Abstract
Magnetic resonance imaging (MRI) with oxygen challenge (T2* OC) uses oxygen as a metabolic biotracer to define penumbral tissue based on CMRO2 and oxygen extraction fraction. Penumbra displays a greater T2* signal change during OC than surrounding tissue. Since timely restoration of cerebral blood flow (CBF) should salvage penumbra, T2* OC was tested by examining the consequences of reperfusion on T2* OC-defined penumbra. Transient ischemia (109±20 minutes) was induced in male Sprague-Dawley rats (n=8). Penumbra was identified on T2*-weighted MRI during OC. Ischemia and ischemic injury were identified on CBF and apparent diffusion coefficient maps, respectively. Reperfusion was induced and scans repeated. T2 for final infarct and T2* OC were run on day 7. T2* signal increase to OC was 3.4% in contralateral cortex and caudate nucleus and was unaffected by reperfusion. In OC-defined penumbra, T2* signal increased by 8.4%±4.1% during ischemia and returned to 3.25%±0.8% following reperfusion. Ischemic core T2* signal increase was 0.39%±0.47% during ischemia and 0.84%±1.8% on reperfusion. Penumbral CBF increased from 41.94±13 to 116.5±25 mL per 100 g per minute on reperfusion. On day 7, OC-defined penumbra gave a normal OC response and was located outside the infarct. T2* OC-defined penumbra recovered when CBF was restored, providing further validation of the utility of T2* OC for acute stroke management.
Keywords: ADC, CBF, imaging, MCAO, T2*
Introduction
The most effective intervention for acute ischemic stroke is reperfusion (Molina and Saver, 2005). As ischemic stroke refers to the sudden loss of blood flow in a cerebral artery due to a blockage, the mechanical or drug-induced restoration of blood flow with its accompanying nutritive delivery by reperfusion enables salvage of previously injured (penumbral) tissue. In 1996, thrombolysis with recombinant tissue plasminogen activator was approved by the US Food and Drug Administration for acute ischemic stroke of <3 hours duration. However, patient ineligibility means that fewer than 10% of all stroke patients can be thrombolyzed (Cocho et al, 2005; Molina and Saver, 2005).
In May 2009, the American Heart Association/American Stroke Association recommended an extension of the window for acute ischemic stroke, approving recombinant tissue plasminogen activator (alteplase) as a treatment up to 4.5 hours after symptom onset. The decision was primarily based on the findings from the third European Cooperative Acute Stroke Study trial, which confirmed a significant reduction in disability at the 90-day time period after recombinant tissue plasminogen activator treatment between 3 and 4.5 hours (Hacke et al, 2008). More recently, a time profile of benefit and harm for alteplase in a pooled analysis of eight randomized trials concluded that one in three patients had improved outcomes when treated between 1 and 3 hours from symptom onset, while one in six benefitted in the 3- to 4.5-hour time window. Significantly, risk may outweigh benefit beyond the 4.5-hour time point (Lees et al, 2010), and this was supported by a previous Cochrane metaanalysis (Wardlaw et al, 2009).
In thrombolysis studies where penumbra was identified by the mismatch between the perfusion deficit and the abnormality on diffusion-weighted imaging (perfusion diffusion mismatch), patients experienced improved clinical outcomes up to 6 hours after symptom onset, compared with the standard noncontrast computed tomography-guided therapy (Köhrmann et al, 2006; Schellinger et al, 2007). This MRI technique, however, has not been validated and is imprecise: DWI lesions have been found to disappear spontaneously or following thrombolysis in both animals and humans (Kidwell et al, 2000). As yet, apparent diffusion coefficient (ADC) values have failed to distinguish between tissue destined to die and the potentially recoverable penumbra (Guadagno et al, 2004). Similarly, the extent of the perfusion deficit is dependent upon the methods and thresholds used to define it, and it may also include benign oligemic tissue that is fated to survive (Butcher et al, 2005).
There is no current MRI technique that accurately detects tissue viability and which could be used in routine clinical practice to identify patients likely to benefit from therapy such as thrombolysis, flow enhancement, or neuroprotection. We hypothesized that alternative techniques that determine tissue metabolic status would represent an advance on current clinical imaging of the penumbra, establishing patient selection criteria for therapeutic strategies outwith current rigid time windows. The oxygen challenge (OC) MRI technique uses a transient hyperoxic challenge to identify changes in deoxyhemoglobin:oxyhemoglobin ratios, detected by T2*-weighted MRI (Santosh et al, 2008). Paramagnetic deoxyhemoglobin and free oxygen in the plasma reduce T2* signal, while diamagnetic oxyhemoglobin has a minimal influence on T2*. Following stroke, penumbral oxidative metabolism (CMRO2) is maintained in the face of reduced cerebral perfusion pressure by increasing oxygen extraction fraction (OEF) (Powers, 1991). This increases the deoxy:oxyhemoglobin ratio in the vasculature, resulting in a decreased T2* signal within penumbra. Increased oxygen delivery during OC will convert deoxyhemoglobin to oxyhemoglobin with a resultant increase in T2* signal, the magnitude of which should be greatest in regions with greatest OEF. T2* maps can be generated that locate and quantify the percentage change in T2* signal throughout the territory of the occluded artery. In addition, the maintenance of this increased signal during the OC and its return back to baseline following OC is consistent with T2* signal change indicating oxygen consumption (Santosh et al, 2008; Robertson et al, 2011). This technique may therefore yield information on oxygen metabolism that more closely correlates with positron emission tomography definitions of the penumbra (Baron et al, 1981).
The aim of the current study was to validate the T2* OC MRI technique based on the consequences of reperfusion. Our stated hypotheses were that T2* OC-defined penumbra should show signs of recovery following early restoration of flow and that its T2* response to OC following reperfusion should resemble the signal change in normal, nonischemic tissue. This was tested acutely after reperfusion and again at day 7. Evidence of tissue recovery in T2* OC-defined penumbra was determined from changes in cerebral blood flow (CBF) and ADC acutely following reperfusion. T2* OC maps during ischemia were also coregistered with T2-defined final infarct to confirm that tissue identified as penumbra had not become incorporated into final infarct.
