Abstract
A central goal in vaccinology is the induction of high and sustained antibody responses. Protein-in-adjuvant formulations are commonly used to achieve such responses. However, their clinical development can be limited by the reactogenicity of some of the most potent pre-clinical adjuvants and the cost and complexity of licensing new adjuvants for human use. Also, few adjuvants induce strong cellular immunity which is important for protection against many diseases, such as malaria. We compared classical adjuvants such as alum to new pre-clinical adjuvants and adjuvants in clinical development such as Abisco®100, CoVaccine HT™, Montanide®ISA720 and SE-GLA, for their ability to induce high and sustained antibody responses and T cell responses. These adjuvants induced a broad range of antibody responses when used in a three-shot protein-in-adjuvant regime using the model antigen ovalbumin and leading blood-stage malaria vaccine candidate antigens. Surprisingly, this range of antibody immunogenicity was greatly reduced when a protein-in-adjuvant vaccine was used to boost antibody responses primed by a human adenovirus serotype 5 (AdHu5) vaccine recombinant for the same antigen. This AdHu5-protein regime also induced a more cytophilic antibody response and demonstrated improved efficacy of merozoite surface protein-1 (MSP-1) protein vaccines against a Plasmodium yoelii blood-stage challenge. This indicates that the differential immunogenicity of protein vaccine adjuvants may be largely overcome by prior immunization with recombinant adenovirus, especially for adjuvants that are traditionally considered poorly immunogenic in the context of subunit vaccination, and may circumvent the need for more potent chemical adjuvants.
Introduction
The use of vaccines has been instrumental in the prevention and control of many infectious diseases. Despite the creation of several efficacious vaccines such as those against smallpox and yellow fever, highly effective vaccines are still lacking for diseases such as malaria and tuberculosis (TB) which cause substantial morbidity and mortality each year (1). Several strategies have been employed towards the development of novel vaccines aimed at these diseases with most focus being placed on subunit vaccines, particularly for vaccines targeting the blood-stage of malaria (2). These subunit vaccines are often aimed at inducing antibody responses and have traditionally comprised recombinant proteins formulated with adjuvants to improve their immunogenicity. However, despite encouraging pre-clinical results, experimental adjuvants can have unacceptable safety profiles in clinical trials(3-5) and to date only six adjuvants have been licensed for use in humans. These adjuvants include aluminum salts/alum (aluminum phosphate and aluminum hydroxide), the oil-in-water emulsion MF59 (from Novartis), virosomes, as well as the AS03 and AS04 adjuvant platform created by GlaxoSmithKline (6). Most currently licensed adjuvants predominantly induce the humoral arm of the immune response, and may therefore be of limited use for diseases, such as TB and malaria, where cellular immunity may be required as an important contributor to protective immunity (7, 8). Similarly, the lack of access to many promising adjuvants developed by some companies has had an adverse effect on vaccine development for difficult diseases, such as TB and malaria, where there is limited commercial interest and very strong immune responses are required for protection. This lack of accessibility and knowledge about the formulation of such adjuvants means that the development of effective human-compatible adjuvants for such diseases remains an urgent priority. Numerous experimental adjuvants are thus being developed that are aimed at inducing strong antibody and T cell responses including TLR agonists, liposomes and novel emulsions(9).However, it is unclear whether these adjuvants will demonstrate reactogenicity profiles that are acceptable for vaccine licensure.
Viral vectored vaccines, although not without their own developmental and regulatory challenges, have been explored as another avenue to generate strong immune responses through subunit vaccination(10). For example, sequential immunizations of recombinant adenovirus human serotype 5 (AdHu5) and modified vaccinia virus Ankara (MVA) vectors, encoding the blood-stage malaria antigen merozoite surface protein-1 42-kDa region(MSP-142),have been shown to generate strong T cell responses as well as high-titer antibodies that are protective against both a lethal Plasmodium yoelii sporozoite and blood-stage challenge (11, 12). The ability of viral vectors to induce strongly both the humoral and cellular arms of the immune system has led to their use in various heterologous prime-boost strategies (13-18).
Adenoviral prime – protein boost (AP) regimes, whereby the two leading subunit vaccine platforms are combined, have more recently been shown to induce improved antibody responses compared to the use of either strategy on its own. We have demonstrated in mice that this AP immunization strategy can lead to improved antibody responses, with a moderate T cell response induced by the adenovirus, when using P. falciparum MSP-1 vaccines (14). These antibody responses were found to be more consistently primed by an adenoviral vector and also induced a more cytophilic antibody response dominated by IgG2a. In agreement with these murine data, non-human primate studies of similar regimes, for candidate malaria and HIV-1 vaccines, have also shown particular promise (15, 19, 20).
Here,we initially compared the potency of several promising adjuvants (both pre-clinical and clinically tested/approvedfor clinical trial) in a head to head manner, in order to provide comparative immunogenicity data on leading adjuvant formulations when administered three times with a protein (PPP regimes). We then extended this work to carry out a detailed characterization of the immunogenicity of AP regimes in comparison to PPP regimes, utilizing the model antigen ovalbumin (OVA) as well as the blood-stage malaria vaccine candidate antigens MSP-1 and erythrocyte binding antigen (EBA)-175(2, 21, 22). We showed that the marked differential immunogenicity of adjuvants seen in PPP regimes can be largely overcome by priming antibody responses with a recombinant adenoviral vector encoding the same antigen, so that weaker adjuvants now perform more comparably to strong adjuvants. Irrespective of the protein adjuvant used, the AP regime induced more cytophilic antibodies and, in the case of using a saponin-based adjuvant, was capable of inducing strong humoral and cellular immunity simultaneously. This consistently improved immunogenicity, particularly when using less potent adjuvants, also translated into improved protective efficacy of MSP-1 vaccines in a P. yoelii blood-stage challenge model in mice.
Materials and Methods
Animals and immunizations
All procedures were performed in accordance with the terms of the UK Animals (Scientific Procedures) Act Project License and were approved by the University of Oxford Animal Care and Ethical Review Committee. 6-8 week old female BALB/c (H-2d) and C57BL/6 (H-2b) mice (Harlan Laboratories, Oxfordshire, UK) were anesthetized before immunization with Isoflo (Abbot Animal Health, UK). All immunizations were administered intramuscularly (i.m.) with vaccine divided equally into each musculus tibialis unless otherwise specified. Immunization doses and intervals varied between experiments and are explained in the text and figure legends. Immune responses were assayed two weeks after each immunization and before the protein vaccine boost in AP regimes.
Viral Vectors and Protein Vaccines
The generation of AdHu5 and MVA viral vectors expressing P. yoelii MSP-142 and MSP-133 as well as P. falciparum MSP-1 (PfM128) has been previously described (11, 16). AdHu5 and MVA viral vectors expressing P. falciparum EBA-175 (F2 region)(23) and OVA were made as described elsewhere (24). The OVA vectors express the full 1188-bp coding sequence of Hen OVA (GenBank Accession #MN205152, http://www.ncbi.nlm.nih.gov/genbank/). An N311Damino acid substitution was carried out to prevent N-linked glycosylation as described elsewhere (25). The final construct was codon optimized for human expression and was synthesized by GeneArt (Regensburg, Germany). For protein vaccinations, Grade VII Ovalbumin was obtained from Sigma Aldrich, UK. P. yoelii MSP-119-GST fusion proteinwas made as previously described using an E. coli expression system (12). P. falciparum EBA-175 (F2 region) and MSP1-19 protein were produced as previously described (26, 27). Endotoxin levels for P. falciparum proteins were measured using the Limulus amebocyte lysate (LAL) gel clot assay according to the manufacturer’s instructions (Salesworth). The endotoxin content of purified P. falciparum EBA-175protein was less than 21EU per 25μg protein and less than 6.7EU per 25μg of P. falciparumMSP1-19protein.
Adjuvants
Adjuvants used in this study were dosed and prepared in low phosphate PBS (<5mM) (Gibco-Invitrogen, UK) as described in Table I. In brief: Abisco®100(28)(Isconova, Sweden) (12μg/dose) was gently mixed with antigen in PBS. Adju-Phos® (Brenntag, Denmark) (75μg Al3+/dose) and Alhydrogel® (Brenntag, Denmark) (75μg Al3+/dose) were combined with antigen in PBS and spun at 4°C for 30 min before administration. CoVaccine HT™(29)(a novel proprietary vaccineadjuvant of Protherics Medicines Development, a BTG International group company, London,UK) was mixed gently 1:1 with antigen in PBS (2mg sucrose fatty acid sulphate esters (SFASE)/dose). Complete and Incomplete Freund’s Adjuvant (C/IFA) (Sigma, UK) were mixed vigorously through vortexing 1:1 with antigen in PBS. CFA was used only once and mice were subsequently boosted with IFA. Immunizations were administered subcutaneously for the C/IFA adjuvants. Montanide® ISA720 (Seppic, France) and antigen in PBS was emulsified using a T10 ULTRA-TURRAX® (IKA®) homogenizer under sterile conditions at 25,000rpm for 6 min keeping the sample on ice in a ratio of 3:7 (Antigen:Adjuvant). Adjuvants based on a stable emulsion (SE) with different TLR agonists incorporated into the emulsion (30)(Infectious Disease Research Institute, USA) (20μg/dose) were mixed with antigen in PBS and vortexed for 30 seconds. All vaccines were kept on ice until administration. For all vaccines the protein dose was incorporated into the PBS fraction of the vaccine. Adsorption of antigen to aluminum based adjuvants was assessed as previously described (15). Using this method OVA was found to adsorb to Alhydrogel®by 89%. OVA only adsorbed to Adju-Phos® by 9% and P. yoelii MSP-119-GST adsorbed to Adju-Phos® by 40% (data not shown).
Table 1.
Adjuvants used throughout the course of the study.
| Adjuvant | Type | Formulation | Development Status |
|---|---|---|---|
| Adju-Phos® | Aluminium Phosphate | 1.5mg/ml – rotated for 30 min at 4°C |
Licensed |
| Alhydrogel® | Aluminium Hydroxide | 1.5mg/ml – rotated for 30 min at 4°C |
Licensed |
| Abisco®100 | Phospholipid, cholesterol and saponin complex. Contains a mixture of Matrix A (QS7) and C (contains QS21) fractions which are purified from Quil A extracts |
12μg/dose – mix by shaking |
Clinical development |
| CoVaccine HT™ | Sucrose fatty acid sulphate esters (SFASE) immobilised on the oil droplets of a submicron emulsion of squalane in water |
1:1 – gently mixed | Clinical development |
| EM01 | Stable oil in water emulsion (SE) | 20μg agonist/dose – vortex for 30 s |
Pre-clinical/research stage |
| EM05 | SE + TLR 4 (GLA) | 20μg agonist/dose – vortex for 30 s |
Clinical development |
| EM012 | SE + TLR4/7/8 (GLA &Iziquimod) | 20μg agonist/dose – vortex for 30 s |
Pre-clinical/research stage |
| EM014 | SE + TLR 4/9 (GLA & CpG ODN 1826) |
20μg agonist/dose – vortex for 30 s |
Pre-clinical/research stage |
| EM020 | SE + TLR 4/7/8/9 (GLA & Iziqumod &CpG ODN 1826) |
20μg agonist/dose – vortex for 30 s |
Pre-clinical/research stage |
| Freund’s adjuvant |
Non-metabolisable oils, Complete Freund’s adjuvant contains mycobacterial derivatives |
1:1 – vortex thoroughly | Experimental |
| Montanide ® ISA720 |
Squalene and refined emulsifier/surfactant based on mannide oleate |
3 Ag:7 ISA - emulsified 6 min on ice using a T10 ULTRA-TURRAX® (IKA®) at 25,000rpm |
Clinical development |
Enzyme Linked Immunosorbent Assay (ELISA)
Total IgG ELISAs were carried out as described previously (12).Optical density (OD) was read at 405nm using a Model 550 Microplate Reader (Bio-Rad, UK). Serum antibody endpoint titers were taken as the x-axis intercept of the dilution curve at an absorbance value three standard deviations greater than the OD405 for serum from a naïve mouse. A standard positive serum sample and naïve serum sample were added as controls for each assay. Naïve mouse serum was negative for antigen-specific responses to all antigens (data not shown). P. yoelii MSP-119 specific antibodies, following immunization of mice with GST-PyMSP-119, were measured using P. yoelii MSP-119-IMX108 protein(31) which does not contain theGST-tag present in the protein used for immunization. P. falciparum MSP-119-specific antibody responses were measured using P. falciparum MSP-119-GST (QKNG) made as previously described in an E. coli expression system (16).
