Abstract
SETTING
Tuberculosis (TB) affected households in impoverished shantytowns, Lima, Peru.
OBJECTIVE
To evaluate socio-economic interventions for strengthening TB control by improving uptake of TB care and prevention services.
DESIGN
Barriers to TB control were characterised by interviews with TB-affected families. To reduce these barriers, a multidisciplinary team offered integrated community and household socio-economic interventions aiming to: 1) enhance uptake of TB care by education, community mobilisation and psychosocial support; and 2) reduce poverty through food and cash transfers, microcredit, microenterprise and vocational training. An interim analysis was performed after the socio-economic interventions had been provided for 2078 people in 311 households of newly diagnosed TB patients for up to 34 months.
RESULTS
Poverty (46% earned <US$1 per day), depression (40%), stigmatisation (77%), and perceived isolation (39%) were common among TB patients (all P < 0.05 vs. non-patients). The project had 100% recruitment, and involved 97% of TB-affected households in regular visits, 71% in community groups, 78% in psychosocial support and 77% in poverty-reduction interventions. The socio-economic interventions were associated with increases in household contact TB screening (from 82% to 96%); successful TB treatment completion (from 91% to 97%); patient human immunodeficiency virus testing (from 31% to 97%); and completion of preventive therapy (from 27% to 87%; all P < 0.0001).
CONCLUSIONS
Socio-economic interventions can strengthen TB control activities.
Keywords: tuberculosis, control, microcredit, poverty, social determinants
TUBERCULOSIS (TB) is the archetypal disease of poverty, and social inequalities undermine TB control.1,2 Poverty predisposes individuals to TB through multiple mechanisms, such as malnutrition,3,4 and TB worsens poverty as it increases expenses and reduces income.5–9 Furthermore, poor TB-affected households often experience stigmatisation, adding barriers to TB control.10–13 Poor people at the greatest risk of TB are therefore, in many settings, also the least able to access TB care.14
Global TB control efforts focus on identifying and curing cases.15 The DOTS-based approach is one of the most effective public health interventions ever implemented.16,17 However, the annual global number of TB cases continues to increase.18 Recent analyses by the World Health Organization19 and others20 have determined that TB rate changes are driven by changes in socio-economic determinants more than by TB control efforts, strengthening a growing consensus that the successes of the DOTS strategy for TB control should be strengthened by socio-economic interventions.2,21,22 There is, however, remarkably little evidence to inform policy makers about how socio-economic interventions should be used to strengthen TB control and reduce TB-related health inequalities.23
Peru has an acclaimed TB control programme but high TB incidence24,25 and multidrug-resistant TB (MDR-TB) rates that have approximately doubled over the last decade to the highest prevalence in the Americas.18 Significant case-finding and treatment access challenges remain in TB hotspots,26 including the shantytowns surrounding Lima.27
The Innovative Socio-economic Interventions Against TB (ISIAT) project aims to develop and evaluate socio-economic interventions to strengthen TB control by improving uptake of TB care and TB prevention services and reducing poverty-related TB risk factors in TB-affected households. Many projects offer socio-economic interventions to support TB-affected households, but this on-going project additionally focuses on rigorously evaluating the feasibility, effectiveness and impact of these interventions to inform policy makers on how to integrate socio-economic interventions with biomedical TB control interventions.
METHODS
Setting
Eight contiguous shantytowns in northern Lima were selected due to high levels of TB, MDR-TB and poverty. This desert region has a registered population of 127 374 that is approximately doubled by migrant workers.
Baseline studies
Baseline studies were used from 2003 to 2007 to characterise potential socio-economic risk factors for TB and barriers to TB control. Qualitative studies used interviews and focus groups to explore perceived barriers to TB control.28 Quantitative studies used questionnaires in the households of newly diagnosed patients, their household contacts and healthy controls, who were randomly selected from the same communities using a census. The measures of poverty, depression, stigma and social capital and the sample sizes are detailed in the Table.