Materials and methods
Rodent Middle Cerebral Artery Occlusion Surgery
Experiments were performed under license from the UK Home Office and were subject to the Animals (Scientific Procedures) Act, 1986. Male Sprague-Dawley rats (306±12 g, n=8, Harlan, Bicester, UK) were initially anesthetized with 5% inhaled isoflurane in an induction chamber at room temperature. Following intubation, animals were artificially ventilated with 2% isoflurane delivered in air, slightly enriched with oxygen (30%) to maintain physiological stability throughout the experiment. Blood gases were maintained within the normal physiological range apart from increased arterial partial pressure of oxygen (Pa2) during the OC. Pa2 was maintained between 35 and 45 mm Hg to minimize cerebrovascular reactivity (Table 1). A rectal thermocouple provided continual monitoring of core body temperature that was maintained at 37°C±0.5°C.
Table 1. Baseline physiological variables for the ischemia scan series and the reperfusion scan series.
|
Physiological data (n=8) | ||||
|---|---|---|---|---|
|
Values during ischemia scans |
Values for reperfusion scans |
|||
| |
Baseline |
During OC |
Baseline |
During OC |
| MABP | 89.4±9 | 98.3±6* | 86.9±9 | 93.5±10** |
| Pa2 (mm Hg) | 41.8±8 | 42.8±7 | ||
| Pa2 (mm Hg) | 90±13 | 89.5±9 | ||
| Blood pH | 7.324±0.05 | 7.302±0.03 | ||
MABP, mean arterial blood pressure
Data are expresses as mean±s.d. *P<0.05 and **P<0.001, Student's paired t-test.
Polyethylene catheters (Portex: external diameter 0.96 mm; internal diameter 0.58 mm; 70 cm long) were placed in femoral arteries, to continuously monitor blood pressure and conduct blood gas analysis. Middle cerebral artery occlusion (MCAO) was achieved by the intraluminal filament technique, using a modified version of Longa et al (1989) technique, where a 5-0 silicon rubber-coated monofilament (diameter 0.12 mm, length 30 mm; diameter with coating 0.31 to 0.35 mm, and coating length ⩾5 mm; http://www.doccol.com) was introduced through the internal carotid artery to the origin of the MCA to preclude flow and induce stroke. The common carotid artery was ligated and the occipital artery branches of the external carotid artery were isolated, ligated, and dissected. The pterygopalatine branch from the internal carotid artery was also ligated. Ischaemia was induced for 109±20 minutes, which reflected the time taken to transfer the animal to the scanner and run the ischaemia scan series. The monofilament was then removed. To ensure complete reperfusion, the ties around the common carotid and pterygopalatine branch were loosened to restore blood flow to MCA territory.
Magnetic Resonance Imaging Scanning
Magnetic resonance imaging data were acquired on a Bruker Biospec (Wikingerstrasse, Karlsruhe, Germany) 7-T/30-cm system equipped with an inserted gradient coil (121 mm ID, 400 mT/m) and a 72-mm birdcage resonator. After stroke surgery, animals were placed prone in a rat cradle, with the head restrained using ear and tooth bars to limit movement, and a linear surface receiver coil (2 cm diameter) placed above the head of the animal.
Scanning Protocol
At ∼1 hour after stroke, animals underwent MRI scanning that comprised DWI to detect ischemic injury, T2* OC to detect penumbra, and arterial spin labeling (ASL) to provide CBF maps of ischemia. Animals were removed from the magnet, and reperfusion was induced by withdrawal of the intraluminal filament. The scanning sequence was then repeated to confirm reperfusion, and to study the consequences of reperfusion on the tissue defined as penumbra from the earlier DWI, T2* OC, and ASL scans.
Ischemia-induced damage will continue to evolve following reperfusion in this model and consequently histology or MRI at 24 hours will not predict final infarct size. Therefore, rats were recovered and rescanned at day 7 to define the final infarct. Choosing this late time point also avoids the confounding effects of brain swelling, which are present during the first days after stroke and improves coregistration of final T2 scans with acute scans. The animals also underwent T2* OC at this time point.
Diffusion-Weighted Imaging and Perfusion-Weighted Imaging
Diffusion-weighted imaging was performed during ischemia and immediately following reperfusion to assess ischemically injured tissue (spin echo (echo planar imaging) TE: 43 milliseconds, TR: 4000.3 milliseconds, in plane resolution of 260 μm, three directions: x, y, z, B values: 0, 1,000 s/mm2, eight slices of 1.5 mm thickness).
Noninvasive quantitative CBF was performed on two coronal slices within the MCA territory during ischemia and immediately following reperfusion using a form of pseudo-continuous ASL based on a train of adiabatic inversion pulses (Moffat et al, 2005). The sequence uses a spin echo (echo planar imaging) module (TE: 20 milliseconds, TR: 7,000 milliseconds, matrix 96 × 96, field of view: 25 × 25 mm2, slice thickness 1.5 mm, 16 averages, 4 shots) preceded by 50 hyperbolic secant inversion pulses in a 3-second train.
DWI and perfusion-weighted imaging were also used to define penumbra from DWI/PWI mismatch (Figure 1Avi) and data were analyzed on two selected coronal slices within the MCA territory. For analysis, the data for the rostral and caudal slices were combined. Arterial spin labeling scans were generated 44±11 and 59±15 minutes after stroke for caudal and rostral slices, respectively. The scanning time for a single ASL slice was ∼6 minutes and two slices were scanned throughout the MCA territory. In addition to this, a T1-weighted image (scan time 10 minutes) was performed to allow quantification of CBF in mL per 100 g per minute.
