10.1053/j.gastro.2006.11.014. [DOI] [PubMed] [Google Scholar]
- [66].Malin SA, Christianson JA, Bielefeldt K, Davis BM. TPRV1 expression defines functionally distinct pelvic colon afferents. J. Neurosci. 2009;29:743–752. doi: 10.1523/JNEUROSCI.3791-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Spencer NJ, Kerrin A, Singer CA, Hennig GW, Gerthoffer WT, McDonnell O. Identification of capsaicin-sensitive rectal mechanoreceptors activated by rectal distension in mice. Neuroscience. 2008;153:518–534. doi: 10.1016/j.neuroscience.2008.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].van den Wijngaard RM, Welting O, Bulmer DC, Wouters MM, Lee K, de Jonge WJ, Boeckxstaens GE. Possible role for TRPV1 in neomycin-induced inhibition of visceral hypersensitivity in rat. Neurogastroenterol. Motil. 2009;21:863–e60. doi: 10.1111/j.1365-2982.2009.01287.x. [DOI] [PubMed] [Google Scholar]
- [69].van den Wijngaard RM, Klooker TK, Welting O, Stanisor OI, Wouters MM, van der Coelen D, Bulmer DC, Peeters PJ, Aerssens J, de Hoogt R, Lee K, de Jonge WJ, Boeckxstaens GE. Essential role for TRPV1 in stress-induced (mast cell-dependent) colonic hypersensitivity in maternally separated rats. Neurogastroenterol. Motil. 2009;21:1107–e94. doi: 10.1111/j.1365-2982.2009.01339.x. [DOI] [PubMed] [Google Scholar]
- [70].Ravnefjord A, Brusberg M, Kang D, Bauer U, Larsson H, Lindström E, Martinez V. Involvement of the transient receptor potential vanilloid 1 (TRPV1) in the development of acute visceral hyperalgesia during colorectal distension in rats. Eur. J. Pharmacol. 2009;611:85–91. doi: 10.1016/j.ejphar.2009.03.058. [DOI] [PubMed] [Google Scholar]
- [71].Sharkey KA, Cristino L, Oland LD, Van Sickle MD, Starowicz K, Pittman QJ, Guglielmotti V, Davison JS, Di Marzo V. Arvanil, anandamide and N-arachidonoyl-dopamine (NADA) inhibit emesis through cannabinoid CB1 and vanilloid TRPV1 receptors in the ferret. Eur. J. Neurosci. 2007;25:2773–2782. doi: 10.1111/j.1460-9568.2007.05521.x. [DOI] [PubMed] [Google Scholar]
- [72].Chu KM, Ngan MP, Wai MK, Yeung CK, Andrews PL, Percie du Sert N, Rudd JA. Olvanil: a non-pungent TRPV1 activator has anti-emetic properties in the ferret. Neuropharmacology. 2010;58:383–391. doi: 10.1016/j.neuropharm.2009.10.002. [DOI] [PubMed] [Google Scholar]
- [73].Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- [74].Masamoto Y, Kawabata F, Fushiki T. Intragastric administration of TRPV1, TRPV3, TRPM8, and TRPA1 agonists modulates autonomic thermoregulation in different manners in mice. Biosci. Biotechnol. Biochem. 2009;73:1021–1027. doi: 10.1271/bbb.80796. [DOI] [PubMed] [Google Scholar]
- [75].Steiner AA, Turek VF, Almeida MC, Burmeister JJ, Oliveira DL, Roberts JL, Bannon AW, Norman MH, Louis JC, Treanor JJ, Gavva NR, Romanovsky AA. Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J. Neurosci. 2007;27:7459–7468. doi: 10.1523/JNEUROSCI.1483-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Gavva NR, Treanor JJ, Garami A, Fang L, Surapaneni S, Akrami A, Alvarez F, Bak A, Darling M, Gore A, Jang GR, Kesslak JP, Ni L, Norman MH, Palluconi G, Rose MJ, Salfi M, Tan E, Romanovsky AA, Banfield C, Davar G. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain. 2008;136:202–210. doi: 10.1016/j.pain.2008.01.024. [DOI] [PubMed] [Google Scholar]