Isotype ELISA
To detect antigen-specific IgG1 and IgG2a responses,plates were coated at a concentration of 2μg/mL with protein overnight at RT as before. A standard curve comprised of isotype purified mouse IgG1 or IgG2a monoclonal antibody (mAb) (eBioscience, UK) was added in duplicate to separate platesat a concentration of 20μg/mL and diluted 3-fold. A positive control of mAb at a dilution of 1:6075 for IgG1 and 1:2025 for IgG2a was also added to each separate plate. After blocking, serum diluted in PBS/T was added in duplicate to the plate for 2 h at RT. Plates were then washed and either biotin anti-mouse IgG1 or IgG2a (BD biosciences) were added to the test plates. Following a30 min incubation with Extravidin Alkaline Phosphatase (Sigma Aldrich, UK) plates were developed using the same reagents as for total IgG ELISA. Plates were developed until the monoclonal positive control reached an OD405 of 1.0.This point was defined as 1 Isotype unit (IU) and IUs were read off the standard curve similar to published methodology (32). Samples were diluted to fall on the linear part of the curve. Low titer samples from the experiments using OVA were diluted 1:100 and were developed according to the same positive control as before. Isotype responses for these samples are reported as OD 405nm.
Avidity ELISA
Antibody avidity was assessed using a sodium thiocyanate (NaSCN)-displacement ELISA as described previously(14). Sera were individually diluted to a level calculated to give a titer of 1:100, based on known total IgG titers,and exposed to an ascending concentration (0–7 M) of the chaotropic agent NaSCN (Sigma Aldrich, UK).Plates were developed as for total IgG. The intercept of the OD405 curve for each sample with the line of 50% reduction of the OD405 in the NaSCN-free well for each sample (i.e. the concentration of NaSCN required to reduce the OD405 to 50% of that without NaSCN) was used as a measure of avidity.
Ex-vivo IFN-γ ELISPOT
IFN-γ ELISPOTs were carried out using PBMC isolated from the blood and spleen as previously described (33). In brief, MAIP ELISPOT plates (Millipore, UK Ltd) were coated with anti-mouse-IFN-γ mAb (Mabtech, UK) at 5μg/mL in carbonate-bicarbonate buffer. Plates were blocked with complete DMEM (from Sigma Aldrich, UK; 10% FBS from Biosera, Ltd; 2mM L-glutamine, 100U/mL penicillin, 100μg/mL streptomycin sulphate all from Invitrogen, UK) for 1 h at 37°C. PBMC and splenocytes were re-suspended in complete medium and counted using a CASY counter (Schärfe Systems, Germany). 50μL of PBMC harvested from the blood were plated into duplicate wells. 50μL peptide diluted in medium plus 0.25 × 106 naïve splenocytes were added to test wells. Medium and naïve splenocytes only were plated into negative control wells. Spleen cells were re-suspended at 1×107cells/mL and 50μL of cells were plated in duplicate. 50μL peptide diluted in complete medium was added to test wells and complete medium alone was added to control wells. OVA-specific CD4+ T cell peptides (ISQAVHAAHAEINEAGR, TEWTSSNVMEERKIKV) (34, 35) were pooled and OVA-specific CD8+ T cell peptide (SIINFEKL) (34) were added at a final concentration of 5μg/mL. Plates were incubated at 37°C, 5% CO2 in a humidified incubator for ~18h. Plates were then washed and incubated with biotinylated anti-mouse-IFN-γ mAb (Mabtech, UK), followed by an incubation with a streptavidin alkaline phosphatase polymer (Mabtech, UK). Spots were developed by addition of color development buffer, and counted using ELISPOT software (Autoimmun Diagnostika, Germany). Results are expressed as spot forming cells (SFC) per million cells. Background responses in media-only wells were subtracted from those measured in peptide-stimulated wells.
Intracellular Cytokine Staining (ICS)
Analysis of the percentage of cytokine producing peripheral blood CD4+ and CD8+ T cells by ICS has previously been described (11). Briefly, cells were stimulated for 5 h with pools of 15mer peptides overlapping by 10 amino acids corresponding to PyMSP-133 at a final concentration of 5 μg/mL for each peptide (11). Cells were surface stained with anti-CD8α PerCP-Cy5.5 and anti-CD4 PB (eBioscience). After permeabilization using Cytofix/Cytoperm (BD Biosciences) cells were stained intracellularly with anti-IFN-γ APC, anti-TNF-α FITC and anti-IL-2 PE (eBioscience). Samples were acquired on a LSRII flow cytometer (BD Bioscience) and analyzed using FlowJo (TreeStar Inc., USA). Background responses in unstimulated cells were subtracted from the stimulated responses prior to analysis.
CD4+ T cell Depletion
CD4+ T cells were depleted using an anti-CD4 GK1.5 (rat IgG2a) mAb purified using protein G affinity chromatography from hybridoma culture supernatants as previously described (11). The degree of CD4+ T cell depletion was assessed by flow cytometry using anti-CD4 PB(clone RM5.4), anti-CD3ε APC and anti-CD8 PerCP-Cy5.5 (eBioscience, UK) in the PBMC of vaccinated depleted mice and unvaccinated control mice on day +1 with respect to challenge on day 0. Depletion was assessed by gating on CD3+CD4+ cells and was shown to be > 98%.
Antibody secreting cell (ASC) assay
Cells isolated from the spleen and bone marrow were tested for ASCs by ELISPOT assay as previously described (14, 36), except thatbone marrow was flushed from dissected femurs with complete DMEM using a 26G needle and passed through a sterile 70μm cell strainer.ELISPOT plates were counted using the AID plate reader software (AID, Cadama Medical) and counts were visually confirmed. Antibody forming spots were relatively large, spherical in size with “fuzzy”granular edges.
Parasites
P. yoelii YM parasitized red blood cell (pRBC) challenges were carried out as previously described (12). Mice were infected by intravenous (i.v.) injection with 105 pRBC. Blood-stage parasitemia was monitored from day three post-challenge by Giemsa-stained thin-blood smear and was calculated as percentage of infected RBC. Mice were considered uninfected if no parasites were observed in 50 fields of view and were sacrificed by a humane method at ≥80% parasitemia.
Statistical Analysis
All statistical analysis was carried out using Prism version 5 (Graphpad, USA). All ELISA titers were log10 transformed prior to analysis. For non-parametric data, a Kruskal-Wallis test with Dunn’s multiple comparison post-test was used to compare more than two groups. A one-way ANOVA was used for multiple comparisons of parametric data with Bonferroni’s multiple comparison post-test for comparison of groups as stated. An un-paired t-test was used to compare the means of two groups for parametric data and a Mann-Whitney U test was used for non-parametric data. A two-way ANOVA with Bonferroni’s multiple comparison post-test was used to explore the effect of two variables.Correlations were tested using Spearman’s rank correlation. p<0.05 was considered significant(* p<0.05, **p<0.01 and *** p<0.001).
Results
Novel adjuvants can improve the immunogenicity of protein vaccines
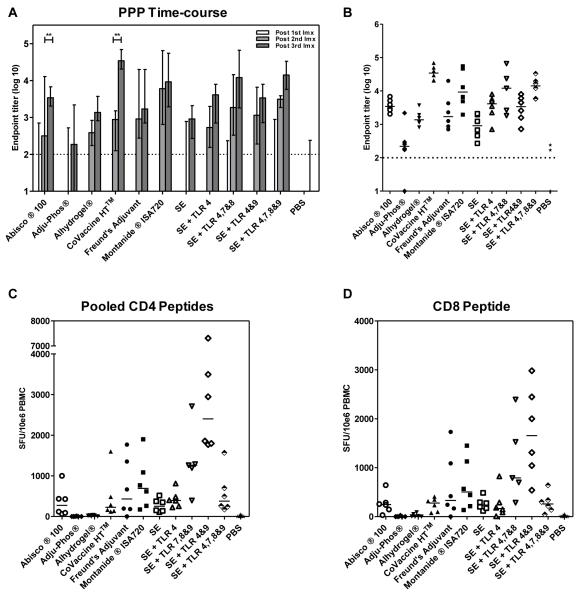
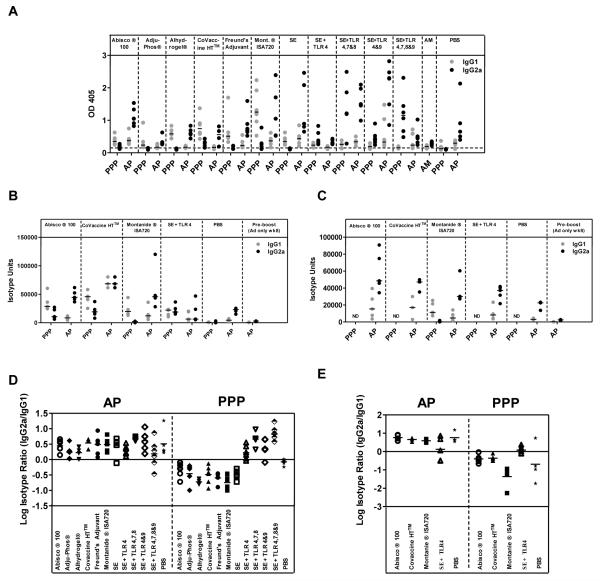
Though numerous adjuvants have been selectively tested with a diverse set of antigens (26, 37-40), a comparative assessment of the immunogenicity of a leading panel of adjuvants is lacking. To address this short-coming, 11 adjuvants (both pre-clinical and clinically tested/approvedfor clinical trial) were assessed in a three shot protein-in-adjuvant regime (PPP regime) using the model antigen OVA. C57BL/6 mice were immunized with three shots of 20μg of OVA, two weeks apart, formulated with adjuvant as described. Serum total IgG titers were assayed two weeks after each immunization in response to OVA protein by ELISA. After one shot of protein-in-adjuvant only some mice in select groups seroconverted (Figure 1A). After a second shot of protein-in-adjuvant, mice in all groups had detectable antibody responses. These IgG titers were significantly boosted by a third shot of protein-in-adjuvant for Abisco®100and CoVaccine HT™(p<0.01 by two-way ANOVA with Bonferroni’s multiple comparison post-test) (Figure 1A). Two weeks after the final immunization a broad range of antibody responses was seen – with a 156-fold difference in median titers observed between the strongest and weakest responding groups. All adjuvants induced significantly higher total IgG titers than OVA in PBS (p<0.05, one-way ANOVA with Bonferroni’s multiple comparison post-test). CoVaccine HT™, Montanide® ISA720, SE + TLR4,7&8 and SE + TLR 4,7,8&9 induced the highest IgG titers. CoVaccine HT™ induced titers surpassingthose induced with Freund’s adjuvant and induced significantly higher IgG titers than the classical aluminum-based adjuvant Adju-Phos® (p<0.05, one-way ANOVA with Bonferroni’s multiple comparison post-test) (Figure 1B). The remaining SE-based adjuvants induced IgG titers comparable to Freund’s adjuvant, with Adju-Phos® (based on aluminum phosphate) inducing by far the lowest titers.Only two mice receiving OVA in PBS seroconverted after three immunizations, though these responses appeared to be transient as they returned to baseline when antibody titers were assayed six weeks after the final immunization (data not shown).