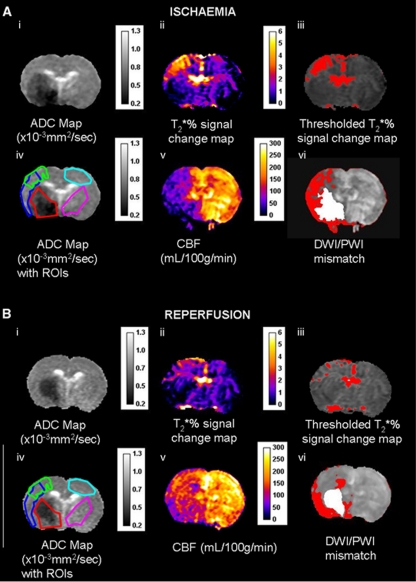
Figure 1.
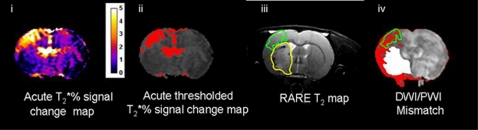
(A) Ischemia scan series and (B) post-reperfusion scan series, with regions of interest (ROIs) superimposed on the apparent diffusion coefficient (ADC) maps (images Aiv and Biv); (ii) T2* oxygen challenge (OC) percentage signal change map, (iii) thresholded T2* OC map, (v) cerebral blood flow (CBF) map (mL per 100 g per minute), and (vi) DWI/PWI overlay (mismatch tissue shown in red). ROIs were defined as follows: green ROI—the penumbra was defined by applying a threshold to display the greatest T2* percentage signal change excluding veins and ventricles (iii). Red ROI—ischemic core within the caudate nucleus, derived from the ADC lesion (i). Sky blue ROI—the contralateral cortex, equivalent to OC-defined penumbra. Cerise ROI—the contralateral caudate nucleus, equivalent to the ADC-derived lesion. Dark blue ROI— DWI/PWI mismatch (vi) derived from the thresholded ADC (i) and CBF maps (v).
T2*-Weighted Imaging
The sequence used to measure T2* changes during OC was a single shot, gradient echo (echo planar imaging) sequence (TE: 20 milliseconds, TR: 10 milliseconds, matrix 96 × 96, field of view: 25 × 25 mm2, eight contiguous slices of 1.5 mm thickness, two averages, temporal resolution 20 seconds, 30 repetitions). Two coronal MRI slices (identified as rostral and caudal slices), which corresponded to territory supplied by the MCA, were selected for analysis. The paradigm for the T2*-weighted OC sequence was 4 minutes breathing air, followed by 6 minutes breathing 100% oxygen. This sequence was repeated at day 7 after stroke.
T2-Weighted Imaging
During acute scanning and at 7 days following reperfusion, a sagittal RARE T2 scan (effective TE: 46.8 milliseconds, TR: 5,000 milliseconds; in plane resolution of 97 μm; 18 slices of 0.5 mm thickness) was performed, in which the rhinal fissure was used as a neuroanatomical landmark to match the geometry as closely as possible with the acute scans. A coronal RARE T2 sequence (effective TE: 46.8 milliseconds, TR: 5,000 milliseconds; in plane resolution of 97 μm; 30 slices of 0.5 mm thickness) enabled T2-derived final infarct measurements.
Magnetic Resonance Imaging Data Analysis
Defining the ischemic penumbra with DWI/PWI mismatch
Quantitative ADCav maps, in units of square millimeters per second, were calculated using the Stejskal–Tanner equation (Stejskal and Tanner, 1965). Apparent diffusion coefficient maps and CBF maps were generated using Image J software. A 16.5% reduction of mean contralateral ADC was used to determine ischemic lesion volume, which has been shown to match closely the final infarct size following permanent MCAO in Sprague-Dawley rats (Lo et al, 1997). Perfusion-weighted imaging was performed on caudal and rostral coronal slices within core MCA territory and the perfusion deficit area was calculated based on a 57% reduction of mean contralateral CBF (Meng et al, 2004). Apparent diffusion coefficient and CBF maps were overlaid to identify the DWI/PWI mismatch area. Diffusion–perfusion mismatch was calculated as the difference between the perfusion deficit and the ADC lesion area on the corresponding slice. Volumes of DWI/PWI mismatch and thresholded T2* OC-defined penumbra were generated from the data from two coronal slices and the neuroanatomical location compared between the two techniques (Figure 5).
T2* oxygen challenge time course data and defining the ischemic penumbra
The time course of the T2* signal change was analyzed from regions of interest (ROIs) (Figure 1Aiv). T2* percentage signal change was calculated from time course graphs (Figures 2A and 2B), where the average baseline signal was subtracted from the peak signal during OC. This value was then divided by the average baseline signal and multiplied by 100. T2* percentage signal change maps were generated using Image J software (http://rsb.info.nih.gov/ij/). The boundaries of penumbral tissue were defined using a threshold based on the empirical rule: the mean plus 2 s.d. of the T2* value of the contralateral hemisphere, excluding the ventricles (see Figure 1Aiii).
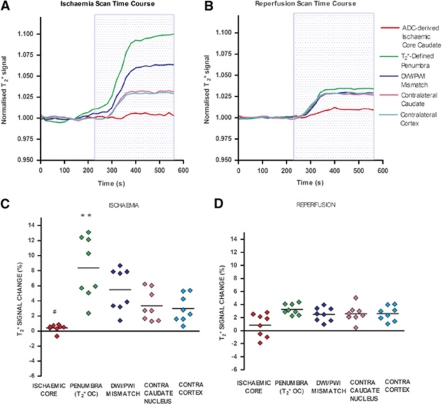
Figure 2.
Echo planar imaging (EPI) T2* signal time course during ischemia (A), and following reperfusion (B), mean T2* percentage signal change from baseline for regions of interest (ROIs) during ischemia (C) and following reperfusion (D). (A, B) Positive T2* signal changes were recorded during oxygen challenge (OC) in contralateral caudate nucleus and cortex, the DWI/PWI mismatch, and the T2* OC-defined penumbra. All data were normalized to the average signal over the 4 minutes before OC from eight animals. The blue box represents the period of 100% oxygen inhalation (OC). (C, D) Horizontal lines represent means. **P<0.01, relative to contralateral cortex ROI. #P<0.05, relative to contralateral caudate nucleus.
