Abstract
It remains unclear whether direct inter-personal contact is processed differently from similar soft touch applied through inanimate objects. We performed a functional MRI (fMRI) experiment in healthy volunteers, whereby activity during gentle stroking or tapping was compared between stimuli delivered using the experimenter’s hand or a velvet stick. Stroking with a hand elicited larger responses than the other three conditions in the contralateral primary and secondary somatosensory areas and posterior insula. The observed effects likely originate from a combination of perceptual differences and cognitive and emotional correlates of contact with another person. This empirical observation indicates that to ensure ecological validity studies of affective touch processing should be performed with stimuli delivered with direct inter-personal contact rather than inanimate objects.
Keywords: Tactile stimulation, Affective touch, Somatosensory system, Functional MRI (fMRI), Insula
Introduction
While inter-personal touch is essential for social interaction, our understanding of how perceptive and contextual information are integrated into emotionally-valenced subjective experiences remains incomplete. Softness and slow movement predispose for recognizing a touch stimulus as pleasant, but subjective pleasurability depends heavily on cognitive and emotional context [1,2].
Inter-personal touch involves myelinated Aβ fibres projecting to the primary somatosensory cortex (area SI) and un-myelinated CT (C-tactile) fibres projecting to the posterior insula [3-5]. The former pathway subserves localization and discriminative functions, whereas the latter may be specifically relevant to the perception of affectively-valenced touch, since CT fibres innervate receptors that are intensely activated by soft stroking-type touch [5-9]. In neuropathy patients with degenerated Aβ fibres, stroking evokes a weak touch sensation reported as pleasant and is associated with activation of the posterior and anterior insula without concomitant engagement of SI or SII [4-5]. However, the central representation of affective touch clearly extends beyond the insula, involving the orbitofrontal and anterior cingulate cortices (OFC and ACC) as demonstrated by studies comparing neutral, pleasant and painful stimuli delivered to the glaborous skin, which is devoid of CT-fibre innervation [10-11].
An important gap in current literature is that, while most existing studies have been performed using brushes or “velvet sticks”, a direct comparison of skin-to-skin vs. indirect human touch is lacking, raising questions about ecological validity. In addition to perceptual-level differences, they may be effects related to the cognitive and emotional context established by awareness of direct contact with another person. For example, recent work has revealed strong differential effects in SI and SII representing whether a participant believes that they are being touched by a male or female hand [12].
In this study, we directly compared pleasant soft stimuli (stroking) with neutral touch (tapping), delivered either directly with a hand or through a “velvet stick”. We hypothesized that somatosensory areas and the insula would show differential responses to stroking vs. tapping, and that this effect would be different between direct and indirect touch.
Methods
Participants and data acquisition
Fourteen right-handed (according to the Edinburgh handedness inventory) healthy female volunteers (age 21.4±2.8 y), free from neurological or psychiatric disorders and not taking psychoactive medication or illicit drugs, were recruited. The study was approved by the Brighton & Sussex Medical School ethics committee.
Imaging was performed at the Clinical Imaging Sciences Centre on a 1.5 T MRI scanner (Magnetom Avanto, Siemens AG, Germany) equipped with a 4-channel head coil. Structural images were obtained through a rapid gradient-echo T1-weighted sequence (1mm3, isotropic voxels, TR=1640 ms and TE=2 ms). Four hundred functional volumes were acquired using an echo-planar (EPI) sequence, including 21 slices having 5 mm-thickness, no gap, 3 × 3 mm in-plane resolution and matrix size 78 × 78; TR and TE were set to 2100 ms and 50 ms.
Stimuli
In the ‘rest’ condition, participants covertly verbalized numbers (1-9) randomly presented on a projection screen at a rate of 0.5 Hz. This condition was chosen because it is attentionally-demanding and therefore prevents sustained recall of the previous touch stimulus. In the touch stimulation conditions, participants were asked to relax and concentrate on the sensory feeling, while passively staring at a fixation cross. They were not prompted about the type of stimulation being applied. The stimuli were arranged in a two-by-two design: one factor was stimulus delivery (touching directly with a hand or with a velvet stick), the other was touch type (stroking or tapping). Stimuli were presented in 30 s blocks and repeated in pseudo-random order 13 times for the rest condition and 5 times for each stimulation condition.