Figure 1. Novel adjuvants can improve the immunogenicity of protein vaccines.
C57BL/6 mice (n= 6 / group) were immunized i.m. with 20μg of OVA formulated in adjuvant. Total IgG titers were measured in the serum in response to OVA protein by ELISA. (A) IgG titers measured two weeks after each immunization (Imx). Median responses are shown with range. ** p<0.01 by two-way ANOVA with Bonferroni’s multiple comparison post-test. (B) IgG titers measured two weeks after the third immunization. Median responses are shown. T cell responses were assayed in the blood against the (C) pooled H-2b CD4+ T cell epitopes and (D) the H-2b CD8+ T cell epitope present in OVA. Median responses are shown. The dotted line indicates the threshold for responses above background in (A) and (B).
T cell responses were also assayed in the blood against the known H-2b CD8+ T cell epitope and pooled CD4+ T cell epitopes present in OVA two weeks after the final immunization. Though the hierarchy of T cell responses induced by the adjuvants differed to that of IgG, it was comparable for CD4+ T cell and CD8+ T cell responses (Figure 1C and D). As previously shown,Alhydrogel® and Adju-Phos® were poor T cell inducers(37). Emulsion based adjuvants such as Freund’s adjuvant and Montanide®ISA720 induced a median of 431 and 692CD4+SFU/106 PBMC respectively with the SE adjuvant containing the combination of TLR agonists 4&9 inducing the most potent T cell responses (median of 2402CD4+SFU/106 PBMC,p<0.05 for CD4+ and CD8+ T cell responses by Kruskal-Wallis test with Dunn’s multiple comparison post-test versus Adju-Phos®, Alhydrogel® and PBS). These data indicate that, not only do protein adjuvants induce T cell and antibody responsesof different magnitudes, but that both the humoral and cellular immune responses induced by more classical protein vaccine adjuvants can be improved upon by using new experimental adjuvants.
A priming immunization with a recombinant adenoviral vector reduces the differential antibody immunogenicity of protein-in-adjuvant vaccines
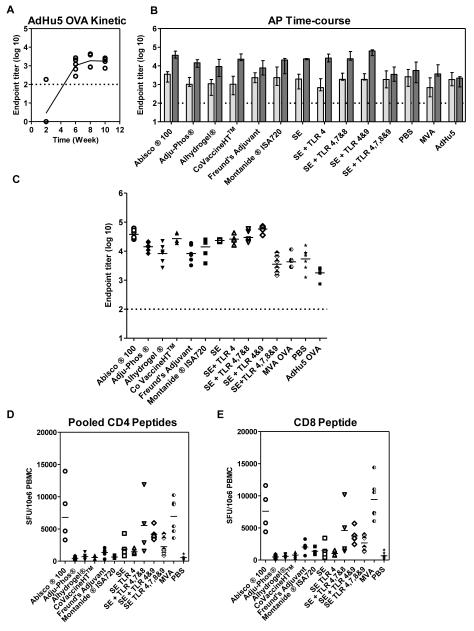
Recently the addition of a protein-in-adjuvant boost following a recombinant adenoviral prime (AP regime) has been shown to enhance antibody titers when compared to protein-in-adjuvant regimes on their own as well as recombinant adenoviral prime – MVA boost regimes (AM regime) (14, 15). C57BL/6 mice were thus primed with 1×1010 viral particles (vp) of a recombinant AdHu5 vector expressing OVA (AdHu5-OVA) and boosted eight weeks later with 20μg of OVA protein-in-adjuvant as prepared previously. The aim was to assess whether a similar effect in terms of improved immunogenicity is seen over a range of different adjuvants. Three control groups primed with AdHu5-OVA and either not boosted, boosted with OVA in PBS or boosted with 1×107 plaque forming units (pfu) MVA-OVA were also included. In the AdHu5-OVA only group, antibody responses peaked at week eight and then plateaued out to week ten, corresponding to two weeks post protein-in-adjuvant boost (Figure 2A). Adenoviral primed antibody responses were significantly boosted by all protein-in-adjuvant vaccines, apart from in the SE + TLR4,7,8&9 group, as well as by MVA-OVA (p<0.01 by two-way ANOVA with Bonferroni’s multiple comparison post-test) (Figure 2B). Analysis of the mean fold change from pre-boost to post-boost IgG titers in individual mice showed that some adjuvants boosted adenoviral primed responses more efficiently than others. SE + TLR 4&9 induced a significantly higher mean fold change in IgG titer than Adju-Phos®, Alhydrogel® and CFA.Abisco®100 also induced a higher mean fold change in titer than Alhydrogel (both: p<0.01, by Kruskal-Wallis testwith Dunn’s multiple comparison post-test on fold change of significantly boosted groups).This indicates that some adjuvants can perform particularly well in the context of an AP immunization regime. However, overall the differential immunogenicity observed following PPP immunization wasgreatly reduced, with most responses across the different groups now being of a comparable and relatively high magnitude (Figure 2C). Only an8-fold difference in median titers was observed within the AP groups that had showed significant boosting. Interestingly using a recombinant adenoviral prime improved the boosting ability of the aluminum-based adjuvant Adju-Phos®, which now induced similar antibody responses to CoVaccine HT™, a potent inducer of antibody responses in the PPP experiments (8-fold increase in the mean, 95% CI: 2-35 for CoVaccine HT™ and 6-fold increase in the mean, 95% CI: 3-12 for Adju-Phos®; p>0.05, one-way ANOVAwith Bonferroni’s multiple comparison post-test). Protein-in-adjuvant boosting also surpassed boosting with MVA-OVA, in agreement with our previous data (14, 15). Surprisingly OVA in PBS also slightly boosted adenoviral primed IgG responses (2-fold increase in the mean, 95% CI: 0.5-10.9).
Figure 2. A priming immunization with a recombinant adenoviral vector reduces the differential antibody immunogenicity of protein-in-adjuvant vaccines.
C57BL/6 mice (n= 6 / group) were primed i.m. with 1×1010 vp of AdHu5-OVA and boosted eight weeks later i.m. with OVA formulated in adjuvant. IgG titers were measured in the serum in response to OVA protein by ELISA. (A) IgG titers measured every two weeks after AdHu5-OVA. Median responses are shown after each immunization. (B) IgG titers measured eight weeks after the AdHu5-OVA prime ( pre-boost) and two weeks after the protein vaccine boost (
pre-boost) and two weeks after the protein vaccine boost ( post-boost). Median responses are shown with range. (C) IgG titers measured two weeks after the protein vaccine. Median responses are shown with individual data points. T cell responses were assayed in the blood against the (D) pooled H-2b CD4+ T cell epitopes and (E) the H-2bCD8+ T cell epitope present in OVA. Median responses are shown. The dotted line indicates the threshold for responses above background in (A) – (C).
post-boost). Median responses are shown with range. (C) IgG titers measured two weeks after the protein vaccine. Median responses are shown with individual data points. T cell responses were assayed in the blood against the (D) pooled H-2b CD4+ T cell epitopes and (E) the H-2bCD8+ T cell epitope present in OVA. Median responses are shown. The dotted line indicates the threshold for responses above background in (A) – (C).
T cell responses were also assessed two weeks after the protein-in-adjuvant boost (Figure 2D and E). Interestingly Abisco®100, in addition to inducing strong IgG titers, induced a median of 6799CD4+SFU/106 PBMC, indicating that AP regimes can impact both the humoral and cellular arm of the immune system. This AP regime induced T cell responses equivalent to AM, which has been optimized for T cell induction (p>0.05 for CD4+ and CD8+ T cell responses by Kruskal-Wallis test with Dunn’s multiple comparison post-test Abisco®100 versus MVA). Overall, these data indicate that the broad range of antibody induction seen with adjuvants following the administration of protein vaccines is greatlyreduced when responses are first primed with an adenoviral vector.
Effect of adenoviral priming is consistent in other mouse strains and with other antigens
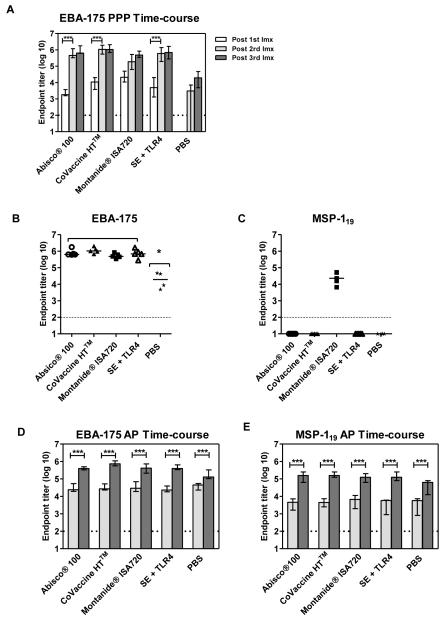
To further investigate the immunogenicity of selected adjuvants and to assess whether the effects seen with OVA are antigen or mouse strain specific, the P. falciparum blood-stage malaria antigens EBA-175 (F2 region) and MSP-1 were also assessed in BALB/c mice immunized with PPP and AP regimes (Figure 3). The MSP-1 C-terminus undergoes proteolytic cleavage during RBC invasion and is cleaved into 33-kDa (MSP-133) and 19-kDa (MSP-119) fragments(41). Antibody responses against MSP-119, but not MSP-133, are protective against blood-stage malaria (11, 42). However, a role has also been reported for MSP-1-specific CD8+ T cells against liver-stage parasites, as MSP-1 is also expressed during late liver-stage infection (11, 43). MSP-133-specific CD4+ T cells have also been shown to provide help for B cells and aid the development of de novo antibody responses(11). EBA-175 binds to sialic acid residues on glycophorin A on the surface of erythrocytes and can mediate invasion by malaria parasites (44). Antibodies induced against this antigen have been shown to inhibit P. falciparum invasion of erythrocytes in vitro(26).Here these two antigens were given as a mixture (10μg of each) formulated with adjuvant as previously described and were administered three weeks apart in the PPP regime. 1×109 vp of AdHu5-MSP-1 and 1×109 vp of AdHu5-EBA-175 were also administered as a mixture in the AP regimeand boosted with protein vaccines eight weeks later as previously. All mice in the PPP groups seroconverted in response to EBA-175 after one immunization (Figure 3A) and followed the same hierarchy as seen after two shots of OVA (with Montanide®ISA720 and CoVaccine HT™ inducing the strongest IgG titers, see Figure 1B). IgG titers were significantly boosted by a second immunization of protein-in-adjuvant (p<0.001, two-way ANOVAwith Bonferroni’s multiple comparison post-test) but not by a third, after which all adjuvants induced comparable antibody responses to EBA-175 indicating that the antibody responses had reached a plateau (p>0.05, two-way ANOVAwith Bonferroni’s multiple comparison post-test) (Figure 3B). Though mice immunized with EBA-175 in PBS had detectable antibody responses, IgG titers in the adjuvant groups were significantly higher (p<0.05, one-way ANOVAwith Bonferroni’s multiple comparison post-test). In agreement with previous data, mice immunized with P. falciparum MSP-119 in Montanide ISA720 had detectable IgG titers to MSP-119 protein (14), however no antibody responses were detected with other adjuvants (Figure 3C).