- [77].Garami A, Shimansky YP, Pakai E, Oliveira DL, Gavva NR, Romanovsky AA. Contributions of different modes of TRPV1 activation to TRPV1 antagonist-induced hyperthermia. J. Neurosci. 2010;30:1435–1440. doi: 10.1523/JNEUROSCI.5150-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Holzer P. Acid-sensitive ion channels and receptors. Handb. Exp. Pharmacol. 2009;194:283–332. doi: 10.1007/978-3-540-79090-7_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kashiba H, Uchida Y, Takeda D, Nishigori A, Ueda Y, Kuribayashi K, Ohshima M. TRPV2-immunoreactive intrinsic neurons in the rat intestine. Neurosci. Lett. 2004;366:193–196. doi: 10.1016/j.neulet.2004.05.069. [DOI] [PubMed] [Google Scholar]
- [80].Zhang L, Jones S, Brody K, Costa M, Brookes SJ. Thermosensitive transient receptor potential channels in vagal afferent neurons of the mouse. Am. J. Physiol. 2004;286:G983–G991. doi: 10.1152/ajpgi.00441.2003. [DOI] [PubMed] [Google Scholar]
- [81].Zhao H, Sprunger LK, Simasko SM. Expression of transient receptor potential channels and two-pore potassium channels in subtypes of vagal afferent neurons in rat. Am. J. Physiol. 2010;298:G212–G221. doi: 10.1152/ajpgi.00396.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat. Neurosci. 2006;9:628–635. doi: 10.1038/nn1692. [DOI] [PubMed] [Google Scholar]
- [83].Ueda T, Yamada T, Ugawa S, Ishida Y, Shimada S. TRPV3, a thermosensitive channel is expressed in mouse distal colon epithelium. Biochem. Biophys. Res. Commun. 2009;383:130–134. doi: 10.1016/j.bbrc.2009.03.143. [DOI] [PubMed] [Google Scholar]
- [84].Brierley SM, Page AJ, Hughes PA, Adam B, Liebregts T, Cooper NJ, Holtmann G, Liedtke W, Blackshaw LA. Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology. 2008;134:2059–2069. doi: 10.1053/j.gastro.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Cenac N, Altier C, Chapman K, Liedtke W, Zamponi G, Vergnolle N. Transient receptor potential vanilloid-4 has a major role in visceral hypersensitivity symptoms. Gastroenterology. 2008;135:937–946. doi: 10.1053/j.gastro.2008.05.024. [DOI] [PubMed] [Google Scholar]
- [86].Sipe WE, Brierley SM, Martin CM, Phillis BD, Cruz FB, Grady EF, Liedtke W, Cohen DM, Vanner S, Blackshaw LA, Bunnett NW. Transient receptor potential vanilloid 4 mediates protease activated receptor 2-induced sensitization of colonic afferent nerves and visceral hyperalgesia. Am. J. Physiol. 2008;294:G1288–G1298. doi: 10.1152/ajpgi.00002.2008. [DOI] [PubMed] [Google Scholar]
- [87].Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol. Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- [88].Cenac N, Altier C, Motta JP, d’Aldebert E, Galeano S, Zamponi GW, Vergnolle N. Potentiation of TRPV4 signalling by histamine and serotonin: an important mechanism for visceral hypersensitivity. Gut. 2010;59:481–488. doi: 10.1136/gut.2009.192567. [DOI] [PubMed] [Google Scholar]
- [89].Suzuki Y, Landowski CP, Hediger MA. Mechanisms and regulation of epithelial Ca2+ absorption in health and disease. Annu. Rev. Physiol. 2008;70:257–271. doi: 10.1146/annurev.physiol.69.031905.161003. [DOI] [PubMed] [Google Scholar]
- [90].Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with Aδ/C-fibers and colocalization with trk receptors. J. Comp. Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- [91].Anand U, Otto WR, Facer P, Zebda N, Selmer I, Gunthorpe MJ, Chessell IP, Sinisi M, Birch R, Anand P. TRPA1 receptor localisation in the human peripheral nervous system and functional studies in cultured human and rat sensory neurons. Neurosci. Lett. 2008;438:221–227. doi: 10.1016/j.neulet.2008.04.007. [DOI] [PubMed] [Google Scholar]