Volumetric Analysis of Penumbra
Volumetric analysis of the perfusion deficit, ADC lesion, DWI/PWI mismatch, and T2* OC-defined penumbra was performed over the rostro-caudal extent of the ASL scans.
Regions of Interest
The researcher responsible for ROI placement in the ischemia scans was blinded to the reperfusion data. Regions of interest were selected according to specific features on the images (Figure 1): (1) ischemic core in caudate nucleus from the thresholded ADC lesion (Figure 1Aiv, red); (2) the contralateral caudate (excluding veins and ventricles) (cerise), manually designated by researcher; (3) penumbra as defined by thresholded T2* percentage signal change (green); (4) equivalent contralateral cortex (sky blue); and (5) DWI/PWI mismatch (dark blue).
For the 7-day data, MRI-defined ROIs were defined and placed within; the final infarct according to the RARE T2 scan; the thresholded T2* OC-defined penumbra identified from the acute ischemic scan series; and two equivalent contralateral ROIs.
Coregistration
To coregister the acute and 7-day scans, linear coregistration was performed using Analyze (AnalyzeDirect, Inc. Overland Park, KS, USA). To align the data, ischemia ASL and T2* images were warped to their corresponding DWI slices, and the reperfusion scan series and 7-day scans were warped to the same DWI slice as the ischemic scan series. The processed data from the T2* OC and thresholded ADC and CBF maps were coregistered to (1) define ROIs and (2) identify the T2* OC-defined penumbral tissue. To correlate the T2-defined infarct with the acute data, the data set at day 7 were also coregistered to the DWI scans generated from the ischemic scan series.
Statistical Analysis
All data are presented as mean±s.d. Data were normally distributed, and as such, mean arterial blood pressure before and during OC was analyzed by Student's paired t-test. T2* signal, ADC, and CBF values in different ROIs were analyzed by one-way analysis of variance followed by Student's paired t-test with a Bonferroni correction for multiple comparisons. A paired t-test was performed to compare changes in T2* signal, ADC, and CBF at the ischemia and post-reperfusion time points. All data were tested to confirm normal distribution using the D'Agostino and Pearson normality test.
Results
Acute Data and Physiological Variables
Oxygen challenge was performed twice, and the mean time to commence OC was 78±15 minutes after MCAO for the scans performed during ischemia and 180±31 minutes for the set of scans performed following reperfusion. Physiological variables were monitored throughout the experiment. Blood pressure and blood gases recorded immediately before OC for both the ischemia and reperfusion scan series were within normal physiological levels (Table 1).
Acute T2* Percentage Signal Change to Oxygen Challenge
During MCAO (ischemia scans), the T2* signal change during OC varied in magnitude across the hemisphere ipsilateral to the stroke (Figures 2A and 2C), with the smallest change in ischemic core and the largest in the dorsolateral cortex (OC-defined penumbra). T2* signal increase in the penumbra ROI was significantly greater than in the contralateral cortical ROI (P<0.01; Figure 2C). In ischemic core caudate nucleus, mean T2* signal during OC was significantly reduced compared with the contralateral caudate nucleus ROI (P<0.05).
Following reperfusion (mean OC scan time=180±31 minutes following stroke onset and 71 minutes from initiation of reperfusion), T2* signal increase in the penumbral ROI reduced significantly from 8.4%±4.1% to 3.25%±0.81% (P<0.001). In the DWI/PWI mismatch ROI, T2* percentage signal changed from 5.48%±2.8% during ischemia to 2.49%±1.04% following reperfusion. There were no significant T2* percentage signal changes from ischemia to reperfusion in any of the other ROIs; T2* percentage signal change in ischemic core caudate nucleus changed from 0.39%±0.47% during ischemia to 0.83%±1.7% following reperfusion, contralateral cortex changed from 2.97%±1.8% to 2.61%±1.1%, and contralateral caudate nucleus changed from 3.35%±2% to 2.59%±1.3%.
Severity of Ischemia and Tissue Viability
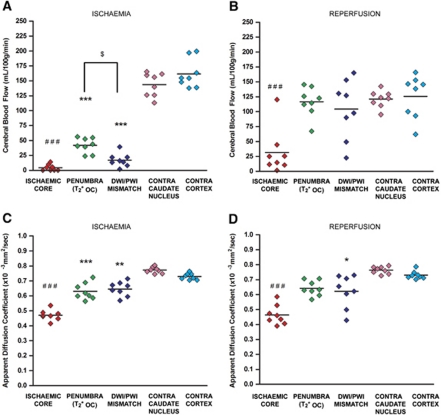
During ischemia, blood flow in the OC-defined penumbra, DWI/PWI mismatch, and ischemic core caudate nucleus were significantly reduced compared with the equivalent contralateral ROIs (Figure 3A; P<0.001). Cerebral blood flow values in the OC-defined penumbra were significantly higher than the DWI/PWI mismatch-defined penumbra (P<0.05). On reperfusion, mean CBF in ischemic core caudate nucleus increased from 4.3±5.3 to 31±74 mL per 100 g per minute, but remained significantly reduced compared with the equivalent contralateral ROI (Figure 3B; P<0.001). Mean CBF in the OC-defined penumbra increased significantly from 41.94±13 to 116.5±25 mL per 100 g per minute (P<0.001; Figure 3B). Mean CBF in DWI/PWI mismatch also increased significantly from 16.8±14 to 104.4±50 mL per 100 g per minute (P<0.001). Blood flow in contralateral caudate nucleus and cortex did not change significantly following reperfusion; 143±20 to 121±14 and 161±24 to 127±36 mL per 100 g per minute, respectively.
