Abstract
Psoriasis is a common chronic skin disorder, but the mechanisms involved in the resolution and clearance of plaques remain poorly defined. We investigated the mechanism of action of UVB, which is highly effective in clearing psoriasis and inducing remission, and tested the hypothesis that apoptosis is a key mechanism. To distinguish bystander effects, equal erythemal doses of two UVB wavelengths were compared following in vivo irradiation of psoriatic plaques; one is clinically effective (311 nm) and one has no therapeutic effect on psoriasis (290 nm). Only 311 nm UVB induced significant apoptosis in lesional epidermis, and most apoptotic cells were keratinocytes. To determine clinical relevance, we created a computational model of psoriatic epidermis. Modeling predicted apoptosis would occur in both stem and transit-amplifying cells to account for plaque clearance; this was confirmed and quantified experimentally. The median rate of keratinocyte apoptosis from onset to cell death was 20 minutes. These data were fed back into the model and demonstrated that the observed level of keratinocyte apoptosis was sufficient to explain UVB-induced plaque resolution. Our human studies combined with a systems biology approach demonstrate that keratinocyte apoptosis is a key mechanism in psoriatic plaques clearance, providing the basis for future molecular investigation and therapeutic development.
Introduction
Psoriasis is a chronic disabling skin disorder affecting 25 million people in North America and Europe (Lowes et al., 2007), and has been estimated to cost the US $1,500 per patient per year (Fowler et al., 2008). The pathogenesis of psoriasis is complex with interplay between genetic predisposition, external environmental triggers, and innate and adaptive immune responses. Recent genetic and functional studies have highlighted the role of innate immunity and cytokine signals, specifically IL-17/IL-22, which appear critical in initiating epidermal remodeling and the formation of psoriatic plaques (Di Cesare et al., 2009; Elder, 2009). Interestingly, genetic and gene array data underscore a fundamental role for the epidermis in psoriasis pathogenesis (de Cid et al., 2009; Wolf et al., 2010). Moreover, psoriatic keratinocytes differ from normal keratinocytes as evidenced by increased proliferative potential (Weinstein et al., 1984), dysregulated intracellular signaling pathways, including calcium signaling (Karvonen et al., 2000) and activated STAT3 (Sano et al., 2005). Transgenic mice with constitutively active STAT3 develop a psoriatic phenotype, but require activated T-cells to be present for this to occur (Sano et al., 2005). These studies indicate that the development of psoriasis is dependent on complex interactions between the innate immune system, dendritic cells, and activated T-cells driving genetically predisposed abnormal keratinocytes.
Despite advances in understanding pathogenesis, less attention has been paid to investigating the mechanisms involved in resolution of psoriatic plaques, which are up to four times thicker than normal uninvolved skin, back to normal architecture in response to therapy. Several different mechanisms have been proposed (Ozawa et al., 1999; Sauder, 2004; McGill et al., 2005; Johnson-Huang et al., 2010), but the evidence is based principally on temporal association of particular observations with stages of plaque resolution or from xenograft mouse models. UVB (280–315 nm) phototherapy remains one of the mainstays of treatment (Menter et al., 2010) and can induce clearance of psoriasis in around 70% of patients (Coven et al., 1997; Gordon et al., 1999; Kirke et al., 2007), often with prolonged remission (Dawe et al., 1998). UVB exerts a multitude of biological effects within skin, but it remains unclear which of these induce clearance of psoriasis.
In this work, we have used an approach, which to our knowledge has not previously been reported, studying the response to equal erythemal doses of a clinically effective wavelength (311 nm) and a proven ineffective wavelength (290 nm; Parrish and Jaenicke, 1981). This allowed us to distinguish potentially important therapeutic actions of UVB from bystander effects. The erythema action spectrum of UVB is similar to the action spectrum of DNA damage (Setlow, 1974) and non-melanoma skin cancer induction (Setlow, 1974; de Gruijl and Van der Leun, 1994), but is distinct from the therapeutic action spectrum of UVB in the clearance of psoriasis (Parrish and Jaenicke, 1981). Moreover, we have developed a model of epidermal homeostasis, which has allowed us to create an in silico model of psoriasis. This was then used to identify key in vivo and in vitro experiments, the results of which were then fed back into the model. Uniquely, the model represents the dynamic development of psoriatic plaques and the response to therapeutic intervention in a stochastic manner. We demonstrate that the observed rate of keratinocyte apoptosis is sufficient to allow psoriatic epidermis to remodel back to normal, and this can account for plaque clearance.
Results
Wavelength dependence of apoptosis in psoriatic epidermis
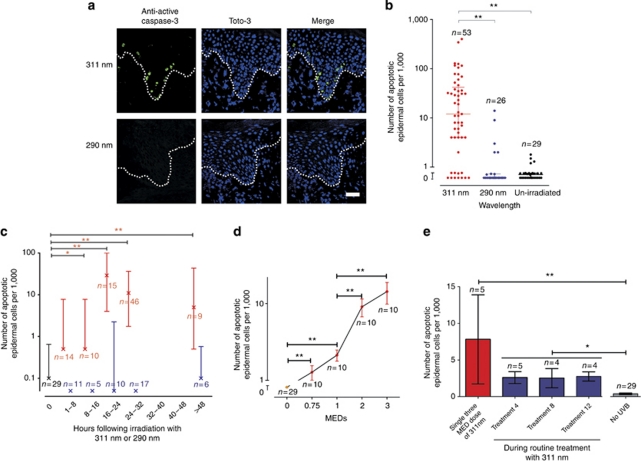
Although psoriatic keratinocytes appear resistant to apoptosis in cell culture (Wrone-Smith et al., 1997), we hypothesized that apoptosis may be involved in the clearance of plaques in response to therapeutic UV irradiation. To test this, we exposed psoriatic plaques to single two to three minimal erythemal doses (MEDs) of 311 and 290 nm UV, and counted cells immunostained with anti-active caspase-3 (indicating apoptosis). The background number of apoptotic cells in un-irradiated lesional psoriatic skin (n=29) was found to be very low (median 0.1 cells per 1,000 nucleated epidermal cells), consistent with previous literature (Laporte et al., 2000). In contrast, Figure 1a shows a significant increase in apoptosis of lesional epidermal cells between 16 and 48 hours following irradiation with 311 nm UVB but not with equally erythemogenic doses of 290 nm (P<0.001). Inter-patient variation in the number of apoptotic cells resulting from a single three MED dose of 311 nm was seen, but measurement error testing showed that this was significantly greater than intra-patient variation (Supplementary Figure S1 online). A within-patient study (n=9) showed inter-patient variation in the time-course of apoptosis (Supplementary Figure S2 online), but overall the number of apoptotic cells induced by 311 nm peaked at 16–24 hours (Figure 1c), and were located in the basal or suprabasal epidermis, consistent with an effect on the proliferative compartment (Figure 1a, Supplementary Figure S3 online). To exclude the possibility that 290 nm induced a different apoptotic time-course, biopsies were examined between 2 and 48 hours, and at no time points were significantly increased numbers of apoptotic cells seen following 290 nm irradiation (Figure 1c).