- [92].Yang J, Li Y, Zuo X, Zhen Y, Yu Y, Gao L. Transient receptor potential ankyrin-1 participates in visceral hyperalgesia following experimental colitis. Neurosci. Lett. 2008;440:237–241. doi: 10.1016/j.neulet.2008.05.093. [DOI] [PubMed] [Google Scholar]
- [93].Brierley SM, Hughes PA, Page AJ, Kwan KY, Martin CM, O’Donnell TA, Cooper NJ, Harrington AM, Adam B, Liebregts T, Holtmann G, Corey DP, Rychkov GY, Blackshaw LA. The ion channel TRPA1 is required for normal mechanosensation and is modulated by algesic stimuli. Gastroenterology. 2009;137:2084–2095. doi: 10.1053/j.gastro.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Kondo T, Obata K, Miyoshi K, Sakurai J, Tanaka J, Miwa H, Noguchi K. Transient receptor potential A1 mediates gastric distention-induced visceral pain in rats. Gut. 2009;58:1342–13. doi: 10.1136/gut.2008.175901. [DOI] [PubMed] [Google Scholar]
- [95].Yu S, Gao G, Peterson BZ, Ouyang A. TRPA1 in mast cell activation-induced long-lasting mechanical hypersensitivity of vagal afferent C-fibers in guinea pig esophagus. Am. J. Physiol. 2009;297:G34–G242. doi: 10.1152/ajpgi.00068.2009. [DOI] [PubMed] [Google Scholar]
- [96].Cattaruzza F, Spreadbury I, Miranda-Morales M, Grady EF, Vanner SJ, Bunnett NW. Transient receptor potential ankyrin-1 has a major role in mediating visceral pain in mice. Am. J. Physiol. 2010;298:G81–G91. doi: 10.1152/ajpgi.00221.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Penuelas A, Tashima K, Tsuchiya S, Matsumoto K, Nakamura T, Horie S, Yano S. Contractile effect of TRPA1 receptor agonists in the isolated mouse intestine. Eur. J. Pharmacol. 2007;576:143–150. doi: 10.1016/j.ejphar.2007.08.015. [DOI] [PubMed] [Google Scholar]
- [98].Purhonen AK, Louhivuori LM, Kiehne K, Kerman KE, Herzig KH. TRPA1 channel activation induces cholecystokinin release via extracellular calcium. FEBS Lett. 2008;582:229–232. doi: 10.1016/j.febslet.2007.12.005. [DOI] [PubMed] [Google Scholar]
- [99].Nozawa K, Kawabata-Shoda E, Doihara H, Kojima R, Okada H, Mochizuki S, Sano Y, Inamura K, Matsushime H, Koizumi T, Yokoyama T, Ito H. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc. Natl. Acad. Sci. USA. 2009;106:3408–3413. doi: 10.1073/pnas.0805323106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Doihara H, Nozawa K, Kawabata-Shoda E, Kojima R, Yokoyama T, Ito H. Molecular cloning and characterization of dog TRPA1 and AITC stimulate the gastrointestinal motility through TRPA1 in conscious dogs. Eur. J. Pharmacol. 2009;617:124–129. doi: 10.1016/j.ejphar.2009.06.038. [DOI] [PubMed] [Google Scholar]
- [101].Kang K, Pulver SR, Panzano VC, Chang EC, Griffith LC, Theobald DL, Garrity PA. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature. 2010;464:597–601. doi: 10.1038/nature08848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Yu S, Ouyang A. TRPA1 in bradykinin-induced mechanical hypersensitivity of vagal C fibers in guinea pig esophagus. Am. J. Physiol. 2009;296:G255–G265. doi: 10.1152/ajpgi.90530.2008. [DOI] [PubMed] [Google Scholar]
- [103].Mitrovic M, Shahbazian A, Bock E, Pabst MA, Holzer P. Chemo-nociceptive signalling from the colon is enhanced by mild colitis and blocked by inhibition of transient receptor potential ankyrin1 channels. Br. J. Pharmacol. 2010;160:1430–1442. doi: 10.1111/j.1476-5381.2010.00794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Matsuda H, Ochi M, Nagatomo A, Yoshikawa M. Effects of allyl isothiocyanate from horseradish on several experimental gastric lesions in rats. Eur. J. Pharmacol. 2007;561:172–181. doi: 10.1016/j.ejphar.2006.12.040. [DOI] [PubMed] [Google Scholar]