Figure 3.
Mean cerebral blood flow (CBF) and apparent diffusion coefficient (ADC) in selected regions of interest (ROIs) during ischemia and following reperfusion. (A, B) Mean CBF during ischemia and following reperfusion. (C, D) Mean ADC during ischemia and following reperfusion, respectively. Horizontal lines represent means. ***P<0.001, **P<0.01 relative to contralateral cortex ROI. ###P<0.001, ##P<0.01, relative to contralateral caudate nucleus ROI. $P<0.05.
During ischemia, ADC values (mean scan time=72±8 minutes after stroke) in the OC-defined penumbra, ischemic core caudate nucleus and DWI/PWI mismatch were significantly reduced compared with the equivalent contralateral ROIs (Figures 1Ai and 3C; P<0.001, P<0.001, and P<0.01, respectively) and remained significantly reduced in the DWI/PWI mismatch and ischemic core caudate nucleus compared with equivalent contralateral ROIs following reperfusion (mean scan time=172±35 minutes after stroke, P<0.05 and P<0.001, respectively), while the OC-defined penumbra was no longer significantly reduced. Mean ADC values were not significantly different between ischemia and reperfusion scans in any of the six ROIs; Figures 3C and 3D).
Day 7 T2-Defined Infarct Volume and Oxygen Challenge T2* Percentage Signal Change
In the surviving animals, infarcts were located subcortically with minimal cortical damage. The surviving animals (n=4) tended to have less brain damage on acute scans than the animals that died prematurely (n=4). Both ipsilateral and contralateral hemispheric volumes were calculated to show that there was no residual edema at day 7 (622±27 and 624±30 mm3 for the ipsilateral and contralateral hemispheric volumes, respectively).
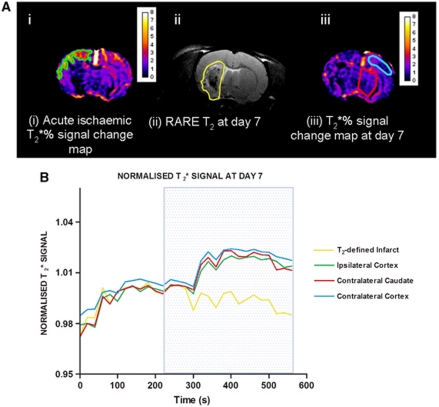
T2* percentage signal change on day 7 in the OC-defined penumbra ROI (1.69%±0.6%) was not significantly different from data for the equivalent contralateral cortex (1.72%±0.6% Figure 4B). The T2* signal change in the T2-defined final infarct ROI was significantly reduced—displaying a negative signal (−0.88%±0.6%)-compared with the equivalent contralateral caudate nucleus (1.72%±0.6%) (P<0.01; Figure 4B).
Figure 4.
T2* percentage signal change at day 1 and day 7 in selected regions of interest (ROIs). (A) MRI ROIs derived from (i) acute T2* percentage signal change maps during ischemia (ipsilateral cortex penumbra—green); (ii) RARE T2 scans at day 7 after stroke (infarct ROI-yellow); and (iii) T2* percentage signal change maps at day 7 (contralateral cortex and caudate—sky blue and red, respectively). ROIs were superimposed onto day 7 T2* percentage signal change maps generated 7 days after stroke to generate EPI T2* signal time course graphs (B). Traces represent the mean normalized signal from four animals. All data were normalized to the average signal over the 4 minutes before oxygen challenge. Blue box represents period of 100% oxygen inhalation.
Evidence of Penumbral Salvage
Coregistration of the T2* OC-defined penumbra ROI (derived from the T2* percentage map during ischemia) onto the RARE T2 scan at day 7 (Figure 5), revealed that this region was not incorporated into the final infarct in any animal, providing evidence that penumbra had been salvaged by reperfusion.
Figure 5.
Comparison of neuroanatomical location of thresholded oxygen challenge (OC)-defined penumbra in relation to T2-defined final infarct and DWI/PWI mismatch. Acute T2* percentage signal change map (i) was thresholded to identify penumbra (ii) and superimposed upon the day 7 RARE T2 (iii)—penumbra outlined in green. The final infarct is outlined in yellow. The T2* OC penumbra region of interest (ROI) was superimposed upon the DWI/PWI mismatch (iv) to compare the difference in the spatial locations of the mismatch and the T2* OC defined penumbra.
Volumetric Analysis of Perfusion Deficit, Apparent Diffusion Coefficient Lesion, and Penumbra
The volume of perfusion deficit, determined at 52.8±14.2 minutes after stroke, was 110±3 mm3 and the ADC lesion volume determined at 72±8 minutes after stroke was 91.6±33 mm3, generating a DWI/PWI mismatch volume of 20.2±15 mm3. The thresholded T2* OC-defined penumbral volume, determined at 78±15 minutes was 17.3±9 mm3. The mismatch- and T2* OC-defined penumbral volumes were ∼22% and 19% of the volume of the ADC-defined ischemic core, respectively. Although the volume of penumbral tissue was similar for the two methods, there were spatial differences with regards to the physical location (Figure 5iv).
The perfusion deficit volume in the four animals that survived to day 7 was 110.3±6 mm3 determined at 53.5±12 minutes after stroke, and the ADC lesion volume was 88.6±21 mm3 determined at 68.3±8.4 minutes after stroke. The DWI/PWI mismatch volume was 25.7±14 mm3 and the thresholded T2* OC-defined penumbra, determined at 82.5±15 minutes was 18.9±8 mm3. The mismatch- and T2* OC-defined penumbral volumes were ∼29% and 22% of the volume of the ADC-defined ischemic core, respectively. The T2-defined final infarct volume was 71.7±22 mm3.