Figure 1.
Significant apoptosis following in vivo irradiation of psoriatic plaques with 311 nm UVB. (a) Representative confocal image of lesional psoriatic epidermis at 18 hours post in vivo irradiation, immunostained with anti-active caspase-3 (green) and Toto-3 (blue). Dotted line shows the basement membrane. Bar=100 μm. (b) Number of anti-active caspase-3 (apoptotic) cells within lesional psoriatic epidermis seen at 16–48 hours following irradiation with either 311 or 290 nm compared with un-irradiated psoriasis. Lines show medians (12/1,000 and 0/1,000 epidermal cells for 311 and 290, respectively) and inter-quartile range. (c) Time-course showing number of apoptotic cells in lesional psoriatic epidermis post UV. Crosses show median number of apoptotic cells per 1,000 epidermal nucleated cells, and inter-quartile range. Red bars represent irradiation with 311 nm, blue represents 290 nm (note median and inter-quartile range is 0 at time-points 0–8, 8–16, and 24–32 hours), and black bar is un-irradiated psoriatic epidermis. (d) Mean (±standard error) number of apoptotic cells in lesional psoriatic epidermis in 10 patients at 24 hours post 311 nm UVB irradiation. Increasing apoptotic response is seen with increasing doses of UVB (means of 1.3, 2.1, 9.1, and 14.3 per 1,000 epidermal cells, respectively), which is significantly different from un-irradiated psoriasis at all doses. (e) Apoptotic response in five patients at 24 hours after a single 311 nm exposure to psoriasis in vivo and at 24 hours after the 4th, 8th, and 12th dose during a routine treatment course. One patient dropped out during treatment because of burning. *P<0.05, **P<0.01. Note the log scale used in figures a, c, and d. MEDs, minimal erythemal doses.
To test whether the apoptotic response following erythemogenic doses of 311 nm are representative of those following routine clinical doses, we measured apoptosis at 24 hours after irradiation of matched psoriatic plaques with single doses of 0.75, 1, 2, and 3 MEDs (Figure 1d). As expected, 2 and 3 MEDs induced a significantly higher rate of apoptosis than 0.75 and 1 MEDs (P<0.001). Importantly, apoptosis induced by 0.75 and 1 MED of 311 nm was significantly greater than un-irradiated psoriasis (P<0.001).
Routine UVB treatment of psoriasis uses repeated sub-erythemogenic doses three times per week for 6–8 weeks. To confirm that epidermal apoptosis occurred during clinical therapy, apoptotic cells were counted in biopsies taken from psoriatic plaques 24 hours following the 4th, 8th, and 12th routine treatment (n=5). Apoptosis was significantly greater (mean of 2.5 cells per 1,000 epidermal cells) compared with un-irradiated psoriasis (P=0.001; n=29; Figure 1e). Linear regression analysis showed no significant correlation between the number of 311 nm-induced apoptotic cells within the epidermis and maximal plaque thickness (P=0.079, r2=0.06, n=43).
Apoptotic cells are predominantly keratinocytes
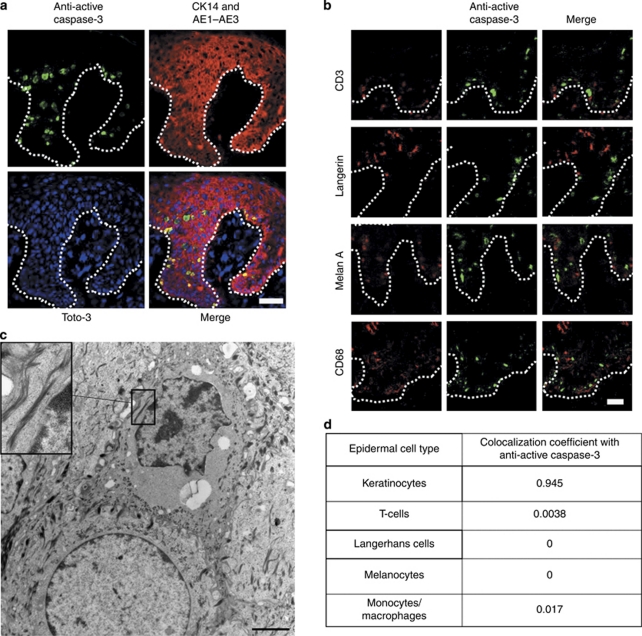
The location, morphology, and double immunostaining of apoptotic cells with specific keratinocyte markers suggested that these cells were predominantly keratinocytes (Figure 2a). However, it has previously been proposed that T-cells within lesional psoriatic skin are differentially susceptible to UVB-induced apoptosis, resulting in a reduction in T-cell-derived cytokines that drive keratinocyte proliferation and thereby leading to plaque resolution (Krueger et al., 1995). To investigate apoptosis in different cell types, epidermal sections were triple labelled with anti-active caspase-3, Toto-3, and specific markers of cell origin, and colocalization coefficients were determined (Figure 2b and d). These data showed that 95% of apoptotic cells within psoriatic epidermis were keratinocytes and 0.4% were T-cells, reflecting the relative proportion of these cells identified within the epidermis. This suggests that keratinocytes and T-cells show a similar susceptibility to UV-induced apoptosis. To rule out the possibility that the time-course of apoptosis differed in T-cells, paired biopsies taken from 4–48 hours were studied, and no significant change in the number of these cells within plaques irradiated with 311 or 290 nm were seen at any time point (P=0.11). Apoptotic cells were rarely seen within the dermis.
Figure 2.