Figure 3. The effect of an adenoviral prime is consistent in other mouse strains and with other antigens.
BALB/c mice (n=5 / group) were immunized twice i.m. three weeks apart with a mixture of 10μg of P. falciparum EBA-175 protein and 10μg of P. falciparum MSP-119 protein. IgG titers were measured in the serum to (A) EBA-175 protein two weeks after each immunization (Imx). Median responses are shown with range. IgG titers were measured in the serum in response to (B) EBA-175 protein and (C) MSP-119(QKNG allele) protein two weeks after the final immunization. Median responses are shown. BALB/c mice were primed i.m. with a mixture of 1×109 vp of AdHu5-MSP-1and AdHu5-EBA-175 and boosted i.m. eight weeks later with a mixture of 10μg of P. falciparum EBA-175 protein and 10μg of P. falciparum MSP-119 protein. IgG titers were measured in the serum in response to (D) EBA-175 protein and (E) MSP-119 (QKNG) protein eight weeks after the prime ( pre-boost) and two weeks after the protein vaccine boost (
pre-boost) and two weeks after the protein vaccine boost ( post-boost). Median responses are shown with range. * p<0.05by one-way ANOVA with Bonferroni’s multiple comparison post-test. ***p<0.001 by two-way ANOVAwith Bonferroni’s multiple comparison post-test.
post-boost). Median responses are shown with range. * p<0.05by one-way ANOVA with Bonferroni’s multiple comparison post-test. ***p<0.001 by two-way ANOVAwith Bonferroni’s multiple comparison post-test.
In accordance with previous data (14, 15), and in contrast to the PPP regimes, mice in the AP groups seroconverted two weeks after the adenoviral prime in response to both antigens (Figure 3D and E). Following a protein immunization, total IgG titers were significantly boosted in response to both antigens by >1 log in all adjuvant groups (16-fold increase in the mean across all adjuvant groups, 95% CI: 13-20 for EBA-175 and 31-fold increase in the mean across all adjuvant groups, 95% CI: 23-41 for MSP-119; p<0.001, two-way ANOVAwith Bonferroni’s multiple comparison post-test). Interestingly, though mice only responded to MSP-119 in Montanide®ISA720 following three shots of protein vaccine (Figure 3C), antibody responses to MSP-119 in mice primed with the adenoviral vector were successfully boosted to high levels by each protein-in-adjuvant vaccine when administered in the AP regime (Figure 3E). There was no significant difference in the mean fold change from pre-boost to post-boost IgG titers in relation to the use of a particular adjuvant with the two different antigens (data not shown). Also, as seen with OVA, adenoviral primed responses were also boosted by protein in PBS (4-fold increase in the mean, 95% CI: 3-7 for EBA-175 and 13-fold increase in the mean, 95% CI: 5-35 for MSP-119; p<0.001, two-way ANOVAwith Bonferroni’s multiple comparison post-test). Taken all together, these data indicate that despite some minor differences, the improved boostability of IgG responses observed following a recombinant adenoviral prime is consistent and can be observed in different mouse strains and when using different antigens.
Longevity of responses
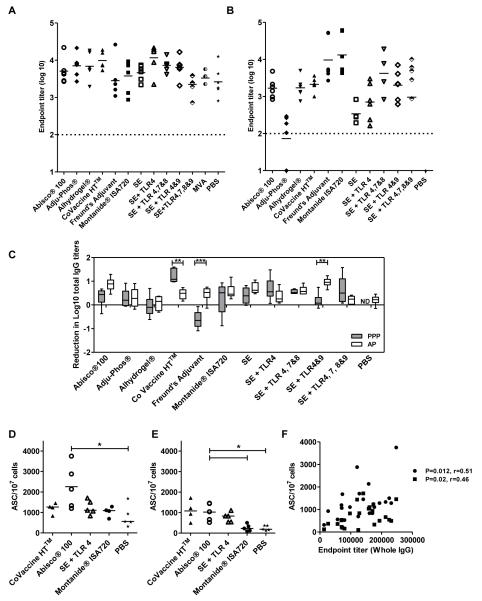
The induction of not only high titer, but also sustained antibody responses is desirable for efficacious vaccines. The longevity of the antibody responses induced by the different adjuvants deployed in PPP and AP regimes were thus measured ten weeks after the final immunization (Figure 4A and B). Antibody responses were generally higher ten weeks post vaccination in the AP groups which is most likely a reflection of initially higher titers eight weeks earlier.The reduction in log10 titers from the peak of the response to the last time point in the OVA system was compared for the different adjuvants administered either in AP or PPP regimes (Figure 4C). There was a mean reduction of 0.3 and 0.5 log10 titers in AP and PPP regimes respectively. A significant difference in the reduction of log10 IgG titers over time between the two regimes was found for 3 of the 11 adjuvants tested (CoVaccine HT™, Freund’s adjuvant and SE + TLR 4&9, p<0.01 two-way ANOVAwith Bonferroni’s multiple comparison post-test). However, in most cases there was no difference in the decline of antibody responses between AP and PPP regimes as has been previously found (14). Thus almost all vaccine-induced IgG, irrespective of method of induction, seems to be subject to the same rate of decay / half-life over time.
Figure 4. Longevity of vaccine-induced IgG responses.
BALB/c and C57BL/6 mice (n=5-6 / group) were immunized as described previously. IgG titers were measured in the serum ten weeks after the final immunization in response to OVA protein in mice immunized with (A) AP regimes and (B) PPP regimes. Median responses are shown. (C) The reduction in log titers was calculated from IgG titers two weeks post the final immunization in each regime and IgG titers ten weeks after the final immunization. ** p<0.01, *** p<0.001 by two-way ANOVA with Bonferroni’s multiple comparison post-test. Median responses are shown with range. Antibody secreting cells (ASC) per 107 cells in mice immunized with AP regimes were quantified in the (D) bone marrow and (E) spleen ten weeks after the last immunization in response to MSP-119 protein. * p<0.05 by Kruskal-Wallis test with Dunn’s multiple comparison post-test. Median responses are shown. (F) IgG titers two weeks after the last immunization were correlated with antibody secreting cells in the spleen (■) and bone marrow(•) to MSP-119 protein for AP regimes. Spearman’s rank correlation co-efficient is shown. The dotted line indicates the threshold for responses above background in (A) and (B). ND = no data.
The levels of plasma cells (antibody secreting cells, ASCs) in the spleen and bone marrow were also investigated to determine if there is a correlation between antibody titers and plasma cells as has been suggested previously (14, 45). In C57BL/6 mice immunized with OVA, ASCs were only detected above baseline in a few mice across different groups (data not shown). In contrast, strong MSP-119 specific ASC responses were detected in the bone marrow (Figure 4D) and spleens (Figure 4E) of BALB/c mice immunized with the AP regime ten weeks after the last immunization, with a trend towards stronger ASCs with Abisco®100. As expected ASC responses were stronger in the bone marrow where long-lived plasma cells are thought to be present in survival niches (46). These ASC levels significantly correlated with peak serum antibody levels as well as antibody levels at the later time point (Figure 4F) as previously reported for this antigen (14). ASC levels were not explored in PPP vaccinated mice because antibody responses were only detectable in the Montanide®ISA720 group for this antigen.
An adenoviral prime skews adjuvants towards the induction of cytophilic antibody isotypes
As adjuvants are known to skew the immune response towards either a Th1-type or Th2-type antibody response (dominated in mice by IgG2a or IgG1 respectively), and as it has previously been shown that viral vector containing regimes induce a more cytophilic antibody response that is maintained better over time (14, 47), the induction of IgG antibody isotypes by AP regimes was compared to PPP regimes. IgG isotype ELISAs were carried out using the serum of mice immunized with PPP or AP regimes with OVA, EBA-175 and MSP-1 vaccines two weeks after the final vaccination. Moderate IgG1 and IgG2a antibodies were induced across the different adjuvants in response to OVA (Figure 5A). The effect of regime on the log isotype ratio was significantas well as the effect of adjuvant (p<0.001, two-way ANOVAwith Bonferroni’s multiple comparison post-test), driven by TLR agonist containing adjuvants which induced a greater ratio of IgG2a:IgG1 in both regimes(Figure 5D). The induction of isotypes was also investigated for MSP-1 and EBA-175 vaccines where the same trend towards a greater induction of IgG2a antibodies was found with AP regimes (Figure 5B and C). Overall, the adjuvants induced comparable isotype responses to both antigens, and SE + TLR4 again induced a greater ratio of IgG2a:IgG1 (as seen in the OVA system) in response to EBA-175 PPP vaccination (Figure 5E). The effect of regime on the log isotype ratio was again significant for EBA-175 (p<0.001, two-way ANOVAwith Bonferroni’s multiple comparison post-test) indicating a skew towards cytophilic antibodies after AP immunization. This was not investigated for MSP-1 vaccines because only mice immunized with Montanide®ISA720 in a PPP regime had detectable antibody responses.
Figure 5. An adenoviral prime skews adjuvants towards the induction of cytophilic antibody isotypes.
BALB/c and C57BL/6 mice (n=5-6 / group) were immunized as described previously with AP and PPP regimes. IgG1 and IgG2a antibody responses were measured in the serum two weeks after the last immunization in response to (A) OVA, (B) EBA-175 and (C) MSP-119(QKNG allele) protein. Median and individualresponses are shown (A-C). IgG2a:IgG1 ratios were calculated for (D) OVA and (E) EBA-175 and log transformed. Mean responses are shown (D-E). The dotted line indicates the threshold for responses above background in (A). ND = no data.