Table.
Baseline study of socio-economic barriers to TB control comparing TB patients, their household contacts and randomly selected community controls prior to the socio-economic interventions*
| TB patients % (n/N) |
Household contacts % (n/N) |
Community controls % (n/N) |
P values |
|||
|---|---|---|---|---|---|---|
| Patients vs. contacts |
Patients vs. controls |
Contacts vs. controls |
||||
| Income <1US$/person/day† | 46 (426/932) | † | 32 (149/471) | † | <0.0001 | † |
| Poverty (high multi dimensional score)‡ | 39 (358/921) | 33 (592/2035) | 25 (114/461) | <0.0001 | <0.0001 | 0.06 |
| Any depression§ | 40 (276/691) | 26 (50/193) | 26 (124/478) | 0.0004 | <0.0001 | 0.9 |
| Severe depression§ | 12 (83/691) | 9 (17/193) | 6 (29/478) | 0.2 | 0.0007 | 0.2 |
| TB stigma experienced¶ | 77 (546/708) | 72 (1026/1429) | § | 0.01 | § | § |
| Severe TB stigma (high stigma score)¶ | 37 (265/708) | 17 (237/1429) | § | <0.0001 | § | § |
| Isolation (low social capital score)# | 39 (401/1029) | 26 (63/239) | 24 (100/424) | 0.0003 | <0.0001 | 0.4 |
All data were assessed by questionnaires in the first interview, except for stigma, which was assessed in the last month of each patient’s treatment. The poverty, stigma and social capital scores were generated using principal component analysis in which non-contributory survey questions were eliminated iteratively. Scores were then generated based on first principal component.29 High and low scores were defined as respectively the highest and lowest terciles. The denominators differ between variables, as not all data were collected from all participants in the baseline studies.
Self-reported income was shared within households and could not therefore be differentiated between patients and their household contacts.
The multi-dimensional poverty score included education, housing conditions, basic services and assets using a total of 13 variables.
Depression was assessed with the 20-question Beck Depression Inventory that was applied during each participant’s first interview by the project team. Standard cut-offs for this questionnaire were used to define any depression and severe depression.
TB-related stigma experienced and perceived in the home and community was assessed using 22 questions. Controls did not have TB and their experience of TB-related stigmatisation was therefore not assessed.
The social capital score summarised 83 questions encompassing the micro, meso and macro-levels of social capital, including support, perceived safety and trust. TB = tuberculosis.
Conceptual framework
A conceptual framework was designed integrating information from baseline studies, TB control literature and opinions of TB stakeholders, including health care workers and TB patients, to characterise how socio-economic interventions may support TB control. The hypothesis underlying intervention development is that social and economic activities may result in defined outputs and outcomes along the TB causal pathway, improving uptake of measures of TB care and prevention services and reducing poverty-related TB risk factors. This conceptual framework is shown in Figure 1.
Figure 1.
Conceptual framework for the ISIAT project. The upper rectangles show the principal project activities, the rounded rectangles show the project outputs, and the shapes at the bottom of the Figure show the project outcomes along the TB causal pathway. Italicised bracketed statements indicate project data shown in Figures 2 and 3. TB = tuberculosis; HIV = human immunodeficiency virus; MDR-TB = multidrug-resistant TB; ISIAT = Innovative Socio-economic Interventions Against Tuberculosis.
Interventions
Interventions in the first community commenced in December 2007, and have since expanded, community by community, to eight shantytowns. This stepwise implementation aims to facilitate impact evaluation and to create an active civil society by introducing interventions simultaneously to geographically clustered TB-affected households. In each community, after interventions commence, all subsequently diagnosed patients and their household contacts are invited to participate. This article presents an interim analysis of data up to the start of October 2010. At this time the first recruited community had received socio-economic interventions for 34 months, and the eighth and last recruited community had received interventions for 3 months.