Discussion
We previously described the OC MRI technique in a focal cerebral ischemia model and compared it with DWI/PWI mismatch and histologically defined neuronal morphology (Santosh et al, 2008). We have also demonstrated feasibility of the technique in clinical use in acute stroke (Dani et al, 2010). Evidence for ongoing metabolism in the T2* OC-defined penumbra was provided by coregistration of [14C]2-deoxyglucose autoradiography with MRI, displaying detailed information on the adjacent tissue compartments within the ischemic hemisphere, which demonstrate markedly different levels of glucose metabolism (Robertson et al, 2011). Depending on the duration and severity of the ischemic insult, at least some of the tissue regarded as penumbra may recover if blood supply is promptly restored. If blood supply is restored and the tissue recovers, it should no longer demonstrate an increased OEF and its response to OC should be similar to nonischemic tissue. We tested the validity of T2* OC by timely restoration of CBF and final infarct measurement to determine tissue salvage.
The aim of this study was to validate the T2* OC MRI technique by identifying penumbral tissue during ischemia and comparing the T2* response to OC in this tissue on reperfusion and 7 days later, when the fate of this tissue was confirmed by a T2 scan. T2* MRI sequences should reflect changes in the penumbra associated with restoration of flow and confirm its amenability to salvage. Evidence of recovery in the OC-defined penumbra was verified acutely by assessing changes in CBF and ADC following reperfusion and T2-derived final infarct volume after 7 days. We propose that the T2* OC technique indirectly identifies penumbra, with its higher OEF influencing deoxy/oxyhaemoglobin ratios and T2* signal. However, we acknowledge that other factors may give rise to an increase in T2* signal in the penumbra. For example, an increased cerebral blood volume in penumbral tissue may increase deoxyhemoglobin in this region, thus magnifying the T2* response.
There are varying definitions of penumbra, which have been devised using different techniques. The penumbra can be described as a region of decreased protein synthesis and preserved adenosine triphosphate (Hossmann, 1993), unlike the ischemic core, which experiences reductions in both protein synthesis and adenosine triphosphate. Following the progressive reduction in blood flow, decreased protein synthesis is one of the first biochemical or molecular changes that can be identified (Dienel et al, 1980; Bergstedt et al, 1993), declining when flow reduces below 50% (Mies et al, 1990). While this method may more accurately delineate penumbra, it requires a terminal autoradiographical technique, which cannot be used in recovery models of stroke. As such, the current definition of penumbra used for the study was tissue with reduced CBF but preserved CMRO2 and raised OEF. Additionally, the pathophysiological mechanisms of ischemic damage differ between permanent and transient MCAO. In permanent MCAO, peri-infarct depolarizations in oligemic tissue are responsible for the evolution of penumbra into the infarct core, whereas in transient MCAO delayed secondary energy failure is implicated in the evolution of damage (Olah et al, 2001).
Acute Data
As expected, during ischemia, the tissue demonstrating the greatest T2* increase was localized to a cortical boundary zone between the MCA and anterior cerebral artery territories, which overlapped the DWI/PWI mismatch area. A smaller T2* response was recorded in nonischemic tissue, with negligible response within ischemic core. These MR findings were consistent with our previous studies (Santosh et al, 2008; Robertson et al, 2011).
Reintroduction of flow into the penumbral region restores arterial oxygen levels and therefore the OEF is expected to reduce. The reduction in T2* signal change in T2* OC-defined penumbra following reperfusion is consistent with our stated hypothesis and provides evidence that the tissue is metabolizing aerobically (Figure 2B). The extent of reperfusion was apparent on CBF maps, which demonstrated that blood flow in penumbral tissue attained levels similar to the contralateral cortex (Figure 3B). However, withdrawal of the intraluminal filament did not universally lead to complete reperfusion, as flow was still compromised in the ADC-defined ischemic core (31.74±38 mL per 100 g per minute; Figure 3B). Tissue recovery following reperfusion was evident in the OC-defined penumbra, in which the ADC value stayed within the normal range (0.63±0.06 × 10−3 mm2/s). Also, reperfusion increased the ADC value of some tissue within the ischemic core to above the predefined viability threshold (shown by change in lesion size following reperfusion) (Figures 1Ai and 1Bi).
Day 7 Data
Our hypothesis stated that reperfused penumbral tissue should exhibit a normal T2* response to OC and this was confirmed on the day 7 T2* response (Figure 4B). Following reperfusion, irreversibly injured (ischemic core) tissue should no longer metabolize oxygen or extract oxygen from the blood. Oxyhemoglobin:deoxyhemoglobin ratios would therefore be expected to remain static during OC. Within the T2-defined infarct on day 7 after stroke, a negligible negative T2* signal change was observed during OC. This is most likely to be due to the presence of paramagnetic free oxygen dissolved within the plasma flowing through nonmetabolizing irreversibly damaged tissue, which will result in a reduction in T2* signal. Therefore, after 7 days, T2* OC enabled reperfused, nonmetabolic tissue to be differentiated from metabolic tissue.
DWI/PWI Mismatch Technique and Its Limitations
Individually, PWI and DWI/ADC provide valuable information on the location and severity of ischemia, and tissue injury, respectively. Combined, they provide an approximate and indirect assessment of the location and size of penumbra. Perfusion-weighted imaging and DWI scans were included in the scanning routine of the current study to provide a reference for comparison with T2* identification of penumbra. However, DWI/PWI mismatch has a number of limitations in detecting penumbra. A number of studies have shown that differentiation between viable and nonviable tissue is difficult, using the diffusion abnormality (Kidwell et al, 2000; Fiehler et al, 2002), which correlates poorly with final infarct (Li et al, 1999). As shown in the current study and by others, DWI-defined lesions may be recoverable following prompt reperfusion in animal models and humans, and may not be destined for infarction (Schlaug et al, 1997; Mintorovitch et al, 1991; Kidwell et al, 2000). Additionally, the perfusion deficit may incorporate tissue with benign oligemia destined to survive (Butcher et al, 2005) and accurate MRI thresholds for defining the perfusion deficit have yet to be determined. As such, the inner and outer margins of the penumbra may not be adequately delineated using the mismatch technique and the region of decreased CBF using PWI frequently overestimates the final lesion size (Kucinski et al, 2005).