The effect of dose and immunization interval on antibody responses
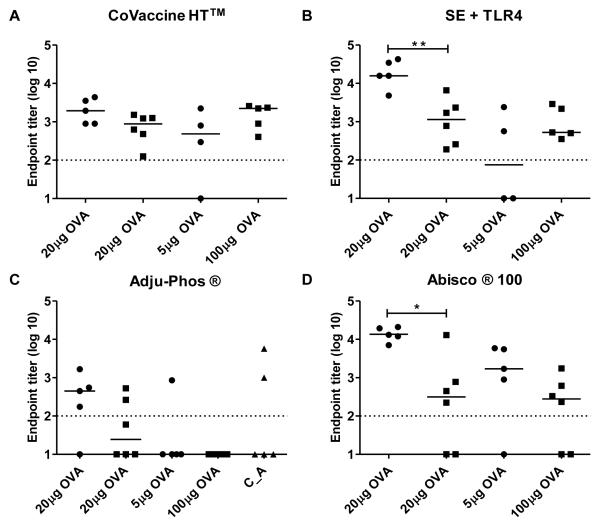
After showing that an adenoviral prime mediates improved boosting of IgG titers by protein-in-adjuvant vaccines, we next sought to address whether a difference in the dose of antigen exposed to the immune system (between protein vaccines and adenoviral vectors), and/or extended immunization intervals might be mediating this effect. In order to address this, C57BL/6 mice were immunized with either 5μg or 20μg of OVA in selected adjuvants at an interval of eight weeks, or immunized with 100μg of OVA two weeks apart. Serum antibody responses were assessed two weeks after the second immunization and compared to responses seen after two shots of 20μg of OVA (see Figure 1A). Antibody responses to OVA in PBS were negative at all doses and antibody responses pre-boost in mice receiving two vaccinations eight weeks apart were also negative (data not shown). For the adjuvants investigated, there was no enhancement of antibody responses with an increased dose of 100μg of OVA given two weeks apart (Figure 6A-D). There was a trend for an improvement of antibody responses using the standard 20μg dose with an extended immunization interval of eight weeks, although this was only significant for Abisco®100 and SE + TLR4&9 (p = 0.0091for Abisco®100 andp =0.0027 for SE + TLR4&9, t-test) (Figure 6B and D). This indicates that a prolonged time interval between immunizations, rather than dose of antigen, may improve antibody induction by a subset of adjuvants when used in protein only PPP regimes. However, these data are insufficient to explain why adjuvants such as Adju-Phos®(Figure 6C) were better able to boost IgG responses in the context of an AP immunization regime. Interestingly, however, priming mice with 20μg OVA in CoVaccine HT™ (a good primer of antibody responses in PPP regimes) followed by a boost 8 weeks later of 20μg of OVA formulated in Adju-Phos® did not result in improved antibody responses (Figure 6C). Overall, these data suggest that the improved boosting of IgG responses, seen with most adjuvants in the AP immunization regime, appears to be inherent to the adenoviral prime, rather than due to differences in immunization schedules and/or antigen dosing.
Figure 6. The effect of protein dose and immunization interval on antibody responses.
C57BL/6 mice (n=5 / group) were immunized twice i.m. with 5μg or 20μg of OVA protein eight weeks (•) apart or with 20μg or 100μg of OVA two weeks (■)apart. Protein was formulated in (A) CoVaccine HT™, (B) SE+TLR4, (C) Adju-Phos® and (D) Abisco®100.In (C) C57BL/6 mice were also immunized with 20μg of OVA in CoVaccine HT™ and boosted eight weeks later with 20μg of OVA in Adju-Phos® (C_A ▲). IgG titers were measured in the serum two weeks after the final immunization. * p=0.0091 and ** p=0.0027 by t-test. Median responses are shown. The dotted line indicates the threshold for responses above background.
AP immunization improves the efficacy of MSP-1 protein vaccines following P. yoelii blood-stage challenge
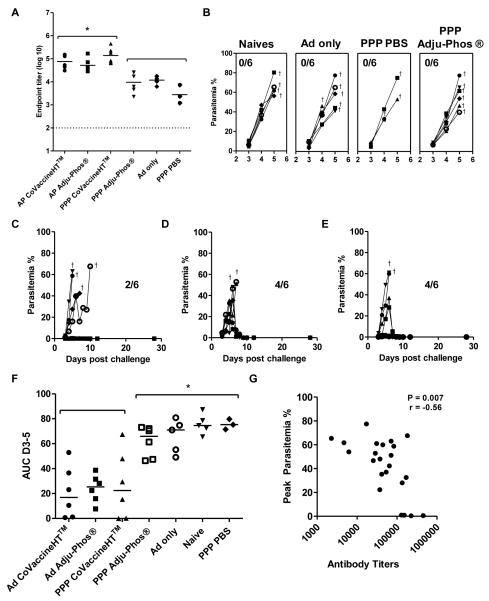
To investigate whether AP regimes could lead to enhanced vaccine efficacy, BALB/c mice were immunized with P.yoelii MSP-1 vaccines as outlined in Figure 7 and subsequently challenged with 105 P. yoelii pRBCs two weeks after the final immunization. The protein vaccines, used here at 1.5μg per dose, were formulated in CoVaccine HT™ and Adju-Phos® as these adjuvants induced very different antibody titers when screened in PPP regimes (Figure 1B). In agreement with the studies of other antigensat higher doses, at the time of challenge, IgG titers were significantly higher in mice immunized with AP regimes and the PPP CoVaccine HT™ regime compared to mice immunized with the PPP Adju-Phos® regime and control vaccines (p<0.05, one-way ANOVAwith Bonferroni’s multiple comparison post-test) (Figure 7A). Following challenge, all mice immunized with control vaccines or the PPP Adju-Phos® regime succumbed to infection (Figure 7B). Survival was seen in four out of six mice (67%) immunized with AP regimes and in two out of six mice (33%) immunized with the PPP CoVaccine HT™ regime (Figure 7C-E). There was a significant reduction in parasitemia, as measured by an area under the curve (AUC) analysis of parasitemia between days 3 and 5, in both AP regimes and the PPP CoVaccine HT™ regime as compared to the PPP Adju-Phos® regime and mice immunized with control vaccines/regimes (Figure 7F). Peak parasitemia was shown to significantlycorrelate with antigen specific totalIgG titers (Figure 7G). As seen with OVA, EBA-175 and P. falciparum MSP-119, more cytophilic IgG2a antibodies were again induced by AP regimes against P. yoelii MSP-119 which were also shown to correlate with protection, but not for IgG1 (Supplementary Figure 1A-C). Antibody avidity was also assessed at the time of challenge. There was a trend towards higher avidity in groups receiving an AP regime compared to a PPP regime, though this was only significant against PPP CoVaccine HT™(p>0.05 by Kruskal-Wallis test with Dunn’s multiple comparison post-test, Supplementary Figure 1D). There was no correlation between antibody avidity and total IgG titers(r=-0.3, p=0.1) or peak parasitemia (r=0.1, p=0.5). Overall thesedata indicate that the improved immunogenicity in terms of antibody responses due to the adenoviral prime can also lead to improved efficacy against a blood-stage challenge.
Figure 7. An adenoviral prime improves efficacy of MSP-1 protein vaccines following P. yoelii blood-stage challenge.
BALB/c mice (n= 3-6 / group) were immunized i.m. with either i) 1.5 μg of P. yoelii MSP-119-GSTprotein in Adju-Phos® or CoVaccine HT™or PBS three weeks apart (PPP); or ii) primed with 1×1010 vp of AdHu5 MSP-142 and either not boosted (Ad only) or boosted eight weeks later with 1.5 μg of P. yoelii MSP-1-GSTprotein in Adju-Phos® or CoVaccine HT™ 19 (AP). IgG titers were measured in the serum in response to (A) P. yoelii MSP-119-IMX108 protein two weeks after the final immunization (day before challenge). * p<0.05 by one-way ANOVA with Bonferroni’s multiple comparison post-test. Median responses are shown. Mice were challenged with 105 pRBCs i.v. and parasitemia was measured as the percentage of infected red blood cells over time. Results are shown in (B) for the naïve unimmunized, Ad only and PPP PBS control groups as well as the PPP Adju-Phos® group; (C) mice immunized with PPP in CoVaccine HT™; (D) mice immunized with AP Adju-Phos®; and (E) mice immunized with AP CoVaccine HT™. Crosses indicate when mice were sacrificed. (F) AUC analysis of parasitemia. * p<0.05 by one-way ANOVA with Bonferroni’s multiple comparison post-test. Median responses are shown. (G) IgG titers measured two weeks after the final immunization in each regime were correlated with percentage peak parasitemia. Spearman’s rank correlation is shown. The dotted line indicates the threshold for responses above background in (A).
AP regime efficacy following P. yoelii blood-stage challenge is not CD4+ T cell dependent
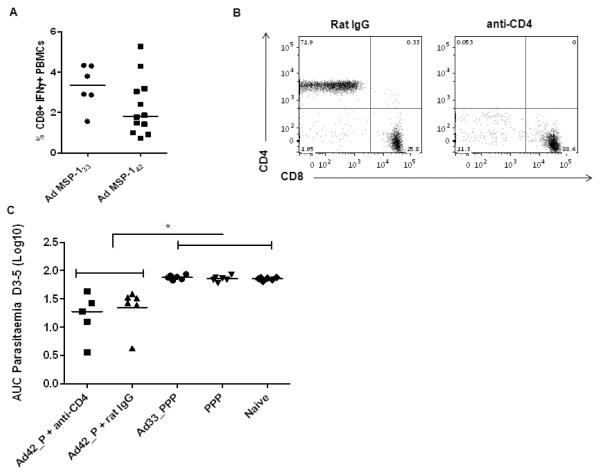
In agreement with previous data, we have shown that antibody responses against PyMSP119associate with protection against blood-stage malaria(12, 38). However, a role has also been shown for CD4+ T cells directed against MSP-133 which can aid the development of de novo anti-parasite antibody responses (11), as well as mediate some control of blood-stage parasitemia (48). To rule out that the improved efficacy of the AP regime was not due to CD4+ T cells directed against the PyMSP-133 fragment included in the AdHu5-PyMSP-142 vaccine, (but absent from the MSP-119 protein vaccine), mice were immunized with the regimes outlined in Figure 8. CD4+ T cells were depleted prior to pRBC challenge in mice receiving an AP Adju-Phos® regime to explore the effect of PyMSP-133 specific T cells in mediation of protection. In a separate group, mice were primed with an AdHu5 expressing PyMSP-133 (11) and boosted with the previously used PPP Adju-Phos® regime in order to determine whether the supplementation of T cell help (in the absence of priming protective PyMSP-119-specific antibodies) through the adenoviral prime could improve this regime. To confirm that the AdHu5 vectors expressing PyMSP-142 and PyMSP-133 induced comparable T cell responses, PBMC from the blood of immunized mice were phenotyped two weeks post-prime in response to an overlapping peptide pool of PyMSP-133. CD8+ IFN-γ+ T cell responses measured two weeks after the prime in response to both vectors were not significantly different (p>0.05, Mann-Whitney test) (Figure 8A) suggesting comparable T cell immunogenicity of the two vectors (as has been observed before following AdHu5-MVA immunization) (11). However, antigen-specific CD4+ T cell responses could not be detected after the single AdHu5 immunization, which was not surprising given these have been shown to be low even after the AdHu5-MVA prime-boost PyMSP-133 regime (11). The depletion of CD4+ T cells was assessed 24 hours post-challenge in the PBMC of depleted mice (Figure 8B) and was over 98% successful. An AUC analysis of parasitemia on days 3-5 revealed that the depletion of CD4+T cells in mice receiving an AP Adju-Phos® regime resulted in no significant difference in comparison to mice receiving the same regime and control rat IgG (p>0.05, Mann-Whitney test, Figure 8C). The same analysis also confirmed a significant difference in the AUC between the AP immunized mice versus mice receiving an AdHu5 MSP-133 priming immunization followed by a PPP Adju-Phos® regime, and the standard PPP Adju-Phos® regime (p<0.05, one-way ANOVA with Bonferroni’s multiple comparison post-test). Taken all together these data indicate that the improved efficacy observed in AP regimes compared to PPP regimes in this challenge system is not due to CD4+T cells that are primed against PyMSP-142 by the adenoviral vector.
Figure 8. Improved efficacy of AP regimes following P. yoelii blood-stage challenge is not CD4+ T cell dependent.