Target population
Interventions focus on TB patients and their household contacts, as they are at high risk of poverty and future TB recurrence and transmission. Household contacts are defined as people spending >2 hours thrice weekly in the home with a TB patient during their illness. Work-place contacts are not considered because stable employment is uncommon in this setting.
Project team
The project team consists of nurses, health promoters, vocational trainers, microcredit/microenterprise officers, a sociologist, a psychologist, a nutritionist, diagnosticians, equity advocates, physicians and logisticians.
Mechanisms of intervention
The main mechanisms of the intervention are household visits, workshops and partnering with existent organisations (e.g., microcredit and training organisations) to connect them with TB-affected households.
Socio-economic activities
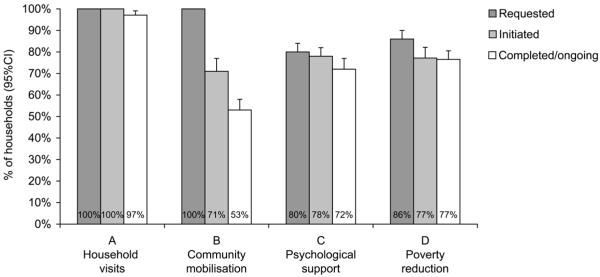
Socio-economic activities are: 1) visits to the homes of all TB-affected households; 2) fortnightly community-mobilisation workshops and other workshops with specific objectives, principally income-generation; 3) psychological counselling, principally for depression and substance abuse; 4) poverty reduction activities involving poverty mitigation with food and cash transfers; and income generation by microenterprise, microcredits and vocational training (Figures 1 and 2). Outputs of these activities are as follows:
Figure 2.
Socio-economic activities that participants requested, that were initiated and completed or are ongoing in the ISIAT project. The numbers in the bars are percentages of the 311 participating households; complete data are available for all 12 measures. For household visits and poverty reduction, the data indicate the % of households that requested (grey), initiated (light grey) and completed or have ongoing (white) activities. For community mobilisation, the white ‘completed/ongoing’ bar indicates the % of households that have participated in more than a quarter of the community workshops that it would have been possible for them to attend. For psychological support, the grey ‘requested’ bar indicates households that either requested psychological support or were identified by the project recruitment questionnaire as having mental health issues (principally depression or substance abuse). CI = confidence interval; ISIAT = Innovative Socio-economic Interventions Against Tuberculosis.
Health promotion
This takes a human rights approach to TB care and aims to help patients overcome psychosocial barriers to increase their uptake of TB-related prevention, diagnosis and treatment. The specific measures of these outputs are shown at the bottom of Figure 1 and in Figure 3: patient health insurance registration (assessed by questionnaire); successful treatment completion; MDR-TB and human immunodeficiency virus (HIV) testing; contact screening and preventive therapy uptake (all assessed by questionnaire and clinical records).
Figure 3.
Outcomes of the first 34 months of socio-economic interventions in the ISIAT project. The numbers in each bar indicate the percentage of participants. For the grey bars ‘pre-intervention (baseline)’ data were available for: A) 216 patients; B) 642 household contacts; C) 1554 patients; D) 190 patients for MDR-TB testing and 72 patients for HIV testing; E) 2829 contacts for preventive therapy initiation and 1116 contacts for preventive therapy completion. For the white ‘after socio-economic intervention’ bars, data were available for: A) 318 patients; B) their 748 household contacts; C) 307 patients; D) 307 patients for MDR-TB testing and 318 patients for HIV testing; E) for 542 contacts for preventive therapy initiation and 441 contacts for preventive therapy completion. * Indicates P < 0.00001 for pre-interventions vs. post-interventions. CI = confidence interval; ISIAT = Innovative Socio-economic Interventions Against Tuberculosis; MDR-TB = multidrug-resistant tuberculosis; HIV = human immunodeficiency virus.