- [105].Doihara H, Nozawa K, Kawabata-Shoda E, Kojima R, Yokoyama T, Ito H. TRPA1 agonists delay gastric emptying in rats through serotonergic pathways. Naunyn Schmiedeberg’s Arch. Pharmacol. 2009;380:353–357. doi: 10.1007/s00210-009-0435-7. [DOI] [PubMed] [Google Scholar]
- [106].Kojima R, Doihara H, Nozawa K, Kawabata-Shoda E, Yokoyama T, Ito H. Characterization of two models of drug-induced constipation in mice and evaluation of mustard oil in these models. Pharmacology. 2009;84:227–233. doi: 10.1159/000236524. [DOI] [PubMed] [Google Scholar]
- [107].Fonfria E, Murdock PR, Cusdin FS, Benham CD, Kelsell RE, McNulty S. Tissue distribution profiles of the human TRPM cation channel family. J. Recept. Signal Transduct. Res. 2006;26:159–178. doi: 10.1080/10799890600637506. [DOI] [PubMed] [Google Scholar]
- [108].Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- [109].Kaske S, Krasteva G, König P, Kummer W, Hofmann T, Gudermann T, Chubanov V. TRPM5, a taste-signaling transient receptor potential ion-channel, is a ubiquitous signaling component in chemosensory cells. BMC Neurosci. 2007;8:49. doi: 10.1186/1471-2202-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Bezençon C, Fürholz A, Raymond F, Mansourian R, Métairon S, Le Coutre J, Damak S. Murine intestinal cells expressing Trpm5 are mostly brush cells and express markers of neuronal and inflammatory cells. J. Comp. Neurol. 2008;509:514–525. doi: 10.1002/cne.21768. [DOI] [PubMed] [Google Scholar]
- [111].Kokrashvili Z, Rodriguez D, Yevshayeva V, Zhou H, Margolskee RF, Mosinger B. Release of endogenous opioids from duodenal enteroendocrine cells requires Trpm5. Gastroenterology. 2009;137:598–606. doi: 10.1053/j.gastro.2009.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Young RL, Sutherland K, Pezos N, Brierley SM, Horowitz M, Rayner CK, Blackshaw LA. Expression of taste molecules in the upper gastrointestinal tract in humans with and without type 2 diabetes. Gut. 2009;58:337–346. doi: 10.1136/gut.2008.148932. [DOI] [PubMed] [Google Scholar]
- [113].Colsoul B, Schraenen A, Lemaire K, Quintens R, Van Lommel L, Segal A, Owsianik G, Talavera K, Voets T, Margolskee RF, Kokrashvili Z, Gilon P, Nilius B, Schuit FC, Vennekens R. Loss of high-frequency glucose-induced Ca2+ oscillations in pancreatic islets correlates with impaired glucose tolerance in Trpm5−/− mice. Proc. Natl. Acad. Sci. USA. 2010;107:5208–5213. doi: 10.1073/pnas.0913107107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Pérez CA, Shigemura N, Yoshida R, Mosinger B, Glendinning JI, Ninomiya Y, Margolskee RF. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem. Senses. 2006;31:253–264. doi: 10.1093/chemse/bjj027. [DOI] [PubMed] [Google Scholar]
- [115].Zhang Z, Zhao Z, Margolskee R, Liman E. The transduction channel TRPM5 is gated by intracellular calcium in taste cells. J. Neurosci. 2007;27:5777–5786. doi: 10.1523/JNEUROSCI.4973-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Rondón LJ, Groenestege WM, Rayssiguier Y, Mazur A. Relationship between low magnesium status and TRPM6 expression in the kidney and large intestine. Am. J. Physiol. 2008;294:R2001–R2007. doi: 10.1152/ajpregu.00153.2007. [DOI] [PubMed] [Google Scholar]