BALB/c mice (n = 6 / group) were immunized i.m. with either 1.5 μg of P. yoelii MSP-119-GSTprotein in Adju-Phos® three weeks apart (PPP), or primed with 1 × 1010 vp of AdHu5-PyMSP-142 (Ad42) and boosted eight weeks later with 1.5 μg of P. yoelii MSP-119-GSTprotein in Adju-Phos®. One group of mice immunized with the AP Adju-Phos® regime was depleted of CD4+ T cells, the other received normal rat IgG as a control. Separately BALB/c mice (n = 6 / group) were primed i.m. with 1 × 1010 vp of AdHu5-PyMSP-133 (Ad33) and boosted with three shots of 1.5 μg of P. yoelii MSP-119-GSTprotein in Adju-Phos® three weeks apart. All mice were challenged with 105 pRBCs i.v. two weeks after the final immunization and parasitemia was measured as the percentage of infected red blood cells over time. (A) The percentage of CD8+ IFN-γ+ T cells was measured by ICS in the PBMC of mice two weeks after the AdHu5 vaccines. Median responses are shown. (B) Representative flow plots from one depleted and control mouse showing the percentage of single and double CD3+ CD4+ and CD3+ CD8+ positive cells. (C) AUC analysis of parasitemia. * p<0.05 by one-way ANOVA with Bonferroni’s multiple comparison post-test. Median and individual responses are shown.
Discussion
The comparative assessment of new vaccine adjuvants remains essential for subunit vaccine development.A panel of 11 leading and accessible vaccine adjuvants (both pre-clinical and clinically tested/approvedfor clinical trial) has been assessed here in a PPP regime and compared to an AP regime for the induction of high and sustained antibody responses.
We have shown that novel adjuvants can induce potent antibody responses surpassing aluminum-based adjuvants and the classical reference adjuvant, Freund’s adjuvant, formulated with OVA in a PPP regime. Though most licensed adjuvants to date predominantly induced the humoral arm of the immune system, we have also shown that the SE + TLR4&9 emulsion induces very strong CD4+ and CD8+ T cell responses with the soluble antigen OVA, as has been shown for TLR agonists coupled to other antigens (49, 50). Interestingly, the differential immunogenicity of IgG titers induced by adjuvants in PPP regimes was greatly reduced when an AdHu5 vector was used to prime antibody responses, although some adjuvants still boosted adenovirus-primed responses more efficiently than others. Importantly, this effect was observed for aluminum-based adjuvants such as Adju-Phos®, which performed poorly in a PPP regime, but which boosted antibody responses primed by an AdHu5 vector as efficiently as the potent adjuvant CoVaccine HT™. Recently, a similar trend was reported when rhesus macaques primed with the simian adenovirus ChAd63 vector expressing the blood-stage malaria apical membrane antigen (AMA)-1 were boosted either with AMA-1 protein formulated in Alhydrogel® or CoVaccine HT™. The use of ChAd63 in this study also suggests that the results here with AdHu5 could potentially be extended to other adenovirus vectors, given the concerns surrounding pre-existing immunity to AdHu5 in humans and its use as a clinical vaccine vector(10). AdHu5 was chosen as a model adenovirus in this study as it has no intellectual property restrictions and induces immunogenicity comparableto simian adenoviruses in pre-clinical models (16, 17).
The improved immunogenicity of adjuvants following an adenoviral prime was also extended to P. falciparum MSP-1 and EBA-175 vaccines in a different strain of mouse, indicating that this effect can be translated to other antigens and is unlikely to be strain-specific. It is possible that the absence of antibody responses to P. falciparum MSP-119 with adjuvants other than with Montanide®ISA720 in a PPP regime could be due to the fact that MSP-119 has been shown to be refractory to antigen processing (51). The observation that thiscan be overcome by Montanide®ISA720 warrants further investigation.
The two vaccine delivery platforms used in this study are inherently different and it is possible that the increased immunogenicity of protein vaccine adjuvants seen following an adenoviral prime is due to an adenoviral vector producing more antigenin vivo at the time of priming than a given dose of protein vaccine. This “dose effect” could prime a quantitatively greater memory B cell response that can be subsequently boosted more effectively. Alternatively, adenoviral vaccines may inherently prime a better quality of memory B cell response, likely related to the profile of innate sensors stimulated by adenoviruses (52, 53), that can be more effectively boosted. Investigations into the amount of antigen produced by viral vectors are limited, although a study by Geiben-Lynn et al. utilizing in vivo imagining of a recombinant AdHu5 expressing luciferase found 100μg of luciferase was expressed one day after an i.m. injection of 1×1010 vp AdHu5 in BALB/c mice (the dose of AdHu5-OVA used here) (54). Dosing studies with OVA in adjuvant however revealed that there was no significant enhancement of antibody priming after giving 100μg of OVA versus 20μg at two week intervals, indicating that it is unlikely that the antigen dose accounted for the better priming ability of adenoviral vectors. Interestingly, an effect was seen in terms of the immunization interval (two weeks versus eight) for Abisco®100 and SE + TLR 4&9 (but not Adju-Phos® or CoVaccine HT™), where a prolonged interval led to an enhancement of responses. This effect has previously been reported for immunizations with viral vectored vaccines and indicates that prolonged prime-boost intervals may allow for optimal development of memory B cells (12, 55). However, priming mice with the potent adjuvant CoVaccine HT™ and boosting mice eight weeks later with Adju-Phos® did not lead to increased IgG responses, which suggests that the priming effect is inherent to the adenovirus vector and that the prime-boost interval alone in the AP regime is unlikely to account for the improved boosting effect seen with most adjuvants.In agreement with the data here, no significant differences were also observed in a Phase Ia clinical trial of an AMA1 vaccine formulated in Alhydrogel + CpG7909 using a 4 week versus 8 week immunization interval (56). It would thus appear that adenoviral primed antibody responses are more easily boosted, which has been suggested previously (14, 15).
The induction of antibody isotypes by vaccine adjuvants was also explored in this study. Th-2 type responses (dominated by IgG1) are thought to function through neutralizing antibodies whereas Th-1 type responses (dominated by IgG2a) are thought to activate complement and function through Fc receptors leading to antibody dependent cellular inhibition (ADCI) as well as phagocytosis. We have found that a more Th-1 type antibody response is induced following an AP regime in comparison to a PPP regime, in agreement with previous data (14). Adjuvants containing TLR 4 agonists were also able to induce a more Th-1 type antibody response in PPP regimes as has been previously shown (49, 57, 58). Surprisingly CoVaccine HT™ did not induce a skewed Th-1 response, despite evidence showing that some of its action is dependent on TLR 4 (59). TLR agonist containing adjuvants also induced relatively strong T cell responses in the PPP OVA regime which may account for the isotype switch as seen in T-dependent antibody responses. The improved induction of CD4+ T cell help (essential for T-dependent antibody responses(60, 61)) by the adenovirus in the AP regimes could also be another explanation for improved B cell priming. A study by Galli et al. has recently demonstrated that the induction of CD4+ T cells following an adjuvanted influenza vaccine predicted the persistence of antibody responses, highlighting a potential link between these two cell types (62).However, data using OVA-specific transgenic CD4+ T cells has indicated that transferred transgenic T cells can only help antigen-experienced (and not naïve) B cells and that the size of the secondary antibody response is restricted by the amount of T cells present at the time of antigen re-exposure (63). Further studies will thus be necessary to confirm whether the induction of better cellular immunity at the time of B cell priming is an important contributing factor.
Following immunization with either the AP or PPP regimes, there appeared to be no difference in the rate of decline of IgG titers as has previously been suggested (14). However, a difference in the rate of decline has been suggested for some IgG isotypes, with IgG2a being shown to be better maintained over time compared to IgG1 (14, 64). It would be interesting to investigate whether this phenomenon is also evident when comparing the different vaccination regimes and adjuvants used here. However, total IgG titers were not evidently better maintained over time in the case of AP regimes, despite the enhanced cytophilic IgG2a antibody response. For P. falciparum MSP-119, we also found a correlation of IgG titers with ASC levels in the spleen and bone marrow for the peak and late time-points assayed, as has previously been shown for this antigen(14). This implies that serum antibody titers may be maintained by long-lived plasma cells (LLPCs), as has been suggested for other antigens(65-67). However, though not explored in this study, serum antibody titers have also been shown to correlate with memory B cell levels, as measured by a cultured ELISPOT assay, for some acute infections and vaccines(65, 68, 69). This suggests that the maintenance of serological antibody titers may be under differential control by these two cell populations. As virally vectored vaccination regimes, as well as AP regimes, have been shown to induce memory B cells (15), the contribution of this cell type to antibody responses and their boostability in AP regimes warrants further investigation.
The AP regime was also shown to lead to improved antibody responses and protection in the P. yoelii model utilizing vaccines formulated with CoVaccine HT™ and Adju-Phos®,and this was not associated with a protective contribution from adenoviral-induced PyMSP1-specific CD4+ T cells. Total IgG and IgG2a titers, but not IgG1 or avidity,were shownto correlate with protection, as has been reported previously for P. yoeliiblood-stage infection(38, 70).It remains of interest to explore whether the induction of a more cytophilic antibody response may account for the increase in protection seen with the AP regime over a PPP regime, although the contribution of different isotypes to protection in this model system is disputed (70, 71).
In summary we have shown that novel emulsion based adjuvants as well as adjuvants containing TLR agonists can induce both strong humoral and cellular immune responses in a classical subunit vaccination approach. More importantly, we have found that the differential immunogenicity of these protein vaccine adjuvants can be largelyovercome through an adenoviral priming immunization. This approach could therefore enhance the clinical immunogenicity and utility of adjuvants that are traditionally considered poorly immunogenic, and circumvent the need for more potent and experimental chemical adjuvants that are currently required to deliver candidate protein vaccines for difficult diseases such as blood-stage malaria.
Supplementary Material
Acknowledgements
We would like to thank S. Biswas, A. Goodman, M. Dicks, A. Spencer, F. Pearson, J. Furze and the Jenner Institute Vector Core Facility for assistance, the Jenner Institute Adjuvant Bank for the provision of adjuvants, as well as F. Hill for the provision ofprotein. We would also like to thank S. Reed and D. Carter from the Infectious Disease Research Institute (IDRI) for the provision of TLR agonist containing SE adjuvants as well as BTG International Group Company for the provision of CoVaccine HT™.
SCdC is a PhD student funded by the EMVDA (European Malaria Vaccine Development Association, a European Commission FP6-funded consortium) [LSHP-CT-2007-037506]. This work was also partly funded by the Wellcome Trust [084113/Z/07/Z], the NIHR Oxford Biomedical Research Centre and TRANSVAC, an EC FP7 infrastructure grant and grants to CEC and VSC from the Department of Biotechnology (DBT), Government of India (GoI) and European Vaccine Initiative (EVI). CEC is supported by a Tata Innovation Fellowship from the Department of Biotechnology, Government of India. AVSH was supported by a Wellcome Trust Principal Research Fellowship. SCG, AVSH and SJD are Jenner Investigators, and SJD is a MRC Career Development Fellow [G1000527].
Abbreviations
- OVA
Ovalbumin
- MSP1
merozoite surface protein 1
- MVA
modified vaccinia virus Ankara
- AdHu5
human adenovirus serotype 5
- ICS
intracellular cytokine staining
- ASC
antibody-secreting cell
- EBA
Erythrocyte binding antigen
- AMA1
apical membrane antigen 1
- ADCI
antibody dependent cellular inhibition
Footnotes
Competing Interests Statement SCdC, EF, ADD, AM, SCG, AVSH and SJD are named inventors on patent applications covering malaria vectored vaccines and immunization regimes. The authors confirm that they have no financial connections with the adjuvant providers.