Colocalization of apoptotic cells within lesional psoriatic epidermis with markers specific for epidermal cell types at 24 hours post irradiation with 311 nm UVB. (a, b) Mid Z-section of a confocal image showing colocalization of apoptotic cells (green) with keratinocyte markers (a) and other epidermal cell types (red; b). Bar=100 μm. CD3 is a marker for T-cells, Langerin for Langerhans cells, Melan A for melanocytes, and CD68 for monocyte/macrophages. Dotted line represents the basement membrane. (c) Transmission electron micrograph showing an apoptotic keratinocyte. Insert shows keratin filaments. Desmosomal junctions between the cells were also seen, confirming cells are keratinocytes. Bar=2 μm. (d) Table summarizing the colocalization coefficients for apoptotic cells and the specific epidermal cell markers. Note that a small number of cells (3.4%) could not be identified by colocalization. These cells were at a late stage of apoptosis, when cell surface markers may be lost.
To confirm UV-induced apoptosis within psoriatic plaques and to confirm that apoptotic cells were predominantly keratinocytes, transmission electron microscopy was performed at 24 hours post irradiation with three MEDs of 311 and 290 nm (n=3). Morphological changes of keratinocyte apoptosis were observed following irradiation with 311 nm only, as evidenced by cell shrinkage and condensation of chromatin. These cells were identified as keratinocytes by the presence of both keratin filaments and desmosomal junctions (Figure 2c).
Wavelength dependence of apoptosis is not seen in vitro
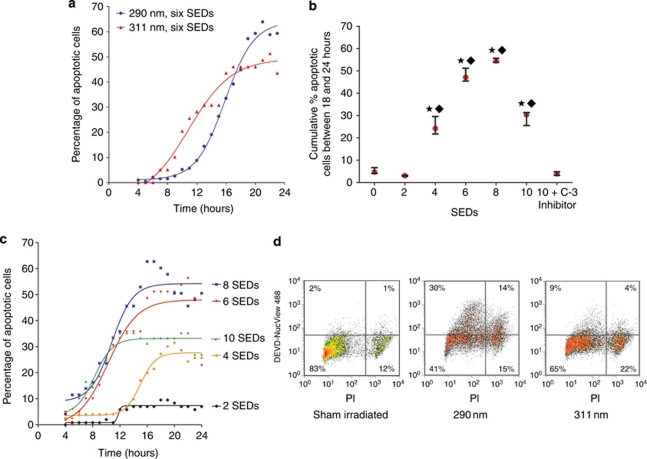
The effect of equal erythemally weighted doses (2–10 standard erythemal doses; SEDs) of 290 and 311 nm UVB were compared in primary cultured keratinocytes (Figure 3). Notably, there was no significant difference in the cumulative proportions of apoptotic cells following 290 and 311 nm irradiation with the real-time, live cell caspase-3 substrate assay (Cen et al., 2008; P>0.05; Figure 3a), and these had a similar time-course of apoptosis, peaking between 16 and 24 hours post irradiation. For 311 nm, doses of six to eight SEDs resulted in the highest levels of apoptosis (Figure 3b) and increasing doses of 311 nm resulted in earlier onset of apoptosis (Figure 3c). Similar results were obtained by flow cytometry at a single time point (24 hours) with 290 nm inducing greater apoptosis than equal erythemally weighted doses of 311 nm (Figure 3d), although this difference was not significant (P=0.165, n=3), but was significantly greater than sham irradiation (P=0.014).
Figure 3.
Apoptosis occurs following irradiation with 311 and 290 nm UVB in primary keratinocytes. (a) A membrane-permeable caspase-3 substrate and live cell imaging was used for detection of onset of apoptosis. A greater proportion of apoptotic cells were seen at 24 hours following irradiation with six SEDs of 290 nm than six SEDs of 311 nm, but the time-course was similar. (b) The highest cumulative number of apoptotic keratinocytes occurred following irradiation with six to eight SEDs of 311 nm UVB. The caspase-3-specific inhibitor successfully blocked caspase-3 activity. Median and inter-quartile ranges are shown for three separate experiments. A significant difference in the cumulative proportion of apoptosis was seen between indicated doses and sham-irradiated cells (★) or irradiated cells incubated with caspase-3 and inhibitor (⧫ P<0.05). (c) Time-course of apoptosis following irradiation with increasing doses of 311 nm. Higher doses (six to eight SEDs) were associated with a quicker rate of onset of apoptosis. (d) Flow cytometry showing the effects of irradiation with UVB at a single time point (24 hours). A greater proportion of apoptotic (DEVD-NucView488 caspase-3+) and dead (PI+) cells are observed with 290 nm than 311 nm. PI, propidium iodide; SED, standard erythemal dose.
Lack of differential effects of 311 and 290 nm on keratinocyte proliferation within plaques
To investigate the potential contribution of reduced epidermal proliferation induced by UVB on plaque clearance, the numbers of Ki67+ epidermal cells were counted between 4 and 48 hours following irradiation with 290 and 311 nm in matched plaques within the same individuals (n=21). No significant difference in Ki67+ cells was observed following irradiation with either 290 nm (P=0.339) or 311 nm (P=0.091) compared with un-irradiated lesional skin (means of 143, 111, and 162 per 1,000 epidermal cells for un-irradiated, 290- and 311-nm-irradiated lesional skin, respectively).
A computational model of psoriatic epidermis
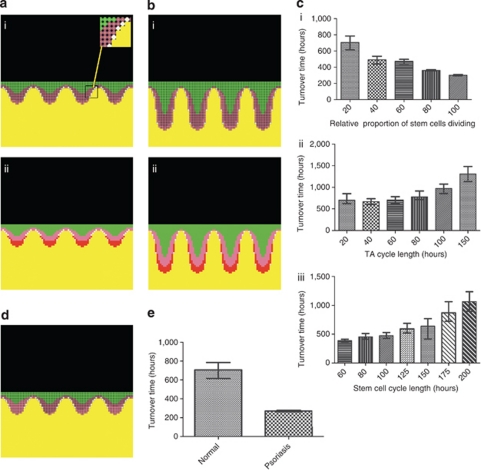
As no suitable mathematical model of psoriatic epidermis exists to date, a stochastic agent-based mathematical model of the non-lesional and psoriatic keratinocyte compartment of the epidermis was created, based on histological observations and previously published experimental data (Figure 4a and b). The model incorporates a threshold switch in the proportion of stem and transit-amplifying (TA) cells that proliferate, which is consistent with an immunological stimulus initiating psoriasis through cytokine signals that induce keratinocyte proliferation. However, once a plaque is established, it is self-propagating and will remain “psoriatic”, even when initial “cytokine stimulus” is completely withdrawn. Iterative simulation of the model allowed rigorous testing and establishment of robust boundaries within which the model remained stable (Figure 4c). In contrast to adjusting cell cycle times, the model showed that increasing the proportion of actively dividing stem and TA cells within “normal” epidermis (Figure 4a–e) were key events contributing to the development of psoriasis. The model showed excellent concordance with existing data, including an absolute increase in both the proliferative and differentiating compartments to levels found in psoriasis, and a reduction in both total epidermal turnover time and transit time of differentiating cells. To attain these parameters, the model predicted that an absolute increase in the number of both stem and TA cells proliferating was required to simulate psoriasis.