Translation of T2* Oxygen Challenge to the Clinic
Recently, Dani et al (2010) demonstrated the first clinical application of OC during T2*-weighted MRI, detecting differences in vascular deoxyhemoglobin levels between tissue compartments following stroke. The percentage signal change maps generated from the OC data could discriminate between gray and white matter on the contralateral hemisphere, consistent with the higher metabolic demand in gray matter. Regions of interest selected within the DWI lesion displayed a reduced T2* percentage signal change compared with the nonischemic hemisphere. With patients scanned in the hyperacute phase, penumbral tissue (defined by DWI/PWI mismatch) had a significantly higher T2* percentage signal change in three out of four patients compared with normal tissue. This increase in metabolic status was less evident in patients scanned at later time points, in line with the likelihood that penumbral tissue may have been recruited into the irreversible ischemic core. These preliminary clinical data support the potential for this novel MRI technique to delineate penumbral tissue in acute stroke by its metabolic status. Crucially, administration of oxygen with T2* scanning can be performed quickly and easily with widely available hardware. This technique may be used acutely or more importantly to detect existing penumbral tissue in patients unable to present within the 3- to 4.5-hour time window for thrombolysis.
Limitations of the Study
Time to reperfusion varied slightly between rats due to the time taken to carry out the MRI scanning protocol during ischemia. The duration of ischemia may have contributed to mortality and explain why in some rats little or no tissue salvage was seen following reperfusion. Reperfusion is associated with considerable brain swelling at the 24- to 48-hour time point. For this reason, only four of the 8 animals survived out to 7 days. This is a drawback of the intraluminal filament model, where the intact skull means there is no control of intracranial pressure.
Summary
An attenuation of the T2* percentage signal change to OC, following restoration of CBF, was consistent with tissue salvage in the OC-defined cortical penumbra. Therefore, cortex identified as penumbra using the OC technique is capable of recovery when blood flow is restored, which provides further validation of the utility of OC MRI for acute stroke management.
The authors declare no conflict of interest.
Footnotes
This work was supported by an MRC Project Grant (G0700439; an MRC Capacity Building Area PhD studentship (to CR); and SINAPSE Collaboration funding (to Md RL-G) http://www.sinapse.ac.uk, a Pooling Initiative funded by the Scottish Funding Council and the Chief Scientist Office of the Scottish Executive.
References
- Baron JC, Bousser MG, Comar D, Soussaline F, Castaigne R. Noninvasive tomographic study of cerebral blood flow and oxygen metabolism in vivo: potentials, limitations and clinical applications in cerebral ischemic disorders. Eur Neurol. 1981;20:273–284. doi: 10.1159/000115247. [DOI] [PubMed] [Google Scholar]
- Bergstedt K, Hu BR, Wieloch T. Postischemic changes in protein synthesis in the rat brain: effects of hypothermia. Exp Brain Res. 1993;95:91–99. doi: 10.1007/BF00229658. [DOI] [PubMed] [Google Scholar]
- Butcher KS, Parsons M, MacGregor L, Barber PA, Chalk J, Bladin C, Levi C, Kimber T, Schultz D, Fink J, Tress B, Donnan G, Davis S. Refinig the perfusion–diffusion mismatch hypothesis. Stroke. 2005;36:1153–1159. doi: 10.1161/01.str.0000166181.86928.8b. [DOI] [PubMed] [Google Scholar]
- Cocho D, Belvis R, Marti-Fabregas J, Molina-Porcel L, Diaz-Manera J, Aleu A, Pagonabarraga J, Garcia-Bargo D, Mauri A, Marti-Vilalta JL. Reasons for exclusion from thrombolytic therapy following acute ischemic stroke. Neurology. 2005;64:719–720. doi: 10.1212/01.WNL.0000152041.20486.2F. [DOI] [PubMed] [Google Scholar]
- Dani KA, Santosh C, Brennan D, McCabe C, Holmes WM, Condon B, Hadley DM, Macrae IM, Shaw M, Muir KW. T2* weighted magnetic resonance imaging with hyperoxia in acute ischemic stoke. Ann Neurol. 2010;68:37–47. doi: 10.1002/ana.22032. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Pulsinelli WA, Duffy TE. Regional protein synthesis in rat brain following acute hemispheric ischemia. J Neurochem. 1980;35:1216–1226. doi: 10.1111/j.1471-4159.1980.tb07878.x. [DOI] [PubMed] [Google Scholar]
- Fiehler J, Foth M, Kucinski T, Knab R, von Bezold M, Weillier C, Zeumer H, Rother J. Severe ADC decreases do not predict irreversible tissue damage in humans. Stroke. 2002;33:79–86. doi: 10.1161/hs0102.100884. [DOI] [PubMed] [Google Scholar]
- Guadagno JV, Warburton EA, Aigbirhio FI, Smielewski P, Fryer TD, Harding S, Price CJ, Gillard JH, Carpenter TA, Baron JC. Does the acute diffusion-weighted imaging represent penumbra as well as core? A combined quantitative PET/MRI voxel-based study. J Cereb Blood Flow Metab. 2004;24:1249–1254. doi: 10.1097/01.WCB.0000141557.32867.6B. [DOI] [PubMed] [Google Scholar]
- Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D, for the ECASS Investigators Thrombolysis with Alteplase 3 to 4.5 Hours after Acute Ischemic Stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- Hossmann KA. Disturbances of cerebral protein synthesis and ischemic cell death. Prog Brain Res. 1993;96:161–177. doi: 10.1016/s0079-6123(08)63265-3. [DOI] [PubMed] [Google Scholar]