References
- 1.WHO; 2009. World Health Statistics 2009. [Google Scholar]
- 2.Goodman AL, Draper SJ. Blood-stage malaria vaccines - recent progress and future challenges. Ann Trop Med Parasitol. 2010;104:189–211. doi: 10.1179/136485910X12647085215534. [DOI] [PubMed] [Google Scholar]
- 3.Roestenberg M, Remarque E, de Jonge E, Hermsen R, Blythman H, Leroy O, Imoukhuede E, Jepsen S, Ofori-Anyinam O, Faber B, Kocken CH, Arnold M, Walraven V, Teelen K, Roeffen W, de Mast Q, Ballou WR, Cohen J, Dubois MC, Ascarateil S, van der Ven A, Thomas A, Sauerwein R. Safety and immunogenicity of a recombinant Plasmodium falciparum AMA1 malaria vaccine adjuvanted with Alhydrogel, Montanide ISA 720 or AS02. PLoS One. 2008;3:e3960. doi: 10.1371/journal.pone.0003960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schubert C. Boosting our best shot. Nat Med. 2009;15:984–988. doi: 10.1038/nm0909-984. [DOI] [PubMed] [Google Scholar]
- 5.Wu Y, Ellis RD, Shaffer D, Fontes E, Malkin EM, Mahanty S, Fay MP, Narum D, Rausch K, Miles AP, Aebig J, Orcutt A, Muratova O, Song G, Lambert L, Zhu D, Miura K, Long C, Saul A, Miller LH, Durbin AP. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS One. 2008;3:e2636. doi: 10.1371/journal.pone.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mbow ML, De Gregorio E, Valiante NM, Rappuoli R. New adjuvants for human vaccines. Curr Opin Immunol. 2010;22:411–416. doi: 10.1016/j.coi.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Hafalla JC, Cockburn IA, Zavala F. Protective and pathogenic roles of CD8+ T cells during malaria infection. Parasite Immunol. 2006;28:15–24. doi: 10.1111/j.1365-3024.2006.00777.x. [DOI] [PubMed] [Google Scholar]
- 8.Caruso AM, Serbina N, Klein E, Triebold K, Bloom BR, Flynn JL. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J Immunol. 1999;162:5407–5416. [PubMed] [Google Scholar]
- 9.Reed SG, Bertholet S, Coler RN, Friede M. New horizons in adjuvants for vaccine development. Trends Immunol. 2009;30:23–32. doi: 10.1016/j.it.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Draper SJ, Heeney JL. Viruses as vaccine vectors for infectious diseases and cancer. Nature reviews. Microbiology. 2010;8:62–73. doi: 10.1038/nrmicro2240. [DOI] [PubMed] [Google Scholar]
- 11.Draper SJ, Goodman AL, Biswas S, Forbes EK, Moore AC, Gilbert SC, Hill AV. Recombinant viral vaccines expressing merozoite surface protein-1 induce antibody- and T cell-mediated multistage protection against malaria. Cell Host Microbe. 2009;5:95–105. doi: 10.1016/j.chom.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Draper SJ, Moore AC, Goodman AL, Long CA, Holder AA, Gilbert SC, Hill F, Hill AV. Effective induction of high-titer antibodies by viral vector vaccines. Nat Med. 2008;14:819–821. doi: 10.1038/nm.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capone S, Reyes-Sandoval A, Naddeo M, Siani L, Ammendola V, Rollier CS, Nicosia A, Colloca S, Cortese R, Folgori A, Hill AV. Immune responses against a liver-stage malaria antigen induced by simian adenoviral vector AdCh63 and MVA prime-boost immunisation in non-human primates. Vaccine. 2010;29:256–265. doi: 10.1016/j.vaccine.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 14.Douglas AD, de Cassan SC, Dicks MD, Gilbert SC, Hill AV, Draper SJ. Tailoring subunit vaccine immunogenicity: maximizing antibody and T cell responses by using combinations of adenovirus, poxvirus and protein-adjuvant vaccines against Plasmodium falciparum MSP1. Vaccine. 2010;28:7167–7178. doi: 10.1016/j.vaccine.2010.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Draper SJ, Biswas S, Spencer AJ, Remarque EJ, Capone S, M. N, Dicks MDJ, Faber BW, Folgori A, Nicosia A, Gilbert SC, Hill AVS. Enhancing Blood-Stage Malaria Subunit Vaccine Immunogenicity in Rhesus Macaques by Combining Adenovirus, Poxvirus and Protein-in Adjuvant Vaccines. Journal of Immunology. 2010;185:7583–7595. doi: 10.4049/jimmunol.1001760. [DOI] [PubMed] [Google Scholar]
- 16.Goodman AL, Epp C, Moss D, Holder AA, Wilson JM, Gao C, Long A, Remarque EJ, Thomas AW, Ammendola V, Colloca S, Dicks MDJ, Biswas S, Seibel D, Van Duivenvoorde LM, Gilbert SC, Hill AVS, Draper SJ. New candidate vaccines against blood-stage P. falciparum malaria: Prime-boost immunization regimes incorporating human and simian adenoviral vectors and poxviral vectors expressing an optimized antigen based on MSP-1. Infection and Immunity. 2010;78:4601–4612. doi: 10.1128/IAI.00315-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reyes-Sandoval A, Berthoud T, Alder N, Siani L, Gilbert SC, Nicosia A, Colloca S, Cortese R, Hill AV. Prime-boost immunization with adenoviral and modified vaccinia virus Ankara vectors enhances the durability and polyfunctionality of protective malaria CD8+ T-cell responses. Infection Immunity. 2010;78:145–153. doi: 10.1128/IAI.00740-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biswas S, Dicks MDJ, Long CA, Remarque EJ, Siani L, Colloca S, Cottingham MG, Holder AA, Gilbert SC, Hill AVS, Draper SJ. Transgene Optimization, Immunogenicity and In Vitro Efficacy of Viral Vectored Vaccines Expressing Two Alleles of Plasmodium falciparum AMA1. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stewart VA, McGrath SM, Dubois PM, Pau MG, Mettens P, Shott J, Cobb M, Burge JR, Larson D, Ware LA, Demoitie MA, Weverling GJ, Bayat B, Custers JH, Dubois MC, Cohen J, Goudsmit J, Heppner DG., Jr. Priming with an adenovirus 35-circumsporozoite protein (CS) vaccine followed by RTS,S/AS01B boosting significantly improves immunogenicity to Plasmodium falciparum CS compared to that with either malaria vaccine alone. Infect Immun. 2007;75:2283–2290. doi: 10.1128/IAI.01879-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vinner L, Therrien D, Wee E, Laursen I, Hanke T, Corbet SL, Fomsgaard A. Immune response in rhesus macaques after mixed modality immunisations with DNA, recombinant adenovirus and recombinant gp120 from human immunodeficiency virus type 1. APMIS. 2006;114:690–699. doi: 10.1111/j.1600-0463.2006.apm_395.x. [DOI] [PubMed] [Google Scholar]
- 21.El Sahly HM, Patel SM, Atmar RL, Lanford TA, Dube T, Thompson D, Sim BK, Long C, Keitel WA. Safety and immunogenicity of a recombinant nonglycosylated erythrocyte binding antigen 175 Region II malaria vaccine in healthy adults living in an area where malaria is not endemic. Clin Vaccine Immunol. 2010;17:1552–1559. doi: 10.1128/CVI.00082-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellis RD, Martin LB, Shaffer D, Long CA, Miura K, Fay MP, Narum DL, Zhu D, Mullen GE, Mahanty S, Miller LH, Durbin AP. Phase 1 trial of the Plasmodium falciparum blood stage vaccine MSP1(42)-C1/Alhydrogel with and without CPG 7909 in malaria naive adults. PLoS One. 2010;5:e8787. doi: 10.1371/journal.pone.0008787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Douglas A, Williams A, Illingworth J, Kamuyu G, Biswas S, Goodman A, Wyllie DH, Crosnier C, Wright G, Osier F, Marsh K, Turner A, Hill AV, Draper SJ. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 24.Goodman AL, Blagborough AM, Biswas S, Wu Y, Hill AVS, Sinden RE, Draper SJ. Submitted for publication.
- 25.Suzuki T, Kitajima K, Emori Y, Inoue Y, Inoue S. Site-specific de-N-glycosylation of diglycosylated ovalbumin in hen oviduct by endogenous peptide: N-glycanase as a quality control system for newly synthesized proteins. Proc Natl Acad Sci U S A. 1997;94:6244–6249. doi: 10.1073/pnas.94.12.6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pattnaik P, Shakri AR, Singh S, Goel S, Mukherjee P, Chitnis CE. Immunogenicity of a recombinant malaria vaccine based on receptor binding domain of Plasmodium falciparum EBA-175. Vaccine. 2007;25:806–813. doi: 10.1016/j.vaccine.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 27.Parween S, Gupta PK, Chauhan VS. Induction of humoral immune response against PfMSP-1(19) and PvMSP-1(19) using gold nanoparticles along with alum. Vaccine. 2011;29:2451–2460. doi: 10.1016/j.vaccine.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 28.Yu H, Jiang X, Shen C, Karunakaran KP, Jiang J, Rosin NL, Brunham RC. Chlamydia muridarum T-cell antigens formulated with the adjuvant DDA/TDB induce immunity against infection that correlates with a high frequency of gamma interferon (IFN-gamma)/tumor necrosis factor alpha and IFN-gamma/interleukin-17 double-positive CD4+ T cells. Infect Immun. 2010;78:2272–2282. doi: 10.1128/IAI.01374-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hilgers LA, Blom AG. Sucrose fatty acid sulphate esters as novel vaccine adjuvant. Vaccine. 2006;24(Suppl 2):S2-81–82. doi: 10.1016/j.vaccine.2005.01.133. [DOI] [PubMed] [Google Scholar]
- 30.Coler RN, Bertholet S, Moutaftsi M, Guderian JA, Windish HP, Baldwin SL, Laughlin EM, Duthie MS, Fox CB, Carter D, Friede M, Vedvick TS, Reed SG. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PLoS One. 2011;6:e16333. doi: 10.1371/journal.pone.0016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogun SA, Dumon-Seignovert L, Marchand JB, Holder AA, Hill F. The oligomerization domain of C4-binding protein (C4bp) acts as an adjuvant, and the fusion protein comprised of the 19-kilodalton merozoite surface protein 1 fused with the murine C4bp domain protects mice against malaria. Infect Immun. 2008;76:3817–3823. doi: 10.1128/IAI.01369-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miura K, Orcutt AC, Muratova OV, Miller LH, Saul A, Long CA. Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria vaccines. Vaccine. 2008;26:193–200. doi: 10.1016/j.vaccine.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore AC, Gallimore A, Draper SJ, Watkins KR, Gilbert SC, Hill AV. Anti-CD25 antibody enhancement of vaccine-induced immunogenicity: increased durable cellular immunity with reduced immunodominance. J Immunol. 2005;175:7264–7273. doi: 10.4049/jimmunol.175.11.7264. [DOI] [PubMed] [Google Scholar]
- 34.Oran AE, Robinson HL. DNA vaccines, combining form of antigen and method of delivery to raise a spectrum of IFN-gamma and IL-4-producing CD4+ and CD8+ T cells. J Immunol. 2003;171:1999–2005. doi: 10.4049/jimmunol.171.4.1999. [DOI] [PubMed] [Google Scholar]
- 35.Maecker HT, Umetsu DT, DeKruyff RH, Levy S. Cytotoxic T cell responses to DNA vaccination: dependence on antigen presentation via class II MHC. J Immunol. 1998;161:6532–6536. [PubMed] [Google Scholar]
- 36.Slifka MK, Ahmed R. Limiting dilution analysis of virus-specific memory B cells by an ELISPOT assay. J Immunol Methods. 1996;199:37–46. doi: 10.1016/s0022-1759(96)00146-9. [DOI] [PubMed] [Google Scholar]