Figure 4.
Development of a mathematical model of normal and psoriatic epidermis based on histological features, differentiation patterns, and epidermal kinetics. (a and bi) Model of normal (a) and psoriatic (b) epidermis with stem cells (white, see insert), actively dividing TA cells (pink), resting TA cells (dark pink), and differentiating cells (green). (a and bii) Two inverted gradients were applied within the model arising from the basement membrane; one maps to the pattern of keratin-10 immunostaining (green) with a specified threshold determining the onset of keratinocyte terminal differentiation; a second gradient contains actively dividing TA cells and stem cells (red), and regulates expansion/contraction of the basement membrane. (c) How adjusting relative proportions of actively dividing stem cells (ci), and TA (cii), or stem cell cycle times (ciii) independently affects turnover time of the whole epidermis. Median and inter-quartile ranges shown; n=50. (d) Model of “normal epidermis” when the number of TA divisions increases from four to five. Note almost all TA cells in the 4th rete ridge are proliferating, but very few in the first, resulting in a patchy distribution of proliferation histologically, not consistent with clinical observation. (e) The turnover times for normal (median 706 hours) and psoriatic (median 270 hours) epidermis used in further experiments. Transit time of differentiated cells (not shown here) reduced from 336 hours to 100 hours.
To model whether UV-induced keratinocyte apoptosis could directly impact on epidermal resolution, it was necessary to determine the rate and duration of keratinocyte apoptosis as well as the differential sensitivity of stem, TA, or differentiating cells to apoptosis. Detection of apoptosis in human tissue in vivo is limited by the availability of the tissue to sampling to determine the time-course of events and the rapid clearance of apoptotic cells. Methods such as immunochemistry can only provide a “snapshot” of what is occurring in the skin at any one time. As real-time imaging of individual cells within human epidermis is not possible in vivo, further experiments were designed to address this question in vitro.
Time of onset and duration of apoptosis
To estimate the functional consequences of UV-induced apoptosis on psoriatic epidermal homeostasis, we measured the time to onset and duration of apoptosis in cultured primary human keratinocytes following UV irradiation, using a live cell caspase-3 assay and propidium iodide (as a marker of cell membrane integrity). Following irradiation with six SEDs of 311 nm, the mean time to caspase-3 activation was 14 hours, and the median duration of apoptosis before the loss of membrane integrity was 20 minutes (inter-quartile range 0–70 minutes; Supplementary Figure S4a online). Interestingly, neighboring cells did not seem to influence each other, and the apoptotic rate and onset was distributed randomly over the imaged areas.
These data were fed back into the model, which predicted that if psoriasis is expected to clear in 75–90% of patients after 6–10 treatment sessions using three MED doses (as seen in the clinical setting following localized irradiation; Feldman et al., 2002; Trehan and Taylor, 2002), then UV must result in apoptosis of ∼15–25% of both stem and TA cells following each irradiation. To test this prediction, intact normal human epidermis was irradiated ex vivo, and the proportion of apoptotic TA cells (α6-integrin+/CD71+) and putative stem cells (α6-integrin+/CD71−) was determined (Tani et al., 2000; Youn et al., 2004). Expression of these cell surface markers changes progressively as the proliferative status of the cell decreases (Webb et al., 2004). Although the majority of apoptotic cells expressed high levels of CD71 and α6-integrin consistent with TA cells (87–89% median 88%), CD71−/α6-integrin+ (putative stem cells) accounted for 3.4–10% (median 8%). To ensure that each population of cells was examined in isolation, three gates were examined from the entire cell population (Supplementary Figure S4b online). UVB was found to cause a mean relative increase in apoptotic cells of 20% in TA cells and 13% in stem cells (Supplementary Figure S4c online). However, as remission can be maintained following phototherapy, apoptosis alone cannot account for TA cells switching to fewer rounds of divisions. The increase in the number of epidermal keratinocytes and T-cells within psoriatic plaques will amplify the local levels of proliferation-inducing cytokines (e.g., IL-20/IL-22) and growth factors (e.g., transforming growth factor α and other members of the epidermal growth factor and hepatocyte growth factor families) by paracrine/autocrine secretion, and thereby contribute to the proliferative potential of epidermal cells (Sano et al., 2005; Wolk et al., 2009). This was represented in the model, by gradually reducing the number of time TA cells divide from five back to four, as the total number of epidermal cells returned to normal.