Poverty reduction
Poverty reduction aims to overcome TB-associated financial challenges, to provide incentives and enable health promotion and to reduce poverty-related TB risk factors.
Crosscutting outputs
Crosscutting outputs that intersect with both the health promotion and poverty reduction interventions aim to be socially just and also to target resources at those most at risk. These include: empowerment, aiming to unite TB-affected people into an active civil society for mutual support; advocacy, with representatives influencing health care policies; and equity, which is promoted by assessing all households with a multidimensional poverty scale and prioritising the poorest, least educated, homeless and unregistered. Biosafety for the project participants and staff is increased by mask use, natural ventilation,30 facilitating early diagnosis of TB/MDR-TB, and administrative controls to ensure that patients are only invited to group activities after they have become non-infectious.
Evaluation
Evaluation includes identifying operational lessons learnt from implementing the project interventions and assessing their effects on outcomes in the TB causal pathway (Figure 1). For the current interim analysis, the outcome variables are compared between the baseline study data vs. the results after up to 34 months of socio-economic interventions. These data are compared using the two-sample z-test of proportions in an intention-to-treat analysis that includes all patients and their household contacts diagnosed within the intervention communities, irrespective of their recruitment and participation. The project is also designed to facilitate future impact evaluation with long-term follow-up to compare between intervention and non-intervention areas the outcomes poverty, equity and TB/MDR-TB rates that are not part of the current interim analysis.
RESULTS
Baseline evaluations
Baseline evaluations are summarised in the Table, demonstrating frequent poverty and depression and that TB patients were significantly more poor, depressed and isolated than controls (all P < 0.001). Household contacts had intermediate values for all these measures. Stigmatisation was common and was significantly more frequently experienced by patients than their household contacts (P = 0.0001).
Intervention population
The intervention population constitutes 311 households in which the National TB Programme (NTP) diagnosed 311 TB patients, all of whom accepted to participate. TB was subsequently diagnosed in 25 (1.4%) of their 1767 participating household contacts. The intervention population for this interim analysis therefore constitutes 2078 individuals, 336 of whom had TB. Four other patients (who lived in four different intervention communities) were excluded, as one was a staff member and three moved to high-altitude locations as soon as they were diagnosed, as they believed that this would cure TB.
Concurrently, in areas not yet receiving interventions, 1043 newly diagnosed TB patients and their 5470 household contacts were interviewed, generating data that may facilitate future project impact evaluation; however, their data do not feature in this article.
Intervention activities
Intervention activities are summarised in Figure 2, which shows the percentage of the 311 participating households that requested, initiated and have on-going/completed participation in each activity.
Psychosocial activities
Household visits are ongoing for 97% of the participants. Community workshops (n = 533) have created groups of TB-affected households in all eight communities. Of these, 71% of households have participated and 53% are currently actively participating in the community workshops. Each community group has elected advocacy representatives to represent them in regional health and political meetings, and a quarter of the ISIAT team are also former TB patients. All newly diagnosed patients undergo psychological assessment; the project psychologist has visited 96% of TB-affected households and has provided counselling to 78% of households, 23% >5 times. The project psychologist has also contributed to most community workshops. The project is facilitating gender empowerment activities, including education, workshops and a mothers’ pooled child care co-operative to help women contribute to household incomes.28
Poverty reduction activities
Poverty reduction activities were requested by 86% of TB-affected households and initiated by 77%.
Microcredit loans
Microcredit loans were requested by 35% of households, 20% initiated them, and 11% have so far r epaid or are on schedule, whereas 9.6% have defaulted or are in arrears. The project has coordinated 117 microcredits involving TB-affected households, 73% as personal (not group) loans. Overall, 12% of households that borrowed once subsequently took out another, larger loan. Microcredits totalled US$50 868, which is equivalent to 132 years of median income in TB-affected households in this community (US$384/person/year).