- 37.Agger EM, Rosenkrands I, Hansen J, Brahimi K, Vandahl BS, Aagaard C, Werninghaus K, Kirschning C, Lang R, Christensen D, Theisen M, Follmann F, Andersen P. Cationic liposomes formulated with synthetic mycobacterial cordfactor (CAF01): a versatile adjuvant for vaccines with different immunological requirements. PLoS One. 2008;3:e3116. doi: 10.1371/journal.pone.0003116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirunpetcharat C, Tian JH, Kaslow DC, van Rooijen N, Kumar S, Berzofsky JA, Miller LH, Good MF. Complete protective immunity induced in mice by immunization with the 19-kilodalton carboxyl-terminal fragment of the merozoite surface protein-1 (MSP1[19]) of Plasmodium yoelii expressed in Saccharomyces cerevisiae: correlation of protection with antigen-specific antibody titer, but not with effector CD4+ T cells. J Immunol. 1997;159:3400–3411. [PubMed] [Google Scholar]
- 39.Ling IT, Ogun SA, Momin P, Richards RL, Garcon N, Cohen J, Ballou WR, Holder AA. Immunization against the murine malaria parasite Plasmodium yoelii using a recombinant protein with adjuvants developed for clinical use. Vaccine. 1997;15:1562–1567. doi: 10.1016/s0264-410x(97)00076-5. [DOI] [PubMed] [Google Scholar]
- 40.Vordermeier HM, Dean GS, Rosenkrands I, Agger EM, Andersen P, Kaveh DA, Hewinson RG, Hogarth PJ. Adjuvants induce distinct immunological phenotypes in a bovine tuberculosis vaccine model. Clin Vaccine Immunol. 2009;16:1443–1448. doi: 10.1128/CVI.00229-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holder AA, Sandhu JS, Hillman Y, Davey LS, Nicholls SC, Cooper H, Lockyer MJ. Processing of the precursor to the major merozoite surface antigens of Plasmodium falciparum. Parasitology. 1987;94(Pt 2):199–208. doi: 10.1017/s0031182000053889. [DOI] [PubMed] [Google Scholar]
- 42.Ahlborg N, Ling IT, Howard W, Holder AA, Riley EM. Protective immune responses to the 42-kilodalton (kDa) region of Plasmodium yoelii merozoite surface protein 1 are induced by the C-terminal 19-kDa region but not by the adjacent 33-kDa region. Infect Immun. 2002;70:820–825. doi: 10.1128/IAI.70.2.820-825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawabata Y, Udono H, Honma K, Ueda M, Mukae H, Kadota J, Kohno S, Yui K. Merozoite surface protein 1-specific immune response is protective against exoerythrocytic forms of Plasmodium yoelii. Infect Immun. 2002;70:6075–6082. doi: 10.1128/IAI.70.11.6075-6082.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Camus D, Hadley TJ. A Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. Science. 1985;230:553–556. doi: 10.1126/science.3901257. [DOI] [PubMed] [Google Scholar]
- 45.Ndungu FM, Cadman ET, Coulcher J, Nduati E, Couper E, Macdonald DW, Ng D, Langhorne J. Functional memory B cells and long-lived plasma cells are generated after a single Plasmodium chabaudi infection in mice. PLoS Pathog. 2009;5:e1000690. doi: 10.1371/journal.ppat.1000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fairfax KA, Kallies A, Nutt SL, Tarlinton DM. Plasma cell development: from B-cell subsets to long-term survival niches. Semin Immunol. 2008;20:49–58. doi: 10.1016/j.smim.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 47.Xiang Z, Gao G, Reyes-Sandoval A, Cohen CJ, Li Y, Bergelson JM, Wilson JM, Ertl HC. Novel, chimpanzee serotype 68-based adenoviral vaccine carrier for induction of antibodies to a transgene product. J Virol. 2002;76:2667–2675. doi: 10.1128/JVI.76.6.2667-2675.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wipasa J, Hirunpetcharat C, Mahakunkijcharoen Y, Xu H, Elliott S, Good MF. Identification of T cell epitopes on the 33-kDa fragment of Plasmodium yoelii merozoite surface protein 1 and their antibody-independent protective role in immunity to blood stage malaria. J Immunol. 2002;169:944–951. doi: 10.4049/jimmunol.169.2.944. [DOI] [PubMed] [Google Scholar]
- 49.Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, Kielland A, Vosters O, Vanderheyde N, Schiavetti F, Larocque D, Van Mechelen M, Garcon N. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol. 2009;183:6186–6197. doi: 10.4049/jimmunol.0901474. [DOI] [PubMed] [Google Scholar]
- 50.Nordly P, Agger EM, Andersen P, Nielsen HM, Foged C. Incorporation of the TLR4 Agonist Monophosphoryl Lipid A Into the Bilayer of DDA/TDB Liposomes: Physico-Chemical Characterization and Induction of CD8(+) T-Cell Responses In Vivo. Pharm Res. doi: 10.1007/s11095-010-0301-9. [DOI] [PubMed] [Google Scholar]
- 51.Hensmann M, Li C, Moss C, Lindo V, Greer F, Watts C, Ogun SA, Holder AA, Langhorne J. Disulfide bonds in merozoite surface protein 1 of the malaria parasite impede efficient antigen processing and affect the in vivo antibody response. Eur J Immunol. 2004;34:639–648. doi: 10.1002/eji.200490004. [DOI] [PubMed] [Google Scholar]
- 52.Huang X, Yang Y. Innate immune recognition of viruses and viral vectors. Hum Gene Ther. 2009;20:293–301. doi: 10.1089/hum.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Q, Muruve DA. Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther. 2003;10:935–940. doi: 10.1038/sj.gt.3302036. [DOI] [PubMed] [Google Scholar]
- 54.Geiben-Lynn R, Greenland JR, Frimpong-Boateng K, Letvin NL. Kinetics of recombinant adenovirus type 5, vaccinia virus, modified vaccinia ankara virus, and DNA antigen expression in vivo and the induction of memory T-lymphocyte responses. Clin Vaccine Immunol. 2008;15:691–696. doi: 10.1128/CVI.00418-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brice GT, Dobano C, Sedegah M, Stefaniak M, Graber NL, Campo JJ, Carucci DJ, Doolan DL. Extended immunization intervals enhance the immunogenicity and protective efficacy of plasmid DNA vaccines. Microbes Infect. 2007;9:1439–1446. doi: 10.1016/j.micinf.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 56.Ellis RD, Mullen GE, Pierce M, Martin LB, Miura K, Fay MP, Long CA, Shaffer D, Saul A, Miller LH, Durbin AP. A Phase 1 study of the blood-stage malaria vaccine candidate AMA1-C1/Alhydrogel with CPG 7909, using two different formulations and dosing intervals. Vaccine. 2009;27:4104–4109. doi: 10.1016/j.vaccine.2009.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Korsholm KS, Petersen RV, Agger EM, Andersen P. T-helper 1 and T-helper 2 adjuvants induce distinct differences in the magnitude, quality and kinetics of the early inflammatory response at the site of injection. Immunology. 129:75–86. doi: 10.1111/j.1365-2567.2009.03164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, Villinger F, Murthy N, Steel J, Jacob J, Hogan RJ, Garcia-Sastre A, Compans R, Pulendran B. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bodewes R, Geelhoed-Mieras MM, Heldens JG, Glover J, Lambrecht BN, Fouchier RA, Osterhaus AD, Rimmelzwaan GF. The novel adjuvant CoVaccineHT increases the immunogenicity of cell-culture derived influenza A/H5N1 vaccine and induces the maturation of murine and human dendritic cells in vitro. Vaccine. 2009;27:6833–6839. doi: 10.1016/j.vaccine.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 60.MacLennan IC, Gulbranson-Judge A, Toellner KM, Casamayor-Palleja M, Chan E, Sze DM, Luther SA, Orbea HA. The changing preference of T and B cells for partners as T-dependent antibody responses develop. Immunol Rev. 1997;156:53–66. doi: 10.1111/j.1600-065x.1997.tb00958.x. [DOI] [PubMed] [Google Scholar]
- 61.Toellner KM, Luther SA, Sze DM, Choy RK, Taylor DR, MacLennan IC, Acha-Orbea H. T helper 1 (Th1) and Th2 characteristics start to develop during T cell priming and are associated with an immediate ability to induce immunoglobulin class switching. J Exp Med. 1998;187:1193–1204. doi: 10.1084/jem.187.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Galli G, Medini D, Borgogni E, Zedda L, Bardelli M, Malzone C, Nuti S, Tavarini S, Sammicheli C, Hilbert AK, Brauer V, Banzhoff A, Rappuoli R, Del Giudice G, Castellino F. Adjuvanted H5N1 vaccine induces early CD4+ T cell response that predicts long-term persistence of protective antibody levels. Proc Natl Acad Sci U S A. 2009;106:3877–3882. doi: 10.1073/pnas.0813390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duffy D, Yang CP, Heath A, Garside P, Bell EB. Naive T-cell receptor transgenic T cells help memory B cells produce antibody. Immunology. 2006;119:376–384. doi: 10.1111/j.1365-2567.2006.02446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jeamwattanalert P, Mahakunkijcharoen Y, Kittigul L, Mahannop P, Pichyangkul S, Hirunpetcharat C. Long-lasting protective immune response to the 19-kilodalton carboxy-terminal fragment of Plasmodium yoelii merozoite surface protein 1 in mice. Clin Vaccine Immunol. 2007;14:342–347. doi: 10.1128/CVI.00397-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 66.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 67.Slifka MK, Matloubian M, Ahmed R. Bone marrow is a major site of long-term antibody production after acute viral infection. J Virol. 1995;69:1895–1902. doi: 10.1128/jvi.69.3.1895-1902.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rohner G. Blanchard, Snape MD, Kelly DF, John T, Morant A, Yu LM, Borkowski A, Ceddia F, Borrow R, Siegrist CA, Pollard AJ. The magnitude of the antibody and memory B cell responses during priming with a protein-polysaccharide conjugate vaccine in human infants is associated with the persistence of antibody and the intensity of booster response. J Immunol. 2008;180:2165–2173. doi: 10.4049/jimmunol.180.4.2165. [DOI] [PubMed] [Google Scholar]
- 69.Crompton PD, Mircetic M, Weiss G, Baughman A, Huang CY, Topham DJ, Treanor JJ, Sanz I, Lee FE, Durbin AP, Miura K, Narum DL, Ellis RD, Malkin E, Mullen GE, Miller LH, Martin LB, Pierce SK. The TLR9 ligand CpG promotes the acquisition of Plasmodium falciparum-specific memory B cells in malaria-naive individuals. J Immunol. 2009;182:3318–3326. doi: 10.4049/jimmunol.0803596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.White WI, Evans CB, Taylor DW. Antimalarial antibodies of the immunoglobulin G2a isotype modulate parasitemias in mice infected with Plasmodium yoelii. Infect Immun. 1991;59:3547–3554. doi: 10.1128/iai.59.10.3547-3554.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rotman HL, Daly TM, Clynes R, Long CA. Fc receptors are not required for antibody-mediated protection against lethal malaria challenge in a mouse model. J Immunol. 1998;161:1908–1912. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.