Apoptosis in epidermal resolution and plaque clearance
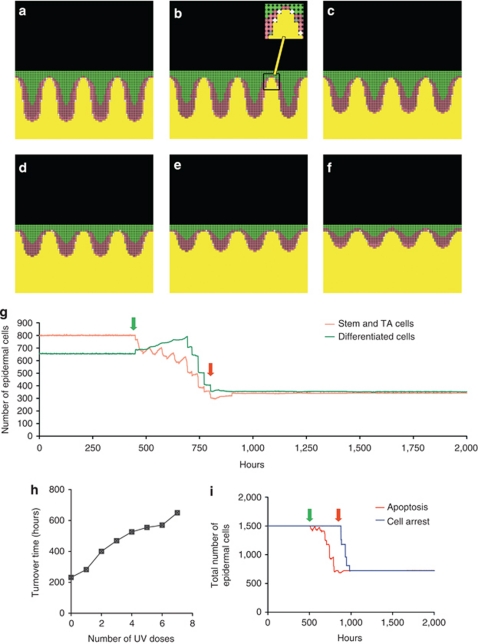
The model showed that repeated doses of three MEDs to lesional (plaque) psoriasis in silico returned the epidermis to normal thickness and morphology following approximately six to eight treatments, and that several treatments were required before any reduction in epidermal thickness was seen (Figure 5a–g). The epidermal turnover time increased following each irradiation, in keeping with the clinical scenario, whereby characteristics of lesional skin return to normal (Figure 5h). Following completion of treatment, remission was maintained (Figure 5g and i). A movie of the model depicting the development and resolution of psoriatic epidermis is available at http://research.ncl.ac.uk/psoroasis/, and is explained in the Supplementary data online. Clearance of psoriatic plaques occurred irrespective of whether or not differentiating cells underwent apoptosis, but apoptosis of both stem and TA cells was necessary to induce timely clearance and remission (Supplementary Figure S5 online). Moreover, sub-erythemogenic irradiation takes ∼25–30 treatments to “clear” psoriasis in the model, consistent with clinical practice (Dawe et al., 1998; Kirke et al., 2007). Together these data suggest that keratinocyte apoptosis may be sufficient to account for plaque clearance in response to UV irradiation.
Figure 5.
Mathematical model showing resolution of psoriatic epidermis in response to three MEDs of 311 nm UVB-induced apoptosis. (a–f) Psoriatic epidermis with elongation of rete ridges and hyperproliferation; different stages of resolution shown until normal state (f) reached; stem cells (white, or gray if apoptosed, see insert), TA cells (pink), and differentiating cells (green). Non-actively dividing stem cells are shown as dark pink. Basement membrane and below is shown in yellow. (g) Progressive reduction in cell numbers following sequential UVB irradiation every 56 hours (equivalent to three times per week). In this experiment, seven doses were required to cause resolution of the psoriasis back to normal. Green arrow shows first irradiation, and red arrow shows final irradiation. (h) Turnover time increases with each dose of UVB as epidermis returns to normal. Irradiation frequency was reduced to 200 hours for the turnover time calculation to allow stability of the model for each measurement. (i) Comparison of time taken for cell numbers to return to normal if apoptosis versus cell cycle arrest is the major mechanism involved in plaque clearance. Note that the time taken for complete resolution following irradiation is significantly prolonged if the mechanism is cell cycle arrest, with a lag time of 336 hours (14 days) from completion of treatment until resolution is completed, although the same number of irradiations were required. Furthermore, in this model remission does not begin until after the UV course is completed, which would not fit with the clinical scenario.
To examine whether cell cycle arrest would also lead to plaque clearance, we substituted cell cycle arrest for apoptosis within the model. Assuming that arrested cells did not re-enter the cell cycle, UV-induced cell cycle arrest eventually led to plaque clearance and remission. In contrast to the apoptotic model, however, there was a substantial lag phase before a reduction in plaque thickness was seen, and it took approximately a further 7–14 days following completion of treatment for plaques to resolve (Figure 5i). This scenario would not fit with the clinical situation, as progressive plaque resolution occurs during a course of UVB treatment without further significant improvement thereafter.
Discussion
UVB wavelengths of 300–313 nm are highly effective at clearing psoriasis (Parrish and Jaenicke, 1981), and phototherapy is one of the very few treatments that can induce complete remission of psoriasis for a period of time after therapy has been withdrawn (Dawe et al., 1998). We have shown that psoriatic keratinocytes within lesional skin undergo apoptosis in response to 311 nm irradiation, but not in response to clinically ineffective 290 nm irradiation. Further, using in silico modeling, we demonstrate that the rate of apoptosis induced appears sufficient to account for plaque resolution during a routine therapy course. These data identify keratinocyte apoptosis as a key mechanism in psoriatic plaque clearance in response to UVB. Moreover, by developing a mathematical model of psoriasis development and resolution in response to UVB, we demonstrate the utility of an integrated systems biology approach to human disease in situations in which in vivo experiments are limited by technical and ethical considerations.
As psoriatic plaques tolerate erythemogenic doses of UVB (Asawanonda et al., 2000), we initially used two to three MED doses to unequivocally demonstrate epidermal keratinocyte apoptosis, the time-course of apoptosis and a differential response to a clinically effective wavelength (311 nm) compared with a clinically ineffective wavelength (290 nm). Although we have used UVB doses of three MEDs in several elements of this study, the results can be extrapolated to the clinical situation for several reasons: first, as psoriasis is relatively resistant to burning, doses of up to three to six MEDs of 308 nm from excimer laser (which has been shown to be of similar efficacy to the 308 nm lamp and 311 nm lamp; Kollner et al., 2005; Goldinger et al., 2006) are used in clinical practice when targeting individual plaques (Menter et al., 2010). Second, multiple MEDs of UVB result in more rapid clearing of psoriasis (Asawanonda et al., 2000; Kollner et al., 2005). Third, we have shown that psoriatic keratinocytes undergo apoptosis in response to sub-erythemogenic (whole-body clinical) doses of 311 nm UVB (0.75 MEDs), and that apoptosis is also seen during routine courses of repeated low-dose treatments. However, three MEDs is a higher dose than would be used for whole-body psoriatic treatment, as it would cause burning of non-lesional skin and unacceptable discomfort.
In normal forearm skin, 290 nm has been shown to penetrate into the lower epidermis ∼14 times less than 314 nm (Meinhardt et al., 2009). It is possible, therefore, that differential penetration of 290 and 311 nm UVB contributes, at least in part, to the differences in number of apoptotic cells observed with these wavelengths in psoriatic epidermis but not in vitro. However, we did not find a correlation between maximal epidermal thickness and the number of apoptotic cells in 311-nm-irradiated psoriatic plaques. UVB (290 nm) clearly penetrates sufficiently to induce DNA damage and non-melanoma skin cancer. Indeed, evidence suggests that 293 nm is a more potent inducer of non-melanoma skin cancer in humans than longer UVB wavelengths (de Gruijl and Van der Leun, 1994), suggesting that differential penetration may not be the only explanation for our findings.