Vocational training
Vocational training was provided by local organisations and was initiated by members of 8.7% of the TB-affected households. Income increased for 3.2% of participating households as a result of vocational training, which cost an average of US$86 per person trained. Members of 0.96% of the households gained formal salaried employment as a result of project-sponsored vocational training. Consequently, the project has shifted emphasis from formal vocational training to subsidising group training during the initiation of microenterprises.
Microenterprise activities
Microenterprise activities are supported by the project by providing advice, expertise, training and facilitation. Examples include raising animals (rabbits and chickens) and home-based manufacturing (e.g., foodstuffs, recycling, greeting cards, knitting, weaving, jewellery, toy and handicraft manufacture). Products are consumed in the household and are sold locally and internationally.
Food and cash transfers
Food and cash transfers constitute an important part of most project activities, and have averaged US$160 in value per household (42% of per capita, 10% of TB-affected household median income). Food (23% of transfer value) is provided at all project events, and food packages are provided in patients’ homes. A project nutritionist has optimised food packages to increase their potential to strengthen immunity against TB. Cash transfers have averaged 13% of transfer value for TB diagnosis and treatment costs supplementary to the main aspects of TB care, which are provided free by the NTP; 25% of transfer value for TB-related transport expenses; and 39% of transfer value for poverty reduction (excluding microcredit loans).
Outcomes
Outcomes (shown in Figure 3) compare the baseline study with interim analysis of the outcomes after up to 34 months of socio-economic interventions. Significant increases from baseline are observed in the following indicators: health insurance registration (from 36% to 98%); household contact TB screening (from 82% to 96%); successful TB treatment completion (from 91% to 97%); uptake of rapid MDR-TB testing (from 67% to 92%); patient HIV testing (from 31% to 97%); preventive therapy initiation (from 39% to 88%); and preventive therapy completion (from 27% to 87%; all P < 0.0001, Figure 3). All 35 patients in the intervention communities with MDR-TB are taking recommended therapy; the median time from laboratory diagnosis of MDR-TB to initiation of appropriate therapy was 41 days (interquartile range 54 days), despite considerable efforts to facilitate more rapid access.
Challenges
Challenges to activities and staff retention include stigma, crime, harsh working conditions and bio-hazard from MDR-TB/XDR-TB (extensively drug-resistant tuberculosis).
DISCUSSION
This project provides evidence that socio-economic interventions can positively impact TB control activities. Implementation of integrated health promotion and poverty reduction interventions by a community-based multidisciplinary team was associated with marked improvements in uptake of TB prevention, diagnosis and treatment services. The importance of these interim findings is emphasised by the baseline evaluations, which demonstrated TB to be associated with poverty, despair, stigmatisation and isolation. Our socio-economic interventions were designed to diminish these barriers to TB care and appeared to considerably increase uptake of the TB control services that the NTP offers free of charge with social and nutritional support.
Most participants engaged in project economic activities that aimed to reduce TB-associated poverty. These economic opportunities were associated with the formation of an active civil society of TB-affected households, with advocacy activities giving the group a collective voice in regional decisions influencing TB care. The resulting community of TB-affected households was provided with education recommending priorities for their response to TB, nutritional incentives to encourage participation, and financial support to subsidise the indirect costs of accessing national TB care. Thus economic and principally nutritional incentives facilitated health promotion, improving access to TB control efforts.
