Abstract
Janus kinase 2 (JAK2) couples ligand activation of cell surface cytokine receptors to the regulation of cellular functions including cell cycle progression, differentiation and apoptosis. It thereby coordinates biological programs such as development and hematopoiesis. Unscheduled activation of JAK2 by point mutations or chromosomal translocations can induce hyperproliferation and hematological malignancies. Typical signal transduction by the JAK2 tyrosine kinase comprises phosphorylation of STAT transcription factors. In this study, we describe the identification of the cyclin-dependent kinase (CDK) inhibitor p27Kip1 as a novel JAK2 substrate. JAK2 can directly bind and phosphorylate p27Kip1. Both, the JAK2 FERM domain and its kinase domain bind to p27Kip1. JAK2 phosphorylates tyrosine residue 88 (Y88) of p27Kip1. We previously reported that Y88 phosphorylation of p27Kip1 by oncogenic tyrosine kinases impairs p27Kip1-mediated CDK inhibition, and initiates its ubiquitin-dependent proteasomal degradation. Consistently, we now find that active oncogenic JAK2V617F reduces p27Kip1 stability and protein levels in patient-derived cell lines harboring the mutant JAK2V617F allele. Moreover, tyrosine phosphorylation of p27Kip1 is impaired and p27Kip1 expression is restored upon JAK2V617F inactivation by small hairpin RNA-mediated knockdown or by the pyridone-containing tetracycle JAK inhibitor-I, indicating that direct phosphorylation of p27Kip1 can contribute to hyperproliferation of JAK2V617F-transformed cells. Activation of endogenous JAK2 by interleukin-3 (IL-3) induces Y88 phosphorylation of p27Kip1, thus unveiling a novel link between cytokine signaling and cell cycle control in non-transformed cells. Oncogenic tyrosine kinases could use this novel pathway to promote hyperproliferation in tumor cells.
Keywords: cell cycle control, CDK inhibitors, p27Kip1, tyrosine kinases, JAK2, JAK2V617F
Introduction
The decision between cell proliferation and cell cycle exit is orchestrated by signaling networks that control the activity of specific cyclin-dependent kinase (CDK) complexes (Morgan, 1997; Malumbres and Barbacid, 2009). Activation of G1-phase CDKs initiates cell cycle entry and promotes cell cycle progression towards S-phase. Misregulated CDKs induce unscheduled proliferation as well as genomic and chromosomal instability (Malumbres and Barbacid, 2009). The CDK inhibitor p27Kip1 has a key role in controlling CDKs and cell proliferation in response to diverse mitogenic or antiproliferative stimuli (Chu et al., 2008). Non-proliferating cells are frequently characterized by elevated p27Kip1 levels that decline upon mitogenic stimulation (Hengst and Reed, 1996; Chu et al., 2008). Binding of p27Kip1 usually inhibits CDK activity. However, p27Kip1 was also found in active CDK complexes and surprisingly even contributes to CDK activation by promoting assembly of active cyclin D/CDK holoenzymes (LaBaer et al., 1997; James et al., 2008; Larrea et al., 2008; Ray et al., 2009). We recently identified a molecular switch that triggers the transition of p27Kip1 from a CDK inhibitor to a potential activator of specific CDKs, thus resulting in the conversion of a tumor suppressor into a potential oncogene. This mechanism is based on the phosphorylation of tyrosine residue 88 (Y88) of p27Kip1, which results in ejection of an inhibitory 310-helix of p27Kip1 from the ATP-binding pocket of the CDK (Grimmler et al., 2007). The induced conformational change permits ATP binding of the CDK and partially activates the p27Kip1-bound kinase (Chu et al., 2007, 2008; Grimmler et al., 2007; James et al., 2008; Larrea et al., 2008; Ray et al., 2009). One substrate of p27Kip1-bound CDK2 is Y88-phosphorylated p27Kip1 itself. Activated CDK2 can now phosphorylate cyclin/CDK-bound Y88-phosphorylated p27Kip1 on threonine 187 (T187) (Grimmler et al., 2007). Phosphorylated T187 is a pivotal core of the phosphodegron recognized by the SCFSkp2 complex, which initiates the ubiquitin-proteasome-dependent degradation of p27Kip1 (Grimmler et al., 2007; Chu et al., 2008; Frescas and Pagano, 2008). The p27Kip1-Y88 phosphorylation is induced upon mitogen stimulation, suggesting that p27Kip1-Y88 phosphorylation may serve as a potent mechanism of direct signal integration. Oncogenes like BCR-Abl use this mechanism to inactivate p27Kip1; however, molecular pathways triggering this mechanism in normal cells remained undefined.
Janus kinase 2 (JAK2) is a non-receptor tyrosine kinase, which can bind with its FERM (band 4.1, ezrin, radixin, moesin) domain to the cytoplasmic tails of various transmembrane cytokine receptors (Parganas et al., 1998). JAK2 activation by trans- or autophosphorylation leads to downstream signaling including activation of signal transducer and activator of transcription 5 (STAT5), phosphoinositol-3-kinase (PI-3 K), mitogen-activated protein kinase or Bcl-2 family members (Baker et al., 2007). Phosphorylated STAT5 proteins enhance the transcription of apoptosis regulatory proteins like BCL-XL and cell cycle regulators including cyclins D1, D2, E and the CDK inhibitor p21Cip1 (Desrivieres et al., 2006). JAK2 has a central role in hematopoiesis by regulating cell survival, proliferation and differentiation (Baker et al., 2007). Constitutive activation of JAK2 can cause oncogenic transformation (Ihle and Gilliland, 2007). Mutation of valine 617 to phenylalanine (JAK2V617F) within its pseudokinase domain is one of the most common activating mutations of JAK2, and JAK2V617F is the most frequent mutation in BCR-Abl negative myeloproliferative disorders, with the highest incidence (95%) in polycythemia vera (PV) (James et al., 2005; Kralovics et al., 2005; Levine et al., 2005; Zhao et al., 2005). Hyperproliferation induced by JAK2V617F involves activation of STAT5 and its downstream targets (Walz et al., 2006; Wernig et al., 2008). It has been reported that transcriptional downregulation of cyclin D2 and upregulation of p27Kip1 is a central mechanism of growth arrest upon JAK2V617F inhibition (Walz et al., 2006). The decrease of p27Kip1 upon JAK2V617F expression correlated with STAT5-induced expression of Skp2, suggesting that the degradation of p27Kip1 could be a consequence of the overexpression of this p27Kip1-directed ubiquitin ligase (Furuhata et al., 2009).
In this study, we describe the identification of p27Kip1 as a novel JAK2 substrate. JAK2 binds to p27Kip1 through its FERM and kinase domains and phosphorylates Y88 of p27Kip1. This leads to partial activation of p27Kip1-bound CDK-cyclin complexes, and permits SCFSkp2-dependent degradation of p27Kip1 (Grimmler et al., 2007). The p27Kip1-Y88 phosphorylation is induced upon JAK2 activation by interleukin-3 (IL-3), and decreased by JAK2 inactivation. Substantial Y88-phosphorylated p27Kip1 was detected in patient-derived JAK2V617F positive hematopoietic cell lines. Inactivation of JAK2V617F reduced Y88-p27Kip1 phosphorylation and resulted in a concomitant increase of p27Kip1 protein and cell cycle arrest. These observations directly connect JAK2-mediated cytokine receptor signaling with the core cell cycle machinery, and uncover a novel pathway that can contribute to hyperproliferation induced by deregulated JAK2 activation.