All stimuli were delivered on the right dorsal forearm by the female experimenter, whom participants had met prior to scanning. The touch stimulation rate was fixed at 0.5 Hz by a tone presented to the experimenter through headphones. Stroking consisted of softly touching the forearm with a force of approx. 250 mN, using either the palm of the right hand or the velvet stick, while moving from the proximal to distal direction to cover a length of approx. 15 cm. Tapping consisted of applying and removing contact to the same region, maintaining approximately equal force at the same rate. The experimenter ensured that the hand used for stimulation had a temperature of approx. 32° C and was free from sweat, cream and debris. The ‘velvet stick’ consisted of a 16×3.5 cm ruler covered with velvet stick and held at a similar temperature. The contact area, approx. 40 cm2, was similar across the four conditions.
After task completion participants rated how pleasant each condition had felt and how well they could distinguish among the four conditions, using a visual analog scale (range −10 to 10, numbers not shown). They also reported whether any stimulation condition was perceived as ticklish.
Data analysis
Functional data were processed using SPM8 (Wellcome Institute of Cognitive Neurology, London, UK). Slice-timing interpolation, head movement correction and co-registration to individual anatomical scans were performed, followed by normalization in Montreal Neurological Institute (MNI) space. Smoothing was applied through an 8 mm FWHM Gaussian kernel. At individual level, statistical maps were generated through a fixed-effects general linear model obtained convolving the experimental box-car with the canonical haemodynamic response function. Movement parameters were included as nuisance regressors. The contrasts of interest were extracted comparing each stimulation condition with rest. Group-level inferences were based on a random-effects model, which was performed as a 2-by-2 analysis of variance (ANOVA), having factors for hand vs. velvet stick and stroking vs. tapping. On group-level maps the significance threshold was set to either p<0.05 FDR-corrected or p<0.001 un-corrected (see below), and the extent threshold 5 voxels.
We also performed regions-of-interest (ROIs) analysis for the anterior (5.9 ml) and posterior (7.2 ml) insula, primary (SI, 4.5 ml, expected location of the hand knob only, z>50 mm) and secondary (SII, 6.5 ml) somatosensory cortices. These ROIs were adapted from the Automated Anatomical Labelling atlas (AAL, [13]). They were considered separately for the two hemispheres and used to measure the average blood-oxygen level-dependent signal percent change (ΔBOLD%) across the four conditions. Similar to the whole-brain analysis, 2-by-2 ANOVAs were performed followed by contrasts between stroking and tapping for hand and velvet stick, and between stroking with hand and all other conditions.
We also assessed correlations between BOLD responses and pleasantness ratings by means of linear regressions on individually-normalized values.
Results
Subjective ratings
Participants could reliably distinguish the four conditions (7.2±2.3, scale range −10 to 10), and all reported that stimulation was not ticklish. Stroking was overall more pleasant than tapping (F(1,13)=27.7, p<0.001) and hand was more pleasant than velvet stick (F(1,13)=5.8, p=0.03), without interaction. Planned t-tests indicated that stroking was more pleasant than tapping for both hand-delivered (6±2.5 vs. 0.8±3.3; t(13)=4.7, p<0.001) and velvet stick-delivered stimuli (2.7±3.8 vs. −1.6±3.7; t(13)=4.8, p<0.001).
Whole-brain analysis
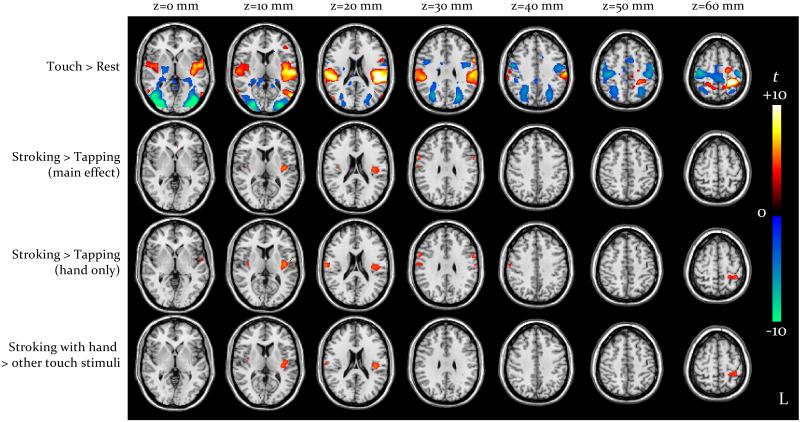
As depicted in Fig. 1 (voxel-level p<0.05, FDR-corrected), overall the four tactile stimulation conditions activated the SI area, predominantly in the left hemisphere, the left posterior temporal lobe, and the SII area and posterior insula bilaterally but predominantly on the left. No clusters were observed within the anterior insula, striatum, cingulate cortex and orbitofrontal cortex. With respect to rest, we observed deactivation of temporal-occipital visual regions, mesial parietal lobe and dorsal premotor areas.