Microcredit has been commended for reducing poverty,31 but also criticised for neglecting the poorest.32–34 Our experiences support both perspectives. Initially, microcredits used the village banking system with shared group responsibility, as personal loans are perceived to be higher risk in this context. Over the first 6 months of the project, microcredits were requested by most households; however, only one household initiated a loan. Focus groups indicated that stigma and financial pressures associated with TB hampered village banking loans; we therefore negotiated personal microcredits that increased uptake. Still, less than a quarter of households initiated microcredits. The principal barrier was reluctance by microcredit organisations to offer high-risk loans to the very poor, especially participants living in informal, unregistered housing or who had bad credit histories. To address this, we established an independent microcredit guarantee fund that assumed all financial risk for unpaid loans. We found microcredit to be a valuable opportunity and incentive, although one that did not optimally serve the poorest TB-affected households. We continue to explore approaches to better serve the economic needs of the poorest TB-affected households, and food transfers play an increasingly important role in the ISIAT project.32,34
The modest uptake of income-generating activities so far contrasts with the observation that these appear to have been powerful incentives for participation and improvements in access to TB care. By combining the requirements of biomedical TB control with economic opportunity and community mobilisation, there has been a marked increase in the uptake of TB control interventions.
We identified an association between TB and severe depression that was a prominent barrier to project activities and TB care. We initially responded by facilitating pharmacological treatment; however, this seemed ineffective and unsustainable. In contrast, the subsequent recruitment of a clinical psychologist to the project team has been of considerable value and is the subject of specific ongoing evaluation.
This interim analysis of an evolving project has several limitations. Although the long-term vision is to work with all of the most vulnerable members of communities, TB risk is difficult to predict, and consequently our evaluation of socio-economic interventions focuses on a well-defined high-risk group, TB-affected households.35 Importantly, the current before vs. after assessment of the socio-economic interventions may be confounded by concurrent incidental changes in the NTP, community development and ascertainment bias by the project team. Planned longer-term assessment of impact by concurrently comparing intervention vs. non-intervention areas should overcome these limitations and offer an opportunity to assess impact on poverty-associated TB risk factors, TB disease and MDR-TB. This planned analysis will also allow the assessment of associations between uptake of the different types of interventions and improvements in outcome measures and analysis of how equitable the project outputs are.
In conclusion, socio-economic interventions were adapted to the needs of TB-affected households living in impoverished peri-urban shantytowns. The socio-economic interventions successfully engaged most TB-affected households in an active civil society that was associated with marked improvements in uptake of TB prevention, diagnosis and treatment, resulting in strengthened TB control.
Acknowledgements
The authors are grateful for the contributions of the entire research team, not all of whom meet the criteria to be co-authors; for expert administrative support from P Maguina, S Carrera and M Rivero; and to the patients who agreed to put aside TB-associated stigma to participate in this project. This research was funded principally by a grant from the Civil Society Challenge Fund of the Department for International Development of the British Government, the charity IFHAD: Innovation For Health And Development and the Wellcome Trust. Members of the project team and specific project activities were also funded by the World Health Organization, the Sir Halley Stewart Trust, the Foundation for Innovative New Diagnostics and the Bill & Melinda Gates Foundation.
Footnotes
Publisher's Disclaimer: Disclaimers and approvals: All personal identifiable and research data were collected with informed written consent by patients and/or their parents/guardians. Ethical approval for research aspects of the project was granted by internationally accredited institutional review boards including Uni versidad Peruana Cayetano Heredia, Lima, Peru. The project and research described were performed with the approval and collaboration of the Peruvian Ministry of Health. These organisations and the funding sources had no decision-making role in the analysis, interpretation or documentation of this research.