Results
p27Kip1 becomes tyrosine phosphorylated upon IL-3 stimulation
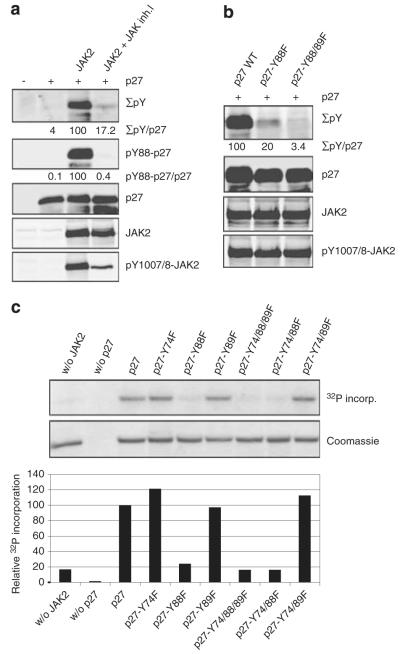
We and others recently reported that serum stimulation can cause p27Kip1 tyrosine phosphorylation (Grimmler et al., 2007; James et al., 2008). Therefore, we aimed to determine mitogenic stimuli that induce this phosphorylation and to identify p27Kip1-phosphorylating kinases. Cytokines are important positive and negative regulators of the cell cycle and can stimulate quiescent cells to proliferate. For example, IL-3 is required to maintain proliferation of different hematopoietic cell types, and its withdrawal leads to cell cycle arrest in G1-phase (Kelvin et al., 1986). Investigating IL-3 induced p27Kip1 modifications in the IL-3-dependent murine pro-B cell line Ba/F3, we observed a strong induction of p27Kip1-Y88 phosphorylation shortly after IL-3 stimulation, whereas no p27Kip1-Y88 phosphorylation was detected upon deprivation of the cytokine (Figure 1a). The p27Kip1-Y88 phosphorylation in response to IL-3 was also observed in IL-3 dependent murine myeloblast 32D cells (Supplementary Figure 1). IL-3 receptor signaling activates JAK2 (Parganas et al., 1998), which was traced by its Y1007/1008 phosphorylation (Feng et al., 1997). JAK2 activation as well as tyrosine phosphorylation of its substrate STAT5A/B was detected 5 min after IL-3 stimulation, slightly preceding the peak of p27Kip1-Y88 phosphorylation (Figure 1a). Activation of Src family kinases, monitored by an antibody recognizing activated Src and related kinases, indicates no change in activity at time points investigated (Figure 1a). The coincidence of JAK2 activation and p27Kip1 tyrosine phosphorylation lead us to investigate whether JAK2 could initiate p27Kip1 phosphorylation. Incubation of Ba/F3 cells with the JAK kinase-specific pyridone-containing tetracycle ‘JAK inhibitor-I’ (Thompson et al., 2002) before IL-3 stimulation, led to a decline in Y1007/1008-phosphorylated JAK2 as well as Y88-phosphorylated p27Kip1 (Figure 1b), indicating that JAK2 activation is a prerequisite for p27Kip1 Y88 phosphorylation.
Figure 1.
IL-3 induces phosphorylation of p27Kip1 on tyrosine 88. (a) Ba/F3 cells were starved (6 h) for IL-3 and stimulated with 5 ng/ml rIL-3 as indicated. Level of p27Kip1-phospho-Y88 immunoprecipitates, JAK2-phospho-Y1007/1008 immunoprecipitates, STAT5A/B-phospho-Y694/699, Src family kinase-phospho-Y416, p27Kip1 and α-tubulin were determined by immunoblot analysis. Representative blots of three independent experiments are shown. (b) Tyrosine-88-phosphorylation of p27Kip1 correlates with JAK2 activation. Ba/F3 cells were starved (6 h) for IL-3 and stimulated with 5 ng/ml recombinant IL-3 for 15 min in the absence and presence of 3 μm JAK inhibitor-I. Immunoblot analysis of p27Kip1-phospho-Y88 immunoprecipitates, JAK2-phospho-Y1007/1008 immunoprecipitates, STAT5A/B-phospho-Y694/699, JAK2, p27Kip1 and α-tubulin as a loading control. Signals decreased to 0.89% for p27Kip1-phospho-Y88, to 1,22% for JAK2-phospho-Y1007/1008 and to 0.28% for STAT5A/B-phospho-Y694/699 after JAK inhibitor treatment of IL-3 stimulated cells (vehicle control=100%; Gel Pro Analyser software, Media Cybernetics, Bethesda, MD, USA). Mouse IgG was used in control IPs (CTRL IP), coupled p27Kip1-phospho-Y88-specific monoclonal antibodies were loaded to exclude unspecific signals of the antibody (CTRL AB). IP, immunoprecipitation; WCE, whole cell extracts.
JAK2 phosphorylates p27Kip1 on tyrosine 88
As p27Kip1-Y88 phosphorylation shortly followed IL-3 stimulation and JAK2 activation (Figure 1a), we investigated whether activation of JAK2 was sufficient to phosphorylate p27Kip1. We transfected 293T cells with p27Kip1 in the presence and absence of JAK2. Coexpression of JAK2 and p27Kip1 resulted in intense tyrosine phosphorylation of p27Kip1, and treatment with the JAK inhibitor-I strongly reduced this phosphorylation (Figure 2a, Supplementary Figure 2) as well as JAK2-Y1007/1008 phosphorylation (Figure 2a). In addition to Y88, p27Kip1 comprises only two additional tyrosine residues, which are also located within its CDK-inhibitory domain. Using p27Kip1 phospho-Y88-specific antibodies, we identified Y88 as major phosphorylation site upon JAK2 expression (Figure 2a) and IL-3 stimulation (Figure 1). The p27Kip1-Y88F and p27Kip1-Y88/89F mutants were poor substrates for JAK2-induced phosphorylation (Figure 2b), indicating that JAK2 preferentially phosphorylates Y88. In all, 80% of p27Kip1 tyrosine phosphorylation is lost if Y88 is exchanged to phenylalanine (Figure 2b). The remaining signal was reduced to 3.4% by mutating Y89 in addition to Y88, indicating that Y89 might be a second, low-affinity phosphorylation site. To investigate if JAK2 can directly phosphorylate p27Kip1, and to determine which tyrosines become phosphorylated, we incubated purified recombinant p27Kip1 with the purified JAK2 kinase domain. Direct phosphorylation of p27Kip1 by JAK2 was observed in kinase assays (Figure 2c). To identify tyrosine residues that can be phosphorylated by JAK2 in vitro, we replaced one, combinations of two or all three tyrosines of p27Kip1 with phenylalanine. Efficient phosphorylation of p27Kip1 by JAK2 required the presence of Y88, whereas mutations of Y89 or Y74 to phenylalanine failed to reduce p27Kip1 phosphorylation (Figure 2c). These data suggest a direct phosphorylation of tyrosine residue 88 of p27Kip1 by JAK2.
Figure 2.