- Kidwell CS, Saver JL, Mattiello J, Starkman S, Vinuela F, Duckwiler D, Gobin YP, Jahan R, Vespa P, Kalafut M, Alger JR. Thrombolytic reversal of acute human cerebral ischemic injury shown by diffusion/perfusion magnetic resonance imaging. Ann Neurol. 2000;47:462–469. [PubMed] [Google Scholar]
- Köhrmann M, Jüttler E, Fiebach J, Huttner H, Siebert S, Schwark C, Ringleb P, Schellinger P, Hacke W. MRI versus CT-based thrombolysis treatment within and beyond the 3 hours time window after stroke onset: a cohort study. Lancet Neurol. 2006;5:661–667. doi: 10.1016/S1474-4422(06)70499-9. [DOI] [PubMed] [Google Scholar]
- Kucinski T, Naumann D, Knab R, Schoder V, Wegener S, Fiehler J, Majumder A, Röther J, Zeumer H. Tissue at risk is overestimated in perfusion-weighted imaging: MR imaging in acute stroke patients without vessel recanalization. Am J Neuroradiol. 2005;26:815–819. [PMC free article] [PubMed] [Google Scholar]
- Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Gotta JC, Albers GW, Kaste M, Marler JR, Hamilton SA, Tilley BC, Davis SM, Donnan GA, Hacke W, for the ECASS, ATLANTIS, NINDS, and EPITHET rt-PA Study Group Investigators Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS and EPITHET trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- Li F, Han SS, Tatlisumak T, Liu KF, Garcia JH, Sotak CH, Fisher M. Reversal of acute apparent diffusion coefficient abnormalities and delayed neuronal death following transient focal cerebral ischemia in rats. Ann Neurol. 1999;46:333–342. doi: 10.1002/1531-8249(199909)46:3<333::aid-ana9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Lo EH, Pierce AR, Mandeville JB, Rosen BR. Neuroprotection with NBQX in rat focal cerebral ischemia. Effects on ADC probability distribution functions and diffusion–perfusion relationships. Stroke. 1997;28:439–447. doi: 10.1161/01.str.28.2.439. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Meng X, Fisher M, Shen Q, Sotak CH, Duong TQ. Characterizing the diffusion/perfusion mismatch in experimental focal cerebral ischemia. Ann Neurol. 2004;55:207–212. doi: 10.1002/ana.10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mies G, Paschen W, Edhardt G, Hossmann KA. Relationship between blood flow, glucose metabolism, protein synthesis, glucose and ATO content in experimentally-induced glioma (RG1 2.2) of rat brain. J Neurooncol. 1990;9:17–28. doi: 10.1007/BF00167064. [DOI] [PubMed] [Google Scholar]
- Mintorovitch J, Moseley ME, Chileuitt L, Shimizu H, Cohen Y, Weinstein PR. Comparison of diffusionand T2-weighted MRI for the early detection of cerebral ischemia and reperfusion in rats. Magn Reson Med. 1991;18:39–50. doi: 10.1002/mrm.1910180106. [DOI] [PubMed] [Google Scholar]
- Moffat BA, Chenevert TL, Hall DE, Rehemtulla A, Ross BD. Continuous arterial spin labeling using a train of adiabatic inversion pulses. J Magn Reson Imaging. 2005;21:290–296. doi: 10.1002/jmri.20268. [DOI] [PubMed] [Google Scholar]
- Molina CA, Saver JL. Extending reperfusion therapy for acute ischemic stroke: emerging pharmacological, mechanical, and imaging strategies stroke. Stroke. 2005;36:2311–2320. doi: 10.1161/01.STR.0000182100.65262.46. [DOI] [PubMed] [Google Scholar]
- Olah L, Wecker S, Hoehn M. Relation of apparent diffusion coefficient changes and metabolic disturbances after 1 hour of focal cerebral ischemia and at different reperfusion phases in rats. J Cereb Blood Flow Metab. 2001;21:430–439. doi: 10.1097/00004647-200104000-00012. [DOI] [PubMed] [Google Scholar]
- Powers WJ. Cerebral hemodynamics in ischemic cerebrovascular disease. Ann Neurol. 1991;29:231–240. doi: 10.1002/ana.410290302. [DOI] [PubMed] [Google Scholar]
- Robertson CA, McCabe C, Gallagher L, Lopez-Gonzalez MdelR, Holmes WM, Condon B, Muir KW, Santosh C, Macrae IM.2011Stroke penumbra defined by an MRI-based oxygen challenge technique: 1. Validation using [14C]2-deoxyglucose autoradiography J Cereb Blood Flow Metabdoi: 10.1038/jcbfm.2011.66 [DOI] [PMC free article] [PubMed]
- Santosh C, Brennan D, McCabe C, Macrae IM, Holmes WM, Graham DI, Gallagher L, Condon B, Hadley DM, Muir KW, Gsell W. Potential use of oxygen as a metabolic biosensor in combination with T2(*)-weighted MRI to define the ischemic penumbra. J Cereb Blood Flow Metab. 2008;28:1742–1753. doi: 10.1038/jcbfm.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellinger PD, Thomalla G, Fiehler J, Köhrmann M, Molina CA, Neumann-Haefelin T, Ribo M, Singer OC, Zaro-Weber O, Sobesky J. MRI-based and CT-based thrombolytic therapy in acute stroke within and beyond established time windows: an analysis of 1210 patients. Stroke. 2007;38:2640–2645. doi: 10.1161/STROKEAHA.107.483255. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Siewert B, Benfield A, Edelman RR, Warach S. Time course of the apparent diffusion coefficient (ADC) abnormality in human stroke. Neurology. 1997;49:113–119. doi: 10.1212/wnl.49.1.113. [DOI] [PubMed] [Google Scholar]
- Stejskal EO, Tanner JE. Spin diffusion measurements: spin echoes in the presence of a time-dependent field gradient. J Chem Phys. 1996;42:288–292. [Google Scholar]
- Wardlaw JM, Murray V, Berge E, del Zoppo GJ.2009Thrombolysis for acute ischaemic stroke Cochrane Database of Systematic Reviews 4Art. No.: CD000213 [DOI] [PubMed]