Comparison of stroking vs. tapping (main effect) revealed greater activation in the left posterior insula (−40, −14, 12), peak t-score 6.2, cluster extent 3.0 ml, cluster-level p<0.001; the converse difference was not observed in any region. There were no main effects of hand vs. velvet stick or interactions between the two factors in any region.
Comparing stroking vs. tapping for hand only revealed a pattern similar to that observed in the main effect, i.e. stroking elicited greater activation than tapping in the left posterior insula (−42, −12, 12), peak t-score 6.3, cluster extent 4.2 ml and cluster-level p<0.001; for this contrast, we additionally observed greater engagement of the left SI area (−30, −40, 61), peak 4.8, extent 1.3 ml and p=0.007, and of the right posterior insula and contiguous regions (62, −12, 28), peak 4.9, extent 2.1 ml and p=0.001.
At the same threshold (voxel-level p<0.05, FDR-corrected), the corresponding contrast for stroking vs. tapping for velvet stick only did not reveal any effect. At the more permissive voxel-level threshold of p<0.001 un-corrected (not shown), greater activation for stroking vs. tapping with a velvet stick became detectable in the posterior insula, predominantly on the left; this activation cluster (−38, −16, 12) corresponded to that obtained for hand-delivered stimuli, but was much smaller (0.2 ml vs. 7.3 ml) and weaker (peak t-score 3.8 vs. 6.3).
Comparison of stroking with hand vs. all other conditions performed as a planned comparison (Fig. 1, voxel-level p<0.05 FDR-corrected) again revealed greater activation in the left posterior insula (−42, −16, 15), peak 5.6, extent 2.9 ml and p<0.001, and in the left SI area (−29, −38, 54), peak 4.8, extent 1.2 ml and p=0.003.
Activity in the left posterior insula (−42, −16, 15) was strongly positively correlated with reported pleasantness (r=0.47, p<0.001); no such correlation was observed for the left SI.
ROI-based analysis
No significant effects were found in the right hemisphere and in anterior insula on either side. As shown in Fig. 2, in the left posterior insula, ΔBOLD% was larger for stroking than tapping (F(1,14)=11.7, p=0.004); there was no difference between hand and velvet stick stimuli, and interaction did not reach statistical significance (p=0.06). Similarly, we found larger ΔBOLD% for stroking than tapping in SI (F(1,14)=13.4, p=0.002) and SII (F(1,14)=6.5, p=0.02). In these regions, however, the interactions were significant (F(1,14)=12.9, p=0.002 for SI and F(1,14)=7.2, p=0.02 for SII): i) the effect of stroking was significant only for hand (t(13)=4.8, p<0.001 for SI and t(13)=4.7, p<0.001 for SII), ii) the difference between hand and velvet stick emerged only during stroking (t(13)=2.8, p=0.01 for SI and t(13)=2.4, p=0.03 for SII) and iii) the comparison of stroking with a hand vs. all other conditions yielded a strongly significant difference (t(13)=4.0, p=0.002 for SI and t(13)=3.5, p=0.004 for SII).
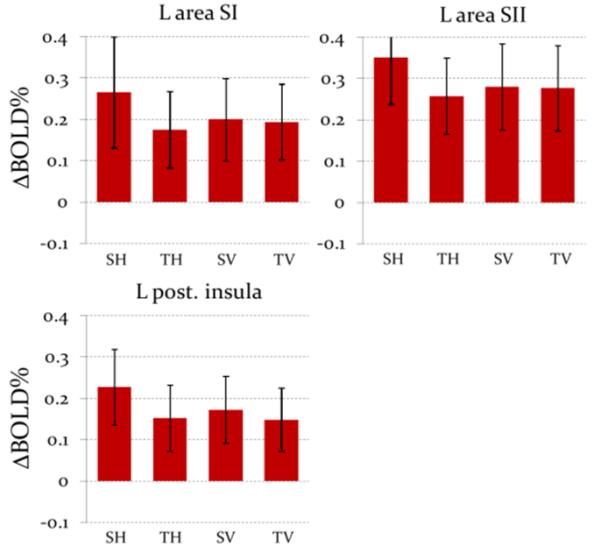
In the left posterior insula ROI, ΔBOLD% was strongly positively correlated with reported pleasantness (r=0.48, p<0.001); similar, but weaker correlations were also observed in SI (r=0.32, p=0.01) and SII (r=0.33, p=0.01).