JAK2 phosphorylates p27Kip1 on tyrosine residue 88 in vitro and in vivo. (a) Incubation of JAK2-transfected 293T cells with 3 μm JAK inhibitor-I for 3 h impairs p27Kip1 tyrosine-88-phosphorylation. HA-p27Kip1 was co-transfected with JAK2 in 293T cells. Extracts were boiled (65 °C; 10 min) to precipitate the majority of the proteins. Levels of the heat stable p27Kip1 and its phosphorylation on tyrosines were simultaneously determined by the Odyssey infrared imaging system. Overall phospho-tyrosine was detected using the 4G10 antibody, and p27Kip1-Y88 phosphorylation was detected by a specific p27Kip1-pY88 monoclonal antibody. Quantitative analyses of these signals (adjusted to 100% for wild-type p27Kip1 when co-expressed with JAK2, and corrected for p27Kip1 expression levels) are listed below as indicated. JAK2 and its tyrosine phosphorylation on residues 1007/1008 were analyzed in whole cell extracts by the Odyssey infrared imaging system. A representative of three independent experiments is shown. (b) Tyrosine 88 is the main JAK2 phosphorylation site. JAK2 was transfected with p27Kip1 and p27Kip1 tyrosine to phenylalanine mutants as indicated. The analysis was performed as described above. (c) JAK2 phosphorylates Y88 of p27Kip1 in vitro. In vitro kinase assays were performed with recombinant purified His-p27Kip1 and His-p27Kip1 mutant proteins as indicated. Proteins were incubated with the kinase domain of JAK2 and γ-32P-ATP. Incorporation of radioactive phosphate into p27Kip1 was detected after SDS–PAGE by autoradiography (upper panel). p27Kip1 loading was confirmed by Coomassie brilliant blue staining (middle panel). Incorporation of 32P was quantified with a Typhoon 9410 scanner and ImageQuant software. Phosphorylation of wt p27Kip1 was set to 100% (lower panel).
p27Kip1 binds to the FERM and the kinase domains of JAK2
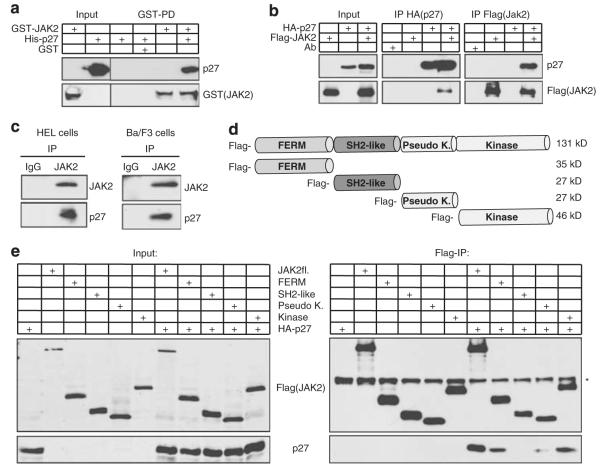
If JAK2 directly phosphorylates p27Kip1, these proteins might associate in a stable complex. This could occur through direct binding or involve additional bridging proteins. To investigate direct binding of JAK2 to p27Kip1, we first analyzed the interaction of purified recombinant His-p27Kip1 with recombinant GST-JAK2 in vitro. To exclude co-purification of interacting eukaryotic proteins, both recombinant proteins were isolated from E.coli. Pull-down of GST-JAK2 on glutathione beads co-precipitated p27Kip1, indicating that both proteins can form a stable complex in vitro (Figure 3a). We next expressed p27Kip1 and JAK2 in 293T cells, where JAK2 co-precipitated with p27Kip1, and p27Kip1 co-precipitated with JAK2 (Figure 3b). To test if the endogenous proteins interact under physiological conditions, we precipitated JAK2 from the mouse Ba/F3 pro-B cells and activated JAK2V617F from the human erythroleukemia cell line HEL. Importantly, endogenous p27Kip1 co-immunoprecipitates with JAK2 as well as with JAK2V617F (Figure 3c). These data support the hypothesis that p27Kip1 is a direct substrate of JAK2, which binds to p27Kip1 and phosphorylates Y88 of p27Kip1. To further characterize the p27Kip1-JAK2 interaction, we investigated binding of p27Kip1 to four domains of JAK2, representing the entire protein: the FERM-, SH2-like-, pseudokinase- and the kinase domain (Figure 3d, (Zhao et al., 1995)). Full-length JAK2, the N-terminal FERM- and the C-terminal kinase domain of JAK2 precipitate p27Kip1 (Figure 3e). A weak interaction was observed with the pseudokinase domain. Immunoprecipitation of p27Kip1 and analysis of the co-precipitated JAK2 fragments confirmed this interaction (data not shown).
Figure 3.
JAK2 directly interacts with p27Kip1 through its FERM and kinase domain. (a) GST and GST-JAK2 fusion proteins isolated from E.coli were bound to glutathione sepharose and incubated with recombinant purified His-p27Kip1. After extensive washing, GST-JAK2-associated p27Kip1 was detected in immunoblots. (b) p27Kip1 interacts with JAK2 in 293T cells. HA-p27Kip1 and Flag-JAK2 were overexpressed and expression was verified by immunoblotting. For the co-preciptiation analysis, HA-p27Kip1 or Flag-JAK2 were immunoprecipitated using tag-specific antibodies. Immunoprecipitated proteins were detected in immunoblots. (c) Endogenous p27Kip1 co-immunoprecipitates with endogenous JAK2 and JAK2V617F in hematopoietic cells. JAK2V617F was immunoprecipitated from HEL cells and JAK2 from IL-3-starved (16 h) Ba/F3 cells and analyzed for co-immunoprecipitated p27Kip1 in immunoblots. (d) Schematic representation of JAK2 domains that are used in (e). p27Kip1 interacts with JAK2, its isolated FERM- and kinase domains. Flag-tagged JAK2 and Flag-tagged JAK2 domains were expressed with HA-p27Kip1 in 293T cells. Their expression was confirmed in immunoblots ((e), left panel, ‘Input’). Flag-tagged proteins were immunoprecipitated with Flag-antibodies and precipitates were analyzed in immunoblots ((e), right panel, ‘Flag-IP’). p27Kip1 co-immunoprecipitates with JAK2, its FERM domain and its kinase domain. A weak interaction could be observed with the pseudokinase domain. The asterisk (*) indicates heavy chain.
Localization of JAK2, p27Kip1 and Y88-phosphorylated p27Kip1
JAK2 is predominantly localized in the cytoplasm and frequently associated with the cytoplasmic tails of cytokine receptors. The p27Kip1 can shuttle between the nucleus and the cytoplasm (Connor et al., 2003), and phosphorylation by several kinases including Akt/PKB leads to its translocation to the cytoplasm (Chu et al., 2008). To investigate if the localization of p27Kip1 and JAK2 overlaps and to determine where Y88-phosphorylated p27Kip1 accumulates, we performed immunofluorescence experiments and biochemical fractionations. Transfection of U2OS and 293T cells with p27Kip1 and JAK2 or JAK2V617F revealed that Y88-phosphorylated p27Kip1 was highly abundant in the cytoplasm (Figure 4a, panels 1, 5, 9, 13), as seen for active JAK2 (Figure 4a, panel 6), or JAK2V617F (Figure 4a, panel 14) (Supplementary Figure 3). p27Kip1 could be detected in the nucleus and the cytoplasm (Figure 4a, panels 2, 10). Endogenous Y88-phosphorylated p27Kip1 of HEL erythroleukemia cells also localized predominantly within the cytoplasm (Figure 4b, panels 1, 3). To confirm the subcellular localization, we fractionated extracts from HEL- and Ba/F3 cells. In line with the immunofluorescence data, endogenous Y88-phosphorylated p27Kip1 was mainly present in the cytosolic fraction, together with p27Kip1 and JAK2V617F (HEL cells) or JAK2 (Ba/F3 cells) (Figures 4c and d).