References
- 1.Hanson C. Tuberculosis, poverty and inequity: a review of the literature and discussion of issues. World Bank; Washington DC, USA: 2002. [Google Scholar]
- 2.Lönnroth K, Castro KG, Chakaya JM, et al. Tuberculosis control and elimination 2010–50: cure, care, and social development. Lancet. 2010;375:1814–1829. doi: 10.1016/S0140-6736(10)60483-7. [DOI] [PubMed] [Google Scholar]
- 3.Lönnroth K, Williams BG, Cegielski P, Dye C. A consistent log-linear relationship between tuberculosis incidence and body mass index. Int J Epidemiol. 2010;39:149–155. doi: 10.1093/ije/dyp308. [DOI] [PubMed] [Google Scholar]
- 4.Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis. 2004;8:286–298. [PubMed] [Google Scholar]
- 5.Lönnroth K, Aung T, Maung W, Kluge H, Uplekar M. Social franchising of TB care through private GPs in Myanmar: an assessment of treatment results, access, equity and financial protection. Health Policy Plan. 2007;22:156–166. doi: 10.1093/heapol/czm007. [DOI] [PubMed] [Google Scholar]
- 6.Pantoja A, Lönnroth K, Lal SS, et al. Economic evaluation of public-private mix for tuberculosis care and control, India. Part II: Cost and cost-effectiveness. Int J Tuberc Lung Dis. 2009;13:705–712. [PubMed] [Google Scholar]
- 7.Pantoja A, Floyd K, Unnikrishnan KP, et al. Economic evaluation of public-private mix for tuberculosis care and control, India. Part I: Socio-economic profile and costs among tuberculosis patients. Int J Tuberc Lung Dis. 2009;13:698–704. [PubMed] [Google Scholar]
- 8.Kemp JR, Mann G, Simwaka BN, Salaniponi FM, Squire SB. Can Malawi’s poor afford free tuberculosis services? Patient and household costs associated with a tuberculosis diagnosis in Lilongwe. Bull World Health Organ. 2007;85:580–585. doi: 10.2471/BLT.06.033167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajeswari R, Balasubramanian R, Muniyandi M, et al. Socio-economic impact of tuberculosis on patients and family in India. Int J Tuberc Lung Dis. 1999;3:869–877. [PubMed] [Google Scholar]
- 10.Atre S, Kudale A, Morankar S, Gosoniu D, Weiss MG. Gender and community views of stigma and tuberculosis in rural Maharashtra, India. Glob Public Health. 2009;13:1–16. doi: 10.1080/17441690903334240. [DOI] [PubMed] [Google Scholar]
- 11.Dhingra VK, Khan S. A sociological study on stigma among TB patients in Delhi. Indian J Tuberc. 2010;57:12–18. [PubMed] [Google Scholar]
- 12.Pungrassami P, Kipp AM, Stewart PW, et al. Tuberculosis and AIDS stigma among patients who delay seeking care for tuberculosis symptoms. Int J Tuberc Lung Dis. 2010;14:181–187. [PMC free article] [PubMed] [Google Scholar]
- 13.Jittimanee SX, Nateniyom S, Kittikraisak W, et al. Social stigma and knowledge of tuberculosis and HIV among patients with both diseases in Thailand. PLoS ONE. 2009;4:e6360. doi: 10.1371/journal.pone.0006360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization . Addressing poverty in TB control: options for national TB control programmes. WHO; Geneva, Switzerland: 2005. WHO/HTM/TB/2005.352. [Google Scholar]
- 15.Raviglione MC, Pio A. Evolution of WHO policies for tuberculosis control, 1948–2001. Lancet. 2002;359:775–780. doi: 10.1016/s0140-6736(02)07880-7. [DOI] [PubMed] [Google Scholar]
- 16.World Bank . World development report 1993: Investing in Health. Oxford University Press; New York, NY, USA: 1993. [Google Scholar]
- 17.Laxminarayan R, Klein E, Dye C, Floyd K, Darley S, Odeji O. Economic benefit of tuberculosis control. Policy research working paper 4295. World Bank; Washington DC, USA: 2007. [Google Scholar]
- 18.World Health Organization . WHO report 2009. Global tuberculosis control: surveillance, planning, financing. WHO; Geneva, Switzerland: 2009. WHO/HTM/TB/2009.411. [Google Scholar]