Discussion
The key novel finding here is that type of touch (stroking vs. tapping) was significantly integrated with skin-to-skin vs. indirect nature, i.e., the difference between stroking and tapping was considerably amplified when participants were touched with hand rather than velvet stick. Even though the interaction terms did not reach significance in the whole-brain analysis, planned contrasts clearly demonstrated that the difference between stroking and tapping was stronger for hand than velvet stick-delivered stimuli. The ROI analyses revealed significant interactions in SI and SII, alongside a marginal effect in the posterior insula.
It is evidently impossible to achieve perfect perceptual matching between stimuli delivered with hand and velvet stick, due to differences in tactile texture. Indeed, in spite of our attempts to match contact area, temperature and stimulation rate as closely as possible, participants could reliably distinguish among the four conditions. This residual perceptual difference had a major effect on activity in SI and SII and, albeit more weakly, also in the insula: as indicated by both whole-brain and ROI-based analyses, stroking with a hand generated larger responses than all other conditions.
It appears plausible that this effect was at least partially mediated by cognitive and emotional correlates of the awareness of being touched directly by another person, rather than by an inanimate object. Such effects on the somatosensory system have, in fact, been previously reported for different experimental designs. For example, Gazzola et al. [12] found that male participants engaged areas SI and SII and the posterior insula more intensely when they believed that they were being touched by a female than a male experimenter. Another study found re-activation of area SI during delivery of reward related to a previously-delivered haptic stimulus [14]. Our observation of similar response patterns across SI, SII and posterior insula is in line with the notion of direct functional integration between these regions, which is demonstrated in the findings of Olausson et al. [4], whereby posterior insula activation was associated with negative BOLD responses in the deafferentated SI area of neuropathy patients.
While this experimental design cannot isolate contextual and perceptual factors, directly comparing naturalistic, hand-delivered stimuli with velvet stick touch is empirically very important, as it informs the question of how relevant existing studies performed with inanimate object touch are in terms of understating affective touch processing.
Our results for the posterior insula are in line with the notion that it embeds a representation of CT fibre afferences and is thereby strongly activated by soft touch of the hairy skin [3-5,10]. Influential models postulate that the anterior insula processes and integrates interoceptive signals from the posterior insula translating them into subjective experiences, and anterior insula engagement has indeed been frequently reported in relation to nociception, taste and thermal stimulation [15-16]. In our task, posterior insula activity strongly correlated with reported pleasantness, in keeping with the view that signals conveyed by CT fibre afferences to this region are key in determining the valence of touch stimuli [5-9]. Activity in SI and SII also correlated with pleasantness, but the effect was weaker and non-significant in whole-brain analyses; this is concordant with the fact that these regions subserve localization and discriminative functions, and are less implicated in the affective component of touch [3-5]. In parallel, absence of activity in the anterior insula suggests that complex affective experiences may not necessarily draw on this region as a major substrate when autonomic engagement is limited. Our results are in fact in line with previous work demonstrating anterior insula engagement during soft touch stimulation in neuropathy patients (likely representing a form of plasticity) but not in healthy controls [3-5,10].
While stroking with a hand was more pleasant than the other conditions, no differential effects were observed in the ventral striatum, indicating that subjective pleasurability was not subserved by mesolimbic dopaminergic activity. Since participants were asked to passively attend to the tactile stimuli, the secondary elements of reward and cognitive processing present in some more complex designs were removed here. It is likely that the lack of activation in the ventral striatum and cingulate cortex, also observed in other similar studies involving passive stimulation, is consequential to this feature [4-5,11].
Conclusion
This study provides the first explicit comparison of skin-to-skin vs. indirect human touch under two different stimulation types, stroking and tapping. The neural response to type of touch, observed in the primary and secondary somatosensory areas as well as in the posterior insula, is significantly modulated depending on whether the participant is touched with a hand or through a velvet stick. Such interaction likely arises from the combination of perceptual differences and cognitive and emotional factors related to the awareness of direct contact with another person, which cannot be isolated through the experimental comparisons performed in this study. At an empirical level, our findings inform the interpretation of existing literature, by demonstrating that stimulation with inanimate objects does not reproduce the same activity pattern expected for realistic, interpersonal affective touch. Further studies of affective touch processing need to be performed direct skin-to-skin contact to ensure ecological validity.