Figure 4.
Tyrosine 88-phosphorylated p27Kip1 is predominantly localized in the cytoplasm. (a) Immunofluorescence analyses of U2OS cells transfected with HA-p27Kip1, Flag-JAK2 or Flag-JAK2V617F as indicated by confocal microscopy. pY88-p27Kip1 is shown in red and pY1007/1008-JAK2/pY1007/1008-JAK2V617F and p27Kip1 are shown in green. DNA of the corresponding cells is stained with Hoechst dye (blue). Panels 1–4 show the localization of p27Kip1 and pY88-p27Kip1, panels 5–8 the localization of pY88-p27Kip1 and pY1007/1008-JAK2 after transfection of p27Kip1 and JAK2. Panels 9–12 show the localization of pY88-p27Kip1 and JAK2V617F and panels 13–16 the localization of pY88-p27Kip1 and pY1007/1008-JAK2 after transfection of p27Kip1 and JAK2V617F. As negative controls, cells transfected with HA-p27Kip1–Y88F and JAK2V617F or cells transfected with HA-p27Kip1 only were stained with antibodies recognizing pY88-p27Kip1 and pY1007/1008-JAK2 (panels 17–24). Scale bar represents 20 μm; (b) Immunofluorescence analysis of endogenous pY88-p27Kip1 (panel 1) and pY1007/1008-JAK2V617F (panel 2) in HEL cells by confocal microscopy. The corresponding overlay is shown in panel 3 and Hoechst staining in panel 4. (c) Endogenous pY88-p27Kip1 is predominantly cytosolic in HEL cells. Whole cell extracts were separated in nuclear, cytosolic and membrane fractions and analyzed by immunoblots as indicated. Lamin A, α-tubulin and IGF-IRβ were used as markers for nuclear, cytoplasmic and membrane fractions. (d) Endogenous pY88-p27Kip1 is predominantly cytosolic in Ba/F3 cells. Analysis as in (c), transcription factor Sp1 was used as control for nuclear proteins. Representative blots of three independent experiments are shown. The asterisk (*) indicates a non-specific band.
Activated JAK2V617F of human leukemia cell lines phosphorylates p27Kip1 and decreases its stability
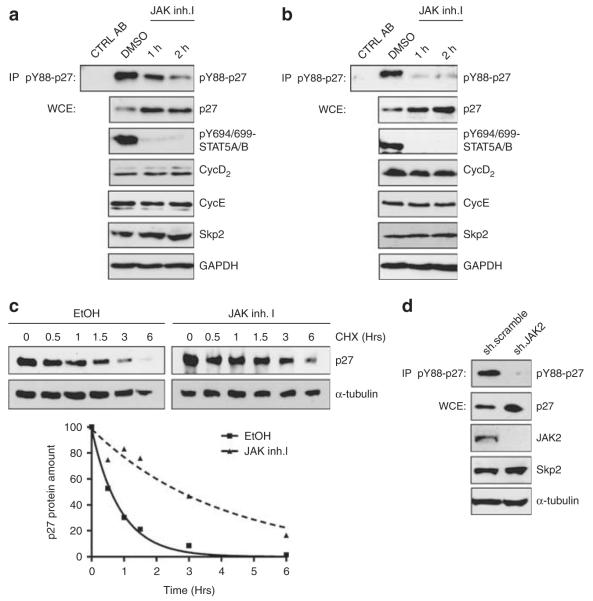
The JAK2V617F mutant transforms hematopoietic progenitors and is a common mutation in myeloproliferative disease (Levine and Gilliland, 2007). JAK2V617F permits IL-independent growth of the IL-3-dependent cell line Ba/F3 (Furuhata et al., 2009). Inhibition of the activated kinase leads to increased p27Kip1 levels and decreased cyclin D2 levels, and arrests human erythroleukemia HEL cells in G1-phase (Walz et al., 2006; Furuhata et al., 2009). To ascertain Y88 phosphorylation of p27Kip1 by JAK2V617F in vivo, we investigated p27Kip1 regulation in two patient-derived leukemia cell lines naturally expressing JAK2V617F: the human erythroleukemia cell line HEL and the acute myeloid leukemia-derived cell line SET2. We observed that p27Kip1 is extensively phosphorylated on Y88 in both cell lines. Inactivation of JAK2 by JAK inhibitor-I strongly decreased p27Kip1-Y88 phosphorylation (Figures 5a and b). Interestingly, an increase in p27Kip1 protein could be observed already 1 h after treatment (Figures 5a and b). We reported earlier that tyrosine-88 phosphorylation of p27Kip1 enhances its SCFSkp2-mediated ubiquitin-proteasomal degradation and decreases its Cdk-cyclin inhibitory potential (Chu et al., 2007, 2008; Grimmler et al., 2007). Consistent with this observation, we now find that p27Kip1 stability is increased upon JAK2 inactivation (Figure 5c, Supplementary Figure 5), and that p27-Y88F leads to a stronger reduction of HEL cells in S-phase than wild-type p27Kip1 (Supplementary Figure 6). It has been proposed that p27Kip1 accumulation by JAK2 inhibition is due to decreased Skp2 expression (Agarwal et al., 2008; Furuhata et al., 2009). We observed that Skp2 levels remained unchanged at early time points of JAK2 inhibition, and only decreased after 4 h of JAK inhibitor-I treatment (Supplementary Figure 4). To exclude that JAK inhibitor-I might act on additional kinases, we used small hairpin RNA-mediated JAK2 knockdown using established small hairpin RNAs (Neilson et al., 2007). Silencing of JAK2 expression resulted in a loss of p27Kip1-Y88 phosphorylation and increased p27Kip1 levels, whereas Skp2 levels remained unchanged (Figure 5d). Consistent with earlier observations (Walz et al., 2006), we observed an increase of cells in G0/G1-phase and a decrease of cells in S-phase, indicating that depletion of JAK2 delays or arrests cells in G0/G1 phase (Supplementary Figure 7). As Skp2 expression remained unchanged, despite the significant loss of p27Kip1 phosphorylation and cell cycle delay/arrest, these data support the model that inhibition of Skp2 expression is not essential for p27Kip1 stabilization after JAK2 inhibition.