Phagocytosis is known to begin before membrane disruption (Dini and Abbro, 2005), although the rate of phagocytosis of apoptotic cells in vivo remains unknown. Our in vitro experiments show that the median time of caspase-3 positivity in individual cells is 20 minutes. The rate of apoptosis seen would be sufficient to allow for clearance of psoriasis in the model if the apoptotic and subsequent phagocytic process took approximately at 6 hours or less to complete. This fits with our data for two reasons: first, we showed significant reduction in the number of apoptotic cells within the epidermis of individual subjects when biopsies were taken just at 6 hours apart (Supplementary Figure S2 online), and second, the apoptotic cells did not appear to migrate into the upper epidermis over the time-course (Supplementary Figure S3 online), in contrast to time-course studies in normal human skin (Qin et al., 2002), suggesting apoptotic cells were rapidly phagocytosed in psoriatic epidermis.
Although clearance can be achieved with many psoriatic therapies, only a few (such as UVB and topical anthralin) can lead to periods of complete remission (Hartman et al., 2002). Psoriatic keratinocytes in culture have previously been shown to be relatively resistant to apoptosis induced by contact inhibition through culture in methylcellulose (Wrone-Smith et al., 1997). However, we have previously demonstrated that anthralin results in apoptosis of cultured keratinocytes (McGill et al., 2005). It is possible that treatments capable of inducing remission act through a different mechanism to other treatments (e.g., immune-modifying agents), and the data presented here along with the anthralin studies (McGill et al., 2005) would support keratinocyte apoptosis as the mechanism directly responsible for resolution of psoriatic plaques following treatment with these modalities.
Although psoriasis appears to be triggered by immunocyte-derived cytokines, the model suggests that plaques can be maintained by an increased proportion of actively dividing stem and TA cells. Interestingly, altering cell cycle times alone did not result in a psoriatic phenotype with a turnover time in keeping with known kinetic parameters. The model predicted that apoptosis of both stem and TA cells would be required to remodel psoriatic plaques back to “normal”, and this was validated experimentally. Apoptosis of differentiating cells did not affect the outcome of the model over time. Creating the model highlighted a number of important questions that were necessary to tackle experimentally. Apoptosis is a dynamic process but is often measured with end-point assays, such as immunohistochemistry. Without understanding the rate of apoptosis, it is impossible to predict the overall impact of this on overall tissue homeostasis, and this question was therefore addressed with live-cell assays.
Overall this study clearly demonstrates that significant apoptosis occurs in psoriatic keratinocytes following irradiation with therapeutic wavelengths of UVB in vivo, and establishes a proof of principal that the rate of apoptosis seen is sufficient to allow resolution of plaques and clearance of psoriasis during a routine therapeutic course. Individual variation in response to treatment in the clinical scenario may depend on factors regulating susceptibility of psoriatic epidermis to apoptosis, and further studies are required to address these. Moreover, identification of keratinocyte apoptosis as a key mechanism involved in psoriatic plaque clearance provides the basis for future drug development and optimization of current therapeutic regimes.
Materials and Methods
Subjects
In all, 64 adult subjects (41 male and 23 female) with psoriasis were recruited from a tertiary referral dermatology clinic following the decision to treat their psoriasis with 311 nm UVB. Exclusion criteria included the use of sun beds or sun exposure to the lower back for the preceding 3 months or use of topical anti-psoriatic treatments (with the exception of emollients) for 2 weeks, and the use of anti-psoriatic systemic therapies for 3 months. This study was approved by the Regional Research Ethics Committee, and all subjects gave written informed consent to participate. The study was conducted according to the declaration of Helsinki principles.
In vivo UVB
Involved (plaque) or uninvolved psoriatic skin was irradiated in a maximum of four 15 mm diameter areas in the sun-protected region of the lower back. Psoriatic plaques were chosen to be of similar size and thickness within each patient, and where possible small plaques were chosen, which could be biopsied in full. For larger plaques, a biopsy was taken from the edge of a plaque. Plaques were irradiated with up to three MED narrowband UVB from a handheld-modified erythema test unit (Hybec, Leicester, UK, fitted with a Philips TL01 lamp (Philips, Amsterdam, The Netherlands); referred to as 311 nm in this paper) or an irradiation monochromator set at a central wavelength of 290 nm (bandwidth 5 nm, measured as full width at half maximum height).
In vitro UVB
Doses of 0–10 SEDs of 311 and 290 nm UVB were used to irradiate primary cultured keratinocytes. An SED is an erythemal weighted dose, whereby one SED is equivalent to an erythemal effective radiant exposure of 100 J m−2 (Diffey et al., 1997). In experiments to determine median time of apoptosis, cells were irradiated with six SEDs of 311 nm.
Biopsies
In each patient, 6-mm punch biopsies were taken from psoriatic plaques or adjacent non-lesional skin before and after irradiation with 311 or 290 nm UVB. A maximum of four biopsies were taken per patient. Biopsies were snap frozen, set in optical coherence tomography (Raymond A Lamb) embedding medium and stored at −80 °C. Details of immunohistochemistry are given in the Supplementary data online.
Electron microscopy
Epidermal sections of 2 mm diameter were processed for transmission electron microscopy, following fixation in 2% gluteraldehyde in Sorensons's phosphate buffer, and secondary fixation in 1% osmium tetraoxide. Samples were then dehydrated in acetone and embedded in resin before analysis.
In vitro time-course of apoptosis following UVB
Normal human foreskin keratinocytes were grown to 70% confluency and irradiated with six SEDs from 311 nm lamps or 290 nm UVB from a monochromator. Cells were then imaged during incubation with NucView 488 caspase-3 substrate (1 μ; Biotium, Hayward, CA) as an indicator of apoptosis and propidium iodide (1 μ; Sigma, Poole, UK) as an indicator of loss of membrane integrity. Further details are given in the Supplementary data online.
Flow cytometric detection of apoptotic cells
Fresh human foreskins were divided into two sections and half of the intact epidermis was irradiated in phosphate-buffered saline with six SEDs 311 nm. Both sections were then incubated in dispase overnight at 4 °C. After 18 hours the epidermis was peeled off the dermis and incubated in trypsin for 10 minutes, then washed twice. Keratinocytes were then incubated with PE-CD49f (α6-integrin) and APC-CD71 (BD Pharmingen, Cambridge, UK) for 45 minutes. Cells were washed and incubated with FITC annexin V for 15 minutes (Molecular Probes, Paisley, UK). A BD LSR11 four color flow cytometer was used to process the cells, and analysis was carried out using Venturi One software (Applied Cytometry, Sheffield, UK).