- 19.Dye C, Lönnroth K, Jaramillo E, Williams BG, Raviglione M. Trends in tuberculosis incidence and their determinants in 134 countries. Bull World Health Organ. 2009;87:683–691. doi: 10.2471/BLT.08.058453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oxlade O, Schwartzman K, Behr MA, et al. Global tuberculosis trends: a reflection of changes in tuberculosis control or in population health? Int J Tuberc Lung Dis. 2009;13:1238–1246. [PubMed] [Google Scholar]
- 21.Hargreaves JR, Boccia D, Evans CA, Adato M, Petticrew M, Porter JD. The social determinants of tuberculosis: from evidence to action. Am J Public Health. 2011;101:654–662. doi: 10.2105/AJPH.2010.199505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lönnroth K, Jaramillo E, Williams BG, Dye C, Raviglione M. Chapter 12. Tuberculosis: the role of risk factors and social determinants. In: Blas E, Kurup AS, editors. Equity, social determinants and public health programmes. World Health Organization; Geneva, Switzerland: 2010. pp. 221–237. [Google Scholar]
- 23.Thim S, Sath S, Sina M, et al. A community-based tuberculosis program in Cambodia. JAMA. 2004;292:566–568. doi: 10.1001/jama.292.5.566-c. [DOI] [PubMed] [Google Scholar]
- 24.Suarez PG, Watt CJ, Alarcon E, et al. The dynamics of tuberculosis in response to 10 years of intensive control effort in Peru. J Infect Dis. 2001;184:473–478. doi: 10.1086/322777. [DOI] [PubMed] [Google Scholar]
- 25.Shin SS, Yagui M, Ascencios L, et al. Scale-up of multidrug-resistant tuberculosis laboratory services, Peru. Emerg Infect Dis. 2008;14:701–708. doi: 10.3201/eid1405.070721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ford CM, Bayer AM, Gilman RH, et al. Factors associated with delayed tuberculosis test-seeking behavior in the Peruvian Amazon. Am J Trop Med Hyg. 2009;81:1097–1102. doi: 10.4269/ajtmh.2009.08-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanghavi DM, Gilman RH, Lescano-Guevara AG, et al. Hyperendemic pulmonary tuberculosis in a Peruvian shantytown. Am J Epidemiol. 1998;148:384–389. doi: 10.1093/oxfordjournals.aje.a009657. [DOI] [PubMed] [Google Scholar]
- 28.Onifade DA, Bayer AM, Montoya R, et al. Gender-related factors influencing tuberculosis control in shantytowns: a qualitative study. BMC Public Health. 2010;10:381. doi: 10.1186/1471-2458-10-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartholomew DJSF, Moustaki I, Galbraith JI. Principal component analysis: the analysis and interpretation of multivariate data for social scientists. Chapman & Hall/CRC; Boca Raton, FL, USA: 2002. pp. 115–142. [Google Scholar]
- 30.Escombe AR, Oeser CC, Gilman RH, et al. Natural ventilation for the prevention of airborne contagion. PLoS Med. 2007;4:e68. doi: 10.1371/journal.pmed.0040068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldberg N. Measuring the impact of microfinance: taking stock of what we know. Grameen Foundation; Washington DC, USA: 2005. [Google Scholar]
- 32.Ahmed SM. Global Forum for Health Research. Global Forum Update on Research for Health Volume 4. Equitable access: research challenges for health in developing countries. Pro-Brook Publishing; London, UK: 2007. Combining health and social protection measures to reach the ultra-poor: experiences of BRAC. [Google Scholar]
- 33.Ahmed SM, Masud A K M Rana. Customized development interventions for the ultra poor: preliminary changes assessment of health and health seeking-behaviour (CFPR/TUP 2002 to 2004) BRAC; Dhaka, Bangladesh: 2005. (CFPR/TUP Working Paper Series No. 7). [Google Scholar]
- 34.Halder SR, Mosley P. Working with the extra-poor: learning from BRAC experiences. J Int Dev. 2004;16:387–406. [Google Scholar]
- 35.Morrison J, Pai M, Hopewell PC. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8:359–368. doi: 10.1016/S1473-3099(08)70071-9. [DOI] [PubMed] [Google Scholar]