Figure 5.
p27Kip1-Y88 phosphorylation is decreased and p27Kip1 stability is increased upon JAK2V617F inhibition or small hairpin RNA-mediated JAK2V617F knockdown. (a) Inhibition of JAK2V617F reduces p27Kip1-Y88 phosphorylation and increases p27Kip1 expression in HEL cells. Cells were incubated with solvent (dimethyl sulfoxide or ethanol, as indicated) or JAK inhibitor-I (JAK inh.I, 3 μm). p27Kip1-pY88 immunoprecipitates, p27Kip1, pY694/699-STAT5A/B, cyclin D2, cyclin E, Skp2, GAPDH were detected by immunoblotting. IP, immunoprecipitation, WCE, whole cell extract. (b) SET-2 cells were analyzed as in (a). (c) p27Kip1 stability increases upon JAK2V617F inhibition. HEL cells were incubated with JAK inhibitor-I (3 μm) or the solvent (ethanol). After 4 h incubation, cycloheximide (CHX) was added to a final concentration of 100 μg/ml and cells were incubated for the indicated periods of time. p27Kip1 expression was determined in immunoblots. The α-tubulin was used as a loading control. Lower panel: immunoblot signals were quantified (Gel Pro Analyser software, Media Cybernetics) and intensities were adjusted to 100% at 0 h and plotted over time. The exponential decay curve, determined by regression analysis, is shown (GraphPad Prism5 software, La Jolla, CA, USA). (d) Knockdown of JAK2V617F in HEL cells reduces p27Kip1-Y88 phosphorylation and increases p27Kip1 levels. HEL cells were electroporated with control (‘scramble’) or JAK2-specific small hairpin RNA (pLKO1.puro plasmids, (Neilson et al., 2007) and incubated for 40 h. p27Kip1-pY88 immunoprecipitates, p27Kip1, JAK2, Skp2 and α-tubulin were detected in immunoblots.
Discussion
We have recently identified p27Kip1 tyrosine phosphorylation as an important molecular mechanism that impairs function and stability of the CDK inhibitor p27Kip1 in the presence of mitogens, leading to partial CDK activation and cell cycle progression (Grimmler et al., 2007; Chu et al., 2008; Ray et al., 2009). Oncogenic tyrosine kinases like BCR-Abl can phosphorylate and inactivate p27Kip1; however, specific kinases phosphorylating p27Kip1 during mitogen-induced cell cycle entry remained unknown. In this study, we describe that cytokine-induced JAK2 activation triggers Y88–phosphorylation of p27Kip1. JAK2 can directly bind and phosphorylate p27Kip1. Thus, the identification of JAK2 as a p27Kip1-Y88-phosphorylating kinase now provides a novel and direct link between cytokine signaling and cell cycle control in untransformed cells.
The p27Kip1 can form a complex with JAK2 that is stable enough to be immunoprecipitated (Figure 3). The FERM domain and the kinase domain of JAK2 bind to p27Kip1. As full-length JAK2 bound p27Kip1 more efficiently than single domains (Figure 3e), both domains seem to cooperate for efficient binding. Activation of JAK2 can trigger diverse biological responses including cell proliferation, differentiation or apoptosis. JAK2 links cytokine stimulation to p27Kip1 phosphorylation, leading to its inactivation and elimination. However, ligand binding to cytokine receptors does not always promote cell proliferation. Depending on the cell type, cytokines like oncostatin M can even prevent cell proliferation and induce p27Kip1 (Halfter et al., 2006). Therefore, activated JAK2 may not always phosphorylate and inactivate p27Kip1. It is tempting to speculate that the ability of JAK2 to bind and inactivate p27Kip1 might be cell type specific and regulated. For example, the p27Kip1–JAK2 interaction may be inhibited by modifications such as phosphorylation or by competing binding partners including various cytokine receptors. Finally, regulation of the subcellular localization of p27Kip1 or active JAK2 may determine the efficiency of p27Kip1 phosphorylation and inactivation by JAK2.
JAK2 associates with the cytoplasmic tail of cytokine receptors, but also with receptor tyrosine kinases and seven transmembrane-spanning receptors (Wallace and Sayeski, 2006). In addition, active JAK2V617F was recently detected in the nucleus (Dawson et al., 2009). Even though Y88-phosphorylated p27Kip1 accumulated in the cytosol under the conditions here, it remains to be determined where JAK2 can phosphorylate p27Kip1. Depending on its cellular context and mode of activation, JAK2 may phosphorylate p27Kip1 in different cellular compartments. Previously, tyrosine-phosphorylated p27Kip1 was detected in the nucleus and it was speculated that tyrosine phosphorylation leads to nuclear translocation of p27Kip1 (Kardinal et al., 2006; Tossidou et al., 2008; Ray et al., 2009). We now observe that endogenous Y88-phosphorylated p27Kip1 in the hematopoietic cell lines HEL and Ba/F3, as well as overexpressed p27Kip1 phosphorylated by JAK2 in U2OS and 293T cells is predominantly cytoplasmic (Figure 4, Supplementary Figure 3), indicating that Y88 phosphorylation alone is insufficient to restrain p27Kip1 to the nucleus.
The p27Kip1 is Y88-phosphorylated in myeloid cell lines expressing the active JAK2V617F variant (Figures 5a and b). Inactivation of JAK2V617F reduces Y88 phosphorylation of p27Kip1 and leads to its concomitant increase and enhanced stability. Our recent observation that Y88 phosphorylation by oncogenic tyrosine kinase BCR-Abl triggers premature Skp2-dependent p27Kip1 proteosomal degradation (Grimmler et al., 2007), is well in line with our observation here that inhibition of oncogenic JAK2V617F can reestablish p27Kip1 stability and expression. In both JAK2V617F-expressing cell lines investigated, increased p27Kip1 protein could already be detected 1 h after JAK inhibitor-I addition, indicating that p27Kip1 stabilization is an immediate consequence of JAK2V617F inactivation. Immediately after IL-3 stimulation (15 and 30 min), we detected an increase of Y88 phosphorylation without a significant decline of p27Kip1 levels (Figure 1). As the half-life of p27Kip1 is significantly longer than these short time points, effects on overall protein level would need more time to build up. Recent reports proposed that JAK2V617F activity leads to decreased p27Kip1 stability by transcriptional induction of Skp2 involving STAT5 (Agarwal et al., 2008; Furuhata et al., 2009). The increase of p27Kip1 after 24-h JAK inhibitor-I treatment correlated with decreased Skp2 expression (Agarwal et al., 2008). In HEL cells, we observed no decline in Skp2 levels for up to 8 h of JAK inhibitor-I treatment (Supplementary Figure 4a), and importantly, p27Kip1 protein levels increase already at earlier time points, when Skp2 level remain unchanged (Figures 5a and b). Skp2 expression itself is regulated during cell cycle progression and the protein is degraded in early G1-phase after ubiquitination by APCCdh1 (Wei et al., 2004; Frescas and Pagano, 2008). As other cell cycle-regulated proteins including cyclin E and cyclin D2 also decline after extended JAK Inhibitor-I incubation (16 h, Supplementary Figure 4b), it cannot be excluded that this decline of Skp2 level might also result from cell cycle synchrony in early G1-phase. In addition, knockdown of JAK2 expression by RNA interference abolished p27Kip1-Y88 phosphorylation and led to accumulation of p27Kip1, with no change in Skp2 protein level (Figure 5d). Together, these data indicate that p27Kip1 regulation by JAK2 can occur independently from Skp2 expression. However, it is clear that Y88-triggered p27Kip1 degradation at the G1/S transition requires Skp2 expression as well as sufficient SCFSkp2 E3 ubiquitin ligase activity (Chu et al., 2008; Frescas and Pagano, 2008). Cells expressing insufficient Skp2 protein, therefore, may require additional induction of Skp2 in order to overcome a p27Kip1-imposed cell cycle arrest and to proliferate.