Mathematical modeling
A two-dimensional-agent-based model was developed using Netlogo 4.0.4 which is a programmable modeling environment suited for complex systems (http://ccl.northwestern.edu/netlogo/. Center for Connected Learning and Computer-Based Modeling, Northwestern University, Evanston, IL). The keratinocyte compartment can be subdivided into stem cell, TA cell, and differentiating cell compartments (Dover and Wright, 1991; Webb et al., 2004), which show distinct patterns of distribution in normal and psoriatic skin. An agent-based model was created by combining known kinetic parameters derived from the literature and observed histological features characteristic of psoriasis. Further details are given in the Supplementary data online.
Statistics
The repeatability of apoptotic cell counts was calculated using measurement error (intra-patient standard deviation), with concordant repeatability was shown using a log (x+1) transformation of the data to a level of 95% confidence. Paired data was analyzed with a paired T-test, and non-parametric data analyzed using Mood's median. The Mann–Whitney U-test was used to compare the number of apoptotic cells induced by 311 and 290 nm, and a one-way analysis of variance was used to compare differences between groups with more than two factors.
Acknowledgments
We thank Brian Diffey for his advice during the initial design of the project; the Newcastle University Electron Microscopy unit and John McGrath and Robin Eady for their assistance with analyzing the electron micrographs; Ian Harvey and Carole Todd for their assistance with flow cytometry experiments and cell culture, respectively; Keith Flanagan for setting up the model webpage; Dave Jones, Derek Mann, and Muzlifah Haniffa for their comments on the manuscript; the phototherapy nurses and all the patients who kindly participated in this study. This work was supported by grants from the Wellcome Trust: Clinical Research Training Fellowship to SW and Research Leave Fellowship to Clinical Academics (NR), Newcastle NHS Special Trustees to DJ, the Psoriasis Association, and the National Institute for Health Research (NIHR) Clinical Research Network. NJR's laboratory/research is also supported by the Newcastle NIHR Biomedical Research Centre.
Glossary
- MED
minimal erythemal dose
- SED
standard erythemal dose
- TA
transit amplifying
Although not directly related to the current study, NJR has received grant/research support from Stiefel UK and Astra Zeneca, and through Newcastle University (non-personal) has participated in advisory boards for the following companies: Schering Plough, Abbott, Janssen-Cilag, and Creabilis. All the other authors state no conflict of interest.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
Supplementary Material
References
- Asawanonda P, Anderson RR, Chang Y, et al. 308-nm excimer laser for the treatment of psoriasis: a dose-response study. Arch Dermatol. 2000;136:619–624. doi: 10.1001/archderm.136.5.619. [DOI] [PubMed] [Google Scholar]
- Cen H, Mao F, Aronchik I, et al. DEVD-NucView488: a novel class of enzyme substrates for real-time detection of caspase-3 activity in live cells. FASEB J. 2008;22:2243–2252. doi: 10.1096/fj.07-099234. [DOI] [PubMed] [Google Scholar]
- Coven TR, Burack LH, Gilleaudeau R, et al. Narrowband UV-B produces superior clinical and histopathological resolution of moderate-to-severe psoriasis in patients compared with broadband UV-B. Arch Dermatol. 1997;133:1514–1522. [PubMed] [Google Scholar]
- Dawe RS, Wainwright NJ, Cameron H, et al. Narrow-band (TL-01) ultraviolet B phototherapy for chronic plaque psoriasis: three times or five times weekly treatment. Br J Dermatol. 1998;138:833–839. doi: 10.1046/j.1365-2133.1998.02221.x. [DOI] [PubMed] [Google Scholar]
- de Cid R, Riveira-Munoz E, Zeeuwen PL, et al. Deletion of the late cornified envelope LCE3B and LCE3C genes as a susceptibility factor for psoriasis. Nat Genet. 2009;41:211–215. doi: 10.1038/ng.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gruijl FR, Van der Leun JC. Estimate of the wavelength dependency of ultraviolet carcinogenesis in humans and its relevance to the risk assessment of a stratospheric ozone depletion. Health Phys. 1994;67:319–325. doi: 10.1097/00004032-199410000-00001. [DOI] [PubMed] [Google Scholar]
- Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129:1339–1350. doi: 10.1038/jid.2009.59. [DOI] [PubMed] [Google Scholar]
- Diffey BL, Jansen CT, Urbach F, et al. The standard erythema dose: a new photobiological concept. Photodermatol Photoimmunol Photomed. 1997;13:64–66. doi: 10.1111/j.1600-0781.1997.tb00110.x. [DOI] [PubMed] [Google Scholar]
- Dini L, Abbro L. Bioeffects of moderate-intensity static magnetic fields on cell cultures. Micron. 2005;36:195–217. doi: 10.1016/j.micron.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Dover R, Wright NA.1991The Cell Proliferation Kinetics of the Epidermis Physiology, Biochemistry, and Molecular Biology of the Skin(Goldsmith LA, ed). Second ed, Vol. 2.Oxford: Oxford University Press; 239–265. [Google Scholar]
- Elder JT. Genome-wide association scan yields new insights into the immunopathogenesis of psoriasis. Genes Immun. 2009;10:201–209. doi: 10.1038/gene.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman SR, Mellen BG, Housman TS, et al. Efficacy of the 308-nm excimer laser for treatment of psoriasis: results of a multicenter study. J Am Acad Dermatol. 2002;46:900–906. doi: 10.1067/mjd.2002.120454. [DOI] [PubMed] [Google Scholar]
- Fowler JF, Duh MS, Rovba L, et al. The impact of psoriasis on health care costs and patient work loss. J Am Acad Dermatol. 2008;59:772–780. doi: 10.1016/j.jaad.2008.06.043. [DOI] [PubMed] [Google Scholar]
- Goldinger SM, Dummer R, Schmid P, et al. Excimer laser versus narrow-band UVB (311 nm) in the treatment of psoriasis vulgaris. Dermatology. 2006;213:134–139. doi: 10.1159/000093852. [DOI] [PubMed] [Google Scholar]