Acknowledgements
The study was supported by a programme grant from the Wellcome Trust to HDC. All data were acquired at the Clinical Imaging Sciences Centre (CISC) of the Brighton & Sussex Medical School (BSMS, Southern Ring Road, BN1 9RY, Falmer, UK). IUK was supported by a research fellowship from the University of Bern (CH) and performed part of the data analysis and manuscript preparation whilst attending at the Fondazione IRCCS Istituto Neurologico “Carlo Besta”.
Footnotes
Conflicts of interest: All authors declare under their own responsibility that they do not have any real or perceived conflicts of interest pertaining to the present study.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Field T. Touch. The MIT press; Cambridge, MA: 2001. [Google Scholar]
- [2].Keysers C, Kaas JH, Gazzola V. Somatosensation in social perception. Nat Rev Neurosci. 2010;11:417–28. doi: 10.1038/nrn2833. [DOI] [PubMed] [Google Scholar]
- [3].Björnsdotter M, Löken LS, Olausson H, Vallbo A, Wessberg J. Somatotopic organization of gentle touch processing in the posterior insular cortex. J. Neurosci. 2009;29:9314–9320. doi: 10.1523/JNEUROSCI.0400-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Olausson H, Cole J, Rylander K, McGlone F, Lamarre Y, Wallin GB, Krämer H, Wessberg J, Elam M, Bushnell CM, Vallbo A. Functional role of unmyelinated tactile afferents in human hairy skin: sympathetic response and perceptual localization. Exp Brain Res. 2008;184:135–140. doi: 10.1007/s00221-007-1175-x. [DOI] [PubMed] [Google Scholar]
- [5].Olausson H, Lamarre Y, Backlund H, Morin C, Wallin BG, Starck G, Ekholm S, Strigo I, Worsley K, Vallbo AB, Bushnell MC. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat. Neurosci. 2002;5:900–904. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- [6].McGlone F, Reilly D. The cutaneous sensory system. Neurosci Biobehav Rev. 2010;34:148–159. doi: 10.1016/j.neubiorev.2009.08.004. [DOI] [PubMed] [Google Scholar]
- [7].Olausson H, Wessberg J, Morrison I, McGlone F, Vallbo A. The neurophysiology of unmyelinated tactile afferents. Neurosci Biobehav Rev. 2010;34:185–9. doi: 10.1016/j.neubiorev.2008.09.011. [DOI] [PubMed] [Google Scholar]
- [8].Löken LS, Wessberg J, Morrison I, McGlone F, Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nat. Neurosci. 2009;12:547–548. doi: 10.1038/nn.2312. [DOI] [PubMed] [Google Scholar]
- [9].Vallbo A, Olausson H, Wessberg J, Norrsell U. A system of unmyelinated afferents for innocuous mechanoreception in the human skin. Brain Res. 1993;628:301–304. doi: 10.1016/0006-8993(93)90968-s. [DOI] [PubMed] [Google Scholar]
- [10].McCabe C, Rolls ET, Bilderbeck A, McGlone F. Cognitive influences on the affective representation of touch and the sight of touch in the human brain. Soc Cogn Affect Neurosci. 2008;3:97–108. doi: 10.1093/scan/nsn005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rolls ET, O’Doherty J, Kringelbach ML, Francis S, Bowtell R, McGlone F. Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cereb. Cortex. 2003;13:308–317. doi: 10.1093/cercor/13.3.308. [DOI] [PubMed] [Google Scholar]
- [12].Gazzola V, Castelli F, Spezio M, Etzel J, Adolphs R, Keysers C. Social modulation of touch representation (abstract). Meeting of the Cognitive Neuroscience Society.2009. [Google Scholar]
- [13].Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated Anatomical Labeling of activations in SPM using a Macroscopic Anatomical Parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- [14].Pleger B, Blankenburg F, Ruff CC, Driver J, Dolan RJ. Reward facilitates tactile judgments and modulates hemodynamic responses in human primary somatosensory cortex. J. Neurosci. 2008;28:8161–8168. doi: 10.1523/JNEUROSCI.1093-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- [16].Craig AD. Interoception: the sense of the physiological condition of the body. Curr. Opin. Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]