The p27Kip1 is a negative regulator of hematopoietic progenitor proliferation. JAK2V617F transforms hematopoietic progenitors in PV patients, causing increased proliferation and maturation (Levine and Gilliland, 2007; Bruchova et al., 2009). Ex vivo culture of primary erythroid progenitors recently revealed that p27Kip1 was increased at certain stages of erythroid differentiation in cells from healthy subjects, whereas p27Kip1 levels remained low in erythroid cells from PV patients. Low p27Kip1 in erythroid PV cells correlated with high proliferation (Bruchova et al., 2009). It is therefore tempting to speculate that p27Kip1-Y88 phosphorylation by JAK2V617F might also have a role in PV development. Initial data indicate that p27Kip1 is extensively phosphorylated on Y88 in PV patients with high levels of active JAK2. Skp2 expression neither correlated with active JAK2 nor with p27Kip1 levels in these primary cells (H Jäkel, D Wolf, L Hengst; unpublished results).
JAK2 inhibitors prove to be effective in controlling hyperproliferation and symptom reduction in PV, although they merely eliminate mutant clones (Verstovsek, 2009). Y88 phosphorylation of p27Kip1 could contribute to JAK2V617F-dependent hyperproliferation and transformation in this and other hematological malignancies. JAK inhibitors may reduce hyperproliferation in part by preventing p27Kip1-Y88 phosphorylation-induced inactivation and degradation.
The phosphorylation of p27Kip1 by JAK2 provides a novel exciting mechanism, by which JAK2 can directly impinge on cell cycle progression in normal and transformed cells. The identification of p27Kip1 as target of JAK2 and JAK2V617F could open new avenues for diagnosis, as well as targeted therapeutic intervention in JAK2-dependent myeloproliferative diseases.
Materials and methods
Cell culture, transfection and cell lysis
HEL and Ba/F3 cells were obtained from American Type Culture Collection (ATCC) and propagated as recommended. The 293T cells were transfected using calcium phosphate. For a 6 cm dish, 2 μg HA-p27 PCDNA3.1 plasmid and 3 μg of JAK2 pCMX plasmid were transfected. For immunoprecipitation experiments, HA-p27 plasmid and Flag-JAK2 plasmid or indicated Flag-JAK2 domains were transfected. 40 h after transfection, cells were washed in ice-cold phosphate buffered saline containing 1 mm pervanadate and lysed in 0.5% NP-40 lysis buffer (50 mm Tris pH 7.5, 150 mm NaCl, 0.5% NP-40, phosphatase inhibitors (Phosphostop, Roche, Mannheim, Germany) and protease inhibitor cocktail (Sigma Aldrich, St Louis, MO, USA)). For immunoblot analysis of p27Kip1 and p27Kip1-Y88 phosphorylation, protein extracts were boiled at 65 °C for 10 min, and the heat labile proteins were precipitated by centrifugation at 16 000 r.p.m. for 15 min. The cleared supernatant containing heat stable proteins such as p27Kip1 (Hengst et al., 1994) was separated by SDS–PAGE. The subsequent immunoblot analyses were performed by using ECL (Pierce, Thermo Fisher Scientific, Waltham, MA, USA) or Odyssey infrared fluorescence detection (Li-Cor, Bad Homburg, Germany) technologies.
Plasmids and RNA interference
Mammalian HA-p27Kip1 and its tyrosine mutants in the pcDNA3.1 vector as well as bacterial His-p27Kip1 and its tyrosine mutants in the pET28a vector were described previously (Grimmler et al., 2007). N- and C-terminus of JAK2 cDNA (a kind gift from W Doppler) was amplified by a two-step PCR and recombined into pDONR207. To obtain the full-length JAK2 the 1.6 kb XhoI-NdeI fragment of the JAK2 cDNA was inserted via ligation into the pDONR-JAK2 plasmid (pENTR-JAK2). To generate expression vectors containing JAK2 fragments, entry vectors were generated via PCR with specific primers and recombination with pDONR207 was performed. The JAK2V617F construct was cloned by a two-step PCR. The PCR fragment and the pENTR-JAK2 plasmid were digested with Bsp119I and XbaI, and the PCR product was ligated into the truncated pENTR-JAK2 plasmid backbone. All expression vectors were generated via recombination with the appropriate destination vector (Gateway technology, Invitrogen, Carlsbad, CA, USA), and verified by sequencing. All primers used for cloning are listed in Supplementary Table 1.
HEL cells were electroporated with control (scramble) pLKO1.puro (Addgene (Cambridge, MA, USA) plasmid #1864 (Sarbassov et al., 2005)) or JAK2-specific small hairpin RNAs pLKO1.puro plasmids (Sigma, TRCN0000003180, as described (Neilson et al., 2007)) with AMAXA Nucleofector (Lonza, Cologne, Germany) according to the manufacturer’s recommendations and incubated for 68 h after electroporation.
Antibodies and reagents
Following antibodies were used: mouse anti-p27Kip1-horseradish peroxidase-coupled antibodies (clone 57, BD Bioscience, Franklin Lakes, NJ, USA), anti-p27Kip1-C19 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-pY1007/1008-JAK2 (Millipore, Billerica, MA, USA), mouse anti-JAK2 (clone 691R5, Invitrogen), rabbit anti-JAK2 antibodies coupled to agarose (Millipore), mouse anti-pY694/699-STAT5A/B (clone 8-5-2, Millipore), rabbit anti-phospho-Src family (Y416) antibody (Cell signaling, Danvers, MA, USA), mouse anti-phosphotyrosine antibodies (clone 4G10, Upstate, Millipore), mouse anti-pY88-p27Kip1 (Grimmler et al., 2007), mouse anti-DDDDK (anti-Flag octapeptide, clone M2, Sigma), mouse monoclonal anti-HA tag antibody (clone 12CA5, Abcam, Cambridge, MA, USA), mouse anti-Skp2 (clone A-2, Santa Cruz), rabbit anti-Cyclin D2 (Santa Cruz), mouse anti-Cyclin E (clone HE12, Santa Cruz), mouse anti-GAPDH (Sigma), mouse anti-α-tubulin (Sigma), mouse anti-LaminA/C (Upstate), rabbit anti-Sp1 (H-225, Santa Cruz) and rabbit anti-IGF-IRβ (C-20, Santa Cruz). Recombinant mouse IL-3 (rIL-3) was purchased from PeproTech (Rocky Hill, NJ, USA) and ‘JAK inhibitor-I’ from Merck (Darmstadt, Germany).