- Gordon PM, Diffey BL, Matthews JN, et al. A randomized comparison of narrow-band TL-01 phototherapy and PUVA photochemotherapy for psoriasis. J Am Acad Dermatol. 1999;41:728–732. doi: 10.1016/s0190-9622(99)70008-3. [DOI] [PubMed] [Google Scholar]
- Hartman M, Prins M, Swinkels OQ, et al. Cost-effectiveness analysis of a psoriasis care instruction programme with dithranol compared with UVB phototherapy and inpatient dithranol treatment. Br J Dermatol. 2002;147:538–544. doi: 10.1046/j.1365-2133.2002.04920.x. [DOI] [PubMed] [Google Scholar]
- Johnson-Huang LM, Suarez-Farinas M, Sullivan-Whalen M, et al. Effective narrow-band UVB radiation therapy suppresses the IL-23/IL-17 axis in normalized psoriasis plaques. J Invest Dermatol. 2010;130:2654–2663. doi: 10.1038/jid.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karvonen SL, Korkiamaki T, Yla-Outinen H, et al. Psoriasis and altered calcium metabolism: downregulated capacitative calcium influx and defective calcium-mediated cell signaling in cultured psoriatic keratinocytes. J Invest Dermatol. 2000;114:693–700. doi: 10.1046/j.1523-1747.2000.00926.x. [DOI] [PubMed] [Google Scholar]
- Kirke SM, Lowder S, Lloyd JJ, et al. A randomized comparison of selective broadband UVB and narrowband UVB in the treatment of psoriasis. J Invest Dermatol. 2007;127:1641–1646. doi: 10.1038/sj.jid.5700767. [DOI] [PubMed] [Google Scholar]
- Kollner K, Wimmershoff MB, Hintz C, et al. Comparison of the 308-nm excimer laser and a 308-nm excimer lamp with 311-nm narrowband ultraviolet B in the treatment of psoriasis. Br J Dermatol. 2005;152:750–754. doi: 10.1111/j.1365-2133.2005.06533.x. [DOI] [PubMed] [Google Scholar]
- Krueger JG, Wolfe JT, Nabeya RT, et al. Successful ultraviolet B treatment of psoriasis is accompanied by a reversal of keratinocyte pathology and by selective depletion of intraepidermal T cells. J Exp Med. 1995;182:2057–2068. doi: 10.1084/jem.182.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte M, Galand P, Fokan D, et al. Apoptosis in established and healing psoriasis. Dermatology. 2000;200:314–316. doi: 10.1159/000018394. [DOI] [PubMed] [Google Scholar]
- Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–873. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- McGill A, Frank A, Emmett N, et al. The anti-psoriatic drug anthralin accumulates in keratinocyte mitochondria, dissipates mitochondrial membrane potential, and induces apoptosis through a pathway dependent on respiratory competent mitochondria. FASEB J. 2005;19:1012–1014. doi: 10.1096/fj.04-2664fje. [DOI] [PubMed] [Google Scholar]
- Meinhardt M, Krebs R, Anders A, et al. Absorption spectra of human skin in vivo in the ultraviolet wavelength range measured by optoacoustics. Photochem Photobiol. 2009;85:70–77. doi: 10.1111/j.1751-1097.2008.00398.x. [DOI] [PubMed] [Google Scholar]
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114–135. doi: 10.1016/j.jaad.2009.08.026. [DOI] [PubMed] [Google Scholar]
- Ozawa M, Ferenczi K, Kikuchi T, et al. 312-nanometer ultraviolet B light (narrow-band UVB) induces apoptosis of T cells within psoriatic lesions. J Exp Med. 1999;189:711–718. doi: 10.1084/jem.189.4.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish JA, Jaenicke KF. Action spectrum for phototherapy of psoriasis. J Invest Dermatol. 1981;76:359–362. doi: 10.1111/1523-1747.ep12520022. [DOI] [PubMed] [Google Scholar]
- Qin JZ, Chaturvedi V, Denning MF, et al. Regulation of apoptosis by p53 in UV-irradiated human epidermis, psoriatic plaques and senescent keratinocytes. Oncogene. 2002;21:2991–3002. doi: 10.1038/sj.onc.1205404. [DOI] [PubMed] [Google Scholar]
- Sano S, Chan KS, Carbajal S, et al. Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat Med. 2005;11:43–49. doi: 10.1038/nm1162. [DOI] [PubMed] [Google Scholar]
- Sauder DN. Mechanism of action and emerging role of immune response modifier therapy in dermatologic conditions. J Cutan Med Surg. 2004;8 (Suppl 3:3–12. doi: 10.1007/s10227-004-0803-3. [DOI] [PubMed] [Google Scholar]
- Setlow RB. The wavelengths in sunlight effective in producing skin cancer: a theoretical analysis. Proc Natl Acad Sci USA. 1974;71:3363–3366. doi: 10.1073/pnas.71.9.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani H, Morris RJ, Kaur P. Enrichment for murine keratinocyte stem cells based on cell surface phenotype. Proc Natl Acad Sci USA. 2000;97:10960–10965. doi: 10.1073/pnas.97.20.10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trehan M, Taylor CR. Medium-dose 308-nm excimer laser for the treatment of psoriasis. J Am Acad Dermatol. 2002;47:701–708. doi: 10.1067/mjd.2002.125075. [DOI] [PubMed] [Google Scholar]
- Webb A, Li A, Kaur P. Location and phenotype of human adult keratinocyte stem cells of the skin. Differentiation. 2004;72:387–395. doi: 10.1111/j.1432-0436.2004.07208005.x. [DOI] [PubMed] [Google Scholar]
- Weinstein GD, McCullough JL, Ross P. Cell proliferation in normal epidermis. J Invest Dermatol. 1984;82:623–628. doi: 10.1111/1523-1747.ep12261462. [DOI] [PubMed] [Google Scholar]
- Wolf R, Mascia F, Dharamsi A, et al. Gene from a psoriasis susceptibility locus primes the skin for inflammation. Sci Transl Med. 2010;2:61ra90. doi: 10.1126/scitranslmed.3001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk K, Haugen HS, Xu W, et al. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J Mol Med. 2009;87:523–536. doi: 10.1007/s00109-009-0457-0. [DOI] [PubMed] [Google Scholar]
- Wrone-Smith T, Mitra RS, Thompson CB, et al. Keratinocytes derived from psoriatic plaques are resistant to apoptosis compared with normal skin. Am J Pathol. 1997;151:1321–1329. [PMC free article] [PubMed] [Google Scholar]
- Youn SW, Kim DS, Cho HJ, et al. Cellular senescence induced loss of stem cell proportion in the skin in vitro. J Dermatol Sci. 2004;35:113–123. doi: 10.1016/j.jdermsci.2004.04.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.