- [117].Kim BJ, So I, Kim KW. The relationship of TRP channels to the pacemaker activity of interstitial cells of Cajal in the gastrointestinal tract. J. Smooth Muscle Res. 2006;42:1–7. doi: 10.1540/jsmr.42.1. [DOI] [PubMed] [Google Scholar]
- [118].Kim BJ, Park KJ, Kim HW, Choi S, Jun JY, Chang IY, Jeon JH, So I, Kim SJ. Identification of TRPM7 channels in human intestinal interstitial cells of Cajal. World J. Gastroenterol. 2009;15:5799–5804. doi: 10.3748/wjg.15.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Quamme GA. Recent developments in intestinal magnesium absorption. Curr. Opin. Gastroenterol. 2008;24:230–235. doi: 10.1097/MOG.0b013e3282f37b59. [DOI] [PubMed] [Google Scholar]
- [120].Mustafa S, Oriowo M. Cooling-induced contraction of the rat gastric fundus: mediation via transient receptor potential (TRP) cation channel TRPM8 receptor and Rho-kinase activation. Clin. Exp. Pharmacol. Physiol. 2005;32:832–838. doi: 10.1111/j.1440-1681.2005.04273.x. [DOI] [PubMed] [Google Scholar]
- [121].Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Tränkner D, Ryba NJ, Zuker CS. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Kataoka S, Yang R, Ishimaru Y, Matsunami H, Sévigny J, Kinnamon JC, Finger TE. The candidate sour taste receptor, PKD2L1, is expressed by type III taste cells in the mouse. Chem. Senses. 2008;33:243–254. doi: 10.1093/chemse/bjm083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Ishimaru Y, Matsunami H. Transient receptor potential (TRP) channels and taste sensation. J. Dent. Res. 2009;88:212–218. doi: 10.1177/0022034508330212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Lee YM, Kim BJ, Kim HJ, Yang DK, Zhu MH, Lee KP, So I, Kim KW. TRPC5 as a candidate for the nonselective cation channel activated by muscarinic stimulation in murine stomach. Am. J. Physiol. 2003;284:G604–G616. doi: 10.1152/ajpgi.00069.2002. [DOI] [PubMed] [Google Scholar]
- [125].Walker RL, Koh SD, Sergeant GP, Sanders KM, Horowitz B. TRPC4 currents have properties similar to the pacemaker current in interstitial cells of Cajal. Am. J. Physiol. 2002;283:C1637–C1645. doi: 10.1152/ajpcell.00266.2002. [DOI] [PubMed] [Google Scholar]
- [126].Torihashi S, Fujimoto T, Trost C, Nakayama S. Calcium oscillation linked to pacemaking of interstitial cells of Cajal: requirement of calcium influx and localization of TRP4 in caveolae. J. Biol. Chem. 2002;277:19191–19197. doi: 10.1074/jbc.M201728200. [DOI] [PubMed] [Google Scholar]
- [127].Lee KP, Jun JY, Chang IY, Suh SH, So I, Kim KW. TRPC4 is an essential component of the nonselective cation channel activated by muscarinic stimulation in mouse visceral smooth muscle cells. Mol. Cells. 2005;20:435–441. [PubMed] [Google Scholar]
- [128].Tsvilovskyy VV, Zholos AV, Aberle T, Philipp SE, Dietrich A, Zhu MX, Birnbaumer L, Freichel M, Flockerzi V. Deletion of TRPC4 and TRPC6 in mice impairs smooth muscle contraction and intestinal motility in vivo. Gastroenterology. 2009;137:1415–1424. doi: 10.1053/j.gastro.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Liu S, Qu MH, Ren W, Hu HZ, Gao N, Wang GD, Wang XY, Fei G, Zuo F, Xia Y, Wood JD. Differential expression of canonical (classical) transient receptor potential channels in guinea pig enteric nervous system. J. Comp. Neurol. 2008;511:847–862. doi: 10.1002/cne.21874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Wong GY, Gavva NR. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: Recent advances and setbacks. Brain Res. Rev. 2009;60:267–277. doi: 10.1016/j.brainresrev.2008.12.006. [DOI] [PubMed] [Google Scholar]