Western blot, co-immunoprecipitation and cell fractionation
For co-precipitation experiments, cells were lysed in immunoprecipitation buffer (50 mm (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (HEPES) pH 7.5, 150 mm NaCl, 1 mm ethylenediaminetetraacetic acid (EDTA), 1 mm EGTA, 10% glycerol, 1% Triton X100, Phosphostop (Roche) and protease inhibitor cocktail (Sigma)) and centrifugated at 16 000 r.p.m. for 20 min. Transfected HA-p27Kip1 was immunoprecipitated with anti-HA antibodies covalently linked to protein A-Sepharose (Immunosorb A; EC Diagnostics AB, Uppsala, Sweden), and Flag-JAK2 was immunoprecipitated with anti-Flag antibodies. After SDS–PAGE, western blot analysis was performed with anti-Flag- and mouse anti-p27Kip1-horseradish peroxidase-coupled antibodies. For co-immunopreciptation analysis of endogenous proteins, JAK2 was immunoprecipitated with anti-JAK2 agarose beads. Immunoblot analyses were performed with mouse anti-JAK2 antibodies and mouse anti-p27Kip1-horseradish peroxidase-coupled antibody. For subcellular fractionation, protein extracts were separated into nuclear, cytoplasmic and membrane compartments (Behrmann et al., 2004). Briefly, cells were incubated in hypotonic lysis buffer and homogenized using a Dounce homogenizer. The nuclei were precipitated by centrifugation at 1000 g, 10 min, and washed twice with lysis buffer. The nuclei were lysed in a detergent-containing buffer (20 mm Tris/HCl pH 7.5, 1% Triton ×100, 280 mm NaCl, 1 mm dithiothreitol, 1mm ethylenediaminetetraacetic acid, protease- and phosphatase inhibitors), and insoluble proteins were precipitated by centrifugation. The crude cytoplasmic fraction was centrifuged at 20 000 g and the pellet (membranes) was solubilized in membrane lysis buffer (20 mm Tris–HCl pH 7.5, 120 mm NaCl, 1% Triton ×100, 1 mm ethylenediaminetetraacetic acid (EDTA), 1mm dithiothreitol , protease and phosphatase inhibitors). The supernatant was once more centrifuged (100 000 g) to obtain the cytosolic fraction.
GST-pulldown assay
Recombinant GST-JAK2 fusion protein was expressed in E.coli, isolated in lysis buffer (137 mm NaCl, 2.7 mm KCl, 1.47 mm KH2PO4, 4.3 mm Na2HPO4 and 0.5% Triton ×100) and purified by binding to gluthathione Sepharose (GE Healthcare, Little Chalfont, UK). GST-JAK2 was incubated with recombinant purified His-p27Kip1 for 90 min. After repeated washing in lysis buffer, bound proteins were eluted in SDS–PAGE at 95 °C and analyzed by western blot.
In vitro kinase assays
Kinase reactions were performed by incubating purified recombinant His-p27Kip1 or His-p27Kip1 tyrosine mutants with the GST-JAK2 kinase domain (amino acid 752–1129 of mouse JAK2; ProQinase, Freiburg, Germany) in kinase buffer (5 mm MgCl2, 2 mm MnCl2, 150 mm NaCl, 20 mm (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid)/KOH pH 7.5, 0.05% NP-40, 1 mm dithiothreitol, 625 μm Na-ortho-vanadate, 50 μm 4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride (AEBSF), 1 μg/μl aprotinin/leupeptin, 100 μm ATP and 10 μCi γ-32P-ATP (NEN)) for 30 min at 35 °C. Proteins were separated by SDS–PAGE, stained with Coomassie brilliant blue and exposed to a Storage Phosphor Screen (GE Healthcare). Incorporation of radioactive phosphate into p27Kip1 was quantified with a Typhoon 9410 scanner (GE Healthcare) and ImageQuant software (GE Healthcare).
Immunofluorescence
U2OS cells were transfected with HA-p27Kip1 or HA-p27Kip1-Y88F and Flag-JAK2 or Flag-JAK2V617F and seeded on glass bottom plates. Cells were fixed with 4% para-formaldehyde for 15 min, permeabilized with 0.1% Triton X-100 and incubated overnight with the primary antibodies (rabbit anti-p27Kip1 C19 (Santa Cruz), rabbit anti-pY1007/1008-JAK2 (Millipore) and mouse anti-pY88-p27Kip1 (Grimmler et al., 2007)). After washing, cells were incubated with fluorescence (Alexa Fluor, Invitrogen) labeled secondary antibodies for 1 h, washed again and finally DNA was detected with Hoechst stain. Confocal image analysis was performed using a TCS SP5 Confocal Laser Scanning Microscope (Leica, Wetzlar, Germany).
Supplementary Material
Acknowledgements
We thank Jakob Troppmair and Justus Duyster for providing cell lines, Wolfgang Doppler for providing plasmids and antibodies and Stephan Geley for providing plasmids. We thank Wolfgang Doppler, Michael Kullmann, Jonathan Vosper and all members of the Hengst lab for support, stimulating discussions and critical reading of the manuscript. The work was funded by the FWF Grants SFB F21-B12 and P18873-B1.
Footnotes
Conflict of interest The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
References
- Agarwal A, Bumm TG, Corbin AS, O’Hare T, Loriaux M, VanDyke J, et al. Absence of SKP2 expression attenuates BCR-ABL-induced myeloproliferative disease. Blood. 2008;112:1960–1970. doi: 10.1182/blood-2007-09-113860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SJ, Rane SG, Reddy EP. Hematopoietic cytokine receptor signaling. Oncogene. 2007;26:6724–6737. doi: 10.1038/sj.onc.1210757. [DOI] [PubMed] [Google Scholar]
- Behrmann I, Smyczek T, Heinrich PC, Schmitz-Van de Leur H, Komyod W, Giese B, et al. Janus kinase (Jak) subcellular localization revisited: the exclusive membrane localization of endogenous Janus kinase 1 by cytokine receptor interaction uncovers the Jak.receptor complex to be equivalent to a receptor tyrosine kinase. J Biol Chem. 2004;279:35486–35493. doi: 10.1074/jbc.M404202200. [DOI] [PubMed] [Google Scholar]
- Bruchova H, Yoon D, Agarwal AM, Swierczek S, Prchal JT. Erythropoiesis in polycythemia vera is hyper-proliferative and has accelerated maturation. Blood Cells Mol Dis. 2009;43:81–87. doi: 10.1016/j.bcmd.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu I, Sun J, Arnaout A, Kahn H, Hanna W, Narod S, et al. p27 phosphorylation by Src regulates inhibition of cyclin E-Cdk2. Cell. 2007;128:281–294. doi: 10.1016/j.cell.2006.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008;8:253–267. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]
- Connor MK, Kotchetkov R, Cariou S, Resch A, Lupetti R, Beniston RG, et al. CRM1/Ran-mediated nuclear export of p27(Kip1) involves a nuclear export signal and links p27 export and proteolysis. Mol Biol Cell. 2003;14:201–213. doi: 10.1091/mbc.E02-06-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MA, Bannister AJ, Gottgens B, Foster SD, Bartke T, Green AR, et al. JAK2 phosphorylates histone H3Y41 and excludes HP1alpha from chromatin. Nature. 2009;461:819–822. doi: 10.1038/nature08448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrivieres S, Kunz C, Barash I, Vafaizadeh V, Borghouts C, Groner B. The biological functions of the versatile transcription factors STAT3 and STAT5 and new strategies for their targeted inhibition. J Mammary Gland Biol Neoplasia. 2006;11:75–87. doi: 10.1007/s10911-006-9014-4. [DOI] [PubMed] [Google Scholar]
- Feng J, Witthuhn BA, Matsuda T, Kohlhuber F, Kerr IM, Ihle JN. Activation of Jak2 catalytic activity requires phosphorylation of Y1007 in the kinase activation loop. Mol Cell Biol. 1997;17:2497–2501. doi: 10.1128/mcb.17.5.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frescas D, Pagano M. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer. 2008;8:438–449. doi: 10.1038/nrc2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhata A, Kimura A, Shide K, Shimoda K, Murakami M, Ito H, et al. p27 deregulation by Skp2 overexpression induced by the JAK2V617 mutation. Biochem Biophys Res Commun. 2009;383:411–416. doi: 10.1016/j.bbrc.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Grimmler M, Wang Y, Mund T, Cilensek Z, Keidel EM, Waddell MB, et al. Cdk-inhibitory activity and stability of p27Kip1 are directly regulated by oncogenic tyrosine kinases. Cell. 2007;128:269–280. doi: 10.1016/j.cell.2006.11.047. [DOI] [PubMed] [Google Scholar]
- Halfter H, Friedrich M, Resch A, Kullmann M, Stogbauer F, Ringelstein EB, et al. Oncostatin M induces growth arrest by inhibition of Skp2, Cks1, and cyclin A expression and induced p21 expression. Cancer Res. 2006;66:6530–6539. doi: 10.1158/0008-5472.CAN-04-3734. [DOI] [PubMed] [Google Scholar]
- Hengst L, Dulic V, Slingerland JM, Lees E, Reed SI. A cell cycle-regulated inhibitor of cyclin-dependent kinases. Proc Natl Acad Sci USA. 1994;91:5291–5295. doi: 10.1073/pnas.91.12.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengst L, Reed SI. Translational control of p27Kip1 accumulation during the cell cycle. Science. 1996;271:1861–1864. doi: 10.1126/science.271.5257.1861. [DOI] [PubMed] [Google Scholar]
- Ihle JN, Gilliland DG. Jak2: normal function and role in hematopoietic disorders. Curr Opin Genet Dev. 2007;17:8–14. doi: 10.1016/j.gde.2006.12.009. [DOI] [PubMed] [Google Scholar]
- James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- James MK, Ray A, Leznova D, Blain SW. Differential modification of p27Kip1 controls its cyclin D-cdk4 inhibitory activity. Mol Cell Biol. 2008;28:498–510. doi: 10.1128/MCB.02171-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardinal C, Dangers M, Kardinal A, Koch A, Brandt DT, Tamura T, et al. Tyrosine phosphorylation modulates binding preference to cyclin-dependent kinases and subcellular localization of p27Kip1 in the acute promyelocytic leukemia cell line NB4. Blood. 2006;107:1133–1140. doi: 10.1182/blood-2005-05-1771. [DOI] [PubMed] [Google Scholar]
- Kelvin DJ, Chance S, Shreeve M, Axelrad AA, Connolly JA, McLeod D. Interleukin 3 and cell cycle progression. J Cell Physiol. 1986;127:403–409. doi: 10.1002/jcp.1041270308. [DOI] [PubMed] [Google Scholar]
- Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- LaBaer J, Garrett MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS, et al. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11:847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- Larrea MD, Liang J, Da Silva T, Hong F, Shao SH, Han K, et al. Phosphorylation of p27Kip1 regulates assembly and activation of cyclin D1-Cdk4. Mol Cell Biol. 2008;28:6462–6472. doi: 10.1128/MCB.02300-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RL, Gilliland DG. JAK-2 mutations and their relevance to myeloproliferative disease. Curr Opin Hematol. 2007;14:43–47. doi: 10.1097/00062752-200701000-00009. [DOI] [PubMed] [Google Scholar]
- Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- Morgan DO. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- Neilson LM, Zhu J, Xie J, Malabarba MG, Sakamoto K, Wagner KU, et al. Coactivation of janus tyrosine kinase (Jak)1 positively modulates prolactin-Jak2 signaling in breast cancer: recruitment of ERK and signal transducer and activator of transcription (Stat)3 and enhancement of Akt and Stat5a/b pathways. Mol Endocrinol. 2007;21:2218–2232. doi: 10.1210/me.2007-0173. [DOI] [PubMed] [Google Scholar]
- Parganas E, Wang D, Stravopodis D, Topham DJ, Marine JC, Teglund S, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–395. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- Ray A, James MK, Larochelle S, Fisher RP, Blain SW. p27Kip1 inhibits cyclin D-cyclin-dependent kinase 4 by two independent modes. Mol Cell Biol. 2009;29:986–999. doi: 10.1128/MCB.00898-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Thompson JE, Cubbon RM, Cummings RT, Wicker LS, Frankshun R, Cunningham BR, et al. Photochemical preparation of a pyridone containing tetracycle: a Jak protein kinase inhibitor. Bioorg Med Chem Lett. 2002;12:1219–1223. doi: 10.1016/s0960-894x(02)00106-3. [DOI] [PubMed] [Google Scholar]
- Tossidou I, Dangers M, Koch A, Brandt DT, Schiffer M, Kardinal C. Tyrosine phosphatase SHP-2 is a regulator of p27(Kip1) tyrosine phosphorylation. Cell Cycle. 2008;7:3858–3868. doi: 10.4161/cc.7.24.7260. [DOI] [PubMed] [Google Scholar]
- Verstovsek S. Therapeutic potential of JAK2 inhibitors. Hematol Am Soc Hematol Educ Program. 2009:636–642. doi: 10.1182/asheducation-2009.1.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace TA, Sayeski PP. Jak2 tyrosine kinase: a mediator of both housekeeping and ligand-dependent gene expression? Cell Biochem Biophys. 2006;44:213–222. doi: 10.1385/CBB:44:2:213. [DOI] [PubMed] [Google Scholar]
- Walz C, Crowley BJ, Hudon HE, Gramlich JL, Neuberg DS, Podar K, et al. Activated Jak2 with the V617F point mutation promotes G1/S phase transition. J Biol Chem. 2006;281:18177–18183. doi: 10.1074/jbc.M600064200. [DOI] [PubMed] [Google Scholar]
- Wei W, Ayad NG, Wan Y, Zhang GJ, Kirschner MW, Kaelin WG., Jr Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature. 2004;428:194–198. doi: 10.1038/nature02381. [DOI] [PubMed] [Google Scholar]
- Wernig G, Gonneville JR, Crowley BJ, Rodrigues MS, Reddy MM, Hudon HE, et al. The Jak2V617F oncogene associated with myeloproliferative diseases requires a functional FERM domain for transformation and for expression of the Myc and Pim protooncogenes. Blood. 2008;111:3751–3759. doi: 10.1182/blood-2007-07-102186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Xing S, Li Z, Fu X, Li Q, Krantz SB, et al. Identification of an acquired JAK2 mutation in polycythemia vera. J Biol Chem. 2005;280:22788–22792. doi: 10.1074/jbc.C500138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Wagner F, Frank SJ, Kraft AS. The amino-terminal portion of the JAK2 protein kinase is necessary for binding and phosphorylation of the granulocyte-macrophage colony-stimulating factor receptor beta c chain. J Biol Chem. 1995;270:13814–13818. doi: 10.1074/jbc.270.23.13814. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.